Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Lines
2.2. Vδ2 Cell Expansion
2.3. Vδ2 Cell Phenotypic Analysis
2.4. Cytokine Production Analysis
2.5. Degranulation and Cytotoxicity
2.6. Immune Repertoire Sequencing Assays
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
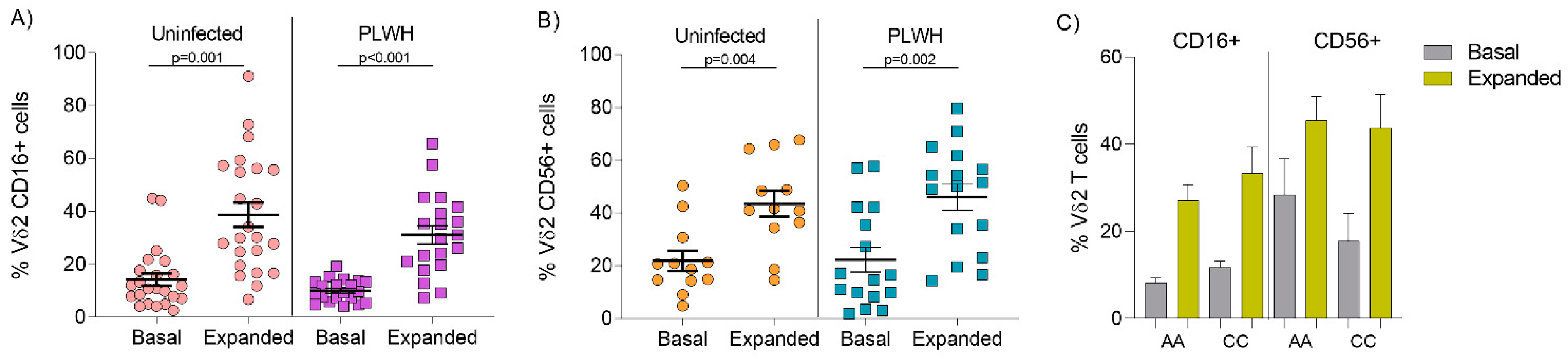
3.2. Comparable Expression of CD16 and CD56 on Vδ2 Cells from Uninfected Donors and PLWH on Stable ART
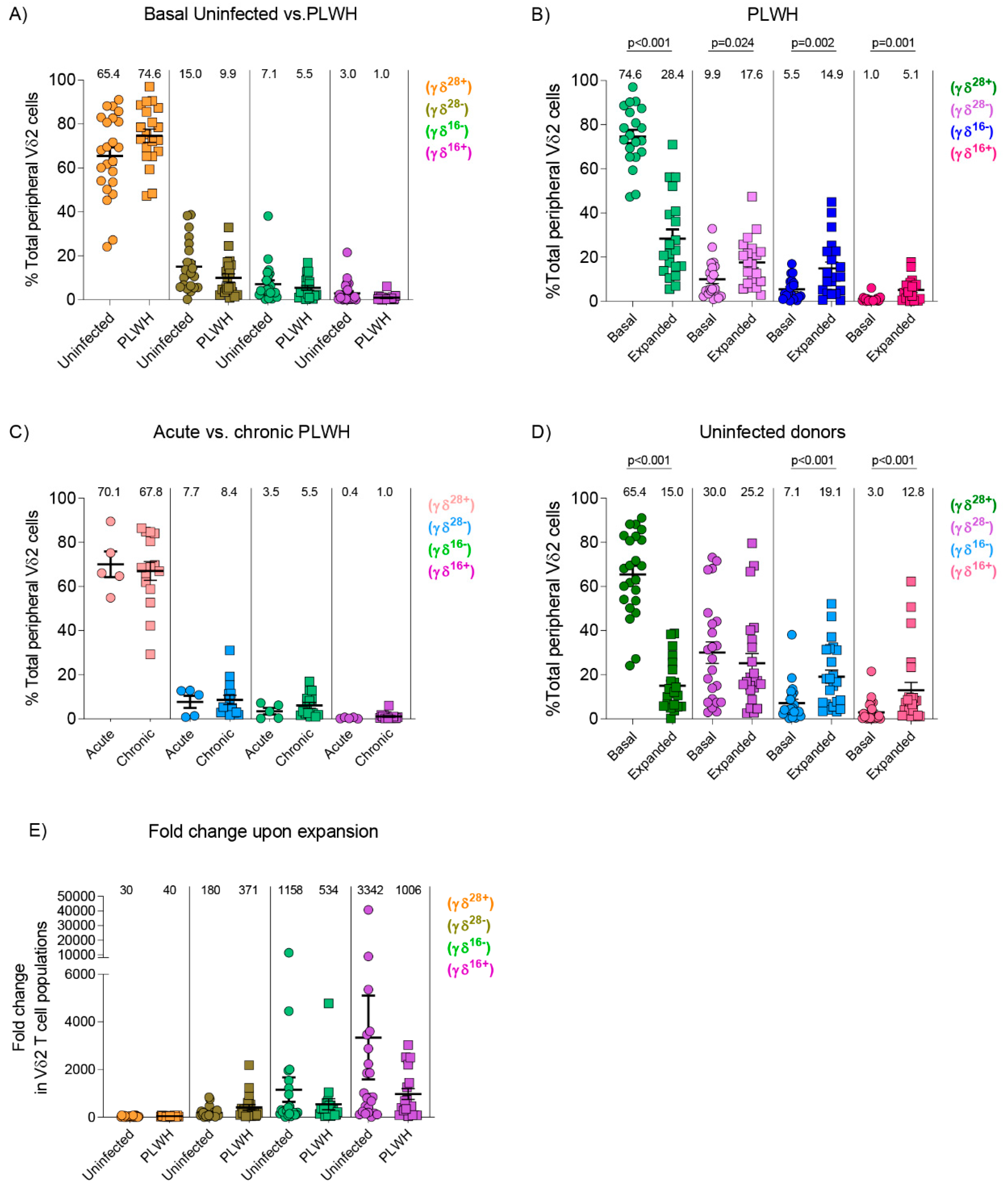
3.3. Similar Composition of Vδ2 Cell Populations in Uninfected Donors and PLWH
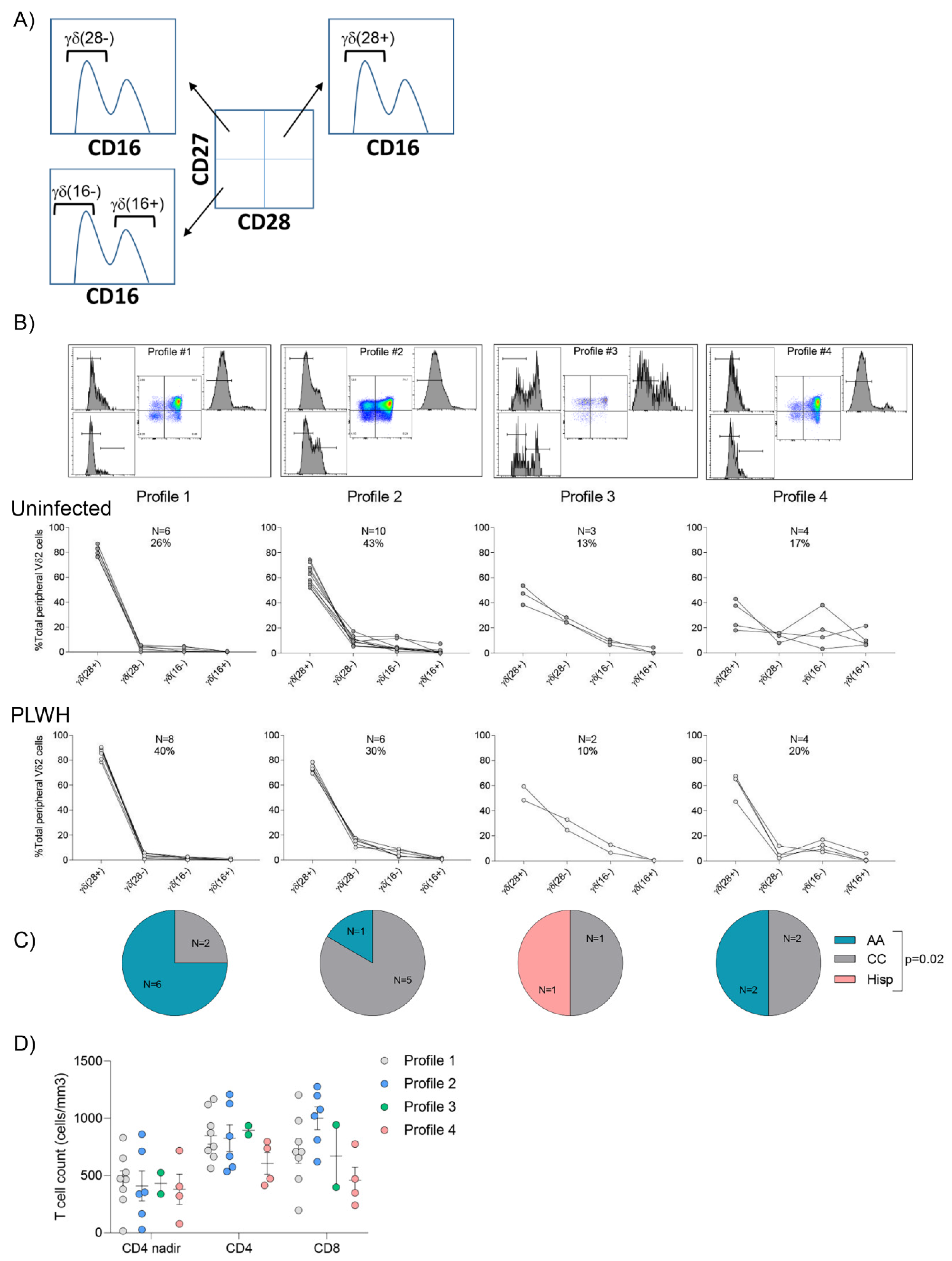
3.4. Vδ2 Cell Populations Are Distributed amongst Several Profiles in Both Uninfected Donors and PLWH
3.5. Cytokine Release after Vδ2 Cell Expansion
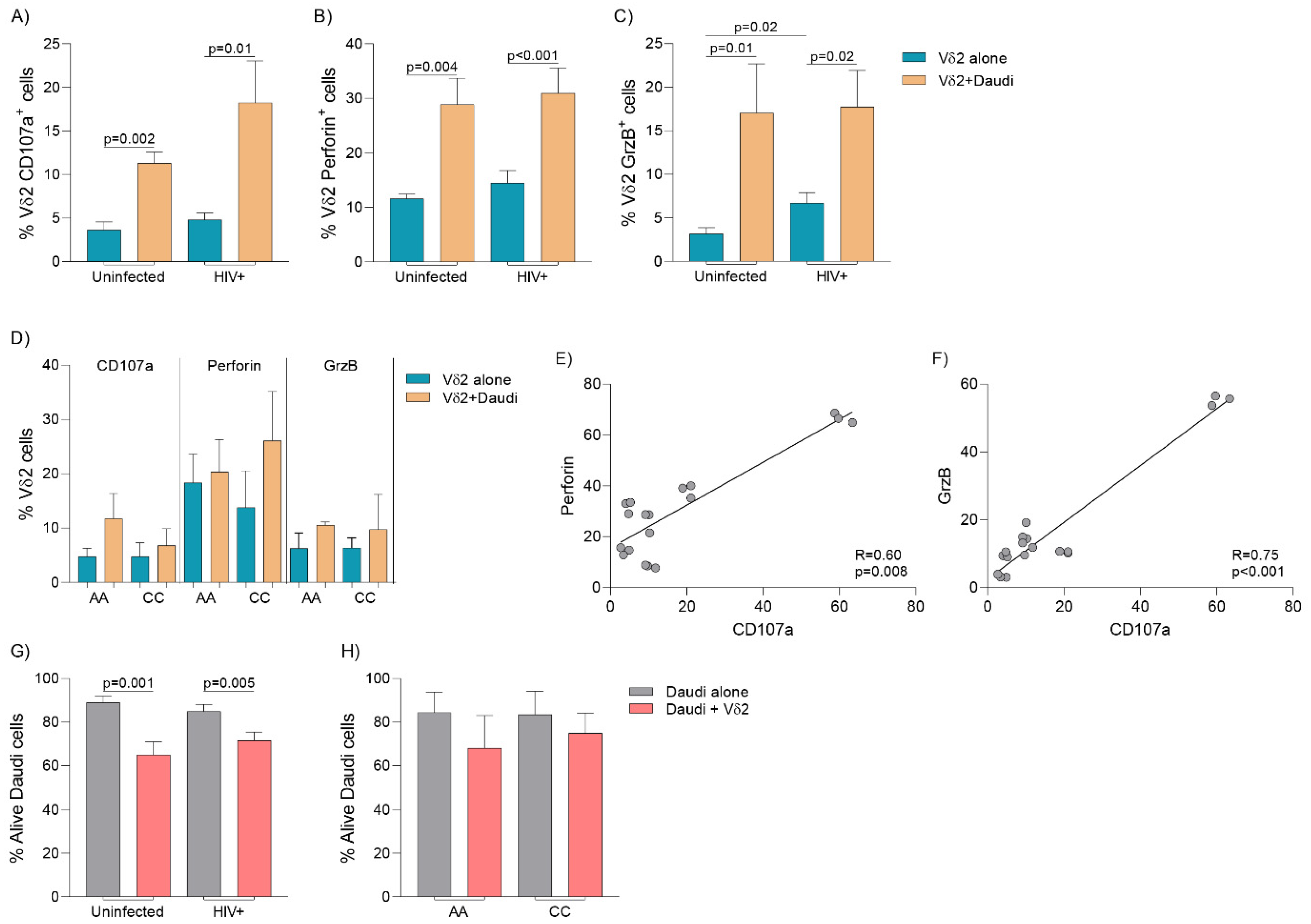
3.6. Cytolytic Capacity and Vδ2 Cell-Mediated Killing
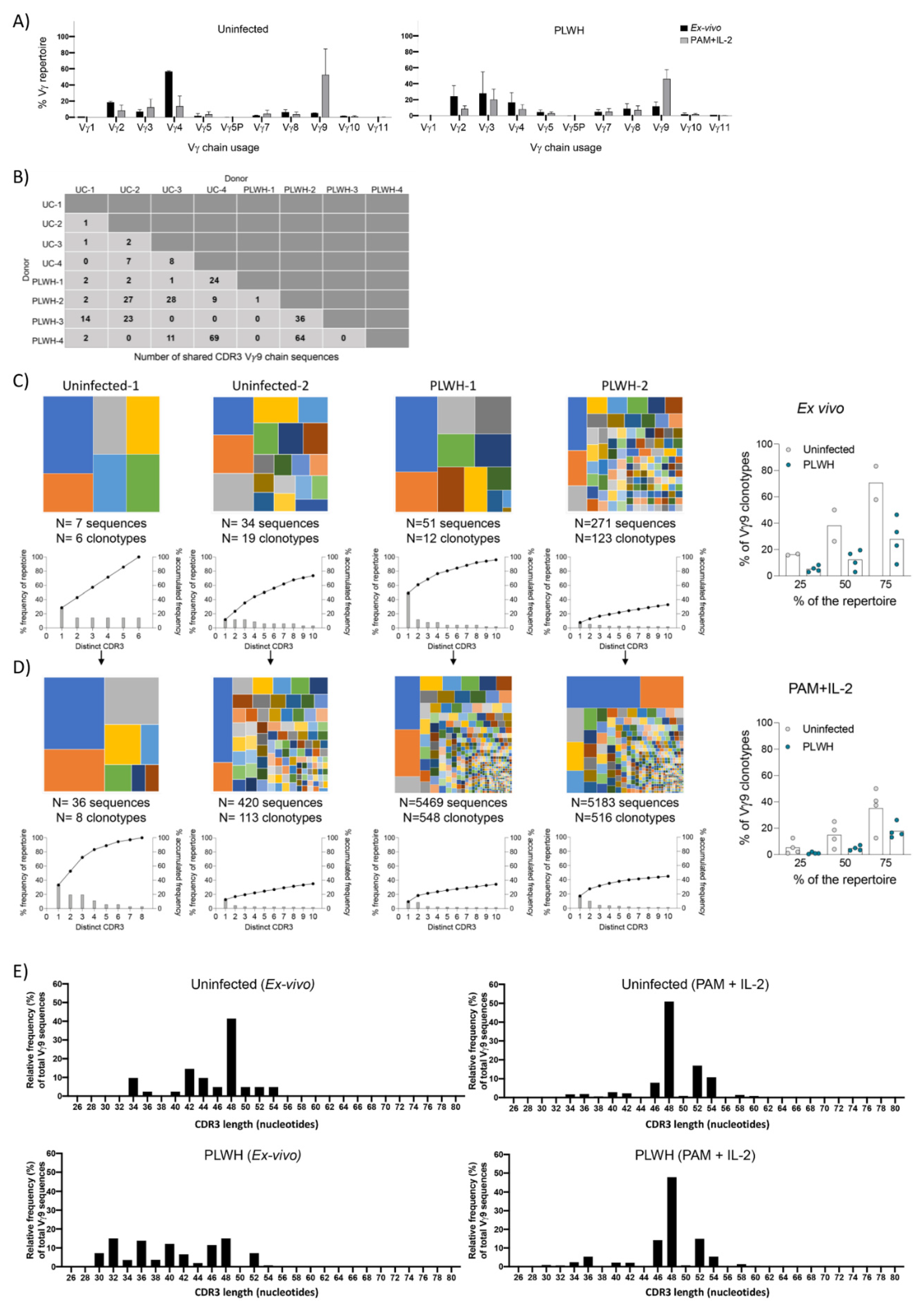
3.7. TCR Repertoire Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, M.B.; McLean, J.; Dialynas, D.P.; Strominger, J.L.; Smith, J.A.; Owen, F.L.; Seidman, J.G.; Ip, S.; Rosen, F.; Krangel, M.S. Identification of a putative second T-cell receptor. Nature 1986, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E. gammadelta T cell receptor ligands and modes of antigen recognition. Arch. Immunol. Ther. Exp. (Warsz.) 2011, 59, 117–137. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Hintz, M.; Reichenberg, A.; Altincicek, B.; Bahr, U.; Gschwind, R.M.; Kollas, A.K.; Beck, E.; Wiesner, J.; Eberl, M.; Jomaa, H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett. 2001, 509, 317–322. [Google Scholar] [CrossRef]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta T-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef]
- Dieli, F.; Gebbia, N.; Poccia, F.; Caccamo, N.; Montesano, C.; Fulfaro, F.; Arcara, C.; Valerio, M.R.; Meraviglia, S.; Di Sano, C.; et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003, 102, 2310–2311. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Z.; Feng, T.; Li, J.; Liu, Y.; Fan, Y.; Lu, Q.; Yin, Z.; Yu, M.; Shen, C.; et al. Human Vgamma9Vdelta2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell Mol. Immunol. 2013, 10, 159–164. [Google Scholar] [CrossRef]
- Hara, T.; Mizuno, Y.; Takaki, K.; Takada, H.; Akeda, H.; Aoki, T.; Nagata, M.; Ueda, K.; Matsuzaki, G.; Yoshikai, Y.; et al. Predominant activation and expansion of V gamma 9-bearing gamma delta T cells in vivo as well as in vitro in Salmonella infection. J. Clin. Investig. 1992, 90, 204–210. [Google Scholar] [CrossRef]
- Costa, G.; Loizon, S.; Guenot, M.; Mocan, I.; Halary, F.; de Saint-Basile, G.; Pitard, V.; Dechanet-Merville, J.; Moreau, J.F.; Troye-Blomberg, M.; et al. Control of Plasmodium falciparum erythrocytic cycle: Gammadelta T cells target the red blood cell-invasive merozoites. Blood 2011, 118, 6952–6962. [Google Scholar] [CrossRef]
- Corvaisier, M.; Moreau-Aubry, A.; Diez, E.; Bennouna, J.; Mosnier, J.F.; Scotet, E.; Bonneville, M.; Jotereau, F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J. Immunol. 2005, 175, 5481–5488. [Google Scholar] [CrossRef]
- Gertner-Dardenne, J.; Fauriat, C.; Vey, N.; Olive, D. Immunotherapy of acute myeloid leukemia based on gammadelta T cells. Oncoimmunology 2012, 1, 1614–1616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Urban, E.M.; Li, H.; Armstrong, C.; Focaccetti, C.; Cairo, C.; Pauza, C.D. Control of CD56 expression and tumor cell cytotoxicity in human Vgamma2Vdelta2 T cells. BMC Immunol. 2009, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liang, H.; Hong, K.; Li, H.; Peng, H.; Zhao, Y.; Jia, M.; Ruan, Y.; Shao, Y. The potential role of CD16+ Vgamma2Vdelta2 T cell-mediated antibody-dependent cell-mediated cytotoxicity in control of HIV type 1 disease. AIDS Res. Hum. Retrovir. 2013, 29, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- James, K.S.; Trumble, I.; Clohosey, M.L.; Moeser, M.; Roan, N.R.; Adimora, A.A.; Joseph, S.B.; Archin, N.M.; Hudgens, M.; Soriano-Sarabia, N. Measuring the contribution of gammadelta T cells to the persistent HIV reservoir. AIDS 2020, 34, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.T.; Sambrano, E.; Maggirwar, S.B.; Soriano-Sarabia, N. Boosting the Immune System for HIV Cure: A γδ T Cell Perspective. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, Z.; Prinz, I.; Dechanet-Merville, J.; Silva-Santos, B.; Kuball, J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 2020, 19, 169–184. [Google Scholar] [CrossRef]
- Cairo, C.; Armstrong, C.L.; Cummings, J.S.; Deetz, C.O.; Tan, M.; Lu, C.; Davis, C.E.; Pauza, C.D. Impact of age, gender, and race on circulating gammadelta T cells. Hum. Immunol. 2010, 71, 968–975. [Google Scholar] [CrossRef]
- Caccamo, N.; Dieli, F.; Wesch, D.; Jomaa, H.; Eberl, M. Sex-specific phenotypical and functional differences in peripheral human Vgamma9/Vdelta2 T cells. J. Leukoc. Biol. 2006, 79, 663–666. [Google Scholar] [CrossRef]
- Ryan, P.L.; Sumaria, N.; Holland, C.J.; Bradford, C.M.; Izotova, N.; Grandjean, C.L.; Jawad, A.S.; Bergmeier, L.A.; Pennington, D.J. Heterogeneous yet stable Vdelta2(+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc. Natl. Acad. Sci. USA 2016, 113, 14378–14383. [Google Scholar] [CrossRef]
- Pauza, C.D.; Poonia, B.; Li, H.; Cairo, C.; Chaudhry, S. gammadelta T Cells in HIV Disease: Past, Present, and Future. Front. Immunol. 2014, 5, 687. [Google Scholar] [CrossRef]
- Casetti, R.; Sacchi, A.; Bordoni, V.; Grassi, G.; Cimini, E.; Besi, F.; Pinnetti, C.; Mondi, A.; Antinori, A.; Agrati, C. In Human Immunodeficiency Virus primary infection, early combined antiretroviral therapy reduced gammadelta T-cell activation but failed to restore their polyfunctionality. Immunology 2019, 157, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Clohosey, M.L.; Whitworth, C.P.; Hudgens, M.; Margolis, D.M.; Soriano-Sarabia, N. gammadelta T cells: An immunotherapeutic approach for HIV cure strategies. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Chaudhry, S.; Cairo, C.; Venturi, V.; Pauza, C.D. The gammadelta T-cell receptor repertoire is reconstituted in HIV patients after prolonged antiretroviral therapy. AIDS 2013, 27, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Sarabia, N.; Sandvold, H.; Jomaa, H.; Kubin, T.; Bein, G.; Hackstein, H. Primary MHC-class II(+) cells are necessary to promote resting Vdelta2 cell expansion in response to (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate and isopentenyl pyrophosphate. J. Immunol. 2012, 189, 5212–5222. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Sarabia, N.; Archin, N.M.; Bateson, R.; Dahl, N.P.; Crooks, A.M.; Kuruc, J.D.; Garrido, C.; Margolis, D.M. Peripheral Vgamma9Vdelta2 T Cells Are a Novel Reservoir of Latent HIV Infection. PLoS Pathog. 2015, 11, e1005201. [Google Scholar] [CrossRef] [PubMed]
- Fisch, P.; Malkovsky, M.; Braakman, E.; Sturm, E.; Bolhuis, R.L.; Prieve, A.; Sosman, J.A.; Lam, V.A.; Sondel, P.M. Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J. Exp. Med. 1990, 171, 1567–1579. [Google Scholar] [CrossRef]
- Autran, B.; Triebel, F.; Katlama, C.; Rozenbaum, W.; Hercend, T.; Debre, P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin. Exp. Immunol. 1989, 75, 206–210. [Google Scholar] [PubMed]
- Chen, Z.W.; Letvin, N.L. Vgamma2Vdelta2+ T cells and anti-microbial immune responses. Microbes Infect 2003, 5, 491–498. [Google Scholar] [CrossRef]
- Li, H.; Pauza, C.D. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood 2011, 118, 5824–5831. [Google Scholar] [CrossRef]
- Juno, J.A.; Eriksson, E.M. gammadelta T-cell responses during HIV infection and antiretroviral therapy. Clin. Transl. Immunol. 2019, 8, e01069. [Google Scholar] [CrossRef]
- Poccia, F.; Boullier, S.; Lecoeur, H.; Cochet, M.; Poquet, Y.; Colizzi, V.; Fournie, J.J.; Gougeon, M.L. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J. Immunol. 1996, 157, 449–461. [Google Scholar]
- Wallace, M.; Scharko, A.M.; Pauza, C.D.; Fisch, P.; Imaoka, K.; Kawabata, S.; Fujihashi, K.; Kiyono, H.; Tanaka, Y.; Bloom, B.R.; et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol. Med. 1997, 3, 60–71. [Google Scholar] [CrossRef]
- Poonia, B.; Pauza, C.D. Gamma delta T cells from HIV+ donors can be expanded in vitro by zoledronate/interleukin-2 to become cytotoxic effectors for antibody-dependent cellular cytotoxicity. Cytotherapy 2012, 14, 173–181. [Google Scholar] [CrossRef]
- Martini, F.; Urso, R.; Gioia, C.; De Felici, A.; Narciso, P.; Amendola, A.; Paglia, M.G.; Colizzi, V.; Poccia, F. gammadelta T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology 2000, 100, 481–486. [Google Scholar] [CrossRef]
- Poccia, F.; Gioia, C.; Martini, F.; Sacchi, A.; Piacentini, P.; Tempestilli, M.; Agrati, C.; Amendola, A.; Abdeddaim, A.; Vlassi, C.; et al. Zoledronic acid and interleukin-2 treatment improves immunocompetence in HIV-infected persons by activating Vgamma9Vdelta2 T cells. AIDS 2009, 23, 555–565. [Google Scholar] [CrossRef]
- Parker, C.M.; Groh, V.; Band, H.; Porcelli, S.A.; Morita, C.; Fabbi, M.; Glass, D.; Strominger, J.L.; Brenner, M.B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J. Exp. Med. 1990, 171, 1597–1612. [Google Scholar] [CrossRef]
- Argentati, K.; Re, F.; Donnini, A.; Tucci, M.G.; Franceschi, C.; Bartozzi, B.; Bernardini, G.; Provinciali, M. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J. Leukoc. Biol. 2002, 72, 65–71. [Google Scholar]
- Casetti, R.; De Simone, G.; Sacchi, A.; Rinaldi, A.; Viola, D.; Agrati, C.; Bordoni, V.; Cimini, E.; Tumino, N.; Besi, F.; et al. Vgamma9Vdelta2 T-Cell Polyfunctionality Is Differently Modulated in HAART-Treated HIV Patients according to CD4 T-Cell Count. PLoS ONE 2015, 10, e0132291. [Google Scholar] [CrossRef]
- Kosub, D.A.; Lehrman, G.; Milush, J.M.; Zhou, D.; Chacko, E.; Leone, A.; Gordon, S.; Silvestri, G.; Else, J.G.; Keiser, P.; et al. Gamma/Delta T-cell functional responses differ after pathogenic human immunodeficiency virus and nonpathogenic simian immunodeficiency virus infections. J. Virol. 2008, 82, 1155–1165. [Google Scholar] [CrossRef][Green Version]
- Bhatnagar, N.; Girard, P.M.; Lopez-Gonzalez, M.; Didier, C.; Collias, L.; Jung, C.; Bollens, D.; Duvivier, C.; Von Platen, C.; Scott-Algara, D.; et al. Potential Role of Vdelta2(+) gammadelta T Cells in Regulation of Immune Activation in Primary HIV Infection. Front. Immunol. 2017, 8, 1189. [Google Scholar] [CrossRef]
- Cummings, J.S.; Cairo, C.; Armstrong, C.; Davis, C.E.; Pauza, C.D. Impacts of HIV infection on Vgamma2Vdelta2 T cell phenotype and function: A mechanism for reduced tumor immunity in AIDS. J. Leukoc. Biol. 2008, 84, 371–379. [Google Scholar] [CrossRef]
- Belkina, A.C.; Starchenko, A.; Drake, K.A.; Proctor, E.A.; Pihl, R.M.F.; Olson, A.; Lauffenburger, D.A.; Lin, N.; Snyder-Cappione, J.E. Multivariate Computational Analysis of Gamma Delta T Cell Inhibitory Receptor Signatures Reveals the Divergence of Healthy and ART-Suppressed HIV+ Aging. Front. Immunol. 2018, 9, 2783. [Google Scholar] [CrossRef]

| N | 20 |
|---|---|
| Age (mean years) | 42.3 |
| Sex (% women) | 5 (25%) |
| Race (%) | |
| African American | 9 (45%) |
| Caucasian | 10 (50%) |
| Hispanic | 1 (2%) |
| Treated in the acute phase | 5 (25%) |
| Median Time on ART (years) | 6 (1.5–7.1) |
| Median CD4 nadir (cells/mm3) | 392 (299–621) |
| Median CD4 count (cells/mm3) | 776 (597–935) |
| Median CD8 count (cells/mm3) | 742 (470–1010) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clohosey, M.L.; Mann, B.T.; Ryan, P.L.; Apanasovich, T.V.; Maggirwar, S.B.; Pennington, D.J.; Soriano-Sarabia, N. Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals. Cells 2020, 9, 2568. https://doi.org/10.3390/cells9122568
Clohosey ML, Mann BT, Ryan PL, Apanasovich TV, Maggirwar SB, Pennington DJ, Soriano-Sarabia N. Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals. Cells. 2020; 9(12):2568. https://doi.org/10.3390/cells9122568
Chicago/Turabian StyleClohosey, Matthew L., Brendan T. Mann, Paul L. Ryan, Tatiyana V. Apanasovich, Sanjay B. Maggirwar, Daniel J. Pennington, and Natalia Soriano-Sarabia. 2020. "Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals" Cells 9, no. 12: 2568. https://doi.org/10.3390/cells9122568
APA StyleClohosey, M. L., Mann, B. T., Ryan, P. L., Apanasovich, T. V., Maggirwar, S. B., Pennington, D. J., & Soriano-Sarabia, N. (2020). Comparable Vδ2 Cell Functional Characteristics in Virally Suppressed People Living with HIV and Uninfected Individuals. Cells, 9(12), 2568. https://doi.org/10.3390/cells9122568





