Cav1.2 Activity and Downstream Signaling Pathways in the Hippocampus of An Animal Model of Depression
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Stress
2.3. Forced Swimming Test
2.4. Sucrose Preference Test
2.5. Three-Chamber Social Interaction Test
2.6. Sample Harvesting
2.7. RT-PCR
2.8. Semi-Quantitative PCR
2.9. Western Blot
2.10. Hippocampal Slices
2.11. Electrophysiology
2.12. Statistical Analysis
3. Results
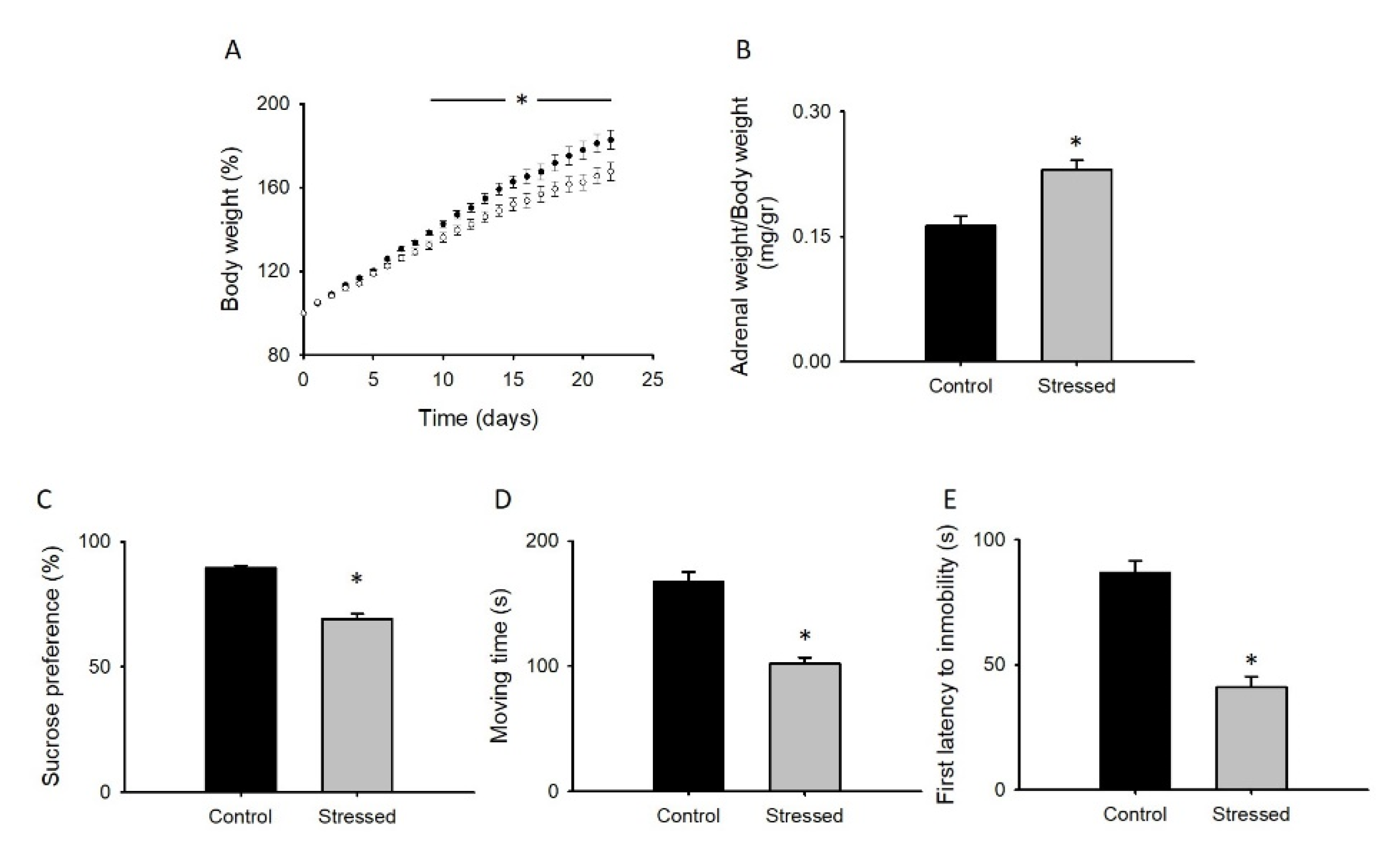
3.1. Behavioral Changes after CRS
3.2. Changes in Cav1.2 after CRS
3.3. Changes in CCAT Activity after CRS
3.4. Changes in CaM Pathways after CRS
3.5. Changes in NFAT Activity after CRS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar]
- DeRubeis, R.J.; Siegle, G.J.; Hollon, S.D. Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Hollon, S.D.; Ponniah, K. A review of empirically supported psychological therapies for mood disorders in adults. Depress. Anxiety 2010, 27, 891–932. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, C.; Duman, R.S. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology 2008, 33, 88–109. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D.; Rooney, R.J.; Thuras, P.D. Expression of GABAA alpha2-, beta1- and epsilon-receptors are altered significantly in the lateral cerebellum of subjects with schizophrenia, major depression and bipolar disorder. Transl. Psychiatry 2013, 3, e303. [Google Scholar] [CrossRef] [PubMed]
- Gargus, J.J. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol. Psychiatry 2006, 60, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D. Calcium channels and channelopathies of the central nervous system. Mol. Neurobiol. 2002, 25, 31–50. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Manji, H.K. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp. Neurol. 2008, 211, 7–10. [Google Scholar] [CrossRef]
- Erk, S.; Meyer-Lindenberg, A.; Linden, D.E.J.; Lancaster, T.; Mohnke, S.; Grimm, O.; Degenhardt, F.; Holmans, P.; Pocklington, A.; Schmierer, P.; et al. Replication of brain function effects of a genome-wide supported psychiatric risk variant in the CACNA1C gene and new multi-locus effects. Neuroimage 2014, 94, 147–154. [Google Scholar] [CrossRef]
- Ferreira, M.A.; O’Donovan, M.C.; Meng, Y.A.; Jones, I.R.; Ruderfer, D.M.; Jones, L.; Fan, J.; Kirov, G.; Perlis, R.H.; Green, E.K.; et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat. Genet. 2008, 40, 1056–1058. [Google Scholar] [CrossRef]
- Roussos, P.; Giakoumaki, S.G.; Georgakopoulos, A.; Robakis, N.K.; Bitsios, P. The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord. 2011, 13, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yoshimizu, T.; Pan, J.Q.; Mungenast, A.E.; Madison, J.M.; Su, S.; Ketterman, J.; Ongur, D.; McPhie, D.; Cohen, B.; Perlis, R.; et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol. Psychiatry 2015, 20, 162–169. [Google Scholar] [CrossRef]
- Bavley, C.C.; Bavley, C.C.; Fischer, D.K.; Rizzo, B.K.; Rajadhyaksha, A.M. Cav1.2 channels mediate persistent chronic stress-induced behavioral deficits that are associated with prefrontal cortex activation of the p25/Cdk5-glucocorticoid receptor pathway. Neurobiol. Stress 2017, 7, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z.D.; Lee, A.S.; Burgdorf, C.E.; Fischer, D.K.; Rajadhyaksha, A.M.; Mok, E.; Rizzo, B.; Rice, R.C.; Singh, K.; Ota, K.T.; et al. Cacna1c in the Prefrontal Cortex Regulates Depression-Related Behaviors via REDD1. Neuropsychopharmacology 2017, 42, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Karst, H.; Joels, M. Brief RU 38486 treatment normalizes the effects of chronic stress on calcium currents in rat hippocampal CA1 neurons. Neuropsychopharmacology 2007, 32, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca(2+) channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Hell, J.W.; Westenbroek, R.E.; Warner, C.; Ahlijanian, M.K.; Prystay, W.; Gilbert, M.M.; Snutch, T.P.; Catterall, W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993, 123, 949–962. [Google Scholar] [CrossRef]
- Deisseroth, K.; Heist, E.K.; Tsien, R.W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 1998, 392, 198–202. [Google Scholar] [CrossRef]
- Murphy, T.H.; Worley, P.F.; Baraban, J.M. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron 1991, 7, 625–635. [Google Scholar] [CrossRef]
- Dolmetsch, R.E.; Pajvani, U.; Fife, K.; Spotts, J.M.; Greenberg, M.E. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 2001, 294, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, P.J.; Dell’Acqua, M.L.; Sather, W.A. Ca2+/calcineurin-dependent inactivation of neuronal L-type Ca2+ channels requires priming by AKAP-anchored protein kinase A. Cell Rep. 2014, 7, 1410–1416. [Google Scholar] [CrossRef]
- Murphy, J.G.; Sanderson, J.L.; Gorski, J.A.; Scott, J.D.; Catterall, W.A.; Sather, W.A.; Dell’Acqua, M.L. AKAP-anchored PKA maintains neuronal L-type calcium channel activity and NFAT transcriptional signaling. Cell Rep. 2014, 7, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.F.; Dell’Acqua, M.L.; Sather, W.A. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 2007, 55, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Deng, X.; Ladenheim, B.; McCoy, M.T.; Cluster, A.; Cai, N.S.; Cadet, J.L. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 2005, 102, 868–873. [Google Scholar] [CrossRef]
- Kipanyula, M.J.; Kimaro, W.H.; Etet, P.F.S. The Emerging Roles of the Calcineurin-Nuclear Factor of Activated T-Lymphocytes Pathway in Nervous System Functions and Diseases. J. Aging Res. 2016, 2016, 5081021. [Google Scholar] [CrossRef]
- Gomez-Ospina, N.; Tsuruta, F.; Barreto-Chang, O.; Hu, L.; Dolmetsch, R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell 2006, 127, 591–606. [Google Scholar] [CrossRef]
- Gomez-Ospina, N.; Panagiotakos, G.; Portmann, T.; Pasca, S.P.; Rabah, D.; Budzillo, A.; Kinet, J.P.; Dolmetsch, R.E. A promoter in the coding region of the calcium channel gene CACNA1C generates the transcription factor CCAT. PLoS ONE 2013, 8, e60526. [Google Scholar] [CrossRef]
- Ge, J.; Ju, Y.; Xue, Z.; Feng, Y.; Huang, X.; Liu, H.; Zhao, S. Distal C terminus of Cav1.2 channels plays a crucial role in the neural differentiation of dental pulp stem cells. PLoS ONE 2013, 8, e81332. [Google Scholar] [CrossRef]
- Schroder, E.; Byse, M.; Satin, J. L-type calcium channel C terminus autoregulates transcription. Circ. Res. 2009, 104, 1373–1381. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Hermosilla, T.; Morales, D.; Encina, M.; Torres-Diaz, L.; Diaz, P.; Sarmiento, D.; Simon, F.; Varela, D. Cavbeta2 transcription start site variants modulate calcium handling in newborn rat cardiomyocytes. Pflugers Arch. 2015, 467, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.T.; Daigle, T.L.; Chen, Q.; Feng, G. Acute brain slice methods for adult and aging animals: Application of targeted patch clamp analysis and optogenetics. Methods Mol. Biol. 2014, 1183, 221–242. [Google Scholar]
- Morales, D.; Hermosilla, T.; Varela, D. Calcium-dependent inactivation controls cardiac L-type Ca(2+) currents under beta-adrenergic stimulation. J. Gen. Physiol. 2019, 151, 786–797. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 6. [Google Scholar] [CrossRef]
- Christel, C.; Lee, A. Ca2+-dependent modulation of voltage-gated Ca2+ channels. Biochim. Biophys. Acta 2012, 1820, 1243–1252. [Google Scholar] [CrossRef]
- Bito, H.; Deisseroth, K.; Tsien, R.W. Ca2+-dependent regulation in neuronal gene expression. Curr. Opin. Neurobiol. 1997, 7, 419–429. [Google Scholar] [CrossRef]
- Sapolsky, R.M. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biol. Psychiatry 2000, 48, 755–765. [Google Scholar] [CrossRef]
- Zhang, J.; Shapiro, M.S. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron 2012, 76, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Maigaard, K.; Hageman, I.; Jorgensen, A.; Jorgensen, M.B.; Wortwein, G. Electroconvulsive stimulations prevent chronic stress-induced increases in L-type calcium channel mRNAs in the hippocampus and basolateral amygdala. Neurosci. Lett. 2012, 516, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Mogilnicka, E.; Czyrak, A.; Maj, J. Dihydropyridine calcium channel antagonists reduce immobility in the mouse behavioral despair test; antidepressants facilitate nifedipine action. Eur. J. Pharmacol. 1987, 138, 413–416. [Google Scholar] [CrossRef]
- Mogilnicka, E.; Czyrak, A.; Maj, J. BAY K 8644 enhances immobility in the mouse behavioral despair test, an effect blocked by nifedipine. Eur. J. Pharmacol. 1988, 151, 307–311. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Consogno, E.; Racagni, G.; Popoli, M. Modifications in brain CaM kinase II after long-term treatment with desmethylimipramine. Neuropsychopharmacology 2001, 24, 21–30. [Google Scholar] [CrossRef]
- Groth, R.D.; Mermelstein, P.G. Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: A role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J. Neurosci. 2003, 23, 8125–8134. [Google Scholar] [CrossRef]
- Graef, I.A.; Mermelstein, P.G.; Stankunas, K.; Neilson, J.R.; Deisseroth, K.; Tsien, R.W.; Crabtree, G.R. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 1999, 401, 703–708. [Google Scholar] [CrossRef]
- Abdul, H.M.; Furman, J.L.; Sama, M.A.; Mathis, D.M.; Norris, C.M. NFATs and Alzheimer’s Disease. Mol. Cell Pharmacol. 2010, 2, 7–14. [Google Scholar]
- Furman, J.L.; Sompol, P.; Kraner, S.D.; Pleiss, M.M.; Putman, E.J.; Dunkerson, J.; Mohmmad Abdul, H.; Roberts, K.N.; Scheff, S.W.; Norris, C.M. Blockade of Astrocytic Calcineurin/NFAT Signaling Helps to Normalize Hippocampal Synaptic Function and Plasticity in a Rat Model of Traumatic Brain Injury. J. Neurosci. 2016, 36, 1502–1515. [Google Scholar] [CrossRef]
- Ni, C.; Li, Z.; Qian, M.; Zhou, Y.; Wang, J.; Guo, X. Isoflurane induced cognitive impairment in aged rats through hippocampal calcineurin/NFAT signaling. Biochem. Biophys. Res. Commun. 2015, 460, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, M.; Jiang, M.; Wang, Y.; Ma, X.; Li, S.; Xie, J. Involvement of calcineurin/NFATc4 pathway in a single-prolonged stress-based rat model of post-traumatic stress disorder. Mol. Biol. Rep. 2019, 46, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.J.; Heine, V.M.; Muller, M.B.; van der Beek, E.M.; Wiegant, V.M.; De Kloet, E.R.; Joels, M.; Fuchs, E.; Swaab, D.F.; Czeh, B. Stress, depression and hippocampal apoptosis. CNS Neurol. Disord. Drug Targets 2006, 5, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Carvalho, S.; Lima, L.; Mota-Pereira, J.; Pimentel, P.; Maia, D.; Correia, D.; Gomes, S.; Cruz, A.; Medeiros, R. FAS -670A>G genetic polymorphism is associated with Treatment Resistant Depression. J. Affect. Disord. 2015, 185, 164–169. [Google Scholar] [CrossRef]
- Liu, D.; Xiao, B.; Han, F.; Wang, E.; Shi, Y. Single-prolonged stress induces apoptosis in dorsal raphe nucleus in the rat model of posttraumatic stress disorder. BMC Psychiatry 2012, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, H.; Xu, X.; Young, D. Predatory stress induces hippocampal cell death by apoptosis in rats. Neurosci. Lett. 2007, 421, 115–120. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Vollmann-Honsdorf, G.K.; Gleisberg, M.; Czeh, B.; De Kloet, E.R.; Fuchs, E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur. J. Neurosci. 2001, 14, 161–166. [Google Scholar] [CrossRef]
- Heine, V.M.; Maslam, S.; Zareno, J.; Joels, M.; Lucassen, P.J. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur. J. Neurosci. 2004, 19, 131–144. [Google Scholar] [CrossRef]
- Campbell, S.; Macqueen, G. The role of the hippocampus in the pathophysiology of major depression. J. Psychiatry Neurosci. 2004, 29, 417–426. [Google Scholar]
- McKernan, D.P.; Dinan, T.G.; Cryan, J.F. “Killing the Blues”: A role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog. Neurobiol. 2009, 88, 246–263. [Google Scholar] [CrossRef]






| Target | Sense | Antisense |
|---|---|---|
| 18S | gggcccgaacgctttacttt | ttgcgccggtccaagaattt |
| CCAT | tcccaagttcatcgaggtca | aaggtaagagggtgccgttg |
| Cav1.2 | ccgctttgactgtttcattgtg | cgaggttgctcagggagttc |
| Cx31.1 | ctcgagcccacgtgaagaaa | actccgactcagctcttttcc |
| FasL | atccctctggaatgggaaga | ccatatctggccagtagtgc |
| KNNQ2 | gcgttcctttagcggtttca | aagactgcggattgcatcct |
| KCNQ3 | gcaaatgccatgccttgaga | aagactgcggattgcatcct |
| Kv4.2 | tgcttcactgcctggtttca | ttcttgcacgctgcctctat |
| Antibody | Type | Dilution | Source | Catalog Number |
|---|---|---|---|---|
| Calmodulin | pAB | 1:200 | ABCAM | ab155550 |
| CaMKII | pAB | 1:1000 | Cell Signaling | 44365 |
| CaMKIV | mAB | 1:100 | Santa Cruz | 55501 |
| Cav1.2 | pAB | 1:200 | Alomone | ACC-003 |
| ERK | mAB | 1:1000 | Cell Signaling | 9102 |
| NFAT4c | pAB | 1:100 | Santa Cruz | SC-13036 |
| pCaMKII-T286 | mAB | 1:1000 | Cell Signaling | 127165 |
| pCaMKIV | pAB | 1:1000 | ABCAM | ab59424 |
| pERK | mAB | 1:1000 | Cell Signaling | 9101 |
| pNFAT4c | mAB | 1:2000 | Santa Cruz | 135770 |
| Tubulin | pAB | 1:3000 | Santa Cruz | SC-20172 |
| Test | Sample Size | |
|---|---|---|
| Control | CRS * | |
| Body weight determination | 24 | 30 |
| Adrenal gland determination | 9 | 9 |
| Forced swim test | 19 | 26 |
| Sucrose preference test | 24 | 30 |
| Social interaction test | 15 | 15 |
| Electrophysiology | 7 | 7 |
| Western Blot | 12 | 12 |
| Semi-quantitative PCR | 12 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, C.; Hermosilla, T.; Hardy, P.; Aballai, V.; Rojas, P.; Varela, D. Cav1.2 Activity and Downstream Signaling Pathways in the Hippocampus of An Animal Model of Depression. Cells 2020, 9, 2609. https://doi.org/10.3390/cells9122609
Moreno C, Hermosilla T, Hardy P, Aballai V, Rojas P, Varela D. Cav1.2 Activity and Downstream Signaling Pathways in the Hippocampus of An Animal Model of Depression. Cells. 2020; 9(12):2609. https://doi.org/10.3390/cells9122609
Chicago/Turabian StyleMoreno, Cristian, Tamara Hermosilla, Paulina Hardy, Víctor Aballai, Patricio Rojas, and Diego Varela. 2020. "Cav1.2 Activity and Downstream Signaling Pathways in the Hippocampus of An Animal Model of Depression" Cells 9, no. 12: 2609. https://doi.org/10.3390/cells9122609
APA StyleMoreno, C., Hermosilla, T., Hardy, P., Aballai, V., Rojas, P., & Varela, D. (2020). Cav1.2 Activity and Downstream Signaling Pathways in the Hippocampus of An Animal Model of Depression. Cells, 9(12), 2609. https://doi.org/10.3390/cells9122609





