Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering
Abstract
1. Pathological Condition of Osteoarthritis
1.1. Social Burden of Osteoarthritis (OA)
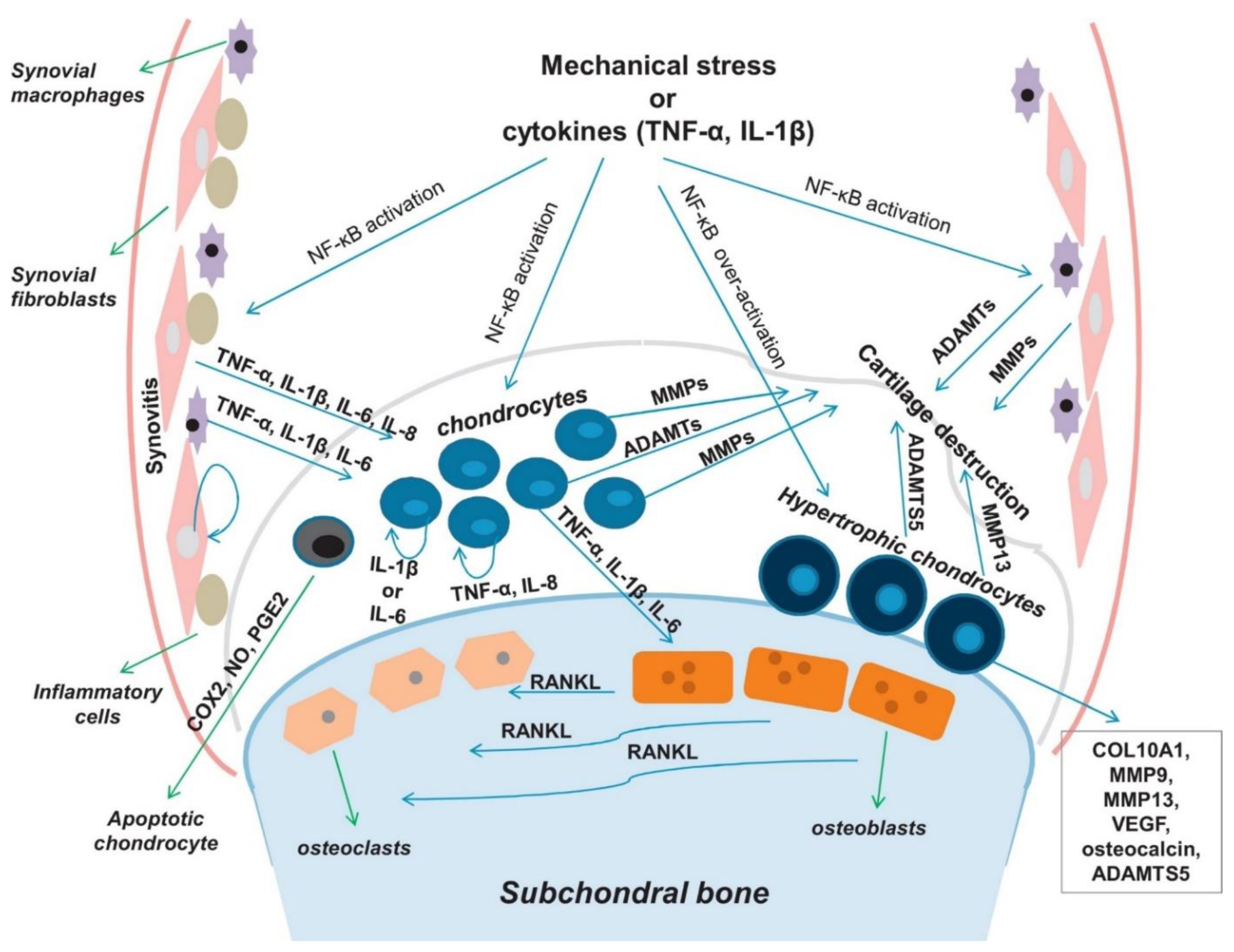
1.2. Pathogenesis of OA
1.3. Current Strategies for OA Treatment
1.3.1. Pharmacological Treatments
1.3.2. Viscosupplementation
1.3.3. Cell-Based Treatments
2. Biomaterials for OA Repair Applications
2.1. Hydrogels for Cartilage Tissue Regeneration
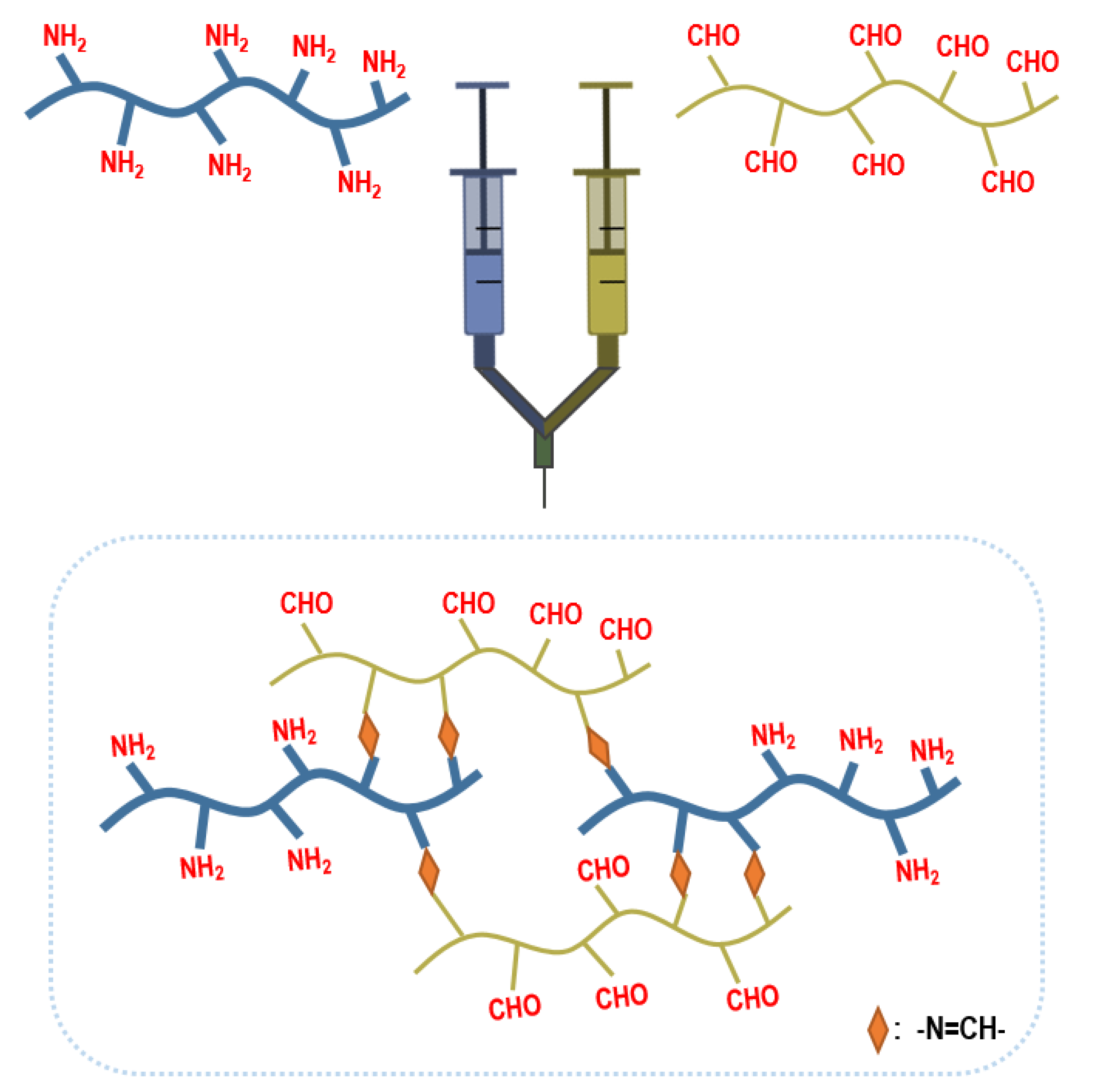
2.1.1. Injectable Hydrogels
2.1.2. Adhesive Hydrogel
2.2. Inflammatory-Modulating Biomaterials for OA
2.2.1. Symptom-Modulating Treatments
2.2.2. Disease-Modifying Treatments
2.2.3. Next-Generation of Immune-Modulating Hydrogels
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Christensen, R. The treatment and prevention of knee osteoarthritis: A tool for clinical decision-making. Expert Opin. Pharmacother. 2009, 10, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E. Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Fautrel, B.; Hilliquin, P.; Rozenberg, S.; Allaert, F.A.; Coste, P.; Leclerc, A.; Rossignol, M. Impact of osteoarthritis: Results of a nationwide survey of 10,000 patients consulting for OA. Joint Bone Spine 2005, 72, 235–240. [Google Scholar] [CrossRef]
- Gabriel, S.E.; Crowson, C.S.; O’Fallon, W.M. Comorbidity in arthritis. J. Rheumatol. 1999, 26, 2475–2479. [Google Scholar]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune modulation to improve tissue engineering outcomes for cartilage repair in the osteoarthritic joint. Tissue Eng. Part B Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Sabzwari, S.R.A.; Vargova, V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs: An Under-Recognized Public Health Issue. Cureus 2017, 9, e1144. [Google Scholar] [CrossRef] [PubMed]
- Rollason, V.; Samer, C.F.; Daali, Y.; Desmeules, J.A. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti- inflammatory drugs: A review. Curr. Drug Metab. 2014, 15, 326–343. [Google Scholar] [CrossRef]
- Nasr, M. In vitro and in vivo evaluation of proniosomes containing celecoxib for oral administration. AAPS Pharm. Sci. Tech. 2010, 11, 85–89. [Google Scholar] [CrossRef]
- Makela, A.L.; Lempiainen, M.; Ylijoki, H. Ibuprofen levels in serum and synovial fluid. Scand. J. Rheumatol. Suppl. 1981, 39, 15–17. [Google Scholar] [CrossRef]
- Netter, P.; Bannwarth, B.; Royer-Morrot, M.J. Recent findings on the pharmacokinetics of non-steroidal anti-inflammatory drugs in synovial fluid. Clin. Pharmacokinet. 1989, 17, 145–162. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyere, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Collaud Basset, S.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. (Hoboken) 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Bellamy, N.; Campbell, J.; Robinson, V.; Gee, T.; Bourne, R.; Wells, G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Colen, S.; van den Bekerom, M.P.; Mulier, M.; Haverkamp, D. Hyaluronic acid in the treatment of knee osteoarthritis: A systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs 2012, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hall, S.; Hanna, F.; Wluka, A.E.; Grant, G.; Marks, P.; Feletar, M.; Cicuttini, F.M. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: A two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.; Hunter, M.; Kolhe, R.; Isales, C.; Hamrick, M.; Fulzele, S. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin. Transl. Med. 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Welch, T.; Mandelbaum, B.; Tom, M. Autologous Chondrocyte Implantation: Past, Present, and Future. Sports Med. Arthrosc. Rev. 2016, 24, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Kramer, J.; Shelton, T.; Merriman, J.; Haus, B. Assessment of Cartilage Growth After Biopsy of Osteochondral Loose Bodies in Adolescent Knees for Use in Autologous Chondrocyte Implantation. J. Pediatr. Orthop. 2018. [Google Scholar] [CrossRef]
- Ruano-Ravina, A.; Jato Diaz, M. Autologous chondrocyte implantation: A systematic review. Osteoarthr. Cartil. 2006, 14, 47–51. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Hegert, C.; Kramer, J.; Hargus, G.; Muller, J.; Guan, K.; Wobus, A.M.; Muller, P.K.; Rohwedel, J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J. Cell Sci. 2002, 115, 4617–4628. [Google Scholar] [CrossRef]
- Nakayama, N.; Duryea, D.; Manoukian, R.; Chow, G.; Han, C.Y. Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J. Cell Sci. 2003, 116, 2015–2028. [Google Scholar] [CrossRef]
- Hwang, N.S.; Varghese, S.; Elisseeff, J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS ONE 2008, 3, e2498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Fu, X.; Yan, L.; Li, S.; Zhao, D.; Wang, X.; Duan, Y.; Yan, Y.; Li, E.; Wu, K.; et al. Transplantation of human ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous osteoarthritis in rhesus macaques. Theranostics 2019, 9, 6587–6600. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Forgács, G.; Sun, W. Biofabrication: Micro- and Nano-Fabrication, Printing, Patterning, and Assemblies; Elsevier/WA, William Andrew is an imprint of Elsevier: Amsterdam The Netherlands; Boston, MA, USA, 2013. [Google Scholar]
- Jin, R.; Teixeira, L.S.; Dijkstra, P.J.; van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010, 31, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Park, K.M.; Joung, Y.K.; Park, K.D.; Do, S.H. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J. Control. Release 2012, 159, 332–337. [Google Scholar] [CrossRef]
- Moreira Teixeira, L.S.; Leijten, J.C.; Wennink, J.W.; Chatterjea, A.G.; Feijen, J.; van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. The effect of platelet lysate supplementation of a dextran-based hydrogel on cartilage formation. Biomaterials 2012, 33, 3651–3661. [Google Scholar] [CrossRef]
- Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Zhang, K.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly(L-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar] [CrossRef]
- Cao, L.; Cao, B.; Lu, C.; Wang, G.; Yu, L.; Ding, J. An injectable hydrogel formed by in situ cross-linking of glycol chitosan and multi-benzaldehyde functionalized PEG analogues for cartilage tissue engineering. J. Mater. Chem. B 2015, 3, 1268–1280. [Google Scholar] [CrossRef]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. Part. A 2015, 21, 757–766. [Google Scholar] [CrossRef]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cheng, A.W.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part. A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
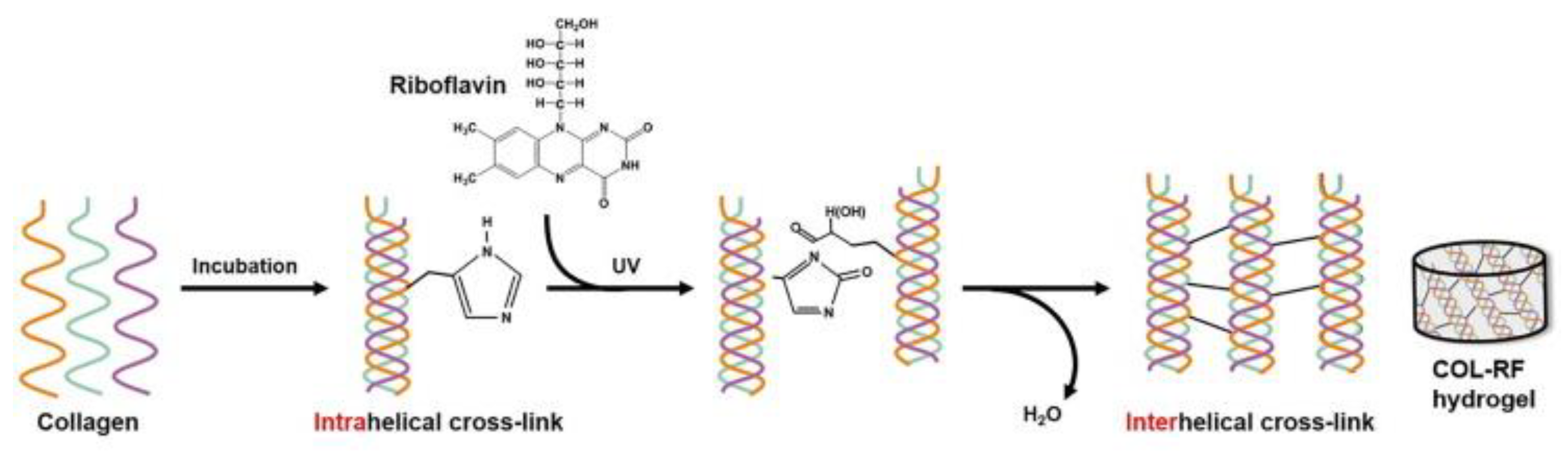
- McCall, A.S.; Kraft, S.; Edelhauser, H.F.; Kidder, G.W.; Lundquist, R.R.; Bradshaw, H.E.; Dedeic, Z.; Dionne, M.J.; Clement, E.M.; Conrad, G.W. Mechanisms of corneal tissue cross-linking in response to treatment with topical riboflavin and long-wavelength ultraviolet radiation (UVA). Invest. Ophthalmol. Vis. Sci. 2010, 51, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Koh, R.H.; Jin, Y.; Kang, B.J.; Hwang, N.S. Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomater. 2017, 53, 318–328. [Google Scholar] [CrossRef]
- Jung, H.H.; Park, K.; Han, D.K. Preparation of TGF-beta1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J. Control. Release 2010, 147, 84–91. [Google Scholar] [CrossRef]
- Chen, J.-P.; Cheng, T.-H. Preparation and evaluation of thermo-reversible copolymer hydrogels containing chitosan and hyaluronic acid as injectable cell carriers. Polymer 2009, 50, 107–116. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef]
- Kim, S.H.; An, Y.H.; Kim, H.D.; Kim, K.; Lee, S.H.; Yim, H.G.; Kim, B.G.; Hwang, N.S. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 2018, 110, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Faust, H.J.; Sommerfeld, S.D.; Rathod, S.; Rittenbach, A.; Ray Banerjee, S.; Tsui, B.M.W.; Pomper, M.; Amzel, M.L.; Singh, A.; Elisseeff, J.H. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 2018, 183, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.T.; Sheu, M.T.; Lin, Y.F.; Lan, J.; Chin, Y.P.; Hsieh, M.S.; Cheng, C.W.; Chen, C.H. Injectable hyaluronic-acid-doxycycline hydrogel therapy in experimental rabbit osteoarthritis. BMC Vet. Res. 2013, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yomota, C.; Okada, S. Development and release characterization of hyaluronan-doxycycline gels based on metal coordination. J. Control. Release 2001, 76, 337–347. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Gao, J.; Zhao, Y.; Zhao, Y.; Guo, L.; Chen, C.; Duan, Z.; Li, P.; Wei, L. Intra-Articular Injection of Cross-Linked Hyaluronic Acid-Dexamethasone Hydrogel Attenuates Osteoarthritis: An Experimental Study in a Rat Model of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 411. [Google Scholar] [CrossRef]
- Petit, A.; Redout, E.M.; van de Lest, C.H.; de Grauw, J.C.; Muller, B.; Meyboom, R.; van Midwoud, P.; Vermonden, T.; Hennink, W.E.; Rene van Weeren, P. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials 2015, 53, 426–436. [Google Scholar] [CrossRef]
- Rivera-Delgado, E.; Djuhadi, A.; Danda, C.; Kenyon, J.; Maia, J.; Caplan, A.I.; von Recum, H.A. Injectable liquid polymers extend the delivery of corticosteroids for the treatment of osteoarthritis. J. Control. Release 2018, 284, 112–121. [Google Scholar] [CrossRef]
- Bedouet, L.; Moine, L.; Pascale, F.; Nguyen, V.N.; Labarre, D.; Laurent, A. Synthesis of hydrophilic intra-articular microspheres conjugated to ibuprofen and evaluation of anti-inflammatory activity on articular explants. Int. J. Pharm. 2014, 459, 51–61. [Google Scholar] [CrossRef]
- Elsaid, K.A.; Ubhe, A.; Shaman, Z.; D’Souza, G. Intra-articular interleukin-1 receptor antagonist (IL1-ra) microspheres for posttraumatic osteoarthritis: In vitro biological activity and in vivo disease modifying effect. J. Exp. Orthop. 2016, 3, 18. [Google Scholar] [CrossRef]
- Whitmire, R.E.; Wilson, D.S.; Singh, A.; Levenston, M.E.; Murthy, N.; Garcia, A.J. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 2012, 33, 7665–7675. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, Y.; He, X.; Yan, J.; Wang, C.; Feng, X. Self-Assembled Raspberry-Like Core/Satellite Nanoparticles for Anti-Inflammatory Protein Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6902–6907. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Volkmer, T.M.; Wang, P.; Lee, L.A.; Wang, Q.; Garcia, A.J. Synthesis of self-assembled IL-1Ra-presenting nanoparticles for the treatment of osteoarthritis. J. Biomed. Mater. Res. A 2016, 104, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.D.; Gouze, E.; Oligino, T.J.; Gouze, J.N.; Watson, R.S.; Levings, P.P.; Bush, M.L.; Dacanay, A.; Nickerson, D.M.; Robbins, P.D.; et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J. Gene Med. 2009, 11, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.S.; Broome, T.A.; Levings, P.P.; Rice, B.L.; Kay, J.D.; Smith, A.D.; Gouze, E.; Gouze, J.N.; Dacanay, E.A.; Hauswirth, W.W.; et al. scAAV-mediated gene transfer of interleukin-1-receptor antagonist to synovium and articular cartilage in large mammalian joints. Gene Ther. 2013, 20, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, C.; Xu, S.; Tang, T.; Dai, K. Direct chitosan-mediated gene delivery to the rabbit knee joints in vitro and in vivo. Biochem. Biophys. Res. Commun. 2006, 341, 202–208. [Google Scholar] [CrossRef]
- Lu, H.D.; Zhao, H.Q.; Wang, K.; Lv, L.L. Novel hyaluronic acid-chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011, 420, 358–365. [Google Scholar] [CrossRef]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef]
- Sun, L.T.; Buchholz, K.S.; Lotze, M.T.; Washburn, N.R. Cytokine binding by polysaccharide-antibody conjugates. Mol. Pharm. 2010, 7, 1769–1777. [Google Scholar] [CrossRef][Green Version]
- Yan, H.; Duan, X.; Pan, H.; Holguin, N.; Rai, M.F.; Akk, A.; Springer, L.E.; Wickline, S.A.; Sandell, L.J.; Pham, C.T. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl. Acad. Sci. USA 2016, 113, E6199–E6208. [Google Scholar] [CrossRef]
- Yang, H.Y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.M.; Huang, J.H.; Oner, F.C.; Dhert, W.J.A.; Kragten, A.H.M.; Willems, N.; Grinwis, G.C.M.; et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Evans, C.H.; Kim, S.; Oligino, T.; Ghivizzani, S.C.; Robbins, P.D. Gene therapy for established murine collagen-induced arthritis by local and systemic adenovirus-mediated delivery of interleukin-4. Arthritis Res. 2000, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Fellowes, R.; Etheridge, C.J.; Coade, S.; Cooper, R.G.; Stewart, L.; Miller, A.D.; Woo, P. Amelioration of established collagen induced arthritis by systemic IL-10 gene delivery. Gene Ther. 2000, 7, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, L.; Atallah, P.; Werner, C.; Freudenberg, U. StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4. Adv. Healthc. Mater. 2016, 5, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Soranno, D.E.; Rodell, C.B.; Altmann, C.; Duplantis, J.; Andres-Hernando, A.; Burdick, J.A.; Faubel, S. Delivery of interleukin-10 via injectable hydrogels improves renal outcomes and reduces systemic inflammation following ischemic acute kidney injury in mice. Am. J. Physiol. Ren. Physiol. 2016, 311, F362–F372. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chen, Q.; Liu, F.; Cui, Z.; Shao, H.; Liu, F.; Ma, A.; Liao, J.; Guo, B.; Guo, Y.; et al. Low molecular weight xanthan gum for treating osteoarthritis. Carbohydr. Polym. 2017, 164, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef]
- Cho, S.Y.; Sim, J.S.; Jeong, C.S.; Chang, S.Y.; Choi, D.W.; Toida, T.; Kim, Y.S. Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol. Pharm. Bull. 2004, 27, 47–51. [Google Scholar] [CrossRef]
- Arlov, O.; Steinwachs, M.; Skjak-Braek, G.; Zenobi-Wong, M. Biomimetic sulphated alginate hydrogels suppress IL-1beta-induced inflammatory responses in human chondrocytes. Eur. Cell Mater. 2017, 33, 76–89. [Google Scholar] [CrossRef]
- Kerschenmeyer, A.; Arlov, O.; Malheiro, V.; Steinwachs, M.; Rottmar, M.; Maniura-Weber, K.; Palazzolo, G.; Zenobi-Wong, M. Anti-oxidant and immune-modulatory properties of sulfated alginate derivatives on human chondrocytes and macrophages. Biomater. Sci. 2017, 5, 1756–1765. [Google Scholar] [CrossRef]
- Hongbin, W.; Jingyuan, D.; Linyun, C.; Yuming, D. Carboxymethylated chitin reduces MMP-1 expression in rabbit ACLT osteoarthritic cartilage. Ann. Rheum Dis. 2004, 63, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Tse, M.J.; Read, E.L.; Liu, W.F. Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. (Camb) 2016, 8, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.U.; Gott, S.C.; Woo, B.W.; Rao, M.P.; Liu, W.F. Micro- and Nanopatterned Topographical Cues for Regulating Macrophage Cell Shape and Phenotype. ACS Appl. Mater. Interfaces 2015, 7, 28665–28672. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zheng, L.; Wang, Y.; Tao, M.; Xie, Z.; Xia, C.; Gu, C.; Chen, J.; Qiu, P.; Mei, S.; et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics 2019, 9, 2439–2459. [Google Scholar] [CrossRef]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef]
- Rasmussen, A.M.; Borelli, G.; Hoel, H.J.; Lislerud, K.; Gaudernack, G.; Kvalheim, G.; Aarvak, T. Ex vivo expansion protocol for human tumor specific T cells for adoptive T cell therapy. J. Immunol. Methods 2010, 355, 52–60. [Google Scholar] [CrossRef]
- Steenblock, E.R.; Fahmy, T.M. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 2008, 16, 765–772. [Google Scholar] [CrossRef]
- Perica, K.; Tu, A.; Richter, A.; Bieler, J.G.; Edidin, M.; Schneck, J.P. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 2014, 8, 2252–2260. [Google Scholar] [CrossRef]
- Prakken, B.; Wauben, M.; Genini, D.; Samodal, R.; Barnett, J.; Mendivil, A.; Leoni, L.; Albani, S. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat. Med. 2000, 6, 1406–1410. [Google Scholar] [CrossRef]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, R.H.; Jin, Y.; Kim, J.; Hwang, N.S. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells 2020, 9, 419. https://doi.org/10.3390/cells9020419
Koh RH, Jin Y, Kim J, Hwang NS. Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells. 2020; 9(2):419. https://doi.org/10.3390/cells9020419
Chicago/Turabian StyleKoh, Rachel H., Yinji Jin, Jisoo Kim, and Nathaniel S. Hwang. 2020. "Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering" Cells 9, no. 2: 419. https://doi.org/10.3390/cells9020419
APA StyleKoh, R. H., Jin, Y., Kim, J., & Hwang, N. S. (2020). Inflammation-Modulating Hydrogels for Osteoarthritis Cartilage Tissue Engineering. Cells, 9(2), 419. https://doi.org/10.3390/cells9020419




