Effect of Dietary Silk Peptide on Obesity, Hyperglycemia, and Skeletal Muscle Regeneration in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Design
2.3. Fasting Blood Glucose Measurement
2.4. Oral Glucose Tolerance Testing
2.5. Measurement of Rectal Temperature
2.6. Grip Strength Test
2.7. Serum Biochemical Analyses
2.8. Histologic and Immunofluorescence Analyses
2.9. Microcomputed Tomography (Micro-CT) Analysis
2.10. Forced Swimming Test (FST)
2.11. Cell Culture and Treatment
2.12. Cell Viability Test
2.13. Oil Red O Staining
2.14. Western Blot Analysis
2.15. Statistical Analysis
3. Results
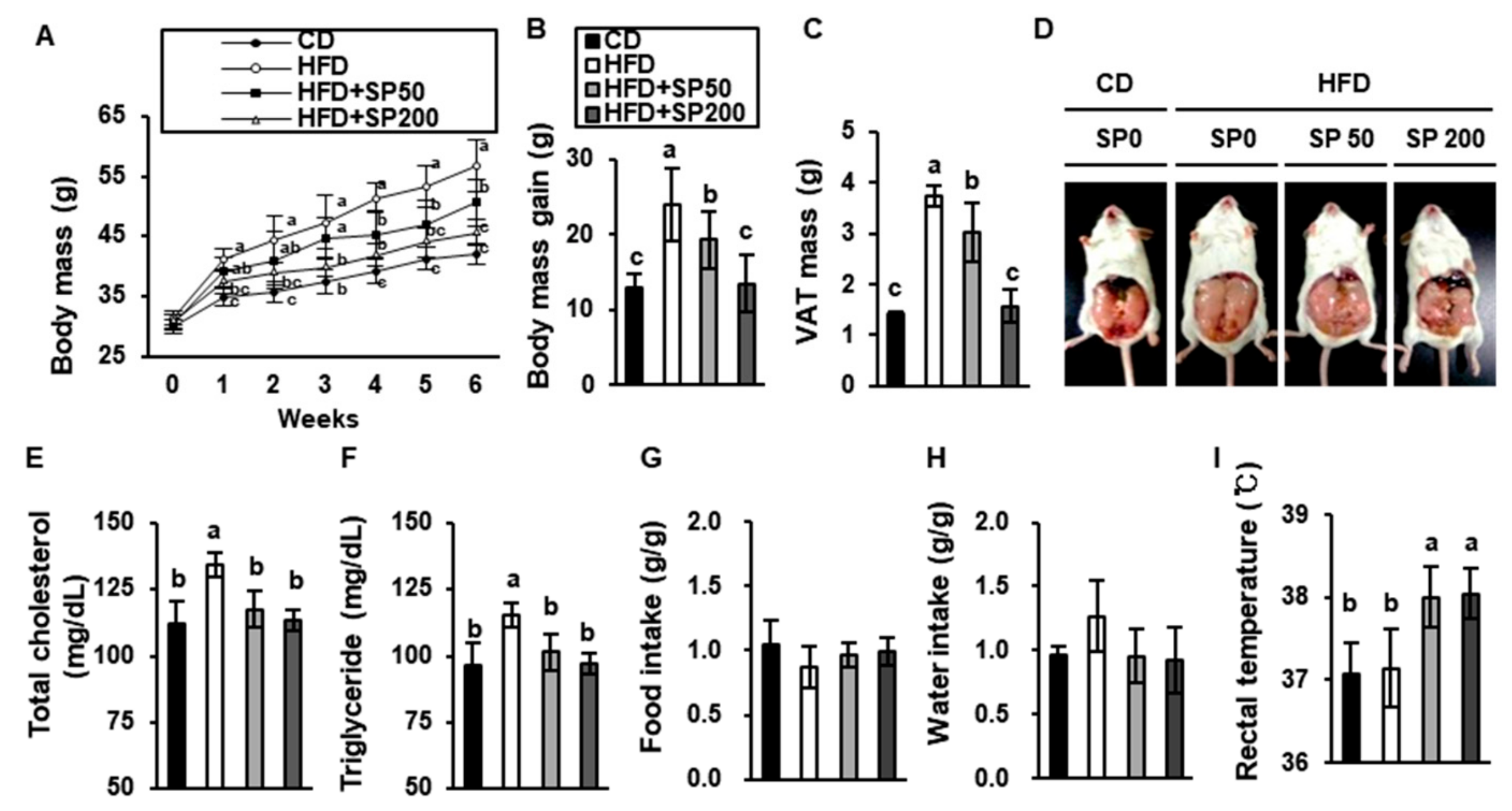
3.1. SP Ameliorates Body Mass Gain in HFD-Fed Mice
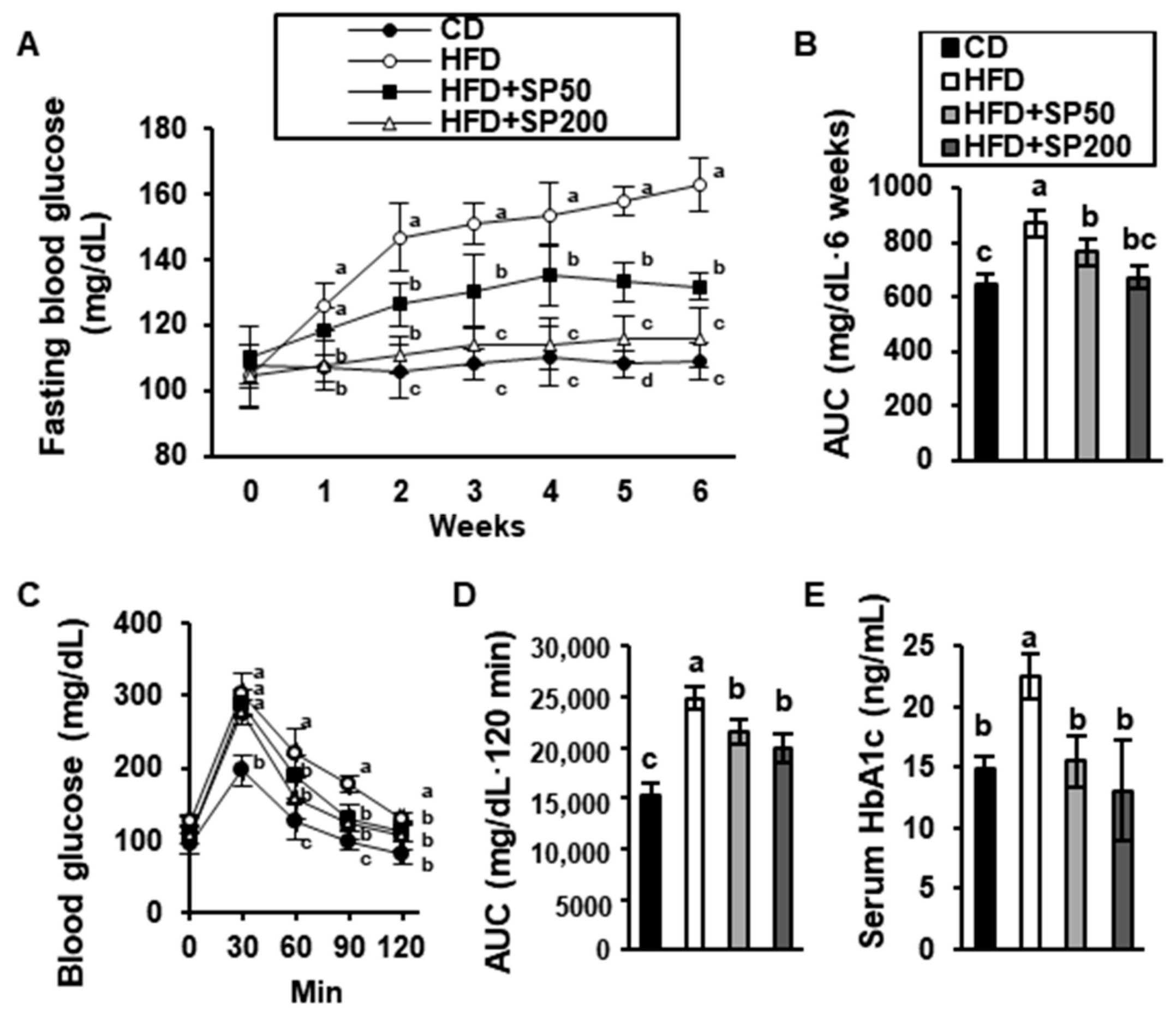
3.2. SP Regulates the Blood Glucose Concentration in HFD-Fed Mice
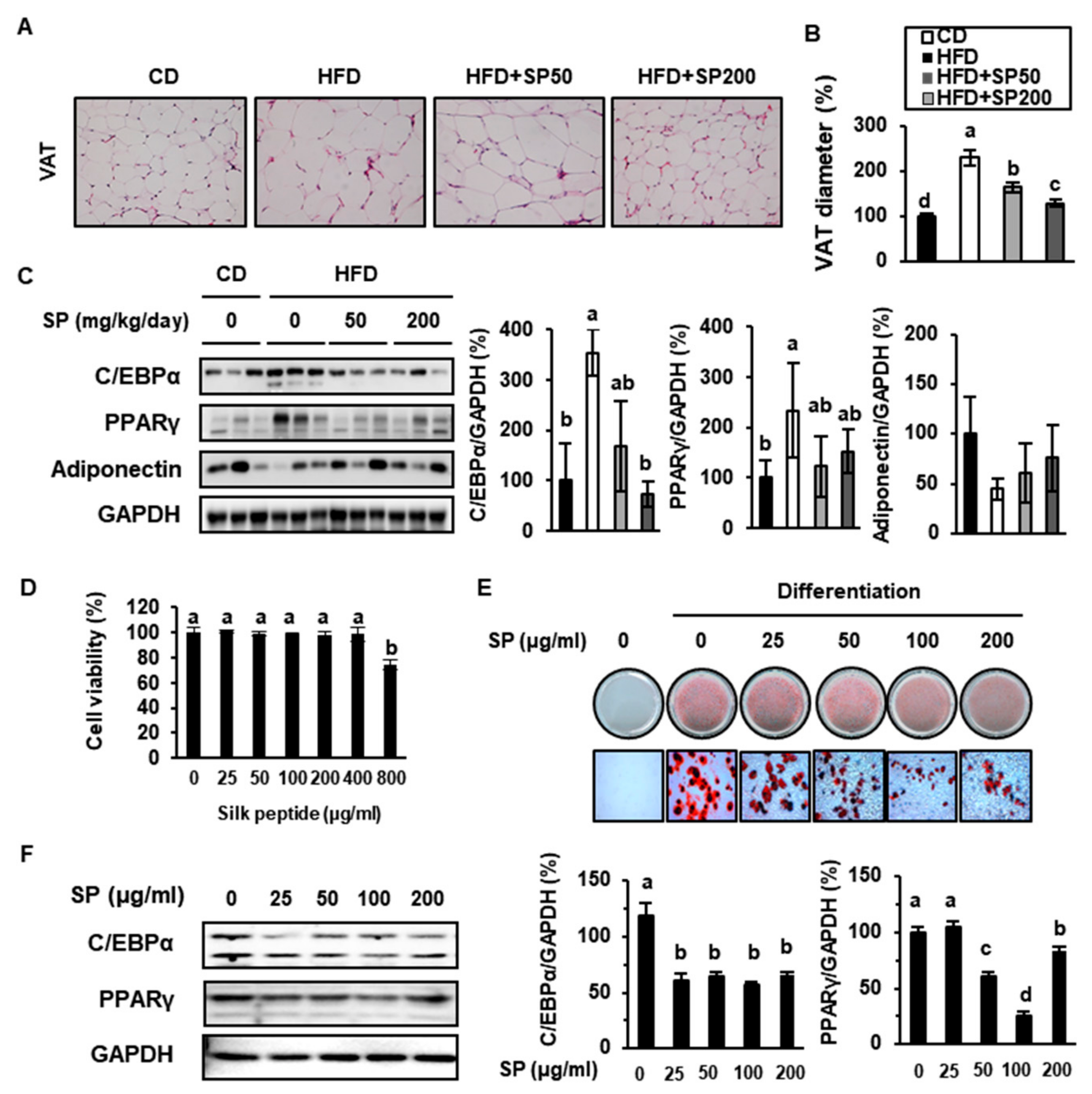
3.3. SP Ingestion Reduces Adiposity in HFD-Fed Mice
3.4. SP Increases Glucose Uptake into Adipose Tissue
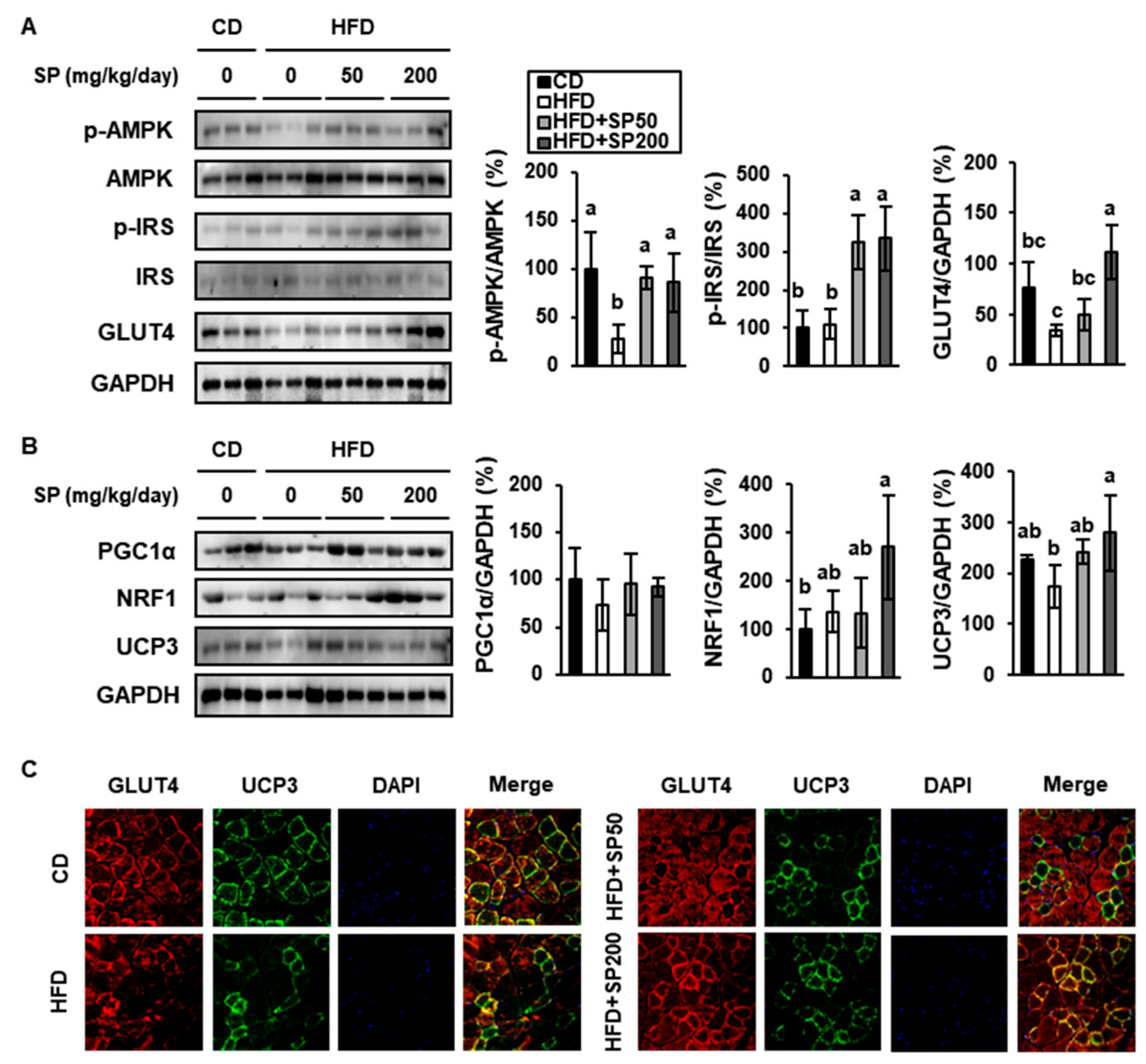
3.5. SP Increases the Phosphorylation of Insulin Signaling Intermediates and Mediators of Energy Consumption in Skeletal Muscle
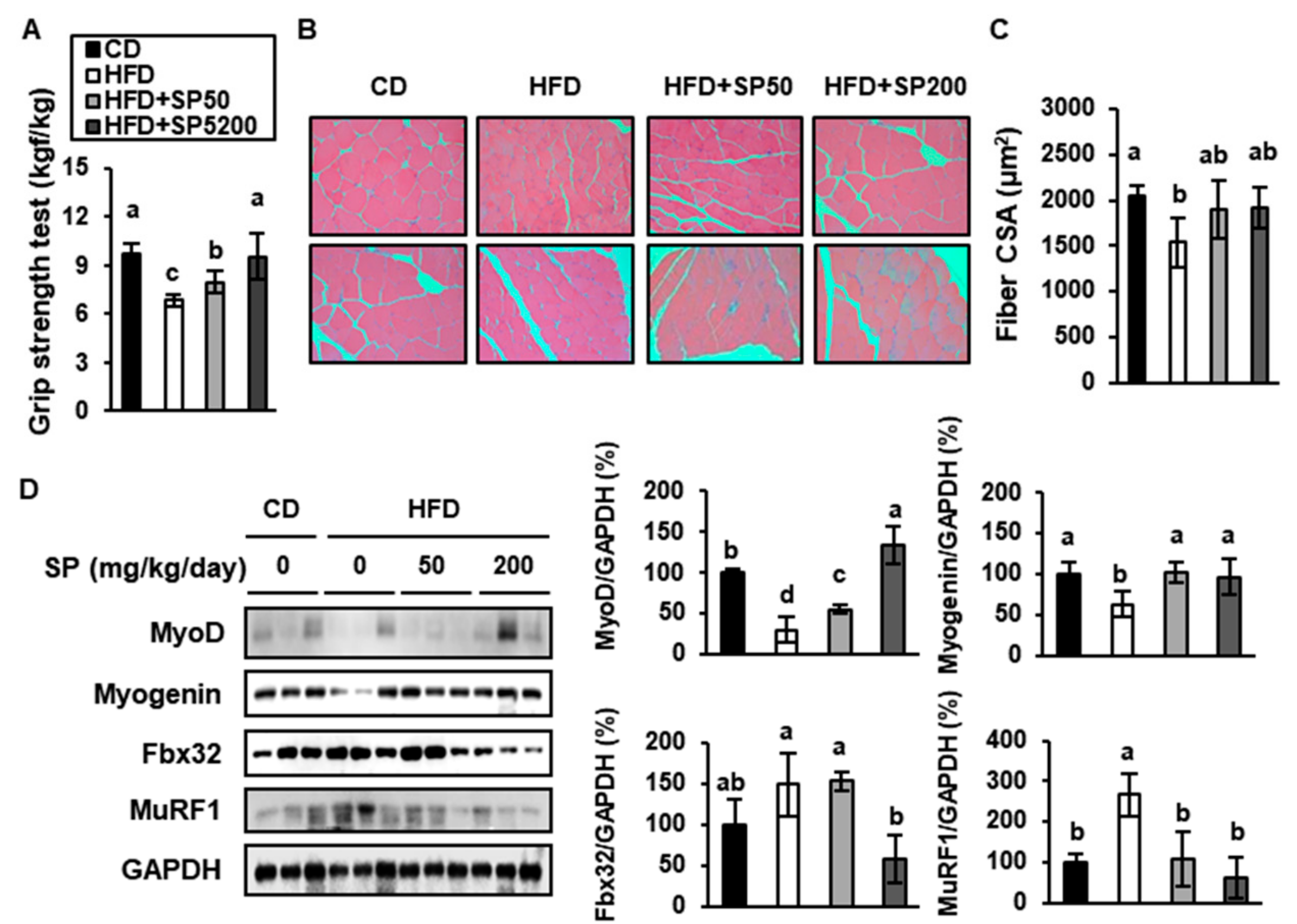
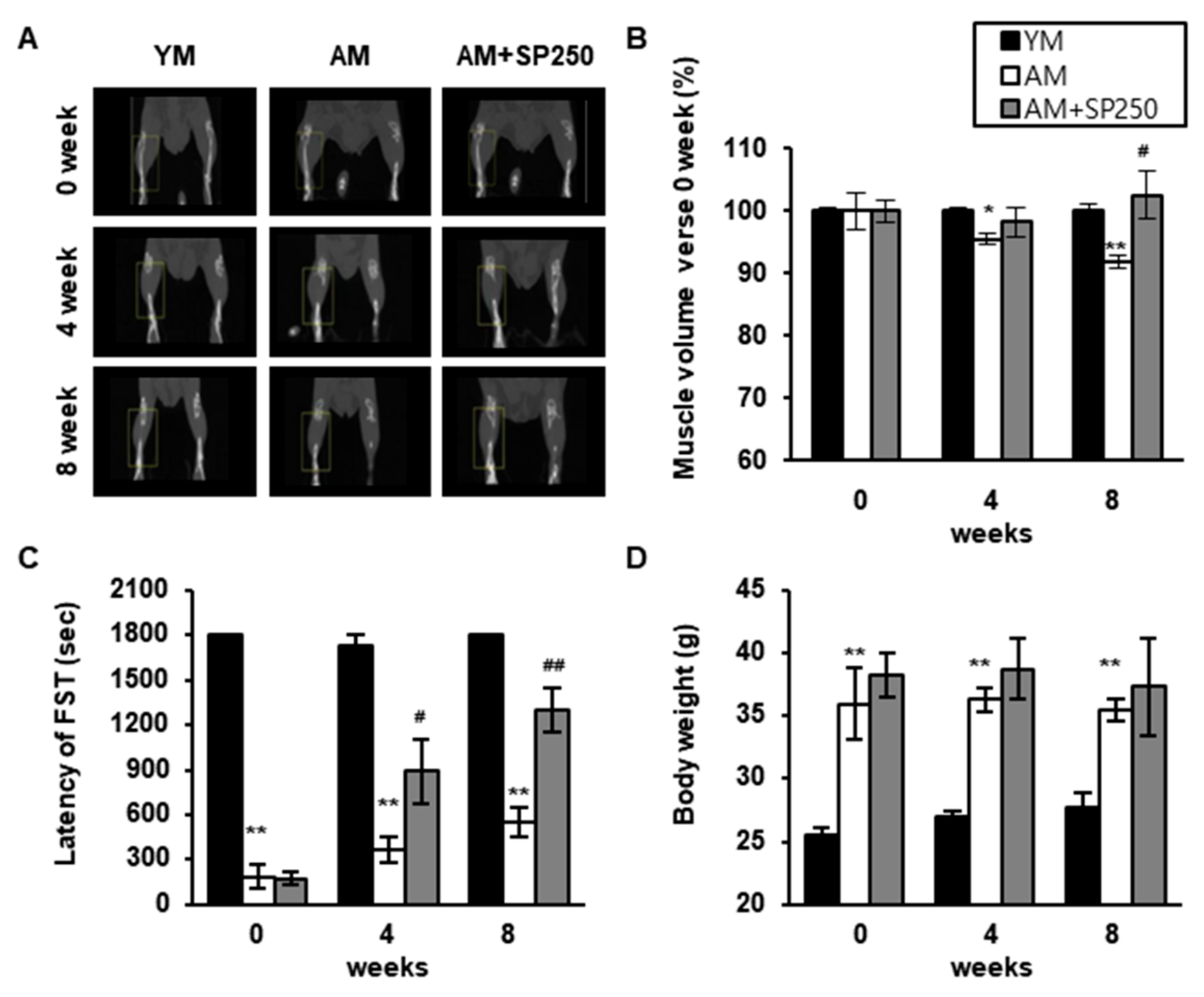
3.6. SP Increases the Expression of Genes Determining Skeletal Muscle Regeneration and Reduces Those Involved in Sarcopenia
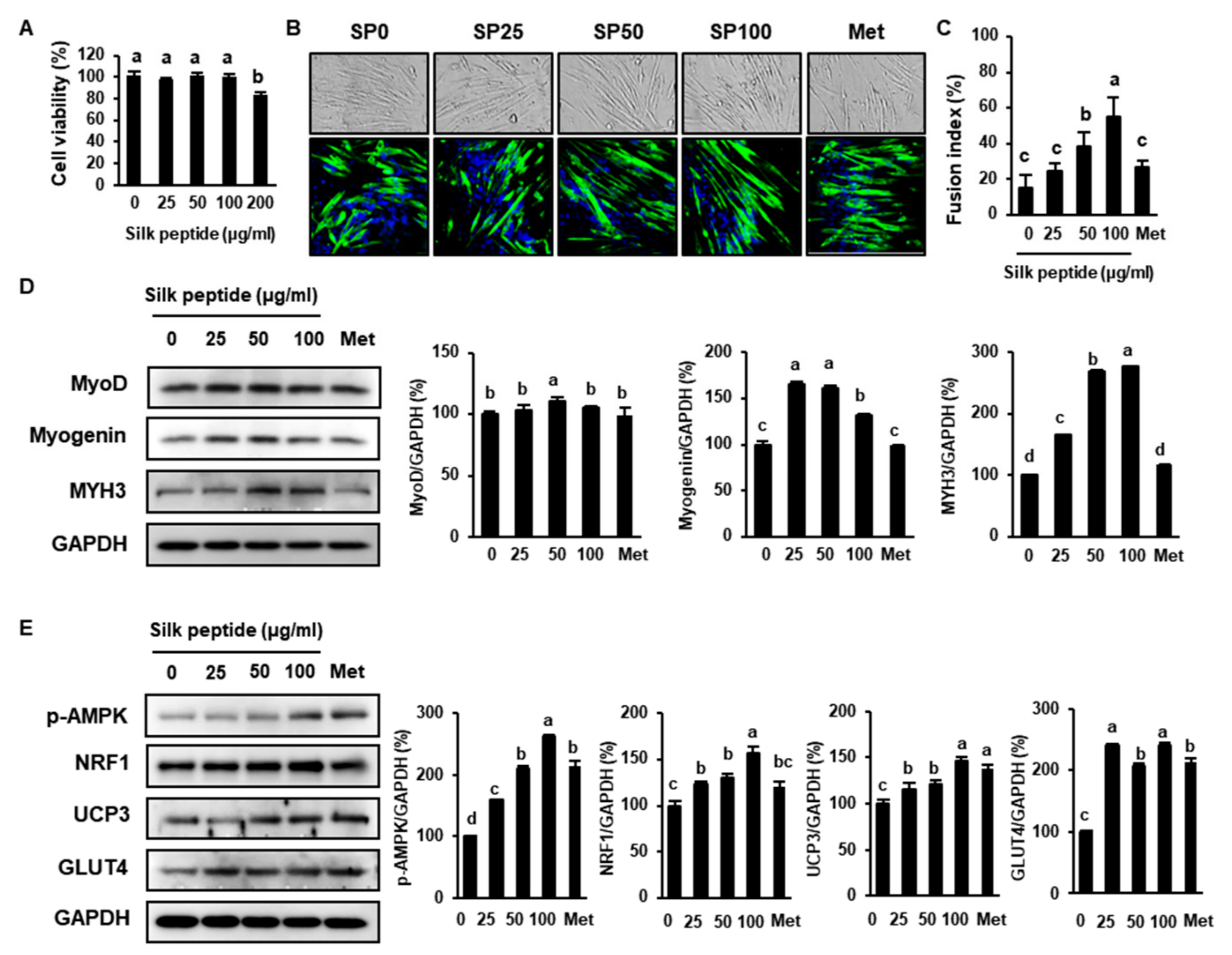
3.7. SP Stimulates Myoblast Differentiation and Glucose Metabolism in C2C12 Cells
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Wulandari, G.P.; Kristina, S.A. Direct and indirect cost of obesity: A systematic review. Glob. J. Health Sci. 2018, 10, 122–131. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottiere, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control CCC 2017, 28, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, Y.-J.; Song, J.-H.; Chei, S.; Lee, B.-Y. Ginsenoside Rg1 promotes browning by inducing UCP1 expression and mitochondrial activity in 3T3-L1 and subcutaneous white adipocytes. J. Ginseng Res. 2019, 43, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Marcadenti, A.; de Abreu-Silva, E.O. Different adipose tissue depots: Metabolic implications and effects of surgical removal. Endocrinol. Nutr. 2015, 62, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zheng, D.; Liu, J.; Fang, L.; Li, Q. Skeletal muscle mass to visceral fat area ratio is an important determinant associated with type 2 diabetes and metabolic syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1399. [Google Scholar] [CrossRef] [Green Version]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, H.S.; Seo, M.J.; Jeon, H.J.; Kim, K.J.; Lee, B.Y. Kaempferol suppresses lipid accumulation by inhibiting early adipogenesis in 3T3-L1 cells and zebrafish. Food Funct. 2015, 6, 2824–2833. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawati, J.A. Diabetes mellitus: A local and global public health emergency! Oman Med. J. 2017, 32, 177. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Kumari, M.; Head, J.; Ritchie, K.; Ancelin, M.-L.; Tabák, A.G.; Brunner, E.; Chaudieu, I.; Marmot, M.G.; Ferrie, J.E. Glycemia, insulin resistance, insulin secretion, and risk of depressive symptoms in middle age. Diabetes Care 2013, 36, 928–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhang, S.; Du, M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr. Res. 2015, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef]
- Bradley, H.; Shaw, C.S.; Worthington, P.L.; Shepherd, S.O.; Cocks, M.; Wagenmakers, A.J. Quantitative immunofluorescence microscopy of subcellular GLUT 4 distribution in human skeletal muscle: Effects of endurance and sprint interval training. Physiol. Rep. 2014, 2, e12085. [Google Scholar] [CrossRef]
- Stockli, J.; Fazakerley, D.J.; James, D.E. GLUT4 exocytosis. J. Cell Sci. 2011, 124, 4147–4159. [Google Scholar] [CrossRef] [Green Version]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. In Glucose Transport; IntechOpen: London, UK, 2018. [Google Scholar]
- Ferreira, G.D.; Germeyer, A.; de Barros Machado, A.; do Nascimento, T.L.; Strowitzki, T.; Brum, I.S.; von Eye Corleta, H.; Capp, E. Metformin modulates PI3K and GLUT4 expression and Akt/PKB phosphorylation in human endometrial stromal cells after stimulation with androgen and insulin. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 175, 157–162. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177. [Google Scholar] [CrossRef]
- Jang, H.C. Sarcopenia, frailty, and diabetes in older adults. Diabetes Metab. J. 2016, 40, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, A.; Ogawa, Y.; Takenoshita, N.; Kaneko, Y.; Hatanaka, H.; Jaime, E.; Fukasawa, R.; Hanyu, H. Decreased muscle strength and quality in diabetes-related dementia. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Rymarz, A.; Zajbt, M.; Jeznach-Steinhagen, A.; Woźniak-Kosek, A.; Niemczyk, S. Body Composition and Biochemical Markers of Nutrition in Non-dialysis-Dependent Chronic Kidney Disease Patients; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Park, S.S.; Kwon, E.-S.; Kwon, K.-S. Molecular mechanisms and therapeutic interventions in sarcopenia. Osteoporos. Sarcopenia 2017, 3, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, K. Exercise-induced PGC-1α transcriptional factors in skeletal muscle. Integr. Med. Res. 2014, 3, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapham, J.C.; Arch, J.R.; Chapman, H.; Haynes, A.; Lister, C.; Moore, G.B.; Piercy, V.; Carter, S.A.; Lehner, I.; Smith, S.A. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature 2000, 406, 415. [Google Scholar] [CrossRef]
- Zhou, M.; Lin, B.-Z.; Coughlin, S.; Vallega, G.; Pilch, P.F. UCP-3 expression in skeletal muscle: Effects of exercise, hypoxia, and AMP-activated protein kinase. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E622–E629. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Progress Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, H.J.; Suh, H.J. Silk protein hydrolysate increases glucose uptake through up-regulation of GLUT 4 and reduces the expression of leptin in 3T3-L1 fibroblast. Nutr. Res. 2011, 31, 937–943. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, H.; Yun, H.-Y.; Kim, B.; Lee, C.-H.; Suh, H.; Lim, K. Silk peptide intake increases fat oxidation at rest in exercised mice. J. Nutr. Sci. Vitaminol. 2013, 59, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Jin, H.; Chei, S.; Lee, J.-Y.; Oh, H.-J.; Lee, B.-Y. Dietary silk peptide prevents high-fat diet-induced obesity and promotes adipose browning by activating AMP-activated protein kinase in mice. Nutrients 2020, 12, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miettinen, T.A. Cholesterol production in obesity. Circulation 1971, 44, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Ginde, A.A.; Cagliero, E.; Nathan, D.M.; Camargo, C.A., Jr. Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J. Gen. Intern. Med. 2008, 23, 1346–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosaee, S.; Khodadost, M.; Esteghamati, A.; Speakman, J.R.; Djafarian, K.; Bitarafan, V.; Shidfar, F. Adiponectin: An indicator for metabolic syndrome. Iran. J. Public Health 2019, 48, 1106–1115. [Google Scholar] [PubMed]
- Chon, J.-W.; Lee, K.-G.; Park, Y.-K.; Park, K.-H.; Yeo, J.-H. Anti-adipogenic Effect of Hydrolysate Silk Fibroin in 3T3-L1 Cells. Int. J. Ind. Entomol. 2010, 21, 169–174. [Google Scholar]
- Ikemoto, S.; Takahashi, M.; Tsunoda, N.; Maruyama, K.; Itakura, H.; Ezaki, O. High-fat diet-induced hyperglycemia and obesity in mice: Differential effects of dietary oils. Metab. Clin. Exp. 1996, 45, 1539–1546. [Google Scholar] [CrossRef]
- Morales, P.E.; Bucarey, J.L.; Espinosa, A. Muscle lipid metabolism: Role of lipid droplets and perilipins. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef]
- Kim, N.; Nam, M.; Kang, M.S.; Lee, J.O.; Lee, Y.W.; Hwang, G.-S.; Kim, H.S. Piperine regulates UCP1 through the AMPK pathway by generating intracellular lactate production in muscle cells. Sci. Rep. 2017, 7, 41066. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brioche, T. Sarcopenia: Mechanisms and Prevention: Role of Exercise and Growth Hormone: Involvement of Oxidative Stress and Glucose-6-Phosphate Dehydrogenase. Ph.D. Thesis, Tabriz University of Medical Sciences, Rennes, France, 2014. [Google Scholar]
- Tang, J.; He, A.; Yan, H.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Tian, G.; Shang, H.; Zhao, H. Damage to the myogenic differentiation of C2C12 cells by heat stress is associated with up-regulation of several selenoproteins. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Banagozar Mohammadi, A.; Sadigh-Eteghad, S.; Torbati, M.; Bagher Fazljou, S.M.; Vatandoust, S.M.; EJ Golzari, S.; Farajdokht, F.; Mahmoudi, J. Identification and applications of neuroactive silk proteins: A narrative review. J. Appl. Biomed. 2019, 17, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Byun, E.-B.; Sung, N.-Y.; Kim, J.-H.; Choi, J.-i.; Matsui, T.; Byun, M.-W.; Lee, J.-W. Enhancement of anti-tumor activity of gamma-irradiated silk fibroin via immunomodulatory effects. Chem. Biol. Interact. 2010, 186, 90–95. [Google Scholar] [CrossRef]
- Kim, D.W.; Hwang, H.S.; Kim, D.-S.; Sheen, S.H.; Heo, D.H.; Hwang, G.; Kang, S.H.; Kweon, H.; Jo, Y.-Y.; Kang, S.W. Effect of silk fibroin peptide derived from silkworm Bombyx mori on the anti-inflammatory effect of Tat-SOD in a mice edema model. Proteins 2011, 12, 13. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.; Sun, Q.; Sun, X.; Wang, Y.; Zhao, P. Preparation and characterization of silver nanoparticles on silk fibroin/carboxymethylchitosan composite sponge as anti-bacterial wound dressing. Bio Med Mater. Eng. 2015, 26, S111–S118. [Google Scholar] [CrossRef] [Green Version]
- Song, D.W.; Kim, S.H.; Kim, H.H.; Lee, K.H.; Ki, C.S.; Park, Y.H. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: Implications for wound healing. Acta Biomater. 2016, 39, 146–155. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Mau, T.; Yung, R. Adipose tissue inflammation in aging. Exp. Gerontol. 2018, 105, 27–31. [Google Scholar] [CrossRef]
- Satoh, T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. Int. J. Mol. Sci. 2014, 15, 18677–18692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Martin, S.A.; Haren, M.T.; Taylor, A.W.; Wittert, G.A. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metab. Clin. Exp. 2009, 58, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhang, T.; Qiu, J.Y.; Wu, X.; Lee, B.Y. Silk amino acid consumption improves anti-diabetic symptoms by potentiating insulin secretion and preventing gut microbiome dysbiosis in non-obese type 2 diabetic animals. Nutrients 2020, 12, 311. [Google Scholar] [CrossRef] [Green Version]
- Kaysen, G.A. Biochemistry and biomarkers of inflamed patients: Why look, what to assess. Clin. J. Am. Soc. Nephrol. 2009, 4, S56–S63. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hwang, J.-T.; Park, H.S.; Kwon, D.Y.; Kim, M.-S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef]
- Min, W.; Wu, M.; Fang, P.; Yu, M.; Shi, M.; Zhang, Z.; Bo, P. Effect of baicalein on GLUT4 translocation in adipocytes of diet-induced obese mice. Cell. Physiol. Biochem. 2018, 50, 426–436. [Google Scholar] [CrossRef] [Green Version]
- Devarshi, P.; McNabney, S.; Henagan, T. Skeletal muscle nucleo-mitochondrial crosstalk in obesity and type 2 diabetes. Int. J. Mol. Sci. 2017, 18, 831. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Ji, L.L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wenz, T. Mitochondria and PGC-1α in aging and age-associated diseases. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Honma, N.; Kobayashi, K.; Jia, L.N.; Hosono, T.; Shindo, K.; Ariga, T.; Seki, T. Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS ONE 2014, 9, e87894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryer, L.G.; Parbu-Patel, A.; Carling, D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.-P.; Alizargar, J.; Bai, C.-H. Resveratrol attenuates high-fat diet-induced obesity and the aging-related sarcopenia mitochondrial dysfunction in skeletal muscle. BioRxiv 2019, 823088. [Google Scholar]
- Rasool, S.; Geetha, T.; Broderick, T.L.; Babu, J.R. High fat with high sucrose diet leads to obesity and induces myodegeneration. Front. Physiol. 2018, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Kang, S.; Meng, X.; Kang, A.; Park, J.; Park, Y.-K.; Jung, H. Effects of rhizome extract of dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in c2c12 myotubes. Molecules 2018, 23, 2023. [Google Scholar] [CrossRef] [Green Version]
- Argilés, J.M.; Campos, N.; Lopez-Pedrosa, J.M.; Rueda, R.; Rodriguez-Mañas, L. Skeletal muscle regulates metabolism via interorgan crosstalk: Roles in health and disease. J. Am. Med Dir. Assoc. 2016, 17, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Megeney, L.A.; Kablar, B.; Garrett, K.; Anderson, J.E.; Rudnicki, M.A. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996, 10, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High fat diet-induced skeletal muscle wasting is decreased by mesenchymal stem cells administration: Implications on oxidative stress, ubiquitin proteasome pathway activation, and myonuclear apoptosis. Oxidative Med. Cell. Longev. 2016, 2016, 9047821. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Organs | Organ Weight (g) | |||
|---|---|---|---|---|
| CD | HFD | HFD + SP50 * | HFD + SP200 * | |
| Visceral white adipose tissue (VAT) | 1.42 ± 0.05 c | 3.74 ± 0.51 a | 3.03 ± 0.57 b | 1.57 ± 0.33 c |
| Subcutaneous white adipose tissue (SAT) | 0.74 ± 0.18 c | 1.67 ± 0.51 a | 1.43 ± 0.44 b | 0.95 ± 0.28 c |
| Brown adipose tissue (BAT) | 0.13 ± 0.03 a | 0.13 ± 0.0.3 a | 0.12 ± 0.04 a | 0.11 ± 0.02 a |
| Liver | 1.59 ± 0.21 a | 1.69 ± 0.15 a | 1.62 ± 0.32 a | 1.46 ± 0.14 a |
| Lung | 0.23 ± 0.03 a | 0.24 ± 0.02 a | 0.22 ± 0.02 a | 0.24 ± 0.02 a |
| Kidney | 0.64 ± 0.08 a | 0.67 ± 0.07 a | 0.63 ± 0.08 a | 0.63 ± 0.04 a |
| Spleen | 0.13 ± 0.02 a | 0.13 ± 0.02 a | 0.13 ± 0.02 a | 0.13 ± 0.06 a |
| Group | Blood Parameter (mg/dL) | |||
|---|---|---|---|---|
| CD | HFD | HFD + SP50 * | HFD + SP200 * | |
| Creatinine | 0.22 ± 0.04 a | 0.25 ± 0.04 a | 0.22 ± 0.03 a | 0.23 ± 0.02 a |
| Aspartate aminotransferase (AST) | 36.67 ± 7.37 a | 41.33 ± 5.51 a | 35.53 ± 9.07 a | 35.33 ± 3.51 a |
| Alanine aminotransferase (ALT) | 70.33 ± 9.50 a | 76.00 ± 5.20 a | 64.67 ± 12.42 a | 62.67 ± 8.33 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Jin, H.; Chei, S.; Oh, H.-J.; Lee, J.-Y.; Lee, B.-Y. Effect of Dietary Silk Peptide on Obesity, Hyperglycemia, and Skeletal Muscle Regeneration in High-Fat Diet-Fed Mice. Cells 2020, 9, 377. https://doi.org/10.3390/cells9020377
Lee K, Jin H, Chei S, Oh H-J, Lee J-Y, Lee B-Y. Effect of Dietary Silk Peptide on Obesity, Hyperglycemia, and Skeletal Muscle Regeneration in High-Fat Diet-Fed Mice. Cells. 2020; 9(2):377. https://doi.org/10.3390/cells9020377
Chicago/Turabian StyleLee, Kippeum, Heegu Jin, Sungwoo Chei, Hyun-Ji Oh, Jeong-Yong Lee, and Boo-Yong Lee. 2020. "Effect of Dietary Silk Peptide on Obesity, Hyperglycemia, and Skeletal Muscle Regeneration in High-Fat Diet-Fed Mice" Cells 9, no. 2: 377. https://doi.org/10.3390/cells9020377
APA StyleLee, K., Jin, H., Chei, S., Oh, H.-J., Lee, J.-Y., & Lee, B.-Y. (2020). Effect of Dietary Silk Peptide on Obesity, Hyperglycemia, and Skeletal Muscle Regeneration in High-Fat Diet-Fed Mice. Cells, 9(2), 377. https://doi.org/10.3390/cells9020377






