Myelin Measurement Using Quantitative Magnetic Resonance Imaging: A Correlation Study Comparing Various Imaging Techniques in Patients with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. MRI Acquisition Protocol
2.3. Acquisition and Processing of SyMRI Data
2.4. Processing of the T1w/T2w Ratio
2.5. Acquisition and Processing of MTsat
2.6. Acquisition and Processing of Radial Diffusivity
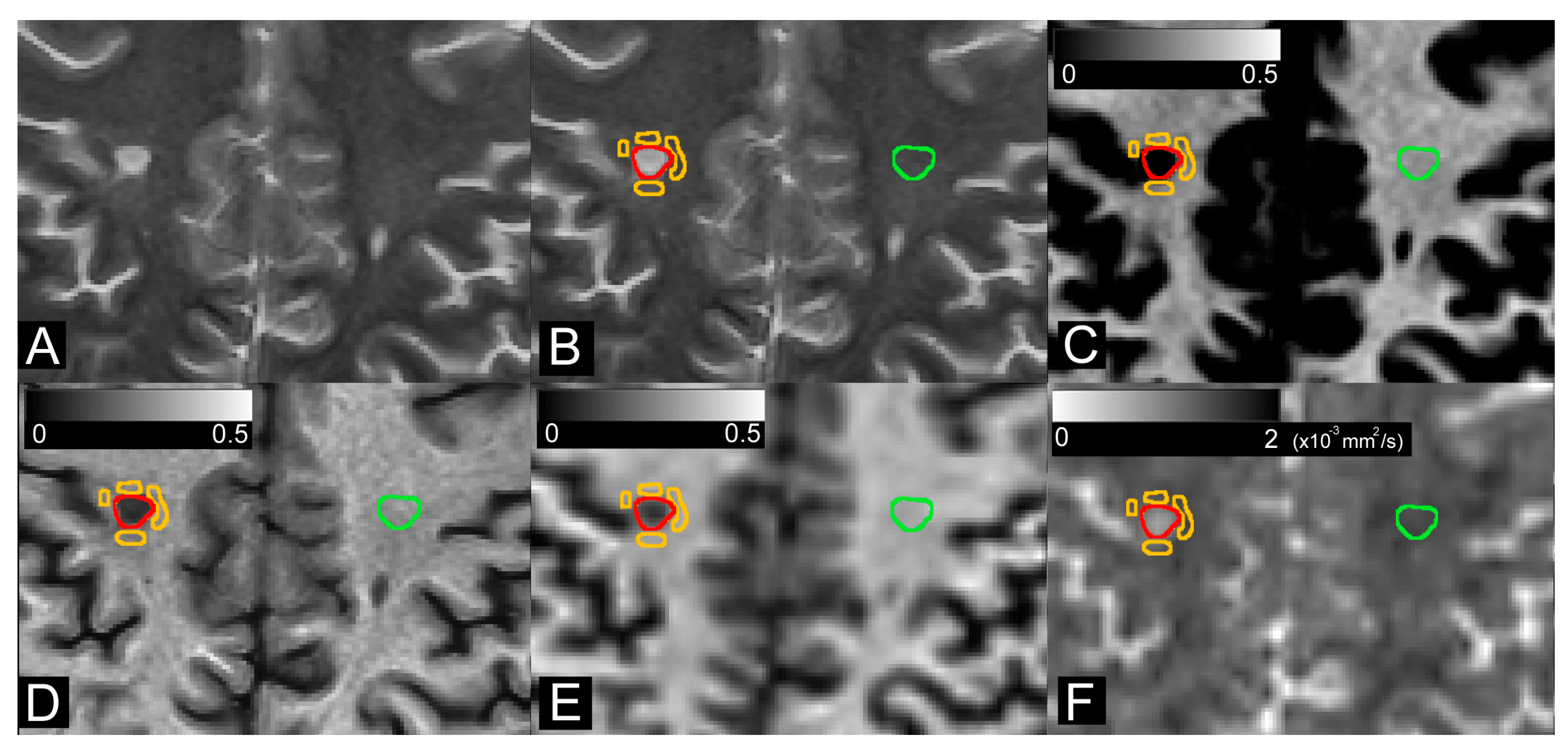
2.7. Image Analysis
2.8. Statistical Analysis
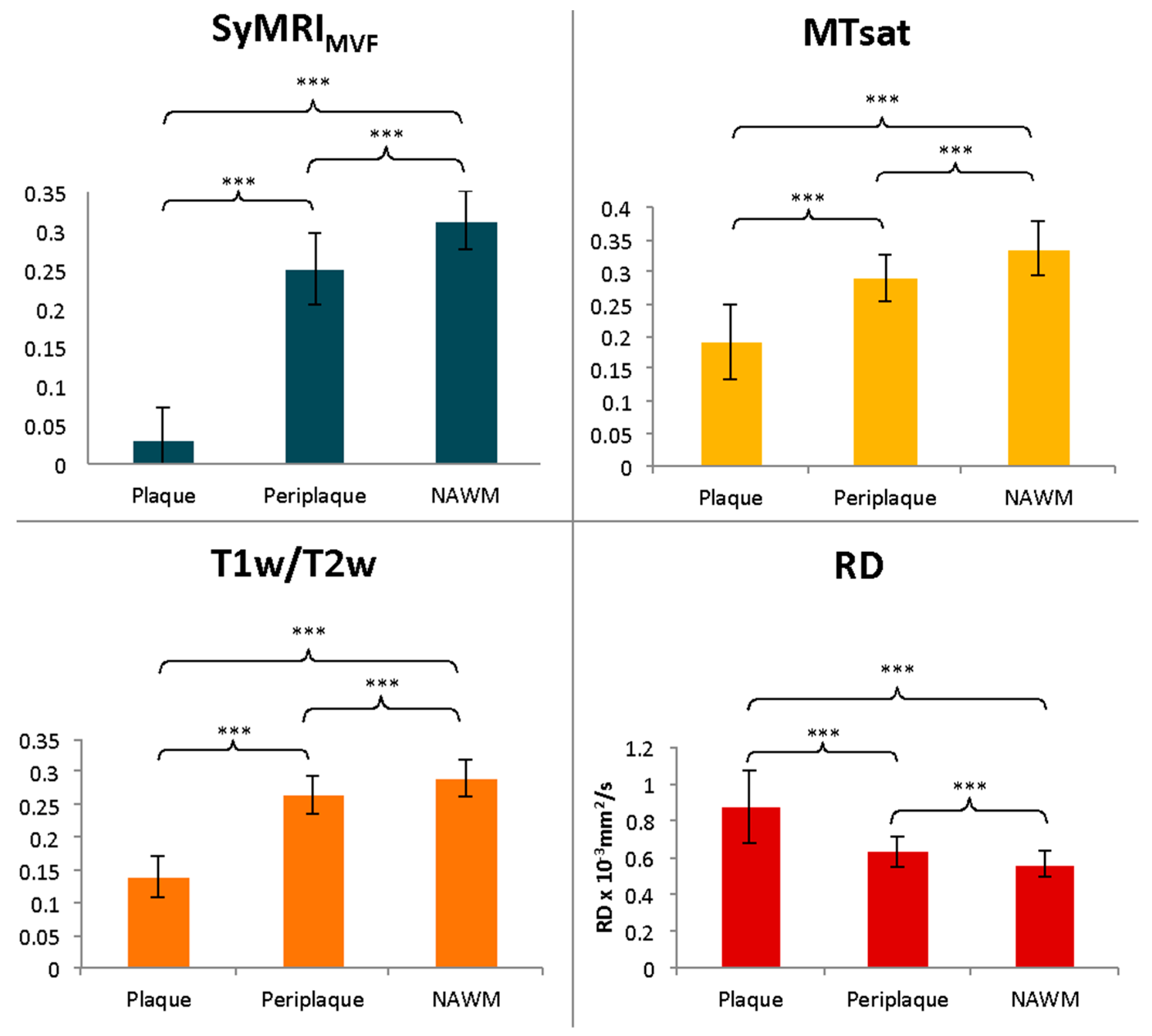
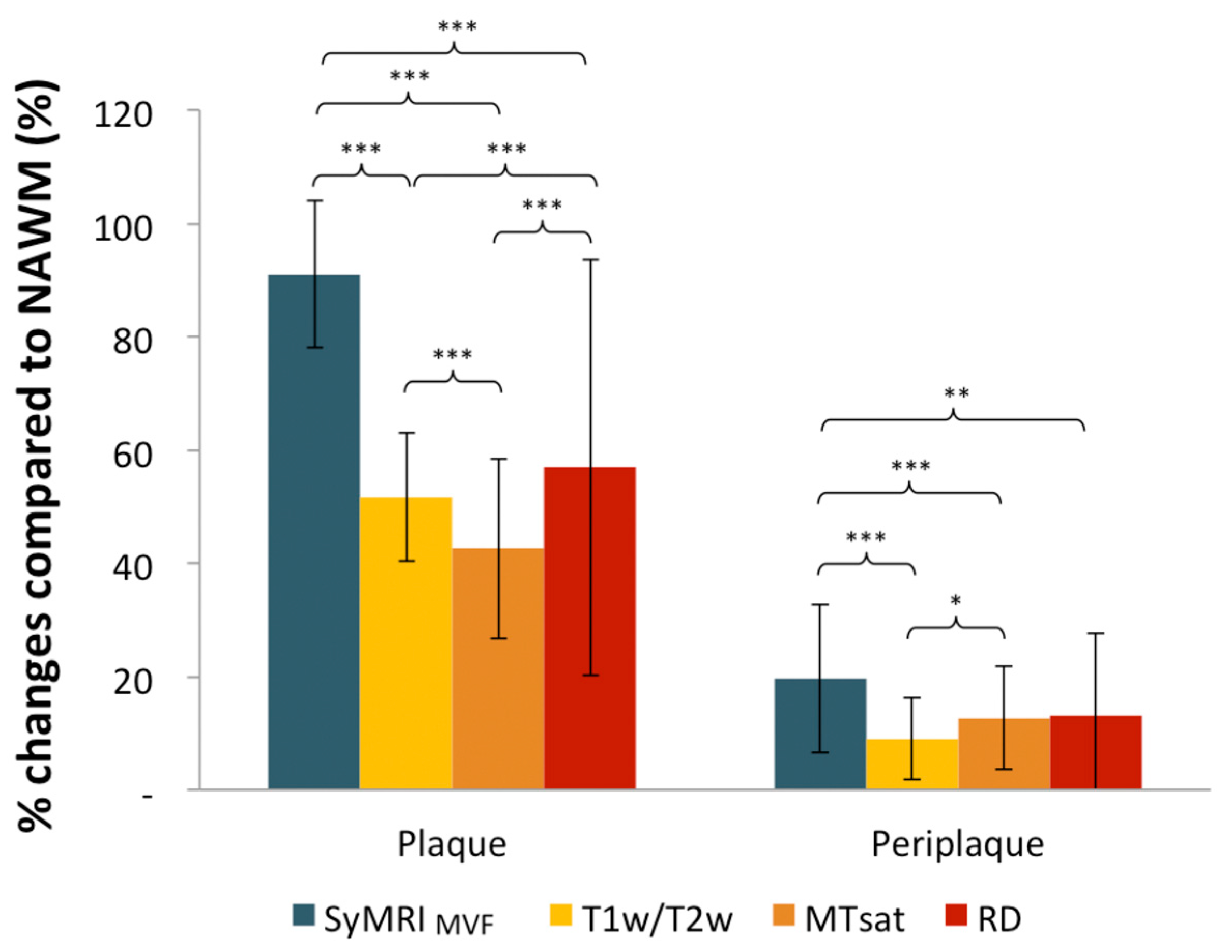
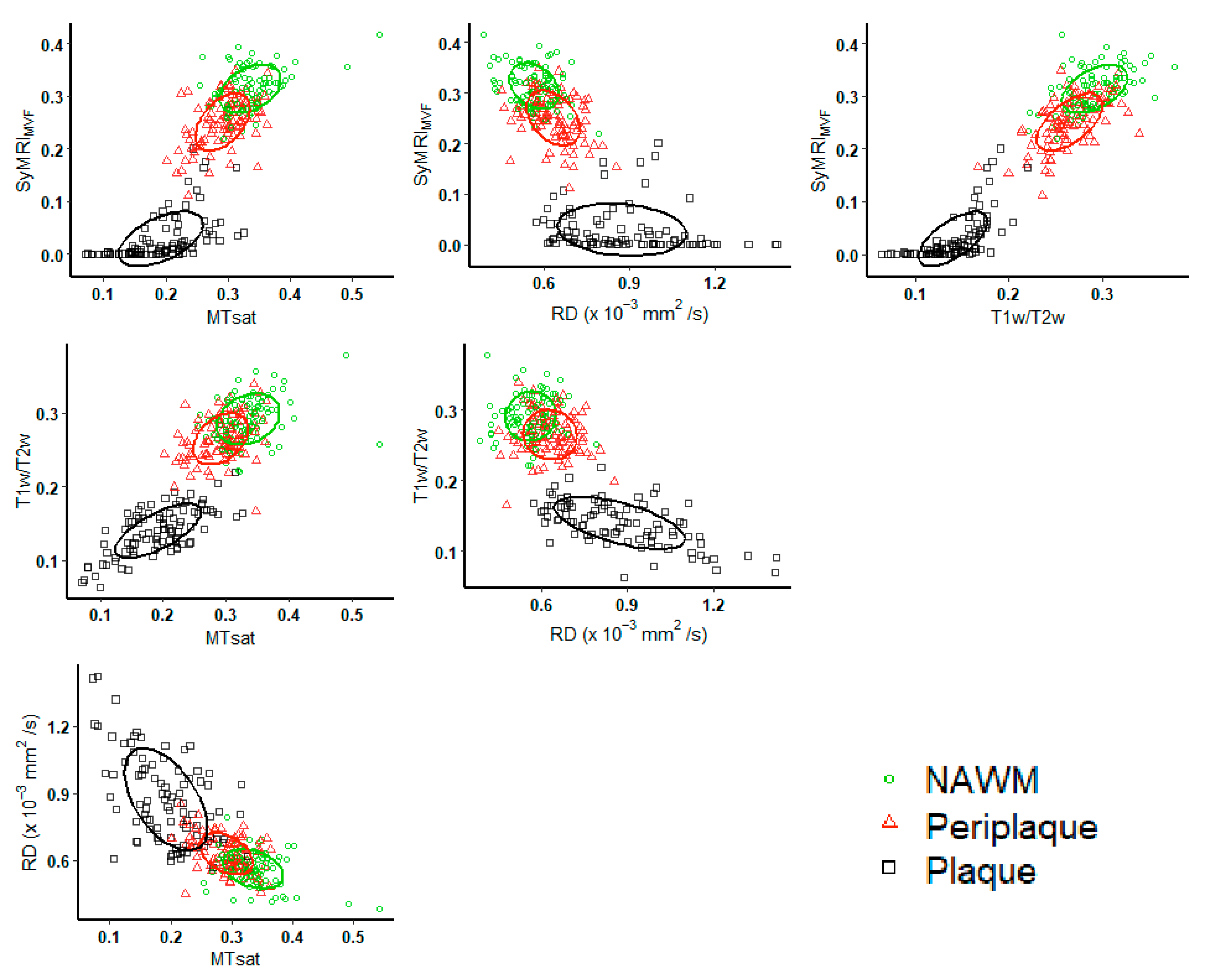
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Hoz, L.; Simons, M. The emerging functions of oligodendrocytes in regulating neuronal network behaviour. BioEssays 2015, 37, 60–69. [Google Scholar] [CrossRef]
- Duval, T.; Stikov, N.; Cohen-Adad, J. Modeling white matter microstructure. Funct. Neurol. 2016, 31, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Heath, F.; Hurley, S.A.; Johansen-Berg, H.; Sampaio-Baptista, C. Advances in noninvasive myelin imaging. Dev. Neurobiol. 2018, 78, 136–151. [Google Scholar] [CrossRef] [PubMed]
- King, E.; Sabatier, M.; Hoque, M.; Kesar, T.; Backus, D.; Borich, M. Myelin status is associated with change in functional mobility following slope walking in people with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Prasloski, T.; Rauscher, A.; MacKay, A.L.; Hodgson, M.; Vavasour, I.M.; Laule, C.; Mädler, B. Rapid whole cerebrum myelin water imaging using a 3D GRASE sequence. NeuroImage 2012, 63, 533–539. [Google Scholar] [CrossRef]
- Stüber, C.; Morawski, M.; Schäfer, A.; Labadie, C.; Wähnert, M.; Leuze, C.; Streicher, M.; Barapatre, N.; Reimann, K.; Geyer, S.; et al. Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. NeuroImage 2014, 93, 95–106. [Google Scholar] [CrossRef]
- Warntjes, M.; Engström, M.; Tisell, A.; Lundberg, P. Modeling the Presence of Myelin and Edema in the Brain Based on Multi-Parametric Quantitative MRI. Front. Neurol. 2016, 7, 16. [Google Scholar] [CrossRef]
- Warntjes, J.B.M.; Leinhard, O.D.; West, J.; Lundberg, P. Rapid magnetic resonance quantification on the brain: Optimization for clinical usage. Magn. Reson. Med. 2008, 60, 320–329. [Google Scholar] [CrossRef]
- Hagiwara, A.; Warntjes, M.; Hori, M.; Andica, C.; Nakazawa, M.; Kumamaru, K.K.; Abe, O.; Aoki, S. SyMRI of the Brain: Rapid Quantification of Relaxation Rates and Proton Density, With Synthetic MRI, Automatic Brain Segmentation and Myelin Measurement. Investig. Radiol. 2017, 52, 647–657. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hori, M.; Yokoyama, K.; Takemura, M.Y.; Andica, C.; Tabata, T.; Kamagata, K.; Suzuki, M.; Kumamaru, K.K.; Nakazawa, M.; et al. Synthetic MRI in the Detection of Multiple Sclerosis Plaques. Am. J. Neuroradiol. 2017, 38, 257–263. [Google Scholar] [CrossRef]
- Warntjes, J.B.M.; Persson, A.; Berge, J.; Zech, W. Myelin Detection Using Rapid Quantitative MR Imaging Correlated to Macroscopically Registered Luxol Fast Blue–Stained Brain Specimens. Am. J. Neuroradiol. 2017, 38, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, R.; Warntjes, M.; Forslin, Y.; Plattén, M.; Uppman, M.; Bergendal, Å.; Piehl, F.; Fredrikson, S.; Kristoffersen-Wiberg, M.; Mainero, C.; et al. Rapid estimation of myelin for diagnostic imaging (REMyDI)- A clinical and histopathological validation in multiple sclerosis. In Proceedings of the 26th Annual Meeting of ISMRM, Paris, France, 16–21 June 2018. [Google Scholar]
- Hagiwara, A.; Hori, M.; Kamagata, K.; Warntjes, M.; Matsuyoshi, D.; Nakazawa, M.; Ueda, R.; Andica, C.; Koshino, S.; Maekawa, T.; et al. Myelin Measurement: Comparison Between Simultaneous Tissue Relaxometry, Magnetization Transfer Saturation Index and T1w/T2w Ratio Methods. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Andica, C.; Hagiwara, A.; Hori, M.; Nakazawa, M.; Goto, M.; Koshino, S.; Kamagata, K.; Kumamaru, K.K.; Aoki, S. Automated brain tissue and myelin volumetry based on quantitative MR imaging with various in-plane resolutions. J. Neuroradiol. 2018, 45, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Hagiwara, A.; Hori, M.; Warntjes, M.; Kamagata, K.; Fukunaga, I.; Andica, C.; Maekawa, T.; Irie, R.; Takemura, M.; et al. Three-dimensional High-resolution Simultaneous Quantitative Mapping of the Whole Brain with 3D-QALAS: An Accuracy and Repeatability Study. Magn. Reson. Imaging 2019, 63, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Saccenti, L.; Andica, C.; Hagiwara, A.; Yokoyama, K.; Takemura, M.Y.; Fujita, S.; Maekawa, T.; Kamagata, K.; Le Berre, A.; Hori, M.; et al. Brain tissue and myelin volumetric analysis in multiple sclerosis at 3T MRI with various in-plane resolutions using synthetic MRI. Neuroradiology 2019, 61, 1219–1227. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hori, M.; Cohen-Adad, J.; Nakazawa, M.; Suzuki, Y.; Kasahara, A.; Horita, M.; Haruyama, T.; Andica, C.; Maekawa, T.; et al. Linearity, Bias, Intrascanner Repeatability and Interscanner Reproducibility of Quantitative Multidynamic Multiecho Sequence for Rapid Simultaneous Relaxometry at 3 T: A Validation Study With a Standardized Phantom and Healthy Controls. Investig. Radiol. 2019, 54, 39–47. [Google Scholar] [CrossRef]
- Hagiwara, A.; Kamagata, K.; Shimoji, K.; Yokoyama, K.; Andica, C.; Hori, M.; Fujita, S.; Maekawa, T.; Irie, R.; Akashi, T.; et al. White Matter Abnormalities in Multiple Sclerosis Evaluated by Quantitative Synthetic MRI, Diffusion Tensor Imaging and Neurite Orientation Dispersion and Density Imaging. Am. J. Neuroradiol. 2019, 40, 1642–1648. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hori, M.; Yokoyama, K.; Nakazawa, M.; Ueda, R.; Horita, M.; Andica, C.; Abe, O.; Aoki, S. Analysis of White Matter Damage in Patients with Multiple Sclerosis via a Novel In Vivo MR Method for Measuring Myelin, Axons and G-Ratio. Am. J. Neuroradiol. 2017, 38, 1934–1940. [Google Scholar] [CrossRef]
- Andica, C.; Hagiwara, A.; Hori, M.; Haruyama, T.; Fujita, S.; Maekawa, T.; Kamagata, K.; Yoshida, M.; Suzuki, M.; Sugano, H.; et al. Aberrant myelination in patients with Sturge-Weber syndrome analyzed using synthetic quantitative magnetic resonance imaging. Neuroradiology 2019, 61, 1387–1395. [Google Scholar] [CrossRef]
- Andica, C.; Hagiwara, A.; Nakazawa, M.; Tsuruta, K.; Takano, N.; Hori, M.; Suzuki, H.; Sugano, H.; Arai, H.; Aoki, S. The Advantage of Synthetic MRI for the Visualization of Early White Matter Change in an Infant with Sturge-Weber Syndrome. Magn. Reson. Med. Sci. 2016, 15, 347–348. [Google Scholar] [CrossRef]
- Wallaert, L.; Hagiwara, A.; Andica, C.; Hori, M.; Yamashiro, K.; Koshino, S.; Maekawa, T.; Kamagata, K.; Aoki, S. The Advantage of Synthetic MRI for the Visualization of Anterior Temporal Pole Lesions on Double Inversion Recovery (DIR), Phase-sensitive Inversion Recovery (PSIR) and Myelin Images in a Patient with CADASIL. Magn. Reson. Med. Sci. 2018, 17, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Scaravilli, F.; Altmann, D.R.; Barker, G.J.; Miller, D.H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 2004, 56, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Gass, A.; Barker, G.; Kidd, D.; Thorpe, J.W.; Macmanus, D.; Brennan, A.; Tofts, P.; Thompson, E.; McDonald, W.I.; Miller, D.H. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann. Neurol. 1994, 36, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Vavasour, I.M.; Laule, C.; Li, D.K.B.; Traboulsee, A.L.; MacKay, A.L. Is the magnetization transfer ratio a marker for myelin in multiple sclerosis? J. Magn. Reson. Imaging 2011, 33, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Graham, S.J. Magnetization transfer in MRI: A review. NMR Biomed. 2001, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Helms, G.; Dathe, H.; Kallenberg, K.; Dechent, P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn. Reson. Med. 2008, 60, 1396–1407. [Google Scholar] [CrossRef]
- Lema, A.; Bishop, C.; Malik, O.; Mattoscio, M.; Ali, R.; Nicholas, R.; Muraro, P.A.; Matthews, P.M.; Waldman, A.D.; Newbould, R.D. A Comparison of Magnetization Transfer Methods to Assess Brain and Cervical Cord Microstructure in Multiple Sclerosis. J. Neuroimaging 2017, 27, 221–226. [Google Scholar] [CrossRef]
- Shafee, R.; Buckner, R.L.; Fischl, B. Gray matter myelination of 1555 human brains using partial volume corrected MRI images. NeuroImage 2015, 105, 473–485. [Google Scholar] [CrossRef]
- Glasser, M.F.; Essen, D.C.V. Mapping Human Cortical Areas In Vivo Based on Myelin Content as Revealed by T1- and T2-Weighted MRI. J. Neurosci. 2011, 31, 11597–11616. [Google Scholar] [CrossRef]
- Grydeland, H.; Walhovd, K.B.; Tamnes, C.K.; Westlye, L.T.; Fjell, A.M. Intracortical Myelin Links with Performance Variability across the Human Lifespan: Results from T1- and T2-Weighted MRI Myelin Mapping and Diffusion Tensor Imaging. J. Neurosci. 2013, 33, 18618–18630. [Google Scholar] [CrossRef]
- Soun, J.E.; Liu, M.Z.; Cauley, K.A.; Grinband, J. Evaluation of neonatal brain myelination using the T1- and T2-weighted MRI ratio. J. Magn. Reson. Imaging 2017, 46, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Stanley, J.A.; Raz, N. Test–retest reliability and concurrent validity of in vivo myelin content indices: Myelin water fraction and calibrated T1w/T2w image ratio. Hum. Brain Mapp. 2017, 38, 1780–1790. [Google Scholar] [CrossRef]
- Righart, R.; Biberacher, V.; Jonkman, L.E.; Klaver, R.; Schmidt, P.; Buck, D.; Berthele, A.; Kirschke, J.S.; Zimmer, C.; Hemmer, B.; et al. Cortical pathology in multiple sclerosis detected by the T1/T2-weighted ratio from routine magnetic resonance imaging. Ann. Neurol. 2017, 82, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Sabisz, A.; Naumczyk, P.; Jodzio, K.; Szurowska, E.; Szarmach, A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front. Neurol. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-K.; Yoshino, J.; Le, T.Q.; Lin, S.-J.; Sun, S.-W.; Cross, A.H.; Armstrong, R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 2005, 26, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Klawiter, E.C.; Schmidt, R.E.; Trinkaus, K.; Liang, H.-F.; Budde, M.D.; Naismith, R.T.; Song, S.-K.; Cross, A.H.; Benzinger, T.L. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage 2011, 55, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.S.W.; Leppert, I.R.; Narayanan, S.; Boudreau, M.; Duval, T.; Cohen-Adad, J.; Pike, G.B.; Stikov, N. Promise and pitfalls of g-ratio estimation with MRI. NeuroImage 2018, 182, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Lipp, I.; Jones, D.K.; Bells, S.; Sgarlata, E.; Foster, C.; Stickland, R.; Davidson, A.E.; Tallantyre, E.C.; Robertson, N.P.; Wise, R.G.; et al. Comparing MRI metrics to quantify white matter microstructural damage in multiple sclerosis. Hum. Brain Mapp. 2019, 40, 2917–2932. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ganzetti, M.; Wenderoth, N.; Mantini, D. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Front. Hum. Neurosci. 2014, 8, 671. [Google Scholar] [CrossRef]
- Andersson, J.; Sotiropoulos, S. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2015, 125, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hori, M.; Yokoyama, K.; Takemura, M.Y.; Andica, C.; Kumamaru, K.K.; Nakazawa, M.; Takano, N.; Kawasaki, H.; Sato, S.; et al. Utility of a Multiparametric Quantitative MRI Model That Assesses Myelin and Edema for Evaluating Plaques, Periplaque White Matter and Normal-Appearing White Matter in Patients with Multiple Sclerosis: A Feasibility Study. Am. J. Neuroradiol. 2017, 38, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.C.; MacFall, J.R.; Provenzale, J.M. Multiple Sclerosis: Diffusion Tensor MR Imaging for Evaluation of Normal-appearing White Matter. Radiology 2002, 222, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.R. Comparing dependent robust correlations. Br. J. Math. Stat. Psychol. 2016, 69, 215–224. [Google Scholar] [CrossRef] [PubMed]
- comp2dcorr. Available online: https://github.com/GRousselet/blog/tree/master/comp2dcorr (accessed on 15 December 2019).
- Benjamini, Y.; Krieger, A.M.; Yekutieli, D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006, 93, 491–507. [Google Scholar] [CrossRef]
- Dziedzic, T.; Metz, I.; Dallenga, T.; König, F.B.; Müller, S.; Stadelmann, C.; Brück, W. Wallerian degeneration: A major component of early axonal pathology in multiple sclerosis. Brain Pathol. 2010, 20, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Moll, N.M.; Rietsch, A.M.; Thomas, S.; Ransohoff, A.J.; Lee, J.-C.; Fox, R.; Chang, A.; Ransohoff, R.M.; Fisher, E. Multiple Sclerosis Normal-Appearing White Matter: Pathology-Imaging Correlations. Ann. Neurol. 2011, 70, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.G.; Barnhart, H.X.; Buckler, A.J.; Choudhury, K.R.; Kondratovich, M.V.; Toledano, A.; Guimaraes, A.R.; Filice, R.; Zhang, Z.; Sullivan, D.C. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat. Methods Med. Res. 2015, 24, 9–26. [Google Scholar] [CrossRef]
- Bells, S.; Morris, D.; Vidarsson, L. Comparison of Linear Combination Filtering to DTI and MTR in Whole Brain Myelin-Water Imaging. In Proceedings of the 15th Annual Meeting of ISMRM, Berlin, Germany, 19–25 May 2007. Abstract 1606. [Google Scholar]
- O’Muircheartaigh, J.; Vavasour, I.; Ljungberg, E.; Li, D.K.B.; Rauscher, A.; Levesque, V.; Garren, H.; Clayton, D.; Tam, R.; Traboulsee, A.; et al. Quantitative neuroimaging measures of myelin in the healthy brain and in multiple sclerosis. Hum. Brain Mapp. 2019, 40, 2104–2116. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Figley, T.D.; Solar, K.G.; Shatil, A.S.; Figley, C.R. Comparisons between multi-component myelin water fraction, T1w/T2w ratio and diffusion tensor imaging measures in healthy human brain structures. Sci. Rep. 2019, 9, 2500. [Google Scholar] [CrossRef]
- Ludwin, S.K. The Pathogenesis of Multiple Sclerosis: Relating Human Pathology to Experimental Studies. J. Neuropathol. Exp. Neurol. 2006, 65, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Barkhof, F. The clinico-radiological paradox in multiple sclerosis revisited. Curr. Opin. Neurol. 2002, 15, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Obberghen, E.V.; Mchinda, S.; Le Troter, A.; Prevost, V.H.; Viout, P.; Guye, M.; Varma, G.; Alsop, D.C.; Ranjeva, J.-P.; Pelletier, J.; et al. Evaluation of the Sensitivity of Inhomogeneous Magnetization Transfer (ihMT) MRI for Multiple Sclerosis. Am. J. Neuroradiol. 2018, 39, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Blystad, I.; Håkansson, I.; Tisell, A.; Ernerudh, J.; Smedby, Ö.; Lundberg, P.; Larsson, E.-M. Quantitative MRI for Analysis of Active Multiple Sclerosis Lesions without Gadolinium-Based Contrast Agent. AJNR Am. J. Neuroradiol. 2016, 37, 94–100. [Google Scholar] [CrossRef]
| MS Patients | |
|---|---|
| No. of Subjects | 21 |
| Mean Age (yr) | 37.9 ± 9.9 |
| Sex (Male/Female) | 2:19 |
| Disease Duration (Mean) (yr) | 8.7 ± 6.5 |
| EDSS Score (range) | 1 (0–2) |
| MTsat | T1w/T2w | RD | |
|---|---|---|---|
| SyMRIMVF | 0.82 [0.77–0.87] *** | 0.89 [0.85–0.92] *** | −0.75 [−0.80–(−0.69)] *** |
| MTsat | 0.80 [0.74–0.85] *** | −0.72 [−0.78–(−0.65)] *** | |
| T1w/T2w | −0.66 [−0.73–(−0.57)] *** |
| MTsat | T1w/T2w | RD | ||
|---|---|---|---|---|
| Plaque | SyMRIMVF | 0.70 [0.58–0.79] *** | 0.78 [0.67–0.86] *** | −0.38 [−0.53–(−0.19)] *** |
| MTsat | 0.64 [0.47–0.77] *** | −0.48 [−0.65–(−0.29)] *** | ||
| T1w/T2w | −0.58 [−0.71–(−0.41)] *** | |||
| Periplaque | SyMRIMVF | 0.45 [0.23–0.60] *** | 0.62 [0.49–0.74] *** | 0.41 [0.23–0.56] *** |
| MTsat | 0.41 [0.22–0.59] *** | 0.40 [0.19–0.59] *** | ||
| T1w/T2w | −0.09 [−0.31–0.13] | |||
| NAWM | SyMRIMVF | 0.33 [0.13–0.51] ** | 0.50 [0.32–0.67] *** | −0.21 [−0.41–(−0.02)] |
| MTsat | 0.28 [0.11–0.47] * | −0.20 [−0.45–0.02] | ||
| T1w/T2w | 0.11 [−0.10–0.29] |
| SyMRIMVF | MTsat | T1w/T2w | RD | ||
|---|---|---|---|---|---|
| Plaque | EDSS | −0.10 [−0.57 to 0.39] | −0.29 [−0.71 to 0.22] | −0.0014 [−0.49 to 0.48] | −0.12 [−0.56 to 0.36] |
| Disease Duration | 0.20 [−0.36 to 0.64] | 0.17 [−0.34 to 0.63] | −0.17 [−0.67 to 0.38] | 0.32 [−0.17 to 0.77] | |
| Periplaque | EDSS | 0.13 [−0.33 to 0.51] | 0.12 [−0.38 to 0.57] | 0.24 [−0.26 to 0.66] | −0.31 [−0.68 to 0.11] |
| Disease Duration | −0.00065 [−0.52 to 0.45] | −0.12 [−0.51 to 0.38] | −0.24 [−0.67 to 0.26] | 0.23 [−0.25 to 0.66] | |
| NAWM | EDSS | 0.45 [0.031 to 0.76] | 0.15 [−0.32 to 0.60] | 0.23 [−0.28 to 0.63] | −0.47 [−0.80 to −0.056] |
| Disease Duration | −0.63 [−0.81 to −0.32] ** | −0.39 [−0.70 to 0.072] | −0.38 [−0.72 to 0.077] | 0.51 [0.11 to 0.81] * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saccenti, L.; Hagiwara, A.; Andica, C.; Yokoyama, K.; Fujita, S.; Kato, S.; Maekawa, T.; Kamagata, K.; Le Berre, A.; Hori, M.; et al. Myelin Measurement Using Quantitative Magnetic Resonance Imaging: A Correlation Study Comparing Various Imaging Techniques in Patients with Multiple Sclerosis. Cells 2020, 9, 393. https://doi.org/10.3390/cells9020393
Saccenti L, Hagiwara A, Andica C, Yokoyama K, Fujita S, Kato S, Maekawa T, Kamagata K, Le Berre A, Hori M, et al. Myelin Measurement Using Quantitative Magnetic Resonance Imaging: A Correlation Study Comparing Various Imaging Techniques in Patients with Multiple Sclerosis. Cells. 2020; 9(2):393. https://doi.org/10.3390/cells9020393
Chicago/Turabian StyleSaccenti, Laetitia, Akifumi Hagiwara, Christina Andica, Kazumasa Yokoyama, Shohei Fujita, Shimpei Kato, Tomoko Maekawa, Koji Kamagata, Alice Le Berre, Masaaki Hori, and et al. 2020. "Myelin Measurement Using Quantitative Magnetic Resonance Imaging: A Correlation Study Comparing Various Imaging Techniques in Patients with Multiple Sclerosis" Cells 9, no. 2: 393. https://doi.org/10.3390/cells9020393
APA StyleSaccenti, L., Hagiwara, A., Andica, C., Yokoyama, K., Fujita, S., Kato, S., Maekawa, T., Kamagata, K., Le Berre, A., Hori, M., Wada, A., Tateishi, U., Hattori, N., & Aoki, S. (2020). Myelin Measurement Using Quantitative Magnetic Resonance Imaging: A Correlation Study Comparing Various Imaging Techniques in Patients with Multiple Sclerosis. Cells, 9(2), 393. https://doi.org/10.3390/cells9020393





