Impact of Melatonin on Skeletal Muscle and Exercise
Abstract
1. Skeletal Muscle Structure and Function in Aging and Diseases
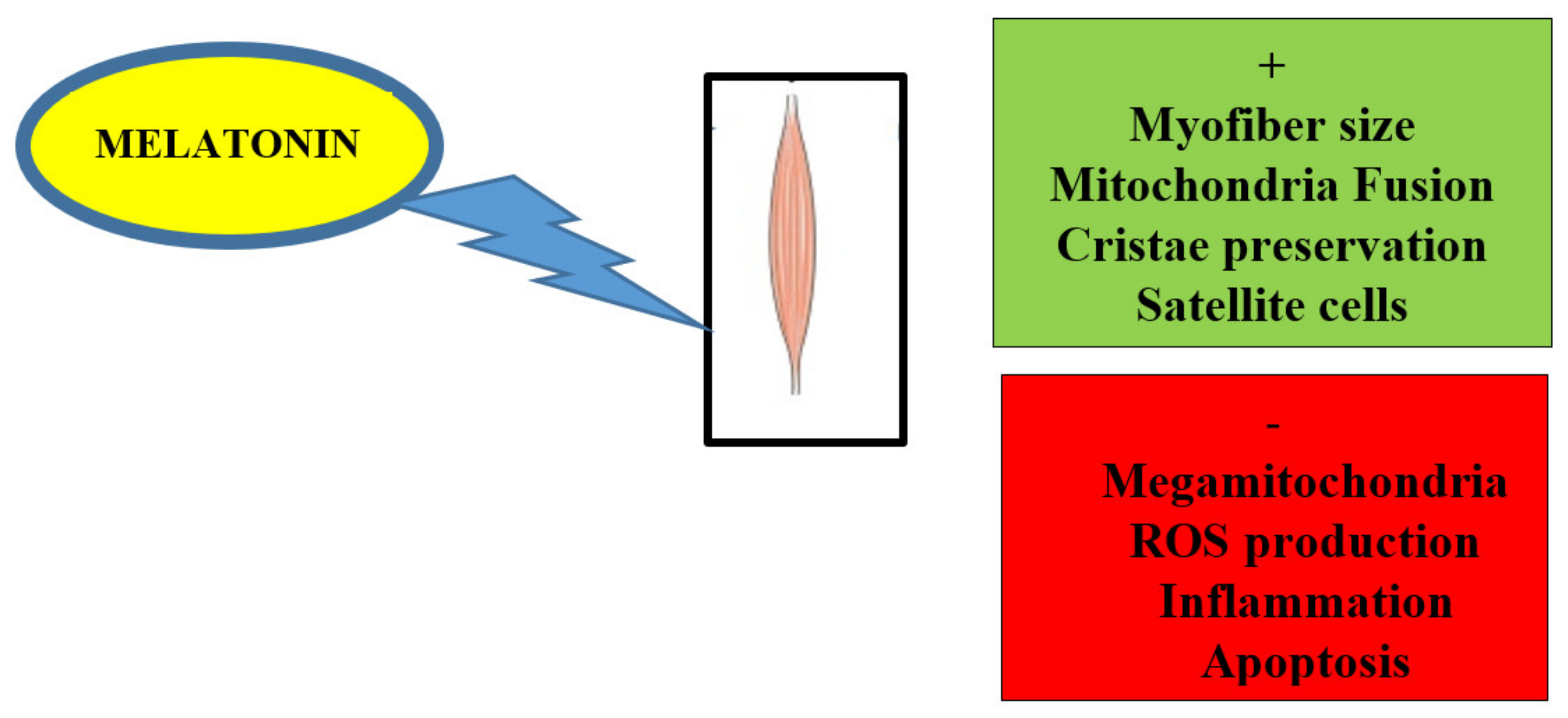
2. Melatonin Alleviates Skeletal Muscle Disorders In Vitro and In Vivo
3. Exercise—an Anti-Aging Strategy that Preserves Mitochondria in Skeletal Muscle
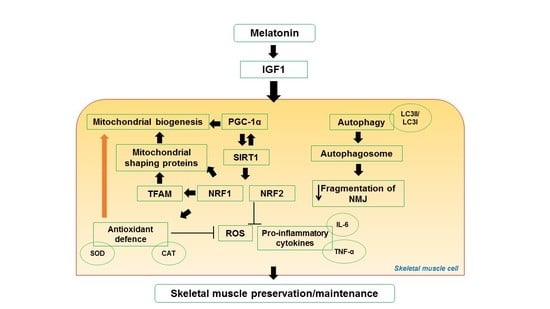
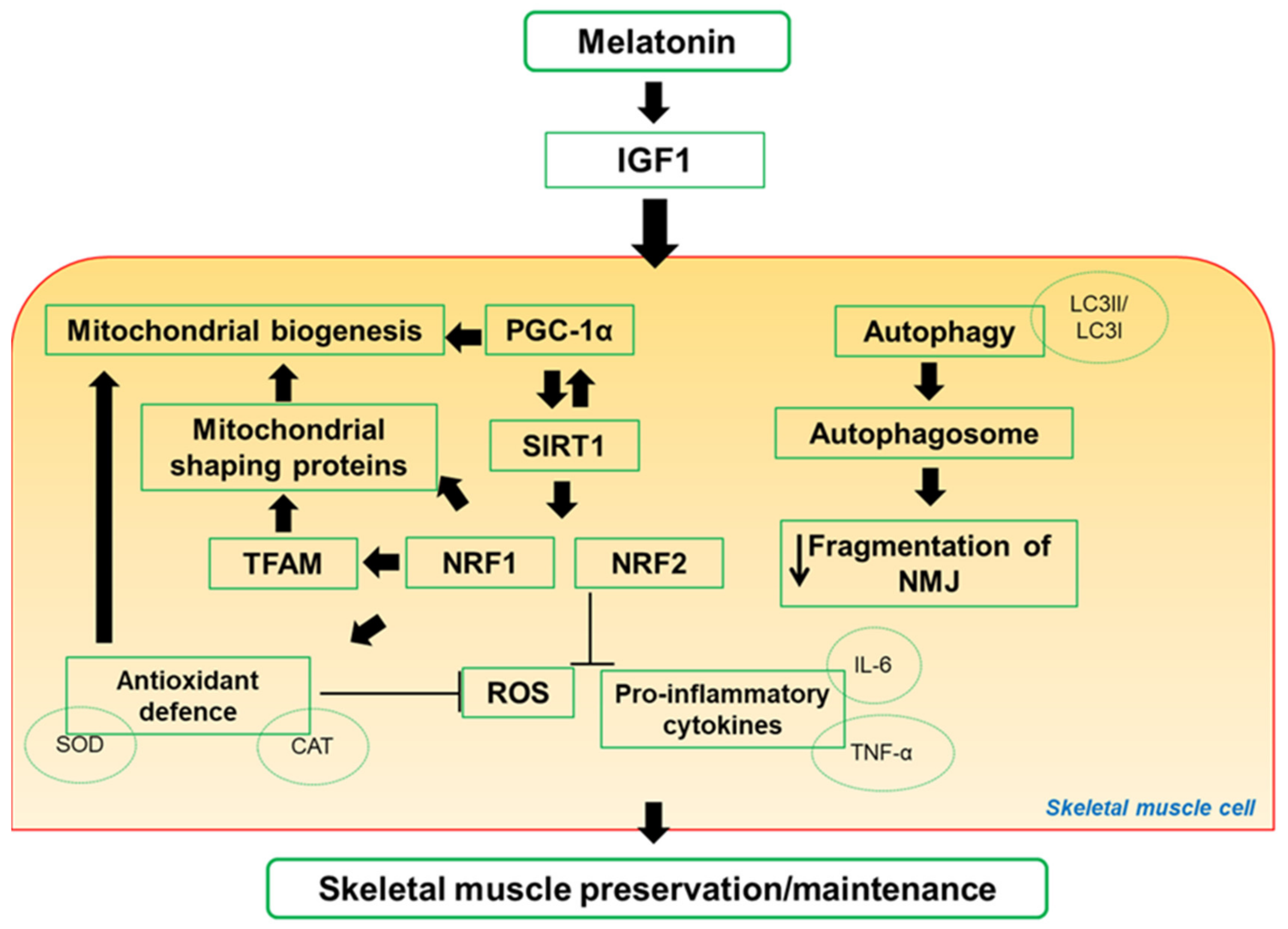
4. Impact of Melatonin on Skeletal Muscle Activity and Exercise
5. The Emerging Concept of the Gut–Muscle Axis—Role of Exercise and Melatonin in the Gut
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janssen, I.; Heymsfield, S.B.; Wang, Z.M.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 1985, 2000, 81–88. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Shadrin, I.; Khodabukus, A.; Bursac, N. Striated muscle function, regeneration, and repair. Cell. Mol. Life Sci. 2016, 73, 4175–4202. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Roman, W.; Gomes, E.R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 2018, 82, 51–56. [Google Scholar] [CrossRef]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Gillies, A.R.; Bushong, E.A.; Deerinck, T.J.; Ellisman, M.H.; Lieber, R.L. Three-dimensional reconstruction of skeletal muscle extracellular matrix ultrastructure. Microsc. Microanal. 2014, 20, 1835–1840. [Google Scholar] [CrossRef][Green Version]
- Hendrickse, P.; Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musaro’, A. Neuromuscolar junction as an entity of nerve-muscle communication. Cells 2019, 8, E906. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.R. The structure of human neuromuscular junctions. Some unanswered molecular questions. Int. J. Mol. Sci. 2017, 18, 2183. [Google Scholar] [CrossRef] [PubMed]
- Vock, R.; Weibel, E.; Hoppeler, H.; Ordway, G.; Weber, J.; Taylor, C. Design of the oxygen and substrate supply to muscle cells. J. Exp. Biol. 1996, 199, 1675–1688. [Google Scholar] [PubMed]
- Boncompagni, S.; Rossi, A.; Micaroni, M.; Beznoussenko, G.; Polishchuk, R.; Dirksen, R.; Protasi, F. Mitochondria are linked to calcium stores in striated muscle by developmentally regulated tethering structures. Mol. Biol. Cell. 2009, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Vitorino, R.; Alves, R.; Appel, H.; Powers, S.; Duarte, J.; Amado, F. Subsarcolemmal and intermyofibrillar mitochondria proteome differences disclose functional specializations in skeletal muscles. Proteomics 2010, 10, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Larsen, S.; Dohlmann, T.; Qvortrup, K.; Helge, J.; Dela, F.; Prats, C. Three-dimensional reconstruction of the human skeletal muscle mitochondrial network as a tool to assess mitochondrial content and structural organization. Acta Physiol. (Oxf.) 2015, 213, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Bleck, C.; Kim, Y.; Willingham, T.; Glancy, B. Subcellular connectomic analyses of energy networks in striated muscle. Nat. Commun. 2018, 9, 5111. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal. Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Glancy, B.; Hartnell, L.; Malide, D.; Yu, Z.; Combs, C.; Connelly, P.; Subramaniam, S.; Balaban, R. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 2015, 523, 617–620. [Google Scholar] [CrossRef]
- Vincent, A.; White, K.; Davey, T.; Philips, J.; Ogden, T.; Lawless, C.; Warren, C.; Hall, M.; Ng, Y.; Falkous, G.; et al. Quantitative 3D mapping of the human skeletal muscle mitochondrial network. Cell Rep. 2019, 26, 996–1009. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell. Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Mishra, P.; Varuzhanyan, G.; Pham, A.; Chan, D. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell. Metab. 2015, 22, 1033–1044. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res.Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. WIREs Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Bourdeau, J.; Sephton, C.; Dutchak, P. Metabolic networks influencing skeletal muscle fiber composition. Front. Cell Dev. Biol. 2018, 6, 125. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, A. The early history of the biochemistry of muscle contraction. J. Gen. Physiol. 2004, 123, 631–641. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Boncompagni, S. The evolution of the mitochondria-to-calcium release units relationship in vertebrate skeletal muscles. J. Biomed. Biotechnol. 2011, 2011, 830573. [Google Scholar] [CrossRef]
- Rossi, A.; Boncompagni, S.; Dirksen, R. Sarcoplasmic reticulum-mitochondrial symbiosis: Bidirectional signaling in skeletal muscle. Exerc. Sport Sci. Rev. 2009, 37, 29–35. [Google Scholar] [CrossRef]
- Ogata, T.; Yamasaki, Y. Ultra-high resolution electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat. Rec. 1997, 248, 214–223. [Google Scholar] [CrossRef]
- Westerblad, H.; Bruton, J.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef]
- Zierath, J.; Hawley, J. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biol. 2004, 2, 1523–1527. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, J.; Kim, H.; Lee, H.; Lim, J.; Choi, S. Sex and fiber-type-related contractile properties in human single muscle fiber. J. Exerc. Rehabil. 2019, 15, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.; Memme, J.; Oliveira, A.; Triolo, M. Maintenance of skeletal muscle mitochondria in health, exercise, and Aging. Ann. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Chan, D. Metabolic regulation of mitochondrial dynamics. J. Cell. Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chomyn, A.; Chan, D. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O. The interplay between mitochondrial dynamics and mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef]
- Gouspillou, G.; Bourdel-Marchasson, I.; Rouland, R.; Calmettes, G.; Biran, M.; Deschodt-Arsac, V.; Miraux, S.; Thiaudiere, E.; Pasdois, P.; Detaille, D.; et al. Mitochondrial energetics is impaired in vivo aged skeletal muscle. Aging Cell 2014, 13, 39–48. [Google Scholar] [CrossRef]
- Picard, M.; Ritchie, D.; Wright, K.; Romestaing, C.; Thomas, M.; Rowan, S.; Taivassalo, T.; Hepple, R. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 2010, 9, 1032–1046. [Google Scholar] [CrossRef]
- Choi, S.J. Age-related functional changes and susceptibility to eccentric contraction-induced damage in skeletal muscle cell. Integr. Med. Res. 2016, 5, 171–175. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.; Picard, M.; St-Jean Pelletier, F.; Sgarioto, N.; Auger, M.; Vallee, J.; Robitaille, R.; St-Pierre, D.; Gouspillou, G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef]
- Delbono, O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr. Aging Sci. 2011, 4, 248–259. [Google Scholar] [CrossRef]
- Jang, Y.; Van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Lim, J.; Miljkovic, I.; Frontera, W. Aging of skeletal muscle fibers. Ann. Rehab. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.; Bahat, G.; Bauer, J.; Boirie, J.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.; Chen, C.; Hood, D. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.; Adhihetty, P.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Hikida, R. Aging changes in satellite cells and their functions. Curr. Aging Sci. 2011, 4, 279–297. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.; Thompson, W.; Kirkland, J.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Zhao, X.; Weisleder, N.; Thornton, A.; Oppong, Y.; Campbell, R.; Ma, J.; Brotto, M. Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 2008, 7, 561–568. [Google Scholar] [CrossRef]
- Sayed, R.; de Leonardis, E.; Guerrero-Martinez, J.; Rahim, I.; Mokhtar, D.; Saleh, A.; Abdalla, K.; Pozo, M.; Escames, G.; López, L.; et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016, 83, 22–30. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Z.; Torigoe, D.; Zhao, J.; Xie, P.; Sugizaki, T.; Sato, M.; Horiguchi, H.; Terada, K.; Kadomatsu, T.; et al. Aging-and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 2019, 14, e0221366. [Google Scholar] [CrossRef]
- Fougere, B.; Boulanger, E.; Nourhashemi, F.; Guyonnet, S.; Cesari, M. Chronic inflammation: Accelerator of biological aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Picca, A.; Marini, F.; Biancolillo, A.; Coelho Junior, H.; Gervasoni, J.; Bossola, M.; Cesari, M.; Onder, G.; Landi, F.; et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: The frialty “cytokinome” at its core. Exp. Gerontol. 2019, 122, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Szentesi, P.; Csernoch, L.; Dux, L.; Keller-Pinter, A. Changes in Redox Signaling in the skeletal muscle with aging. Oxid. Med. Cell. Long. 2019, 2019, 4617801. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A. Mitophagy as a new therapeutic target for sarcopenia. Acta Physiol. 2019, 225, e13219. [Google Scholar] [CrossRef]
- Sheard, P.; Anderson, R. Age-related loss of muscle fibers is highly variable among mouse skeletal muscles. Biogerontology 2012, 13, 157–167. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.; van Kranenburg, J.; Verdijk, L.; van Loon, L. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Del Campo, A.; Contreras-Hernandez, I.; Castro-Sepulveda, M.; Campos, C.; Figueroa, R.; Tevy, M.; Eisner, V.; Casas, M.; Jainovich, E. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging 2018, 10, 34–55. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial fusion, fission and cristae remodeling as key mediators of cellular function. Ann. Rev. Physiol. 2018, 78, 505–531. [Google Scholar] [CrossRef]
- Romanello, V.; Sandri, M. Mitochondrial quality control and muscle mass maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox control of skeletal muscle regeneration. Antiox. Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Li, M.; Shoelson, S.; Zhang, N.; Reddick, R.; Musi, N. NF-kB regulates muscle development and mitochondrial function. J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Robinson, M.; Nair, K. Skeletal muscle aging and the mitochondrion. Trends Endocrinol. Metab. 2013, 24, 247–256. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.; Vina, J.; Ji, L. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1 alpha overexpression in vivo. Free Rad. Biol. Med. 2019, 130, 361–368. [Google Scholar] [CrossRef]
- Huang, D.; Fan, S.; Chen, X.; Yan, X.; Zhang, X.; Ma, B.; Yu, D.; Xiao, W.; Zhuang, C.; Yu, Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp. Gerontol. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Ciciliot, S.; Schiaffino, S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr. Pharm. Des. 2010, 16, 906–914. [Google Scholar] [CrossRef]
- Abeles, A.; Pillinger, M.; Solitar, B.; Abeles, M. Narrative review: The pathophysiology of fibromyalgia. Ann. Intern. Med. 2007, 146, 726–734. [Google Scholar] [CrossRef]
- Chung, C.P.; Titova, D.; Oeser, A.; Randels, M.; Avalos, I.; Milne, G.L.; Morrow, J.D.; Stein, C. Oxidative stress in fibromyalgia and its relationship to symptoms. Clin. Rheumatol. 2009, 28, 435–438. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E.; Culic, O.; Carrión, A.M.; de Miguel, M.; Díaz-Parrado, E.; Pérez-Villegas, E.M.; Bullón, P.; Battino, M.; Sánchez-Alcazar, J.A. NLRP3 inflammasome is activated in fibromyalgia: The effect of coenzyme Q10. Antioxid. Redox Signal. 2014, 20, 1169–1180. [Google Scholar] [CrossRef]
- Picard, M.; Hepple, R.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Guilbert, A.; Guillot, M.; Talha, S.; Lejay, A.; Meyer, A.; Kindo, M.; Wolff, V.; Bouitbir, J.; Zoll, J.; et al. Muscles susceptibility to ischemia-reperfusion injuries depends on fiber type specific antioxidant level. Front. Physiol. 2017, 8, 52. [Google Scholar] [CrossRef]
- Guiraud, S.; Aartsma-Rus, A.; Vieira, N.; Davies, K.; Van Ommen, G.; Kunkel, L. The pathogenesis and therapy of muscular dystrophies. Ann. Rev. Genom. Human Genet. 2015, 16, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Hardeland, R.; Manchester, L.; Paredes, S.; Korkmaz, A.; Sainz, R.; Mayo, J.; Fuentes-Broto, L.; Reiter, R. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tan, D.; Rosales, C.; Manchester, L. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini-Rev. Med. Chem. 2013, 13, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.; Petrosillo, G. Protective role of melatonin in mitochondrial dysfunction and related disorders. Arch. Toxicol. 2015, 89, 923–939. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.; Rosales-Corral, S.; Galano, A.; Zhou, X.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Reiter, R.; Tan, D.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.; Lima-Cabello, E.; Lopez, L.; Rosales-Corral, S.; Tan, D.; Reiter, R. Extra-pineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.; Xu, D.; Li, H. Dietary sources and bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernandez-Ruiz, J. The potential of Phytomelatonin as a nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.; Konturek, S. Melatonin and aging: Prospects for human treatment. J. Physiol. Pharmacol. 2011, 62, 13–19. [Google Scholar] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro-Inflammatory and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Lee, D. Urine melatonin levels are inversely associated with sarcopenia in postmenopausal women. Menopause 2014, 21, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Oner, J.; Oner, H.; Sahin, Z. Melatonin is as effective as testosterone in the prevention of soleus muscle atrophy induced by castration in rats. Anat. Rec. 2008, 29, 448–455. [Google Scholar] [CrossRef]
- Rodriguez, M.; Escames, G.; Lopez, L.; Garcia, J.; Ortiz, F.; Lopez, A.; Acuña-Castroviejo, D. Melatonin administration prevents cardiac and diaphragmatic mitochondrial oxidative damage in senescence-accelerated mice. J. Endocrinol. 2007, 194, 637–643. [Google Scholar] [CrossRef]
- Dardevet, D.; Remond, D.; Peyron, M. Muscle wasting and resistance of muscle anabolism: The “anabolic threshold concept” for adapted nutritional strategies during sarcopenia. Sci. World J. 2012, 2012, 269531. [Google Scholar] [CrossRef]
- McBride, M.; Foley, K.; D’Souza, D.; Li, Y.; Lau, T.; Hawke, T.; Schertzer, J. The NLRP3 inflammasome contributes to sarcopenia and lower muscle glycolytic potential in old mice. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E222–E232. [Google Scholar] [CrossRef]
- Sayed, R.; Fernandez-Ortiz, M.; Diaz-Casado, M.; Rusanova, I.; Rahim, I.; Escames, G.; Lopez, L.; Mokhtar, D.; Acuña-Castroviejo, D. The protective effect of melatonin against age-associated, sarcopenia-dependent tubular aggregate formation, lactate depletion, and mitochondrial changes. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1330–1338. [Google Scholar] [CrossRef]
- Sayed, R.; Fernandez-Ortiz, M.; Diaz-Casado, M.; Aranda-Martinez, P.; Fernandez-Martinez, J.; Guerra-Librero, A.; Escames, G.; Lopez, L.; Alsaadawy, R.; Acuña-Castroviejo, D. Lack of NLRP3 inflammasome activation reduces age-dependent sarcopenia and mitochondrial dysfunction, favoring the prophylactict effect of melatonin. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, B.; Baraldi, F.; Sampaio, I.; Bomfim, L.; Queiroz, A.; Passos, M.; Carneiro, E.; Alberici, R.; Amaral, F.; Cipolla-Neto, J.; et al. Melatonin prevents mitochondria dysfunction and insulin resistance in rat skeletal muscle. J. Pineal Res. 2014, 57, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Rodella, L.; Nardo, L.; Giugno, L.; Cocchi, M.; Borsani, E.; Reiter, R.; Rezzani, R. A comparison of melatonin and α-lipoic acid in the induction of antioxidant defences in L6 rat skeletal muscle cells. AGE 2015, 37, 83. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Yim, S.; Chung, J.; Yoon, K.; Kang, I.; Cho, Y.; Balk, H. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J. Pineal Res. 2006, 41, 67–72. [Google Scholar] [CrossRef]
- Salucci, S.; Battistelli, M.; Baldassarri, V.; Burini, D.; Falcieri, E.; Burattini, S. Melatonin prevents mitochondrial dysfunctions and death in differentiated skeletal muscle cells. Microsc. Res. Tech. 2017, 80, 1174–1181. [Google Scholar] [CrossRef]
- Quan, X.; Wang, J.; Liang, C.; Zheng, H.; Zhang, L. Melatonin inhibits tunicamycin-induced endoplasmic reticulum stress and insulin resistance in skeletal muscle cells. Biochem. Biophys Res. Commun. 2015, 463, 1102–1107. [Google Scholar] [CrossRef]
- Hibaoui, Y.; Roulet, E.; Ruegg, U. Melatonin prevents oxidative stress-mediated mitochondrial permeability transition and death in skeletal muscle cells. J. Pineal Res. 2009, 47, 238–252. [Google Scholar] [CrossRef]
- Coto-Montes, A.; Boga, J.; Tan, D.; Reiter, R. Melatonin as a potential agent in the treatment of sarcopenia. Int. J. Mol. Sci. 2016, 17, 1771. [Google Scholar] [CrossRef]
- Rondanelli, M.; Peroni, G.; Gasparri, C.; Infantino, V.; Nichetti, M.; Cuzzoni, G.; Spadaccini, D.; Perna, S. Is a combination of melatonin and amino acids useful to sarcopenic elderly patients? A randomized trial. Geriatrics 2019, 4, 4. [Google Scholar] [CrossRef]
- Romanello, V.; Scalabrin, M.; Albiero, M.; Blaauw, B.; Scorrano, L.; Sandri, S. Inhibition of the fission machinery mitigates OPA1 impairment in adult skeletal muscles. Cells 2019, 8, 597. [Google Scholar] [CrossRef]
- Favaro, G.; Romanello, V.; Varanita, T.; Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-mediated mitochondrial shape controls calcium homeostasis and muscle mass. Nature Communication 2019, 10, 2576. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Knight, K.; Dowsing, B.; Zhang, B.; Phan, L.; Hurley, J.; Morrison, W.; Stewart, A. Localization of inducible nitric oxide synthase to mast cells during ischemia/reperfusion injury of skeletal muscle. Lab. Invest. 2000, 80, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, X.; Stephenson, L.; Baynosa, R.; Khiabani, K.; Zamboni, W. Microcirculatory effects of melatonin in rat skeletal muscle after prolonged ischemia. J. Pineal. Res. 2005, 39, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, X.; Stephenson, L.; Zhang, X.; Khiabani, K.; Zamboni, W. Melatonin attenuates I/R–induced mitochondrial dysfunction in skeletal muscle. J. Surg. Res. 2011, 171, 108–113. [Google Scholar] [CrossRef]
- Qazi, T.; Duda, G.; Ort, M.; Perka, C.; Geissler, S.; Winkler, T. Cell therapy to improve regeneration of skeletal muscle injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Mehanna, R.; Soliman, G.; Hassaan, P.; Sharara, G.; Abdel-Moneim, R. Protective role of melatonin on skeletal muscle injury in rats. Int. J. Clin. Exp. Med. 2017, 10, 1490–1501. [Google Scholar]
- Stratos, I.; Richter, N.; Li, Z.; Zechner, D.; Mittlemeier, T.; Vollmar, B.; Riotter, R. Melatonin restores muscle regeneration and enhances muscle function after crush injury in rats. J. Pineal Res. 2012, 52, 62–70. [Google Scholar] [CrossRef]
- Ostjen, C.; Sakuno Rosa, C.; Minuzzo Hartmann, R.; Goncalves Schemitt, E.; Raskopf Colares, J.; Possa Marroni, N. Anti-inflammatory and antioxidant effect of melatonin on recovery from muscular trauma induced in rats. Exp. Mol. Pathol. 2019, 106, 52–59. [Google Scholar] [CrossRef]
- Caumo, W.; Hidalgo, M.; Soyuza, A.; Torres, I.; Antunes, L. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J. Pain Res. 2019, 12, 545–556. [Google Scholar] [CrossRef]
- Favero, G.; Trapletti, V.; Bonomini, F.; Stacchiotti, A.; Lavazza, A.; Rodella, L.; Rezzani, R. Oral supplementation of melatonin protects against fibromyalgia-related skeletal muscle alterations in reserpine-induced myalgia rats. Int. J. Mol. Sci. 2017, 18, 1389. [Google Scholar] [CrossRef]
- Favero, G.; Bonomini, F.; Franco, C.; Rezzani, R. Mitochondrial dysfunction in skeletal muscle of a fibromyalgia model: The potential benefits of melatonin. Int. J. Mol. Sci. 2019, 20, 765. [Google Scholar] [CrossRef] [PubMed]
- Woodman, K.; Coles, C.; Lamande’, S.; White, J. Nutraceuticals and their potential to treat Duchenne muscular dystrophy: Separating the credible from the conjecture. Nutrients 2016, 8, 713. [Google Scholar] [CrossRef] [PubMed]
- Hibaoui, Y.; Reutenauer-Patte, J.; Patthey-Vaudens, O.; Ruegg, U.; Dorchies, O. Melatonin improves function of the dystrophic mdx5cv mouse, a model for Duchenne muscular dystrophy. J. Pineal Res. 2011, 51, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Mc Cormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Nilsson, M.; Tarnopolsky, M. Mitochondria and Aging-The role of exercise as a countermeasure. Biology 2019, 8, 40. [Google Scholar] [CrossRef]
- Seo, D.; Lee, S.; Kim, N.; Ko, K.; Rhee, D.; Han, J. Age-related changes in skeletal muscle mitochondria: The role of exercise. Int. Med. Res. 2016, 5, 182–186. [Google Scholar] [CrossRef]
- Distefano, G.; Goodpaster, B. Effects of exercise and aging on skeletal muscle. Cold Spring Harb. Perspect. Med. 2018, 8, a029785. [Google Scholar] [CrossRef]
- Balan, E.; Schwalm, C.; Naslain, D.; Nielsen, H.; Francaux, M.; Deldicque, L. Regular endurance exercise promotes fission, mitophagy, and oxidative phosphorylation in human skeletal muscle independently of age. Front. Physiol. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Laurin, J.; Reuid, J.; Lawrence, M.; Miller, B. Long-term aerobic exercise preserves muscle mass and function with age. Curr. Opin. Physiol. 2019, 10, 70–74. [Google Scholar] [CrossRef]
- Drake, J.; Yan, Z. Mitophagy in maintaining skeletal muscle mitochondrial proteostasis and metabolic health with ageing. J. Physiol. 2017, 20, 6391–6399. [Google Scholar] [CrossRef]
- Always, S.; Mohamed, J.; Myers, M. Mitochondria initiate and regulate sarcopenia. Exerc. Sport Sci. Rev. 2017, 45, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L. Skeletal muscle adaptations with age, inactivity, and therapeutic exercise. J. Orthop. Sports Phys. Ther. 2002, 32, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Ouchi, N.; Izumiya, Y.; Bernardo, B.; Le Brasseur, N.; Walsh, K. Glycolytic fast-twitch muscle fiber restoration counters adverse age-related changes in body composition and metabolism. Aging Cell 2013, 13, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Diaz, V.; Soldini, L.; Haider, T.; Thomassen, M.; Nordsborg, N.; Gassmann, M.; Lundby, C. Fast-twitch glycolytic skeletal muscle is predisposed to age-induced impairments in mitochondrial function. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1010–1022. [Google Scholar] [CrossRef]
- Crupi, A.; Nunnelee, J.; Taylor, D.; Thomas, A.; Vit, J.; Riera, C.; Gottlieb, R.; Goodridge, H. Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging. Aging 2018, 10, 3327–3352. [Google Scholar] [CrossRef]
- Menshikova, E.; Ritov, V.; Fairfull, L.; Ferrell, R.; Kelley, D.; Goodpaster, B. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J. Gerontol. Biol. Sci. 2006, 61A, 534–540. [Google Scholar] [CrossRef]
- Gan, Z.; Fu, T.; Kelly, D.; Vega, R. Skeletal muscle mitochondrial remodeling in exercise and diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef]
- Coen, P.; Music, R.; Hinkley, J.; Miller, B. Mitochondria as a target for mitigating sarcopenia. Front. Physiol. 2019, 9, 1883. [Google Scholar] [CrossRef]
- Ahmetov, I.; Vinogradova, O.; Williams, A. Gene polymorphism and fiber-type composition of human skeletal muscle. Int. J. Sport Nutrit. Exerc. Metab. 2012, 22, 292–303. [Google Scholar] [CrossRef]
- Flück, M.; Kramer, D.; Fitze, D.; Kasper, S.; Franchi, M.; Valdivieso, P. Cellular aspects of muscle specialization demonstrate genotype-phenotype interaction effects in athletes. Front. Physiol. 2019, 10, 526. [Google Scholar] [CrossRef]
- Valdivieso, P.; Vaughan, D.; Laczko, E.; Broglioli, M.; Waldron, S.; Rittweger, J.; Flück, M. The metabolic response of skeletal muscle to endurance exercise is modified by the ACE-I/D gene polymorphism and training state. Front. Physiol. 2017, 8, 993. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Erlich, A.; Hood, D. Role of Parkin and endurance training on mitochondrial turnover in skeletal muscle. Skelet. Muscle 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Erlich, A.; Crilly, M.; Hood, D. Parkin is required for exercise-induced mitophagy in muscle: Impact of aging. Am. J. Physiol. Metab. 2018, 315, E404–E415. [Google Scholar] [CrossRef] [PubMed]
- Erlich, A.; Hood, D. Mitophagy regulation in skeletal muscle: Effect of endurance exercise and age. J. Science Sport Exerc. 2019. [Google Scholar] [CrossRef]
- Erlich, A.; Brownlee, D.; Beyfuss, K.; Hood, D. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1α-dependent manner. Am. J. Physiol. Cell Physiol. 2018, 314, C62–C72. [Google Scholar] [CrossRef]
- Settembre, C.; Ballabio, A. TFEB regulates autophagy: An integrated coordination of cellular degradation and recycling processes. Autophagy 2011, 7, 1379–1381. [Google Scholar] [CrossRef]
- Vainshtein, A.; Tryon, L.; Pauly, M.; Hood, D. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef]
- Uguccioni, G.; Hood, D. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E361–E371. [Google Scholar] [CrossRef]
- Carter, H.; Kim, Y.; Erlich, A.; Zarrin-Khat, D.; Hood, D. Autophagy and mitophagy flux in young and aged skeletal muscle following chronic contractile activity. J. Physiol. 2018, 596, 3567–3584. [Google Scholar] [CrossRef]
- Triolo, M.; Hood, D. Mitochondrial breakdown in skeletal muscle and the emerging role of the lysosomes. Arch. Biochem. Biophys. 2019, 661, 66–73. [Google Scholar] [CrossRef]
- Preisler, N.; Laforêt, P.; Madsen, K.; Husu, E.; Vissing, C.; Hedermann, G.; Galbo, H.; Lindberg, C.; Vissing, J. Skeletal muscle metabolism during prolonged exercise in Pompe disease. Endocrin. Connect. 2017, 6, 384–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van den Berg, L.; Favejee, M.; Wens, S.; Kruijshaar, M.; Praet, S.; Pijnappel, W.; van Doorn, P.; Bussmann, J.; van der Ploeg, A. Exercise training in adults with Pompe disease: The effects on pain, fatigue, and functioning. Arch. Phys. Med. Rehabil. 2015, 96, 817–822. [Google Scholar] [CrossRef]
- Mcleod, J.; Stokes, T.; Phillips, S. Resistance exercise training as a primary countermeasure to age-related chronic disease. Front. Physiol. 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, R. Exercise-induced myokines with therapeutic potential for muscle wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Standford, K.; Goodyear, L. Muscle-adipose tissue cross talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef]
- Tanimura, Y.; Aoi, W.; Takanami, Y.; Kawai, Y.; Mizushima, K.; Naito, Y.; Yoshikawa, T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol. Rep. 2016, 4, e12828. [Google Scholar] [CrossRef]
- Izumiya, Y.; Bina, H.; Ouchi, N.; Akasaki, Y.; Kharitonenkov, A.; Walsh, K. FGF21 is an Akt-regulated myokine. FEBS Lett. 2008, 582, 3805–3810. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.; Zhang, L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Szuhany, K.; Bugatti, M.; Otto, M. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine response to high-intensity interval vs resistance exercise: An individual approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Trovato, E.; Di Felice, V.; Barone, R. Extracellular vesicles: Delivery vehicles of myokines. Front. Physiol. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.; Wallis, M.; Polit, D.; Steele, M.; Shum, D.; Morris, N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomized controlled trial. Age and Ageing 2014, 43, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Suire, C.; Eitan, E.; Shaffer, N.; Tian, Q.; Studenki, S.; Mattson, M.; Kapogiannis, D. Walking speed decline in older adults is associated with elevated pro-BDNF in plasma extracellular vesicles. Exp. Gerontol. 2017, 98, 209–216. [Google Scholar] [CrossRef]
- Hastings, M.; O’Neill, J.; Maywood, E. Circadian clocks: Regulators of endocrine and metabolic rhythms. J. Endocrinol. 2007, 195, 187–198. [Google Scholar] [CrossRef]
- De Goede, P.; Wefers, J.; Brombacher, E.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Doherty, R.; Madigan, S.; Warrington, G.; Ellis, J. Sleep and nutrition interactions: Implications for athletes. Nutrients 2019, 11, 822. [Google Scholar] [CrossRef]
- Welsh, D.; Takahashi, J.; Kay, S. Suprachismatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Yanar, K.; Simsek, B.; Cakatay, U. Integration of Melatonin related redox homeostasis, aging, and circadian rhythm. Rejuvenation Res. 2019, 22, 409–417. [Google Scholar] [CrossRef]
- Nakamura, T.; Takasu, N.; Nakamura, W. The suprachiasmatic nucleus: Age-related decline in biological rhythms. J. Physiol. Sci. 2016, 66, 367–374. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin: Countering chaotic time cues. Front. Endocrinol. 2019, 10, 391. [Google Scholar] [CrossRef]
- Kandalepas, P.; Mitchell, J.; Gillette, M. Melatonin signal transduction pathways require E-Box-Mediated transcription of Per 1 and Per 2 to reset the SCN clock at dusk. PLoS ONE 2016, 11, e0157824. [Google Scholar] [CrossRef] [PubMed]
- Huang, R. The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in physiology or medicine. Biomed. J. 2018, 41, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Harfmann, B.; Schroeder, E.; Esser, K. Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythms 2015, 30, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Ma, K. Circadian clock regulation of skeletal muscle growth and repair. F1000 Res. 2016, 5, 1549. [Google Scholar] [CrossRef]
- Vitale, J.; Bonato, M.; La Torre, A.; Banfi, G. The role of the molecular clock in promoting skeletal muscle growth and protecting against sarcopenia. Int. J. Mol. Sci. 2019, 20, 4318. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C.; Murgia, M. Fiber type divesity in skeletal muscle explored by mass spectrometry-based single fiber proteomics. Histology Histopathol. 2019. [Google Scholar] [CrossRef]
- Mirizio, G.; Nunes Mendes, R.; Castillo Figueroa, A.; de Sousa Junior, I.; Pimentel Ferreira, A.; Vieira, E. The impact of physical exercise on the skeletal muscle clock genes. Kinesiology 2018, 50, 5–18. [Google Scholar]
- Melancon, M.; Lorrain, D.; Dionnbe, I. Sleep depth and continuity before and after chronic exercise in older man: Electrophysiological evidence. Physiol. Behav. 2015, 140, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Carlson, l.; Pobocik, K.; Lawrence, M.; Brazeau, D.; Koch, A. Influence of exercise time of day on salivary melatonin responses. Int. J. Sports Physiol. Perf. 2019, 14, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; Ozturk, G.; Baño-Otalora, B.; Pozo, M.; Madrid, J.; Reiter, R.; Serrano, E.; Concepcion, M.; Acuña-Castroviejo, D. Exercise and melatonin in humans: Reciprocal benefits. J. Pineal Res. 2012, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Maegawa, T.; Iwamoto, J.; Sakai, T.; Otaki, N.; Kataoka, H.; Kurumatani, N. Melatonin secretion and muscle strength in elderly individuals: A cross-sectional study of the HEIJO-KYO Cohort. J. Gerontol. A Biol. Med. Sci. 2016, 71, 1235–1240. [Google Scholar] [CrossRef]
- Thrift, A.; Xiao, L.; Patel, S.; Tworoger, S.; Mc Tiernan, A.; Duggan, C. Effects of physical activity on melatonin levels in previously sedentary men and women. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1696–1699. [Google Scholar] [CrossRef][Green Version]
- De Aquino, L.; dos Santos Thomatieli, R.; Antunes Moreira, H.; Behn, C.; Viscor, G.; Lira Santos, F.; Bittar, I.; Caris Venticinque, A.; Tufik, S.; De Mello, M. Melatonin and sleep responses to normobaric hypoxia and aerobic physical exercise: A randomized controlled trial. Physiol. Behav. 2018, 196, 95–103. [Google Scholar] [CrossRef]
- Mendes, C.; Lopes Sousa, A.; Do Amaral Gaspar, F.; Peliciari-Garcia, R.; de Oliveira Turati, A.; Hirabara, S.; Scialfa, F.; Cipolla-Neto, J. Adaptations of the aging animal to exercise: Role of daily supplementation with melatonin. J. Pineal Res. 2013, 55, 229–239. [Google Scholar] [CrossRef]
- Ochoa, J.; Diaz-Castro, J.; Kajarabille, N.; Garcia, C.; Guisado, I.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef]
- Borges da Silva, L.; Dermargos, A.; Pinto da Silva Junior, E.; Weimann, E.; Herling Lambertucci, R.; Hatanaka, E. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal Res. 2015, 58, 166–172. [Google Scholar] [CrossRef]
- Trionfante, C.; Davis, G.; Farney, T.; Miskowiec, R.; Nelson, A. A pre-exercise dose of melatonin can alter substrate use during exercise. Int. J. Exer. Sci. 2017, 10, 1029–1037. [Google Scholar]
- Czuczejko, J.; Sielski, L.; Wozniak, B.; Wozniak, A.; Szewczyk-Golec, K. Melatonin supplementation improves oxidative and inflammatory state in the blood of professional athletes during the preparatory period for competitions. Free Rad. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Farjallah, M.; Hammouda, O.; Mahmoud, L.; Graja, A.; Ghattassi, K.; Boudaya, M.; Jammoussi, K.; Sahnoun, Z.; Souissi, N. Melatonin supplementation ameliorates oxidative stress, antioxidant status and physical performances recovery during soccer training camp. Biol. Rhythm Res. 2018. [Google Scholar] [CrossRef]
- Leonardo-Mendonça, R.; Ocaña-Wilhelmi, J.; de Haro, T.; de Teresa-Galvan, C.; Guerra-Hernandez, E.; Rusanova, I.; Fernandez-Ortiz, M.; Sayed, R.; Escames, G.; Acuña-Castroviejo, D. The benefit of a supplement with the antioxidant melatonin on redox status and muscle damage in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Franco, M.; Planells, E.; Quintero, B.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G.; Molina-Lopez, J. Effect of melatonin supplementation on antioxidant status and DNA damage in high intensity trained athletes. Int. J. Sport Med. 2017, 38, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.; Reiter, R. Melatonin therapy for blunt trauma and strenuous exercise: A mechanism involving cytokines, NFkB, Akt, MAFBX and MURF-1. J. Sport Sci. 2018, 36, 1897–1901. [Google Scholar] [CrossRef]
- Beck, W.; Botezelli, J.; Pauli, J.; Rochete Ropelle, E.; Gobatto, C. Melatonin has an ergogenic effect but does not prevent inflammation and damage in exhaustive exercise. Sci. Rep. 2015, 5, 18065. [Google Scholar] [CrossRef]
- Beck, W.; Scariot, P.; Gobatto, C. Melatonin is an ergogenic aid for exhaustive aerobic exercise only during the wakefulness period. Int. J. Sport Med. 2016, 37, 71–76. [Google Scholar] [CrossRef]
- Andersen, L.; Gogenur, I.; Rosenberg, J.; Reiter, R. The safety of melatonin in humans. Clin. Drug Invest. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation during skeletal muscle regeneration and tissue remodeling: Application to exercise-induced muscle damage management. Immunol. Cell Biol. 2016, 94, 140–145. [Google Scholar] [CrossRef]
- Lopez-Flores, M.; Luque-Nieto, R.; Costa Moreira, O.; Suarez-Iglesias, D.; Villa-Vicente, J. Effects of melatonin on sports performance: A systematic review. JEP Online 2018, 21, 121–138. [Google Scholar]
- Roach, G.; Sargent, C. Interventions to minimize jet lag after westward and eastward flight. Front. Physiol. 2019, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Cheikh, M.; Hammouda, O.; Gaamouri, N.; Driss, T.; Chamari, K.; Cheikh, R.; Dogui, M.; Souissi, N. Melatonin ingestion after exhaustive late-evening exercise improves sleep quality and quantity, and short-term performances in teenage athletes. Chronobiol. Int. 2018, 35, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Holder, A.; Robertson, C.; Gant, N.; Drust, B.; Reilly, T.; Waterhouse, J. Effects of melatonin on the thermoregulatory responses to intermittent exercise. J. Pineal Res. 2005, 39, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, W.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2019, 60, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D. Are melatonin doses employed clinically adequate for melatonin-induced cytoprotection? Melatonin Res. 2019, 2, 106–132. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut-muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Grosicki, G.; Fielding, R.; Lustgarten, M. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for gut-muscle axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef]
- Genton, L.; Mareschal, J.; Charretier, Y.; Lazarevic, V.; Binndels, L.; Schrenzel, J. Targeting the gut microbiota to treat cachexia. Front. Cell. Infect. Microbiol. 2019, 9, 305. [Google Scholar] [CrossRef]
- Lustgarten, M. The role of the gut microbiome on skeletal muscle mass and physical function: 2019 Update. Front. Physiol. 2019, 10, 1435. [Google Scholar] [CrossRef]
- Heintz, C.; Mair, W. You are what you host: Microbiome modulation of the aging process. Cell 2014, 156, 408–411. [Google Scholar] [CrossRef]
- Picca, A.; Fanelli, F.; Calvani, R.; Mule’, G.; Pesce, V.; Sisto, A.; Pantanelli, C.; Bernabei, R.; Landi, F.; Marzetti, E. Gut dysbiosis and muscle aging: Searching for novel targets against sarcopenia. Mediat. Inflamm. 2018, 2018, 7026198. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.; Strowig, T.; Thaiss, C.; Kau, A.; Eisenbarth, S.; Jurczak, M.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.; Lynch, D.; O’Toole, P. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016, 10, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.; Delzenne, N. Muscle wasting: The gut microbiota a a new therapeutic target? J. Biochem. Cell Biol. 2013, 45, 2186–2190. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.; Koh, G.; Nagy, A.; Semenkovich, C.; Gordon, J. The gut microbiota as an environmental factor that regulates fat storage. PNAS 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Backhed, F.; Manchester, J.; Semenkovich, C.; Gordon, J. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. PNAS 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Yan, H.; Diao, H.; Xiao, Y.; Wenxia, L.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Mao, X.; Luo, Y.; et al. Gut microbiota can transfer fiber characteristics and lipid metabolic profiles of skeletal muscle from pigs to germ-free mice. Sci. Rep. 2016, 6, 31786. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Rza, M.; Martin, K.; Kundu, P.; Cox, L.; Selkrig, J.; Posma, J.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Manickam, R.; Oh, H.; Tan, C.; Paramalingam, E.; Wahli, W. Metronidazole causes skeletal muscle atrophy and modulates muscle chronometabolism. Int. J. Mol. Sci. 2018, 19, 2418. [Google Scholar] [CrossRef]
- Fielding, R.; Reeves, A.; Jasuja, R.; Liu, C.; Barrett, B.; Lustgarten, M. Muscle strenght is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed]
- Mailing, L.; Allen, J.; Buford, T.; Fields, C.; Woods, J. Exercise and the gut microbiome: A review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Huang, W.; Chen, Y.; Chuang, H.; Chiu, C.; Huang, C. Investigation of the effects of microbiota on exercise physiological adaptation, performance, and energy utilization using a Gnotobiotic animal model. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise modifies the gut microbiota with positive health effects. Oxid. Med. Cell. Long. 2017, 2017, ID3831972. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.; Chavkin, T.; MacDonald, T.; Tung, A.; Pham, L.; Wibowo, M.; Wurth, R.; Punthambaker, S.; Tierney, B.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Allen, J.; Mailing, L.; Niemiro, G.; Moore, R.; Cook, M.; White, B.; Holscher, H.; Wood, J. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Hu, B.; Huang, W.; Yuan, C.; Zou, L. Response of gut microbiota to metabolite changes induced by endurance exercise. Front. Microbiol. 2018, 9, 765. [Google Scholar] [CrossRef]
- Dalton, A.; Mermier, C.; Zuhl, M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes 2019, 10, 555–568. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports 2018, 28, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Nay, K.; Jollet, M.; Goustard, B.; Baati, N.; Vernus, B.; Pontones, M.; Lefeuvre-Orfila, L.; Bendavid, C.; Ruè, O.; Mariadassou, M.; et al. Gut bacteria are critical for optimal muscle function: A potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab 2019, 317, E158–E171. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Gut dysbiosis dysregulates central and systemic homeostasis via decreased melatonin and suboptimal mitochondria functioning: Pathoetiological and pathophysiological implications. Melat. Res. 2019, 2, 70–85. [Google Scholar] [CrossRef]
- Paulose, J.; Wright, J.; Patel, A.; Cassone, V. Human gut bacteria are sensitive to melatonin and express endogenous circadiam rhythmicity. PLoS ONE 2016, 11, e0146643. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Engstler, A.; Sellmann, C.; Ziegenhardt, D.; Landmann, M.; Kanuri, G.; Lounis, H.; Schröder, M.; Vetter, W.; Bergheim, I. Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: Role of melatonin and lipid peroxidation. Br. J. Nutr. 2016, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, J.; Hong, F.; Wang, S.; Jin, X.; Xue, T.; Jia, L.; Zhai, Y. Melatonin prevents obesity through modulatrion of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sports Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Wosinska, L.; Cotter, P.; O’Sullivan, O.; Guiname, C. The potential impact of probiotics on the gut microbiome of athletes. Nutrients 2019, 11, 2270. [Google Scholar] [CrossRef]
- Lochlainn, M.; Bowyer, R.; Steves, C. Dietary protein and muscle in aging people: The potential role of the gut microbiome. Nutrients 2018, 10, 929. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Z.; Chen, L.; Zhang, Y.; Cheng, Q.; Nüssler, A.; Bao, W.; Liu, L.; Yang, W. Prospective views for whey protein and/or resistance training against age-related sarcopenia. Aging Dis. 2019, 10, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Camera, D. Anabolic heterogeneity following resistance training: A role for circadian rhythm? Front. Physiol. 2018, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Jackson, M.; Kastenmuller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.; Small, K.; Bell, J.; Steves, C.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
| Healthy Middle-Aged | Age-Related Muscle Diseases | References | |
|---|---|---|---|
| Myotubes size | Regular | Reduced | [48,50,57] |
| Satellite cells | Present | Reduced/Absent | [6,42,47] |
| Mitophagy | Normal | Aberrant | [55,59,60,65] |
| Neuromuscular junction | Regular | Absent | [9,10,12,41] |
| Triads/Calcium flux | Regular/Present | Disrupted/Absent | [26,27,49,74] |
| Mitochondria size/number | Regular Fission/Fusion | Megamitochondria Abnormal Fission/Fusion | [32,54,56,66] |
| Inflammation | Absent | Present | [3,52,62,70] |
| ROS formation | Absent/Minimal | High | [53,69,73] |
| ATP production | High | Limited | [61] |
| Microcirculation | Effective | Disrupted | [71,72,74] |
| Subjects/Cells | Dose | Times of Administration | Reference-Muscle Type or Exercise |
|---|---|---|---|
| Wistar albino rats | 6 mg/kg s.c. | 5 weeks | [86] Soleus |
| SAMP8 mice | 10 mg/kg oral (water) | 10 months | [87] Diaphragm |
| C57BL/6J mice | 10 mg/kg oral (chow) | 2 months | [90] Gastrocnemius |
| NLRP3 KO mice | 10 mg/kg oral (chow) | 2 months | [91] Gastrocnemius |
| Pinealectomized Wistar rats | 0.5 mg/kg oral (water) | 45 days | [92] |
| L6 cells | 10 nM | 24 h | [93] |
| C2C12 | 1–10 nM | 20 min | [94] |
| C2C12 cells | 100 mM | 12–24 h | [95] |
| C2C12 cells | 100 nM | 16 h | [96] |
| Primary muscle cells | 1–100 µM | 24 h | [97] |
| Elderly patients | 1 mg/day oral | 4 weeks | [99] |
| Sprague-Dawley rats | 10 mg/kg i.p. | 30 min prior and immediately after reperfusion | [103] Cremaster |
| Sprague-Dawley rats | 10 mg/kg i.v. | 10 min prior and 10 min after reperfusion | [104] Gracilis |
| Wistar rats | 10 mg/kg i.p. | 4–14 days | [106] Soleus |
| Wistar rats | 10 mg/kg i.p. | 1–14 days | [107] Soleus |
| Wistar rats | 20 mg/kg i.p. | 7 days | [108] |
| Sprague-Dawley rats | 2.5 mg/kg 5 mg/kg oral (water) | 1–2 months | [110] Gastrocnemius |
| Sprague-Dawley rats | 5 mg/kg oral (water) | 2 months | [111] Gastrocnemius |
| Wistar rats | 1 mg/kg oral (water) | 16 weeks | [177] Treadmill running |
| Adult men | 15 mg oral | Before starting exercise | [178] High intensity run |
| Wistar rats | 20 mg/kg i.p. | Immediately after or 2 h after exercise | [179] Treadmill running |
| Adult subjects | 6 mg oral | Before starting exercise | [180] 30 min graded exercise |
| Football players | 5 mg oral | 30 days | [181] Preparatory training |
| Professionalsoccer players | 6 mg oral | 6 days | [182] Intensive training |
| Adult athletes | 100 mg oral | 4 weeks | [183] Resistance training |
| Adult athletes | 20 mg oral | 2 weeks | [184] High Intensity training |
| Wistar rat | 10 mg/kg i.p. | 2 days after exercise | [186] Incremental swimming |
| Teenage athletes | 10 mg oral | After exercise | [192] Exhaustive exercise |
| Adult subjects | 2.5 mg oral | Before exercise | [193] Intermittent running |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of Melatonin on Skeletal Muscle and Exercise. Cells 2020, 9, 288. https://doi.org/10.3390/cells9020288
Stacchiotti A, Favero G, Rodella LF. Impact of Melatonin on Skeletal Muscle and Exercise. Cells. 2020; 9(2):288. https://doi.org/10.3390/cells9020288
Chicago/Turabian StyleStacchiotti, Alessandra, Gaia Favero, and Luigi Fabrizio Rodella. 2020. "Impact of Melatonin on Skeletal Muscle and Exercise" Cells 9, no. 2: 288. https://doi.org/10.3390/cells9020288
APA StyleStacchiotti, A., Favero, G., & Rodella, L. F. (2020). Impact of Melatonin on Skeletal Muscle and Exercise. Cells, 9(2), 288. https://doi.org/10.3390/cells9020288