Extracellular Vesicles from Hyperammonemic Rats Induce Neuroinflammation and Motor Incoordination in Control Rats
Abstract
1. Introduction
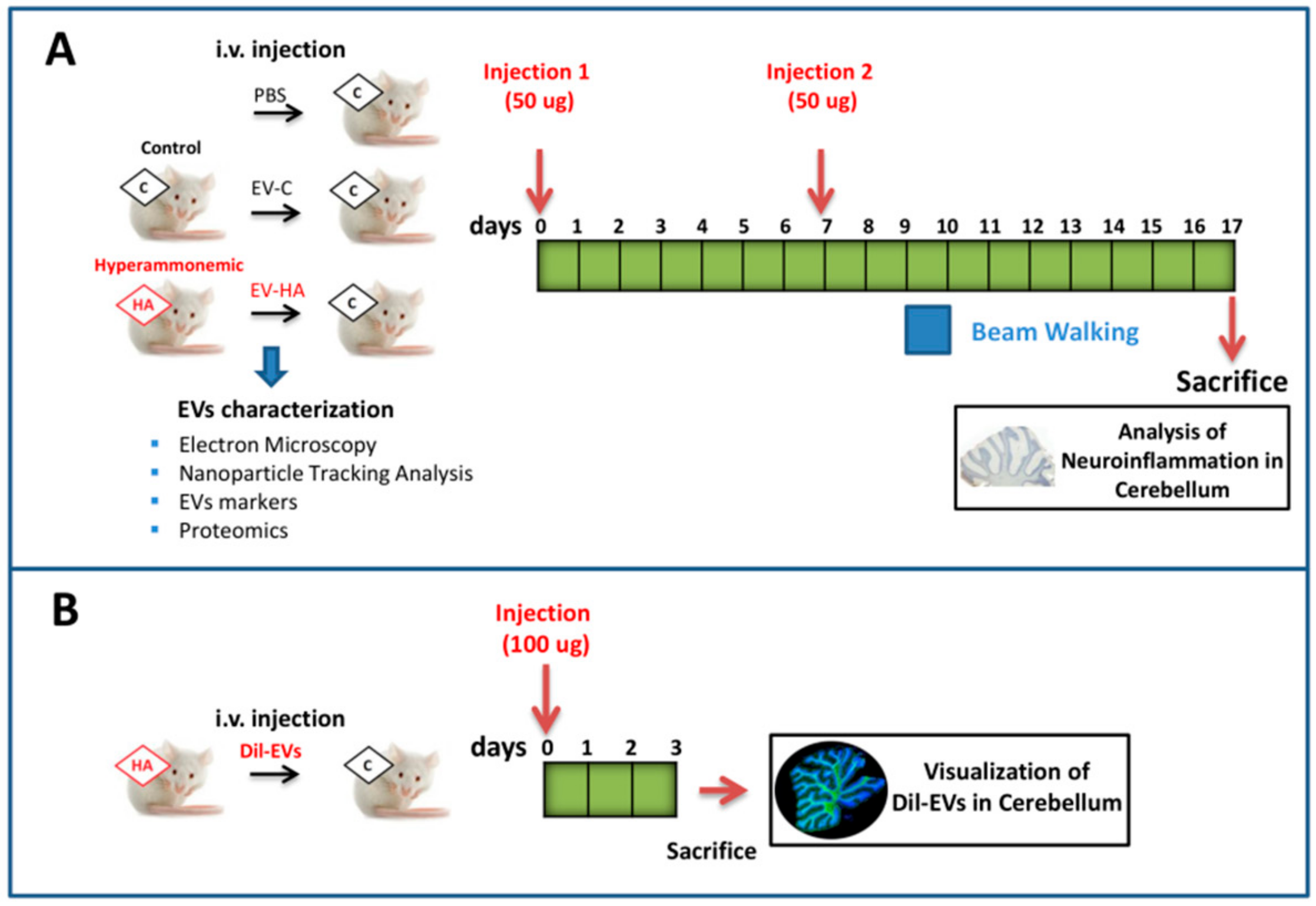
2. Materials and Methods
3. Results
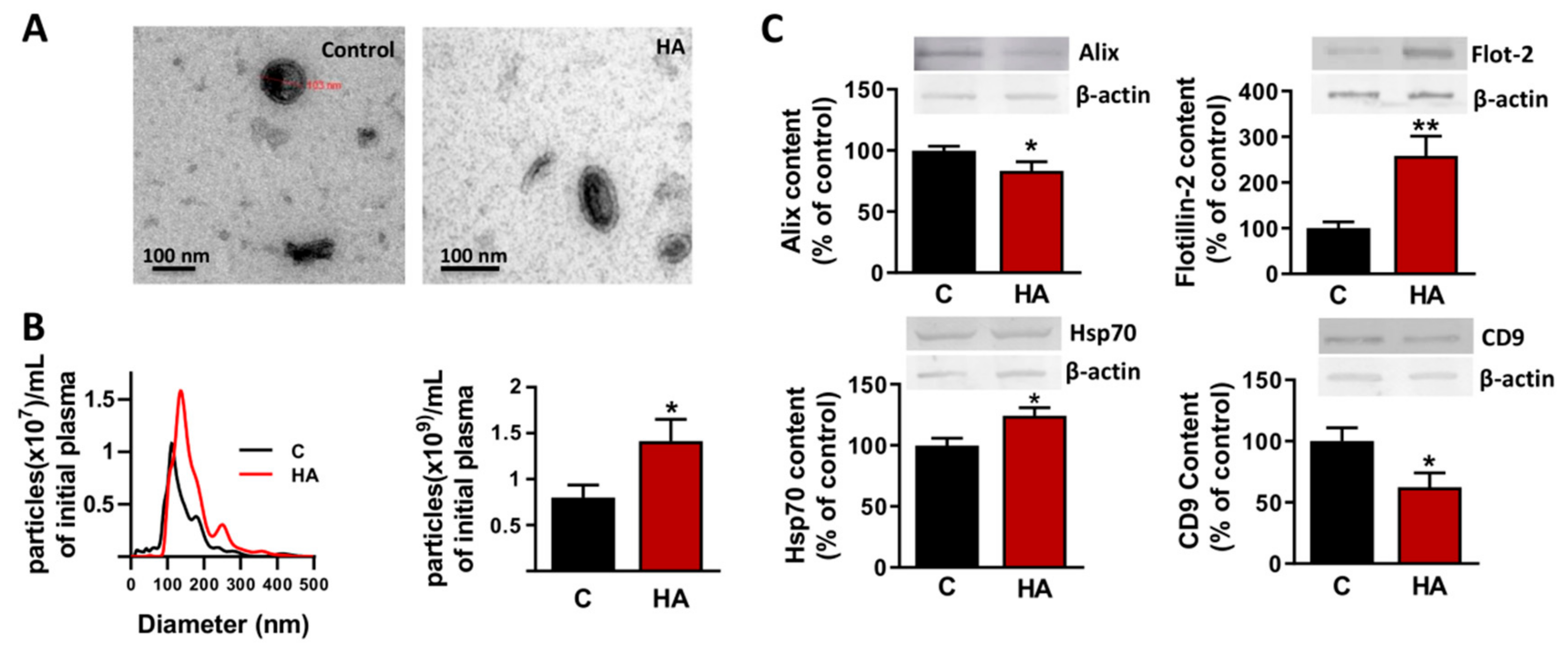
3.1. Chronic Hyperammonemia Alters the Amount and Protein Cargo of evs in Plasma
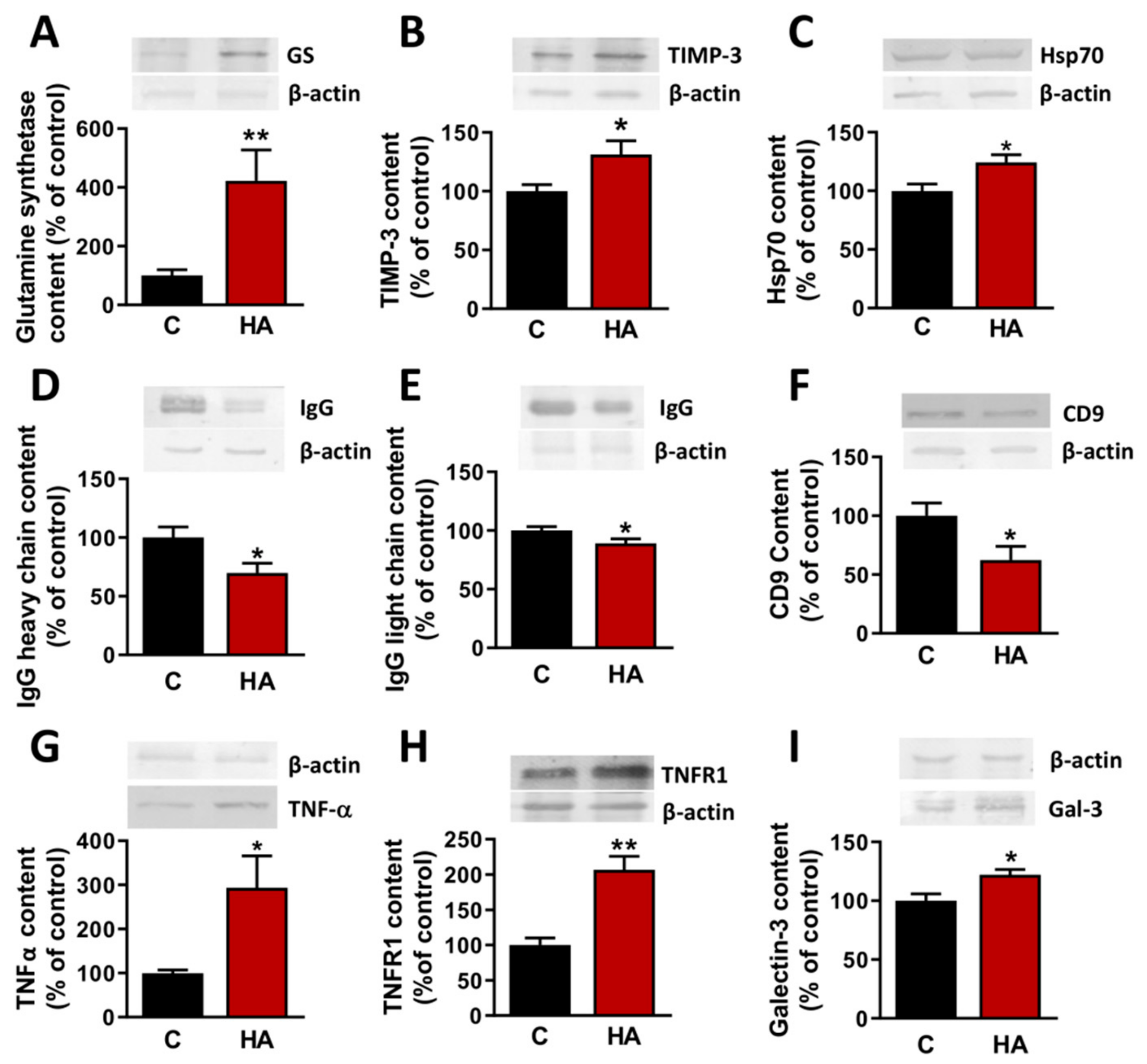
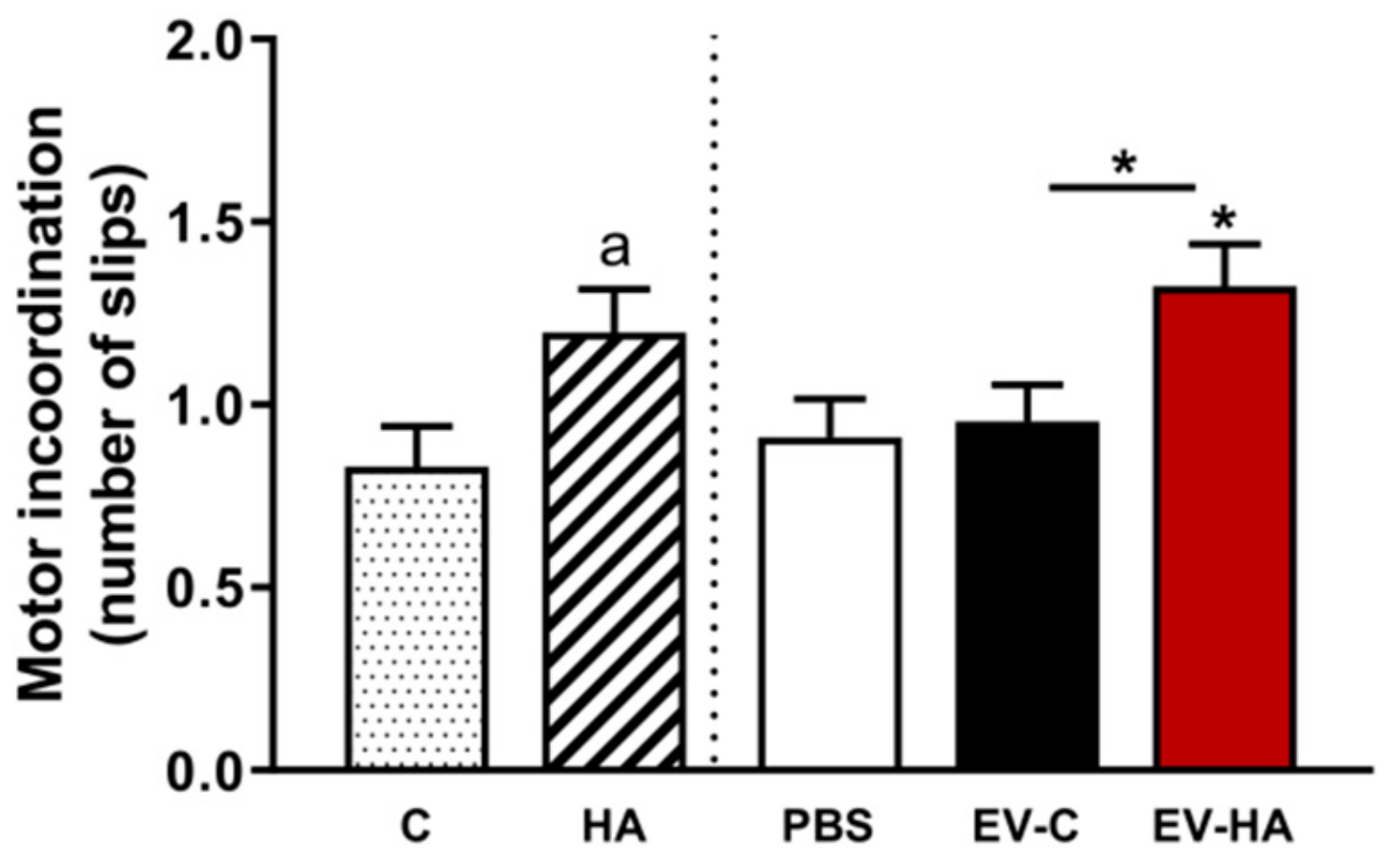
3.2. Injection of EVs from Hyperammonemic Rats Induces Neuroinflammation in Cerebellum in Control Rats
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shawcross, D.L.; Davies, N.A.; Williams, R.; Jalan, R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J. Hepatol. 2004, 40, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Shawcross, D.L.; Wright, G.; Olde Damink, S.W.; Jalan, R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab. Brain Dis. 2007, 22, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, C.; Piedrafita, B.; Serra, M.A.; Del Olmo, J.A.; Urios, A.; Rodrigo, J.M.; Felipo, V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 2009, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Urios, A.; Montesinos, E.; Molina, I.; Garcia-Torres, M.L.; Civera, M.; Del Olmo, J.A.; Ortega, J.; Martinez-Valls, J.; Serrano, J.O.; et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 2012, 27, 51–58. [Google Scholar] [CrossRef]
- Felipo, V. Hepatic encephalopathy: Effects of liver failure on brain function. Nat. Rev. Neurosci. 2013, 14, 851–858. [Google Scholar] [CrossRef]
- Dadsetan, S.; Balzano, T.; Forteza-Vila, J.; Agusti, A.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Hernandez-Rabaza, V.; Gomez-Gimenez, B.; ElMlili, N.; Llansola, M.; et al. Infliximab reduces peripheral inflammation, neuroinflammation, and extracellular GABA in the cerebellum and improves learning and motor coordination in rats with hepatic encephalopathy. J. Neuroinflammation 2016, 13, 245. [Google Scholar] [CrossRef]
- Dadsetan, S.; Balzano, T.; Forteza, J.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Hernandez-Rabaza, V.; Gil-Perotín, S.; Núñez, L.C.; García-Verdugo, J.-M.; Agusti, A.; et al. Reducing Peripheral Inflammation with Infliximab Reduces Neuroinflammation and Improves Cognition in Rats with Hepatic Encephalopathy. Front. Mol. Neurosci. 2016, 9, 106. [Google Scholar] [CrossRef]
- Mangas-Losada, A.; García-García, R.; Urios, A.; Escudero-García, D.; Tosca, J.; Giner-Durán, R.; Serra, M.A.; Montoliu, C.; Felipo, V. Minimal hepatic encephalopathy is associated with expansion and activation of CD4+CD28-, Th22 and Tfh and B lymphocytes. Sci. Rep. 2017, 7, 6683. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A.; Balzano, T.; Hernández-Rabaza, V.; Malaguarnera, M.; Llansola, M.; Felipo, V. Increasing extracellular cGMP in cerebellum in vivo reduces neuroinflammation, GABAergic tone and motor incoordination in hyperammonemic rats. Brain Behav. Immun. 2018, 69, 386–398. [Google Scholar] [CrossRef]
- Balzano, T.; Dadsetan, S.; Forteza, J.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Malaguarnera, M.; Gil-Perotin, S.; Núñez, L.C.; Casanova, B.; Castro-Quintas, A.; et al. Chronic hyperammonemia induces peripheral inflammation that leads to cognitive impairment in rats: Reversal by anti-tnfa treatment. J. Hepatol. 2019. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A.; Llansola, M.; Montoliu, C.; Malaguarnera, M.; Balzano, T.; Taoro-Gonzalez, L.; García-García, R.; Mangas-Losada, A.; Izquierdo-Altarejos, P.; Arenas, Y.M.; et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: Underlying mechanisms and therapeutic implications. Acta Physiol. 2019, 226, e13270. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A. Cognitive and motor perturbations in elderly with longstanding diabetes mellitus. Nutrition 2014, 30, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.; Selim, Z.I.; Elattar, A.M.; Elserogy, Y.M.; Ahmed, E.A.; Mohamed, H.O. Assessment of biocorrelates for brain involvement in female patients with rheumatoid arthritis. Clin. Rheumatol. 2012, 31, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Spyridaki, E.C.; Simos, P.G.; Avgoustinaki, P.D.; Dermitzaki, E.; Venihaki, M.; Bardos, A.N.; Margioris, A.N. The association between obesity and fluid intelligence impairment is mediated by chronic low-grade inflammation. Br. J. Nutr. 2014, 112, 1724–1734. [Google Scholar] [CrossRef]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef]
- Da Matta, S.M.; Janaina Matos, M.; Kummer, A.M.; Barbosa, I.G.; Teixeira, A.L.; Silva, A.C. Cognitive alterations in chronic kidney disease: An update. Braz. J. Nephrol. 2014, 36, 241–245. [Google Scholar] [CrossRef]
- Boyer, L.; Richieri, R.; Dassa, D.; Boucekine, M.; Fernandez, J.; Vaillant, F.; Padovani, R.; Auquier, P.; Lançon, C. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Res. 2013, 210, 381–386. [Google Scholar] [CrossRef]
- Garretti, F.; Agalliu, D.; Lindestam Arlehamn, C.S.; Sette, A.; Sulzer, D. Autoimmunity in Parkinson’s Disease: The Role of α-Synuclein-Specific T Cells. Front. Immunol. 2019, 10, 303. [Google Scholar] [CrossRef]
- Takeda, S.; Sato, N.; Morishita, R. Systemic inflammation, blood–brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: Relevance to pathogenesis and therapy. Front. Aging Neurosci. 2014, 6, 171. [Google Scholar] [CrossRef]
- Ridder, K.; Keller, S.; Dams, M.; Rupp, A.K.; Schlaudraff, J.; Del Turco, D.; Starmann, J.; Macas, J.; Karpova, D.; Devraj, K.; et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014, 12, e1001874. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, B.; Kuypers, S.; Van den Akker, N.M.S.; Molin, D.G.M.; Michiels, L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front. Immunol. 2018, 9, 1789. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Wong, W.-Y.; Lee, M.L.; Cho, W.C.-S.; Yee, B.K.; Kwan, Y.W.; Tai, W.C.-S. Exosomes in Inflammation and Inflammatory Disease. Proteomics 2019, 19, e1800149. [Google Scholar] [CrossRef]
- Gupta, A.; Pulliam, L. Exosomes as mediators of neuroinflammation. J. Neuroinflammation 2014, 11, 68. [Google Scholar] [CrossRef]
- Tsilioni, I.; Theoharides, T.C. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflammation 2018, 15, 239. [Google Scholar] [CrossRef]
- Sproviero, D.; La Salvia, S.; Giannini, M.; Crippa, V.; Gagliardi, S.; Bernuzzi, S.; Diamanti, L.; Ceroni, M.; Pansarasa, O.; Poletti, A.; et al. Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients. Front. Neurosci. 2018, 12, 487. [Google Scholar] [CrossRef]
- Han, C.; Xiong, N.; Guo, X.; Huang, J.; Ma, K.; Liu, L.; Xia, Y.; Shen, Y.; Li, J.; Jiang, H.; et al. Exosomes from patients with Parkinson’s disease are pathological in mice. J. Mol. Med. 2019, 97, 1329–1344. [Google Scholar] [CrossRef]
- Riazifar, M.; Mohammadi, M.; Pone, E.J.; Yeri, A.; Lässer, C.; Ségaliny, A.I.; McIntyre, L.L.; Shelke, G.; Hutchins, E.; Hamamoto, A.; et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019, 13, 6670–6688. [Google Scholar] [CrossRef]
- Canales-Aguirre, A.; Reza-Zaldivar, E.E.; Sapiéns, M.A.H.; Gutiérrez-Mercado, Y.K.; Sandoval-Ávila, S.; Gomez-Pinedo, U.; Márquez-Aguirre, A.L.; Vazquez-Mendez, E.; Padilla-Camberos, E. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1626–1634. [Google Scholar] [CrossRef]
- Vilaça-Faria, H.; Salgado, A.J.; Teixeira, F.G. Mesenchymal Stem Cells-derived Exosomes: A New Possible Therapeutic Strategy for Parkinson’s Disease? Cells 2019, 8, E118. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Miñana, M.D.; Grisolía, S. Long term ingestion of ammonium increases acetylglutamate and urea levels without affecting the amount of carbamyl phosphate synthase. Eur. J. Biochem. 1988, 176, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30. [Google Scholar] [CrossRef]
- Shevchenko, A.; Jensen, O.N.; Podtelejnikov, A.V.; Sagliocco, F.; Wilm, M.; Vorm, O.; Mortensen, P.; Shevchenko, A.; Boucherie, H.; Mann, M. Linking genome and proteome by mass spectrometry: Large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 1996, 93, 14440–14445. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, H.; Jin, M.; Yang, X.; Ji, H.; Jiang, Y.; Zhang, H.; Wu, F.; Wu, G.; Lai, X.; et al. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell. Physiol. Biochem. 2018, 47, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Usano, A.; Cauli, O.; Agustí, A.; Felipo, V. Pregnenolone sulfate restores the glutamate-nitric-oxide-cGMP pathway and extracellular GABA in cerebellum and learning and motor coordination in hyperammonemic rats. ACS Chem. Neurosci. 2014, 5, 100–105. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13, 261. [Google Scholar] [CrossRef]
- Manakeng, K.; Prasertphol, P.; Phongpao, K.; Chuncharunee, S.; Tanyong, D.; Worawichawong, S.; Svasti, S.; Chaichompoo, P. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent β-thalassemia/HbE patients with pulmonary arterial hypertension. Ann. Hematol. 2019, 98, 281–288. [Google Scholar] [CrossRef]
- Dziechciowski, M.; Zapala, B.; Skotniczny, K.; Gawlik, K.; Pawlica-Gosiewska, D.; Piwowar, M.; Balajewicz-Nowak, M.; Basta, P.; Solnica, B.; Pitynski, K. Diagnostic and prognostic relevance of microparticles in peripheral and uterine blood of patients with endometrial cancer. Ginekol. Pol. 2018, 89, 682–687. [Google Scholar] [CrossRef]
- Liu, Q.; Xiang, Y.; Yuan, S.; Xie, W.; Li, C.; Hu, Z.; Wu, N.; Wu, L.; Yu, Z.; Bai, L.; et al. Plasma exosome levels in non-small-cell lung cancer: Correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018, 22, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pardo, F.; Villalobos-Labra, R.; Sobrevia Toledo, F.; Sobrevia, L. Extracellular vesicles in obesity and diabetes mellitus. Mol. Asp. Med. 2018, 60, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.B.A.; Armitage, J.; Teo, T.H.; Ong, N.E.; Shin, H.; Moodley, Y.P. Elevated levels of circulating exosome in COPD patients are associated with systemic inflammation. Respir. Med. 2017, 132, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J.; et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflammation 2018, 15, 8. [Google Scholar] [CrossRef]
- Thomas, L.; Pasquini, L.A. Galectin-3 mediated Glial Crosstalk Drives Oligodendrocyte Differentiation and (Re)myelination. Front. Cell. Neurosci. 2018, 12, 297. [Google Scholar] [CrossRef]
- Bänfer, S.; Schneider, M.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.-P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef]
- Wesley, U.V.; Vemuganti, R.; Ayvaci, E.R.; Dempsey, R.J. Galectin-3 enhances angiogenic and migratory potential of microglial cells via modulation of integrin linked kinase signaling. Brain Res. 2013, 1496, 1–9. [Google Scholar] [CrossRef]
- Burguillos, M.A.; Svensson, M.; Schulte, T.; Boza-Serrano, A.; Garcia-Quintanilla, A.; Kavanagh, E. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015, 10, 1626–1638. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Li, A.; Tan, C.; Liu, X. Exosomes play roles in sequential processes of tumor metastasis. Int. J. Cancer 2019, 144, 1486–1495. [Google Scholar] [CrossRef]
- Hu, J.L.; Wang, W.; Lan, X.L.; Zeng, Z.C.; Liang, Y.S.; Yan, Y.R.; Song, F.Y.; Wang, F.F.; Zhu, X.H.; Liao, W.; et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Mol. Cancer 2019, 18, 91. [Google Scholar] [CrossRef]
- Hosseini-Beheshti, E.; Grau, G.E.R. Extracellular vesicles as mediators of immunopathology in infectious diseases. Immunol. Cell Biol. 2018, 96, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Ciregia, F.; Urbani, A.; Palmisano, G. Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front. Mol. Neurosci. 2017, 10, 276. [Google Scholar] [CrossRef] [PubMed]




| Down-Regulated Proteins | |||
| Protein Name | P-Value | Protein Name | P-Value |
| Serine (Or cysteine) peptidase inhibitor, clade C (Antithrombin) | 0.011 | Pyruvate kinase PKM | 0.025 |
| Apolipoprotein H | 0.042 | Complement component C6 | 0.005 |
| Cfh protein | 0.042 | Complement C3 | 0.015 |
| Carboxypeptidase B2 | 0.007 | Gelsolin | 0.008 |
| Hemopexin | 0.028 | Apolipoprotein A-IV | 0.001 |
| Complement component factor h-like 1 | 0.009 | Alpha-2 antiplasmin | 0.005 |
| Rat apolipoprotein E protein | 0.002 | RCG33981, isoform CRA | 0.005 |
| Complement C8 gamma chain | 0.014 | Mannan-binding lectin serine protease 1 | 0.003 |
| Inter alpha-trypsin inhibitor, heavy chain 4 | 0.016 | Tetraspanin-CD9 | 0.039 |
| Vitronectin | 0.019 | Proteasome subunit beta | 0.006 |
| Complement factor B | 0.017 | C9 protein | 0.050 |
| Coagulation factor XIII B chain | 0.035 | Fetub protein | 0.013 |
| Complement factor H | 0.002 | Complement component C8 beta chain | 0.017 |
| Retinoic acid receptor responder (Tazarotene induced) | 0.010 | Serine (Or cysteine) proteinase inhibitor, clade A (Alpha-1 antiproteinase, antitrypsin), member 4 | 0.002 |
| Prothrombin | 0.018 | Ig gamma-2B chain C region | 0.036 |
| Complement factor I | 0.008 | Ig gamma-2C chain C region | 0.039 |
| Kininogen-1 | 0.021 | Kallikrein B, plasma 1 | 0.026 |
| MCG1038839 | 0.002 | Complement C4B (Chido blood group) | 0.026 |
| Macrophage stimulating 1 (Hepatocyte growth factor-like) | 0.005 | RCG55135, isoform CRA_b | 0.019 |
| Apolipoprotein B-100 | 0.010 | Complement C7 | 0.048 |
| LOC299567 protein (Fragment) | 0.019 | Apolipoprotein C-II (Predicted) | 0.007 |
| Coagulation factor XII | 0.034 | Inter-alpha-trypsin inhibitor heavy chain H3 | 0.039 |
| Complement C8 alpha chain | 0.013 | MASP-3 protein (Fragment) | 0.014 |
| Insulin-like growth factor binding protein complex acid-labile subunit | 0.001 | Inter-alpha trypsin inhibitor, heavy chain 1 | 0.033 |
| Carboxypeptidase N catalytic chain | 0.013 | Inter-alpha-trypsin inhibitor heavy chain 2 | 0.032 |
| Serine (Or cysteine) peptidase inhibitor, clade C (Antithrombin) | 0.011 | Peroxidasin | 0.014 |
| Apolipoprotein H | 0.042 | Reelin | 0.018 |
| Up-regulated proteins | |||
| Protein Name | P-Value | Protein Name | P-Value |
| Polymeric immunoglobulin receptor | 0.009 | Myeloperoxidase | 0.011 |
| CD5 antigen-like | 0.002 | Major urinary protein | 0.002 |
| Ig kappa chain V19-17-like | 0.042 | Brain cDNA, clone MNCb-5810, tissue inhibitor of metalloproteinase 3 (Timp3) | 0.010 |
| RCG21066 | 0.010 | Urinary protein 1 | 0.035 |
| Collectin sub-family member 11 | 0.039 | Ab2-001 | 0.030 |
| Ceruloplasmin | 0.015 | Thrombospondin 1 | 0.005 |
| C4b-binding protein beta chain | 0.047 | Coagulation factor XI | 0.001 |
| Urinary protein 2 | 0.042 | Ficolin (Collagen/fibrinogen domain containing) 1 | 0.0003 |
| Heat shock protein family A (Hsp70) member 5 | 0.024 | Fc fragment of IgG-binding protein | 0.001 |
| Thrombospondin-4 | 0.004 | Galectin-3-binding | 0.001 |
| Glutamine synthetase | 0.016 | Alpha-2-macroglobulin | 0.0003 |
| HGF activator | 0.031 | Ficolin-2 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo-Altarejos, P.; Cabrera-Pastor, A.; Gonzalez-King, H.; Montoliu, C.; Felipo, V. Extracellular Vesicles from Hyperammonemic Rats Induce Neuroinflammation and Motor Incoordination in Control Rats. Cells 2020, 9, 572. https://doi.org/10.3390/cells9030572
Izquierdo-Altarejos P, Cabrera-Pastor A, Gonzalez-King H, Montoliu C, Felipo V. Extracellular Vesicles from Hyperammonemic Rats Induce Neuroinflammation and Motor Incoordination in Control Rats. Cells. 2020; 9(3):572. https://doi.org/10.3390/cells9030572
Chicago/Turabian StyleIzquierdo-Altarejos, Paula, Andrea Cabrera-Pastor, Hernan Gonzalez-King, Carmina Montoliu, and Vicente Felipo. 2020. "Extracellular Vesicles from Hyperammonemic Rats Induce Neuroinflammation and Motor Incoordination in Control Rats" Cells 9, no. 3: 572. https://doi.org/10.3390/cells9030572
APA StyleIzquierdo-Altarejos, P., Cabrera-Pastor, A., Gonzalez-King, H., Montoliu, C., & Felipo, V. (2020). Extracellular Vesicles from Hyperammonemic Rats Induce Neuroinflammation and Motor Incoordination in Control Rats. Cells, 9(3), 572. https://doi.org/10.3390/cells9030572






