Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering
Abstract
:1. Pathological Condition of Osteoarthritis
1.1. Social Burden of Osteoarthritis (OA)
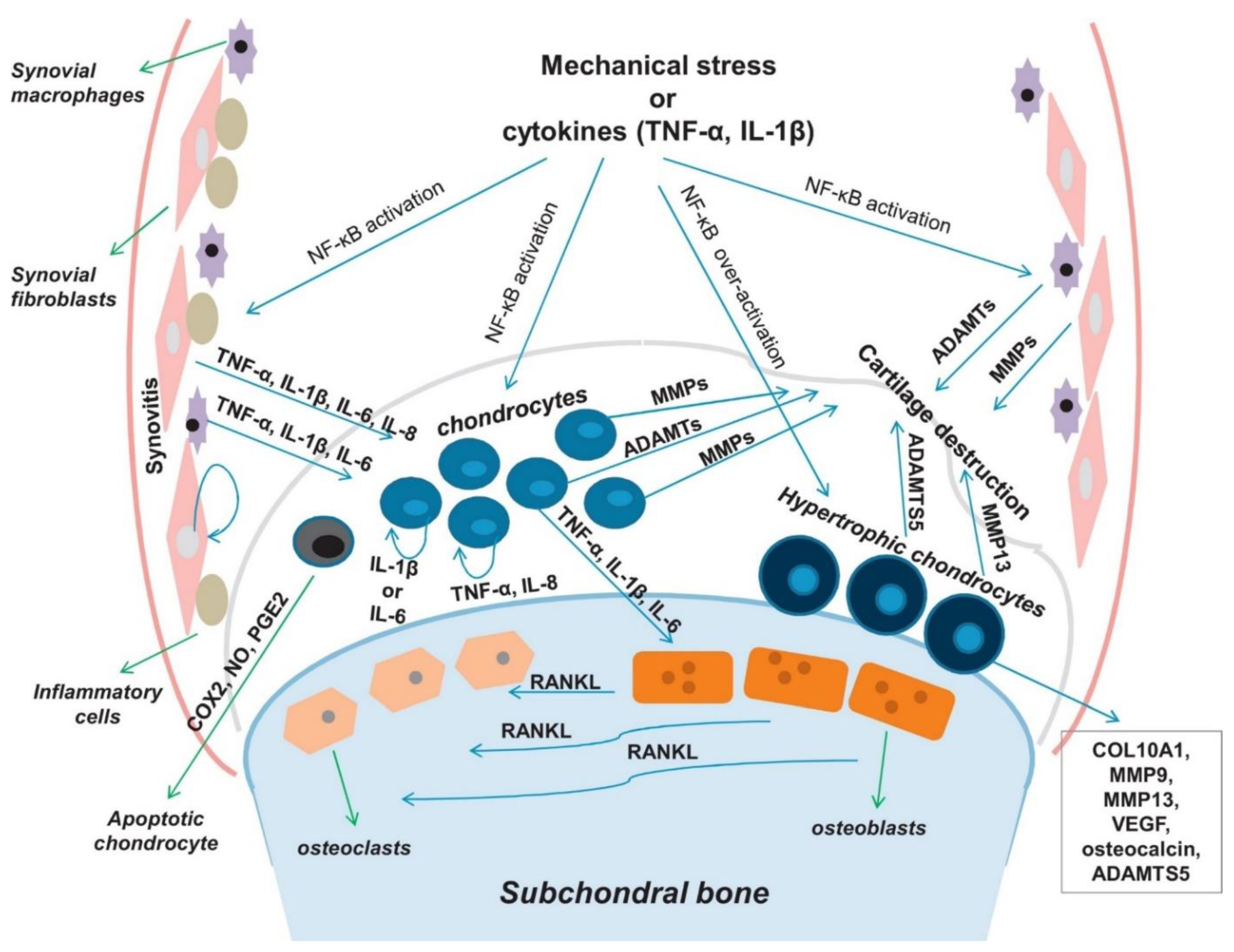
1.2. Pathogenesis of OA
1.3. Current Strategies for OA Treatment
1.3.1. Pharmacological Treatments
1.3.2. Viscosupplementation
1.3.3. Cell-Based Treatments
2. Biomaterials for OA Repair Applications
2.1. Hydrogels for Cartilage Tissue Regeneration
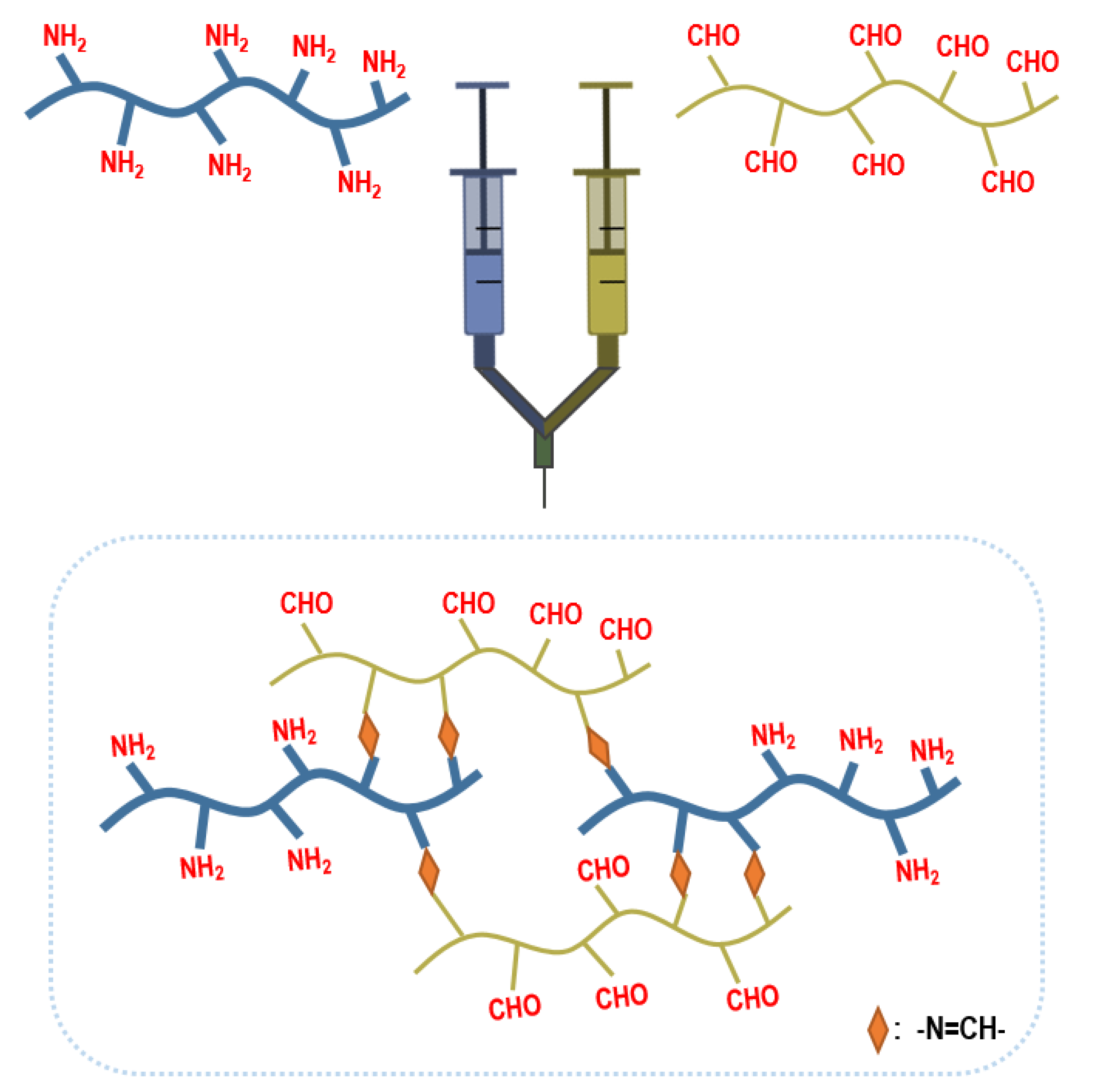
2.1.1. Injectable Hydrogels
2.1.2. Adhesive Hydrogel
2.2. Inflammatory-Modulating Biomaterials for OA
2.2.1. Symptom-Modulating Treatments
2.2.2. Disease-Modifying Treatments
2.2.3. Next-Generation of Immune-Modulating Hydrogels
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliddal, H.; Christensen, R. The treatment and prevention of knee osteoarthritis: A tool for clinical decision-making. Expert Opin. Pharmacother. 2009, 10, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Fautrel, B.; Hilliquin, P.; Rozenberg, S.; Allaert, F.A.; Coste, P.; Leclerc, A.; Rossignol, M. Impact of osteoarthritis: Results of a nationwide survey of 10,000 patients consulting for OA. Joint Bone Spine 2005, 72, 235–240. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Crowson, C.S.; O’Fallon, W.M. Comorbidity in arthritis. J. Rheumatol. 1999, 26, 2475–2479. [Google Scholar]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune modulation to improve tissue engineering outcomes for cartilage repair in the osteoarthritic joint. Tissue Eng. Part B Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sabzwari, S.R.A.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollason, V.; Samer, C.F.; Daali, Y.; Desmeules, J.A. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti- inflammatory drugs: A review. Curr. Drug Metab. 2014, 15, 326–343. [Google Scholar] [CrossRef]
- Nasr, M. In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. AAPS Pharm. Sci. Tech. 2010, 11, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Makela, A.L.; Lempiainen, M.; Ylijoki, H. Ibuprofen levels in serum and synovial fluid. Scand. J. Rheumatol. Suppl. 1981, 39, 15–17. [Google Scholar] [CrossRef]
- Netter, P.; Bannwarth, B.; Royer-Morrot, M.J. Recent findings on the pharmacokinetics of non-steroidal anti-inflammatory drugs in synovial fluid. Clin. Pharmacokinet. 1989, 17, 145–162. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyere, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. (Hoboken) 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [Green Version]
- Colen, S.; van den Bekerom, M.P.; Mulier, M.; Haverkamp, D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hall, S.; Hanna, F.; Wluka, A.E.; Grant, G.; Marks, P.; Feletar, M.; Cicuttini, F.M. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: A two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.; Hunter, M.; Kolhe, R.; Isales, C.; Hamrick, M.; Fulzele, S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin. Transl. Med. 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, T.; Mandelbaum, B.; Tom, M. Autologous Chondrocyte Implantation: Past, Present, and Future. Sports Med. Arthrosc. Rev. 2016, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Kramer, J.; Shelton, T.; Merriman, J.; Haus, B. Assessment of Cartilage Growth After Biopsy of Osteochondral Loose Bodies in Adolescent Knees for Use in Autologous Chondrocyte Implantation. J. Pediatr. Orthop. 2018. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Jato Diaz, M. Autologous chondrocyte implantation: A systematic review. Osteoarthr. Cartil. 2006, 14, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Hegert, C.; Kramer, J.; Hargus, G.; Muller, J.; Guan, K.; Wobus, A.M.; Muller, P.K.; Rohwedel, J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J. Cell Sci. 2002, 115, 4617–4628. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, N.; Duryea, D.; Manoukian, R.; Chow, G.; Han, C.Y. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J. Cell Sci. 2003, 116, 2015–2028. [Google Scholar] [CrossRef] [Green Version]
- Hwang, N.S.; Varghese, S.; Elisseeff, J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE 2008, 3, e2498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Fu, X.; Yan, L.; Li, S.; Zhao, D.; Wang, X.; Duan, Y.; Yan, Y.; Li, E.; Wu, K.; et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics 2019, 9, 6587–6600. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgács, G.; Sun, W. Biofabrication: Micro- and Nano-Fabrication, Printing, Patterning, and Assemblies; Elsevier/WA, William Andrew is an imprint of Elsevier: Amsterdam The Netherlands; Boston, MA, USA, 2013. [Google Scholar]
- Jin, R.; Teixeira, L.S.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Park, K.M.; Joung, Y.K.; Park, K.D.; Do, S.H. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J. Control. Release 2012, 159, 332–337. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Leijten, J.C.; Wennink, J.W.; Chatterjea, A.G.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 2012, 33, 3651–3661. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly(L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. Part. A 2015, 21, 757–766. [Google Scholar] [CrossRef]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Cheng, A.W.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part. A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.; Clement, E.M.; Conrad, G.W. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest. Ophthalmol. Vis. Sci. 2010, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
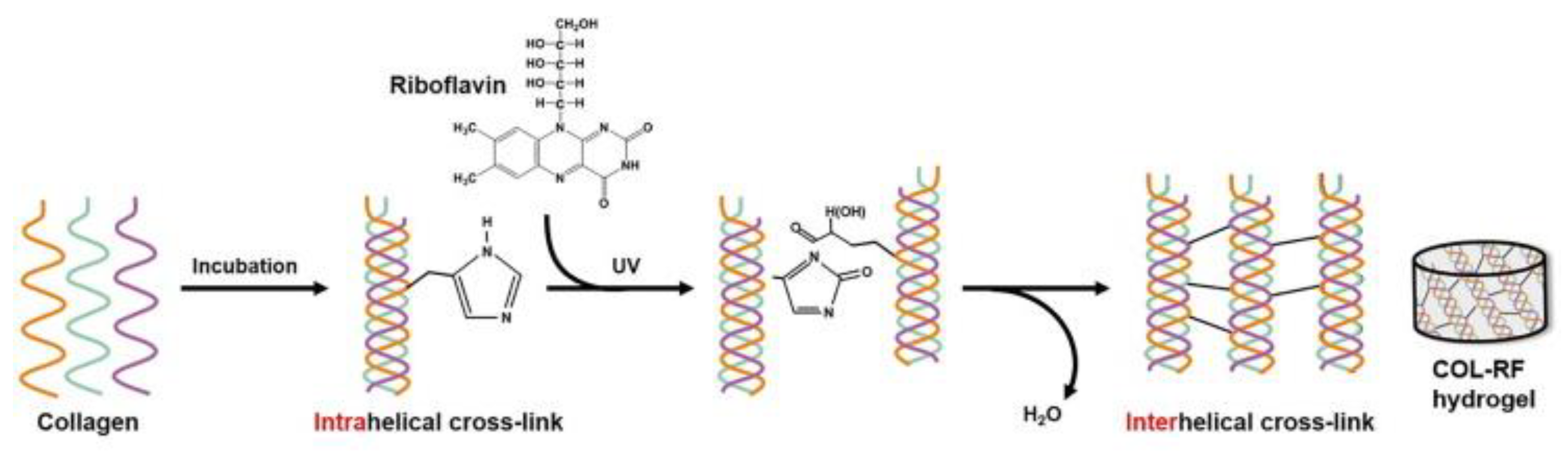
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kang, B.J.; Hwang, N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017, 53, 318–328. [Google Scholar] [CrossRef]
- Jung, H.H.; Park, K.; Han, D.K. Preparation of TGF-beta1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J. Control. Release 2010, 147, 84–91. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Kim, S.H.; An, Y.H.; Kim, H.D.; Kim, K.; Lee, S.H.; Yim, H.G.; Kim, B.G.; Hwang, N.S. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 2018, 110, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Faust, H.J.; Sommerfeld, S.D.; Rathod, S.; Rittenbach, A.; Ray Banerjee, S.; Tsui, B.M.W.; Pomper, M.; Amzel, M.L.; Singh, A.; Elisseeff, J.H. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018, 183, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Sheu, M.T.; Lin, Y.F.; Lan, J.; Chin, Y.P.; Hsieh, M.S.; Cheng, C.W.; Chen, C.H. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. BMC Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, T.; Yomota, C.; Okada, S. Development and release characterization of hyaluronan-doxycycline gels based on metal coordination. J. Control. Release 2001, 76, 337–347. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Gao, J.; Zhao, Y.; Zhao, Y.; Guo, L.; Chen, C.; Duan, Z.; Li, P.; Wei, L. Intra-Articular Injection of Cross-Linked Hyaluronic Acid-Dexamethasone Hydrogel Attenuates Osteoarthritis: An Experimental Study in a Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 411. [Google Scholar] [CrossRef]
- Petit, A.; Redout, E.M.; van de Lest, C.H.; de Grauw, J.C.; Muller, B.; Meyboom, R.; van Midwoud, P.; Vermonden, T.; Hennink, W.E.; Rene van Weeren, P. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials 2015, 53, 426–436. [Google Scholar] [CrossRef]
- Rivera-Delgado, E.; Djuhadi, A.; Danda, C.; Kenyon, J.; Maia, J.; Caplan, A.I.; von Recum, H.A. Injectable liquid polymers extend the delivery of corticosteroids for the treatment of osteoarthritis. J. Control. Release 2018, 284, 112–121. [Google Scholar] [CrossRef]
- Bedouet, L.; Moine, L.; Pascale, F.; Nguyen, V.N.; Labarre, D.; Laurent, A. Synthesis of hydrophilic intra-articular microspheres conjugated to ibuprofen and evaluation of anti-inflammatory activity on articular explants. Int. J. Pharm. 2014, 459, 51–61. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Ubhe, A.; Shaman, Z.; D’Souza, G. Intra-articular interleukin-1 receptor antagonist (IL1-ra) microspheres for posttraumatic osteoarthritis: In vitro biological activity and in vivo disease modifying effect. J. Exp. Orthop. 2016, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Whitmire, R.E.; Wilson, D.S.; Singh, A.; Levenston, M.E.; Murthy, N.; Garcia, A.J. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 2012, 33, 7665–7675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Tang, Y.; He, X.; Yan, J.; Wang, C.; Feng, X. Self-Assembled Raspberry-Like Core/Satellite Nanoparticles for Anti-Inflammatory Protein Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6902–6907. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; Garcia, A.J. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, J.D.; Gouze, E.; Oligino, T.J.; Gouze, J.N.; Watson, R.S.; Levings, P.P.; Bush, M.L.; Dacanay, A.; Nickerson, D.M.; Robbins, P.D.; et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J. Gene Med. 2009, 11, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, R.S.; Broome, T.A.; Levings, P.P.; Rice, B.L.; Kay, J.D.; Smith, A.D.; Gouze, E.; Gouze, J.N.; Dacanay, E.A.; Hauswirth, W.W.; et al. scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene Ther. 2013, 20, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yu, C.; Xu, S.; Tang, T.; Dai, K. Direct chitosan-mediated gene delivery to the rabbit knee joints in vitro and in vivo. Biochem. Biophys. Res. Commun. 2006, 341, 202–208. [Google Scholar] [CrossRef]
- Lu, H.D.; Zhao, H.Q.; Wang, K.; Lv, L.L. Novel hyaluronic acid-chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef]
- Sun, L.T.; Buchholz, K.S.; Lotze, M.T.; Washburn, N.R. Cytokine binding by polysaccharide-antibody conjugates. Mol. Pharm. 2010, 7, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Duan, X.; Pan, H.; Holguin, N.; Rai, M.F.; Akk, A.; Springer, L.E.; Wickline, S.A.; Sandell, L.J.; Pham, C.T. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl. Acad. Sci. USA 2016, 113, E6199–E6208. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.M.; Huang, J.H.; Oner, F.C.; Dhert, W.J.A.; Kragten, A.H.M.; Willems, N.; Grinwis, G.C.M.; et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Evans, C.H.; Kim, S.; Oligino, T.; Ghivizzani, S.C.; Robbins, P.D. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000, 2, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellowes, R.; Etheridge, C.J.; Coade, S.; Cooper, R.G.; Stewart, L.; Miller, A.D.; Woo, P. Amelioration of established collagen induced arthritis by systemic IL-10 gene delivery. Gene Ther. 2000, 7, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, L.; Atallah, P.; Werner, C.; Freudenberg, U. StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv. Healthc. Mater. 2016, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Soranno, D.E.; Rodell, C.B.; Altmann, C.; Duplantis, J.; Andres-Hernando, A.; Burdick, J.A.; Faubel, S. Delivery of interleukin-10 via injectable hydrogels improves renal outcomes and reduces systemic inflammation following ischemic acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 2016, 311, F362–F372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, G.; Chen, Q.; Liu, F.; Cui, Z.; Shao, H.; Liu, F.; Ma, A.; Liao, J.; Guo, B.; Guo, Y.; et al. Low molecular weight xanthan gum for treating osteoarthritis. Carbohydr. Polym. 2017, 164, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Sim, J.S.; Jeong, C.S.; Chang, S.Y.; Choi, D.W.; Toida, T.; Kim, Y.S. Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol. Pharm. Bull. 2004, 27, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Arlov, O.; Steinwachs, M.; Skjak-Braek, G.; Zenobi-Wong, M. Biomimetic sulphated alginate hydrogels suppress IL-1beta-induced inflammatory responses in human chondrocytes. Eur. Cell Mater. 2017, 33, 76–89. [Google Scholar] [CrossRef]
- Kerschenmeyer, A.; Arlov, O.; Malheiro, V.; Steinwachs, M.; Rottmar, M.; Maniura-Weber, K.; Palazzolo, G.; Zenobi-Wong, M. Anti-oxidant and immune-modulatory properties of sulfated alginate derivatives on human chondrocytes and macrophages. Biomater. Sci. 2017, 5, 1756–1765. [Google Scholar] [CrossRef]
- Hongbin, W.; Jingyuan, D.; Linyun, C.; Yuming, D. Carboxymethylated chitin reduces MMP-1 expression in rabbit ACLT osteoarthritic cartilage. Ann. Rheum Dis. 2004, 63, 369–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.D.; Tse, M.J.; Read, E.L.; Liu, W.F. Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. (Camb) 2016, 8, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.U.; Gott, S.C.; Woo, B.W.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef]
- Rasmussen, A.M.; Borelli, G.; Hoel, H.J.; Lislerud, K.; Gaudernack, G.; Kvalheim, G.; Aarvak, T. Ex vivo expansion protocol for human tumor specific T cells for adoptive T cell therapy. J. Immunol. Methods 2010, 355, 52–60. [Google Scholar] [CrossRef]
- Steenblock, E.R.; Fahmy, T.M. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 2008, 16, 765–772. [Google Scholar] [CrossRef]
- Perica, K.; Tu, A.; Richter, A.; Bieler, J.G.; Edidin, M.; Schneck, J.P. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 2014, 8, 2252–2260. [Google Scholar] [CrossRef]
- Prakken, B.; Wauben, M.; Genini, D.; Samodal, R.; Barnett, J.; Mendivil, A.; Leoni, L.; Albani, S. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat. Med. 2000, 6, 1406–1410. [Google Scholar] [CrossRef]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells 2020, 9, 419. https://doi.org/10.3390/cells9020419
Koh RH, Jin Y, Kim J, Hwang NS. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells. 2020; 9(2):419. https://doi.org/10.3390/cells9020419
Chicago/Turabian StyleKoh, Rachel H., Yinji Jin, Jisoo Kim, and Nathaniel S. Hwang. 2020. "Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering" Cells 9, no. 2: 419. https://doi.org/10.3390/cells9020419
APA StyleKoh, R. H., Jin, Y., Kim, J., & Hwang, N. S. (2020). Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells, 9(2), 419. https://doi.org/10.3390/cells9020419




