Endothelial Protease Activated Receptor 1 (PAR1) Signalling Is Required for Lymphocyte Transmigration across Brain Microvascular Endothelial Cells
Abstract
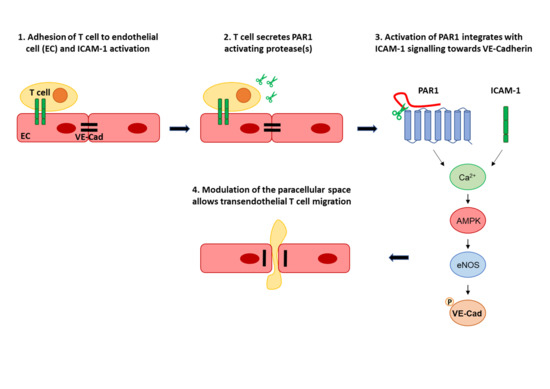
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Endothelial Cell Culture
2.4. Lymphocyte Coculture, Adhesion and Migration Assays In Vitro
2.5. RT-PCR
2.6. siRNA Knockdown of PAR1
2.7. Immunoblotting
2.8. VE-Cadherin Plasmids
2.9. Data Analysis and Statistics
3. Results
3.1. Endothelial PAR-1 Is Required for Lymphocyte Migration across Rat Brain Microvascular ECs
3.2. PAR1 Activation Leads to MAPK, AMPK and eNOS Activation in Brain Microvascular ECs
3.3. Transendothelial Migration Requires Ca2+, AMPK, eNOS and VEC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| aPC | activated protein C |
| ATIII | anti-thrombin III |
| BBB | blood brain barrier |
| BMVEC | brain microvascular endothelial cell |
| EAE | experimental autoimmune encephalomyelitis |
| EC | endothelial cell |
| eNOS | endothelial nitric oxide synthase |
| GPCR | G protein-coupled receptor |
| LFA-1 | lymphocyte function-associated antigen 1 |
| Mpr-NH2 | Mercaptopropionyl-Phe-Cha-Arg-Lys-Pro-Asn-Asp-Lys-NH2 |
| tcY-NH2 | trans-Cinnamoyl-Tyr-Pro-Gly-Lys-Phe-NH2 |
| PAR | protease-activated receptor |
| PLN | peripheral lymph node |
| S1P | sphingosine 1-phosphate |
| TEER | transendothelial electrical resistance |
| TEM | transendothelial migration |
References
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Heasman, S.J.; Alvarez, J.I.; Prat, A.; Lyck, R.; Engelhardt, B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol. Appl. Neurobiol. 2011, 37, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.A. The regulation of transendothelial migration: New knowledge and new questions. Cardiovasc. Res. 2015, 107, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Beraud, E.; Balzano, C.; Zamora, A.J.; Varriale, S.; Bernard, D.; Ben-Nun, A. Pathogenic and non-pathogenic T lymphocytes specific for the encephalitogenic epitope of myelin basic protein: Functional characteristics and vaccination properties. J. Neuroimmunol. 1993, 47, 41–53. [Google Scholar] [CrossRef]
- Pryce, G.; Male, D.; Campbell, I.; Greenwood, J. Factors controlling T-cell migration across rat cerebral endothelium in vitro. J. Neuroimmunol. 1997, 75, 84–94. [Google Scholar] [CrossRef]
- Oppenheimer-Marks, N.; Davis, L.S.; Bogue, D.T.; Ramberg, J.; Lipsky, P.E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J. Immunol. 1991, 147, 2913–2921. [Google Scholar]
- Etienne-Manneville, S.; Manneville, J.B.; Adamson, P.; Wilbourn, B.; Greenwood, J.; Couraud, P.O. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J. Immunol. 2000, 165, 3375–3383. [Google Scholar] [CrossRef]
- Martinelli, R.; Gegg, M.; Longbottom, R.; Adamson, P.; Turowski, P.; Greenwood, J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol. Biol. Cell 2009, 20, 995–1005. [Google Scholar] [CrossRef]
- Dragoni, S.; Hudson, N.; Kenny, B.A.; Burgoyne, T.; McKenzie, J.A.; Gill, Y.; Blaber, R.; Futter, C.E.; Adamson, P.; Greenwood, J.; et al. Endothelial MAPKs Direct ICAM-1 Signaling to Divergent Inflammatory Functions. J. Immunol. 2017, 198, 4074–4085. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Adamson, P.; Wilbourn, B.; Etienne-Manneville, S.; Calder, V.; Beraud, E.; Milligan, G.; Couraud, P.-O.; Greenwood, J. Lymphocyte trafficking through the blood-brain barrier is dependent on endothelial cell heterotrimeric G-protein signaling. FASEB J. 2002, 16, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Katritch, V.; Cherezov, V.; Stevens, R.C. Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 2012, 33, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 1–24. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Hempel, D.; Sierko, E.; Tucker, S.C.; Honn, K.V. Protease-activated receptors (PARs)--biology and role in cancer invasion and metastasis. Cancer Metastasis Rev. 2015, 34, 775–796. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Vanlandewijck, M.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 2018, 5, 180160. [Google Scholar] [CrossRef]
- Han, X.; Nieman, M.T. The domino effect triggered by the tethered ligand of the protease activated receptors. Thromb. Res. 2020, 196, 87–98. [Google Scholar] [CrossRef]
- Willis Fox, O.; Preston, R.J.S. Molecular basis of protease-activated receptor 1 signaling diversity. J. Thromb. Haemost. 2020, 18, 6–16. [Google Scholar] [CrossRef]
- Alabanza, L.M.; Bynoe, M.S. Thrombin induces an inflammatory phenotype in a human brain endothelial cell line. J. Neuroimmunol. 2012, 245, 48–55. [Google Scholar] [CrossRef][Green Version]
- Brailoiu, E.; Shipsky, M.M.; Yan, G.; Abood, M.E.; Brailoiu, G.C. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res. 2017, 1657, 167–175. [Google Scholar] [CrossRef]
- Ukropec, J.A.; Hollinger, M.K.; Salva, S.M.; Woolkalis, M.J. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 2000, 275, 5983–5986. [Google Scholar] [CrossRef]
- Niessen, F.; Furlan-Freguia, C.; Fernández, J.A.; Mosnier, L.O.; Castellino, F.J.; Weiler, H.; Rosen, H.; Griffin, J.H.; Ruf, W. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood 2009, 113, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, C.; Riewald, M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 2005, 105, 3178–3184. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, Y.R.; Ahn, S.M.; Lee, S.K.; Shin, H.K.; Choi, B.T. Protease activated receptor-1 antagonist ameliorates the clinical symptoms of experimental autoimmune encephalomyelitis via inhibiting breakdown of blood-brain barrier. J. Neurochem. 2015, 135, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.; Pryce, G.; Devine, L.; Male, D.K.; dos Santos, W.L.C.; Calder, V.L.; Adamson, P. SV40 large T immortalised cell lines of the rat blood-brain and blood-retinal barriers retain their phenotypic and immunological characteristics. J. Neuroimmunol. 1996, 71, 51–63. [Google Scholar] [CrossRef]
- Hudson, N.; Powner, M.B.; Sarker, M.H.; Burgoyne, T.; Campbell, M.; Ockrim, Z.K.; Martinelli, R.; Futter, C.E.; Grant, M.B.; Fraser, P.A.; et al. Differential apicobasal VEGF signaling at vascular blood-neural barriers. Dev. Cell 2014, 30, 541–552. [Google Scholar] [CrossRef]
- Turowski, P.; Martinelli, R.; Crawford, R.; Wateridge, D.; Papageorgiou, A.P.; Lampugnani, M.G.; Gamp, A.C.; Vestweber, D.; Adamson, P.; Dejana, E.; et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J. Cell Sci. 2008, 121, 29–37. [Google Scholar] [CrossRef]
- Zhao, P.; Metcalf, M.; Bunnett, N.W. Biased signaling of protease-activated receptors. Front. Endocrinol. (Lausanne) 2014, 5, 67. [Google Scholar] [CrossRef]
- Russo, A.; Soh, U.J.; Trejo, J. Proteases display biased agonism at protease-activated receptors: Location matters! Mol. Interv. 2009, 9, 87–96. [Google Scholar] [CrossRef]
- Wessel, F.; Winderlich, M.; Holm, M.; Frye, M.; Rivera-Galdos, R.; Vockel, M.; Linnepe, R.; Ipe, U.; Stadtmann, A.; Zarbock, A.; et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat. Immunol. 2014, 15, 223–230. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Hailfinger, S.; Rebeaud, F.; Thome, M. Adapter and enzymatic functions of proteases in T-cell activation. Immunol. Rev. 2009, 232, 334–347. [Google Scholar] [CrossRef]
- van Daalen, K.R.; Reijneveld, J.F.; Bovenschen, N. Modulation of Inflammation by Extracellular Granzyme A. Front. Immunol. 2020, 11, 931. [Google Scholar] [CrossRef]
- Lee, P.R.; Johnson, T.P.; Gnanapavan, S.; Giovannoni, G.; Wang, T.; Steiner, J.P.; Medynets, M.; Vaal, M.J.; Gartner, V.; Nath, A. Protease-activated receptor-1 activation by granzyme B causes neurotoxicity that is augmented by interleukin-1beta. J. Neuroinflamm. 2017, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Haile, Y.; Carmine-Simmen, K.; Olechowski, C.; Kerr, B.; Bleackley, R.C.; Giuliani, F. Granzyme B-inhibitor serpina3n induces neuroprotection in vitro and in vivo. J. Neuroinflamm. 2015, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Zeglinski, M.R.; Granville, D.J. Granzymes in cardiovascular injury and disease. Cell. Signal. 2020, 76, 109804. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G. Novel Insight into the in vivo Function of Mast Cell Chymase: Lessons from Knockouts and Inhibitors. J. Innate Immun. 2020, 12, 357–372. [Google Scholar] [CrossRef]
- Göbel, K.; Asaridou, C.M.; Merker, M.; Eichler, S.; Herrmann, A.M.; Geuß, E.; Ruck, T.; Schüngel, L.; Groeneweg, L.; Narayanan, V.; et al. Plasma kallikrein modulates immune cell trafficking during neuroinflammation via PAR2 and bradykinin release. Proc. Natl. Acad. Sci. USA 2019, 116, 271–276. [Google Scholar] [CrossRef]
- Schulze-Topphoff, U.; Prat, A.; Prozorovski, T.; Siffrin, V.; Paterka, M.; Herz, J.; Bendix, I.; Ifergan, I.; Schadock, I.; Mori, M.A.; et al. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nat. Med. 2009, 15, 788–793. [Google Scholar] [CrossRef]
- Alberelli, M.A.; De Candia, E. Functional role of protease activated receptors in vascular biology. Vascul. Pharmacol. 2014, 62, 72–81. [Google Scholar] [CrossRef]
- Mihara, K.; Ramachandran, R.; Renaux, B.; Saifeddine, M.; Hollenberg, M.D. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J. Biol. Chem. 2013, 288, 32979–32990. [Google Scholar] [CrossRef]
- Allingham, M.J.; van Buul, J.D.; Burridge, K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J. Immunol. 2007, 179, 4053–4064. [Google Scholar] [CrossRef] [PubMed]






| Activating Protease (gene) | Expression in T Cells | Inhibition by ATIII ‡ | ||
|---|---|---|---|---|
| hCD4+ (Th1) * | mCD4+ † | |||
| PAR1 | Thrombin13, 27, 28 (f2) | no | no | |
| Activated Protein C13, 27, 28 (proc) | very low | no | weak, but § | |
| Factor VIIa13 (f7) | no | no | ||
| Factor Xa13, 27, 28 (f10) | no | no | ||
| Trypsin13, 28 (prss1,2,3) | very low (prss1) | yes (prss2) | yes, but ¶ | |
| Plasmin13, 27, 28 (plg) | no | no | ||
| MMPs13, 27, 28 (mmp1,2,3,8,9,12,13) | yes (mmp9) | yes (mmp1,2,8,9) | no | |
| Neutrophil Elastase13, 27 (elane) | no | no | ||
| Proteinase-313, 27 (prtn3) | no | no | ||
| Granzymes13, 27, 28 (gzma,gzmb,gzmk) | high | yes | yes | |
| Chymase13 (cma1) | no | yes | yes # | |
| Cathepsin G13 (ctsg) | no | no | ||
| Kallikreins13 (klk4,5,6,14) | yes (klk14) | no | yes | |
| Calpain-113 (capn1) | high | yes | no ** | |
| PAR2 | Trypsin13, 27, 28 (prss1,2,3) | very low (prss1) | yes (prss2) | yes, but ¶ |
| Tryptase13, 27, 28 (tpsab1) | no | no | ||
| Factor VIIa27, 28 (f7) | no | no | ||
| Factor Xa27, 28 (f10) | no | no | ||
| Kallikreins13, 27, 28 (klk4,5,6,14) | yes (klk14) | no | yes | |
| Neutrophil Elastase13, 27 (elane) | no | no | ||
| Proteinase-313, 27 (prtn3) | no | no | ||
| Cathepsins13, 27 (ctss, ctsg) | high (ctss) | yes (ctss) | n/d | |
| Granzyme A28 (gzma) | high | yes | yes | |
| Matripase28 (st14) | no | yes | yes | |
| Thrombin13 (f2) | no | no | ||
| Activated Protein C13 (proc) | very low | no | weak, but § | |
| Chymase13 (cma1) | no | yes | yes | |
| Plasmin13 (plg) | no | no | ||
| Testisin13 (prss21) | yes | no | n/d | |
| Calpain-213 (capn2) | high | yes | no ** | |
| PAR3 | Thrombin13, 27, 28 (f2) | no | no | |
| Activated Protein C13, 27 (proc) | very low | no | weak, but § | |
| Factor Xa13 (f10) | no | no | ||
| Trypsin13 (prss1,2,3) | very low (prss1) | yes (prss2) | yes, but ¶ | |
| PAR4 | Thrombin13, 27, 28 (f2) | no | no | |
| Trypsin13, 27, 28 (prss1,2,3) | very low (prss1) | yes (prss2) | yes, but ¶ | |
| Factor Xa28 (f10) | no | no | ||
| Plasmin27, 28 (plg) | no | no | ||
| Cathepsin G13, 27, 28 (ctsg) | no | no | ||
| MASP127 (masp1) | no | no | ||
| Kallikrein1413, 28 (klk14) | yes | no | yes | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragoni, S.; Papageorgiou, A.; Araiz, C.; Greenwood, J.; Turowski, P. Endothelial Protease Activated Receptor 1 (PAR1) Signalling Is Required for Lymphocyte Transmigration across Brain Microvascular Endothelial Cells. Cells 2020, 9, 2723. https://doi.org/10.3390/cells9122723
Dragoni S, Papageorgiou A, Araiz C, Greenwood J, Turowski P. Endothelial Protease Activated Receptor 1 (PAR1) Signalling Is Required for Lymphocyte Transmigration across Brain Microvascular Endothelial Cells. Cells. 2020; 9(12):2723. https://doi.org/10.3390/cells9122723
Chicago/Turabian StyleDragoni, Silvia, Anna Papageorgiou, Caroline Araiz, John Greenwood, and Patric Turowski. 2020. "Endothelial Protease Activated Receptor 1 (PAR1) Signalling Is Required for Lymphocyte Transmigration across Brain Microvascular Endothelial Cells" Cells 9, no. 12: 2723. https://doi.org/10.3390/cells9122723
APA StyleDragoni, S., Papageorgiou, A., Araiz, C., Greenwood, J., & Turowski, P. (2020). Endothelial Protease Activated Receptor 1 (PAR1) Signalling Is Required for Lymphocyte Transmigration across Brain Microvascular Endothelial Cells. Cells, 9(12), 2723. https://doi.org/10.3390/cells9122723