Mast Cell Functions Linking Innate Sensing to Adaptive Immunity
Abstract
1. Introduction
2. Innate MC Functions in Peripheral Tissues Fostering Adaptive Responses
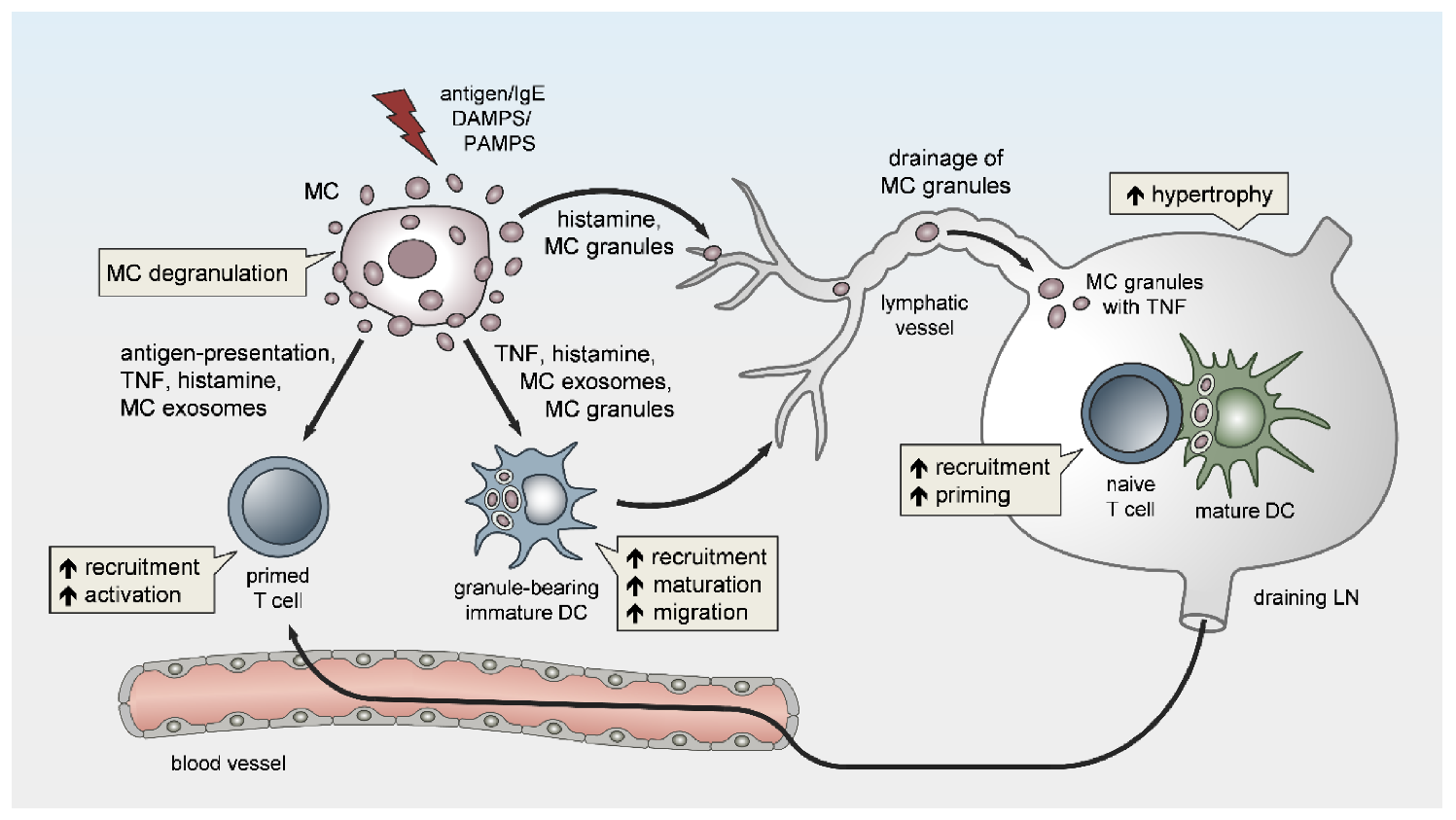
3. MC Functions in LN Conditioning and Hypertrophy
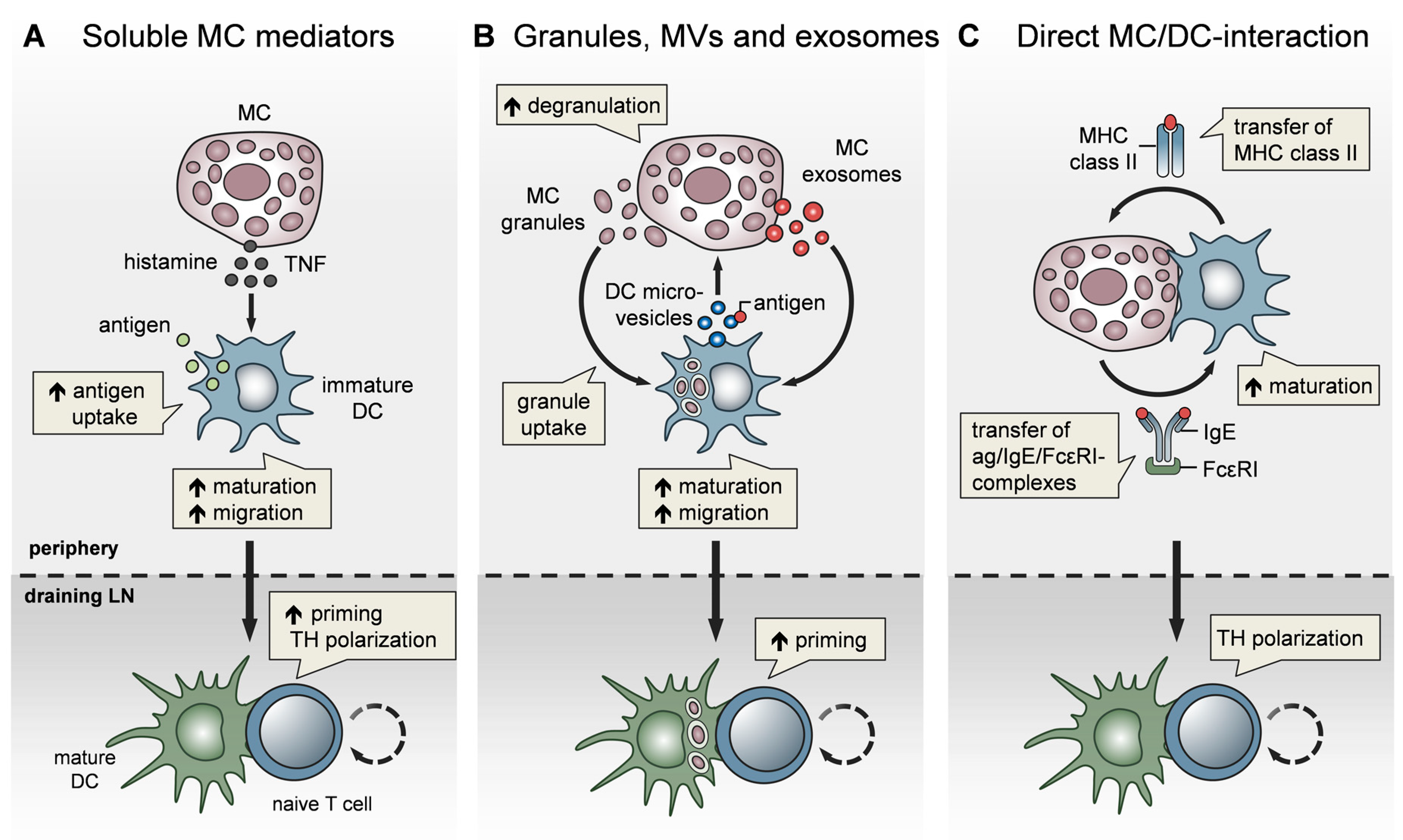
4. MCs Affect Adaptive Immunity via the Modulation of Dendritic Cells
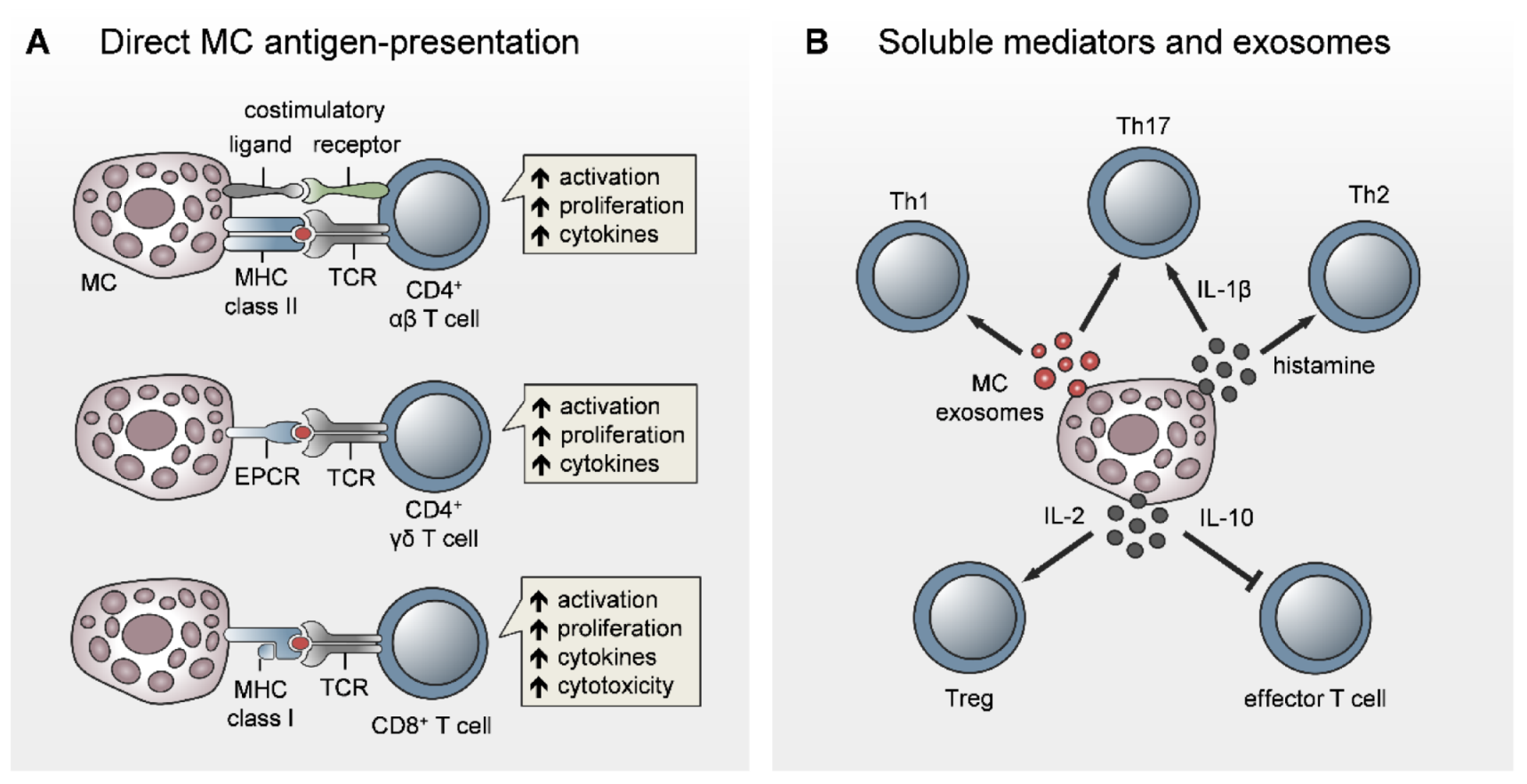
5. Direct Role for MCs in T-Cell Activation
5.1. Antigen-Presenting Capacity of MCs
5.2. MC Modulation of T-Cell Priming, Differentiation, and Polarization
6. MC Functions in B-Cell Activation
7. MCs Orchestrate Effector Cell Recruitment to Inflamed Tissues
8. The Adjuvant Effect of MC Activators in Vaccination
9. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Migalovich-Sheikhet, H.; Friedman, S.; Mankuta, D.; Levi-Schaffer, F. Novel identified, receptors on mast cells. Front. Immunol. 2012, 3, 238. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Frossi, B.; Mion, F.; Sibilano, R.; Danelli, L.; Pucillo, C.E.M. Is it time for a new classification of mast cells? What do we know about mast cell heterogeneity? Immunol. Rev. 2018, 282, 35–46. [Google Scholar] [CrossRef]
- Abraham, S.N.; John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Piliponsky, A.M.; Romani, L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol. Rev. 2018, 282, 188–197. [Google Scholar] [CrossRef]
- Hoppe, A.; Katsoulis-Dimitriou, K.; Edler, H.J.; Dudeck, J.; Drube, S.; Dudeck, A. Mast cells initiate the vascular response to contact allergens by sensing cell stress. J. Allergy Clin. Immunol. 2020, 145, 1476–1479.e3. [Google Scholar] [CrossRef]
- Olivera, A.; Beaven, M.A.; Metcalfe, D.D. Mast cells signal their importance in health and disease. J. Allergy Clin. Immunol. 2018, 142, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Valitutti, S. New roles and controls of mast cells. Curr. Opin. Immunol. 2018, 50, 39–47. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Rönnberg, E.; Melo, F.R.; Pejler, G. Mast cell proteoglycans. J. Histochem. Cytochem. 2012, 60, 950–962. [Google Scholar] [CrossRef]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Kohler, A.; Peschke, K.; Voehringer, D.; Waskow, C.; Krieg, T.; et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine induces vascular hyperpermeability by increasing blood flow and endothelial barrier disruption in vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Ballesteros-Martinez, C.; Mendez-Barbero, N.; Montalvo-Yuste, A.; Jensen, B.M.; Gomez-Cardenosa, A.; Klitfod, L.; Garrido-Arandia, M.; Alvarez-Llamas, G.; Pastor-Vargas, C.; Vivanco, F.; et al. Endothelial Regulator of Calcineurin 1 Promotes Barrier Integrity and Modulates Histamine-Induced Barrier Dysfunction in Anaphylaxis. Front. Immunol. 2017, 20, 1323. [Google Scholar] [CrossRef]
- Mendez-Barbero, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Jensen, B.M.; Gutiérrez-Muñoz, C.; Tome-Amat, J.; Garrido-Arandia, M.; Díaz-Perales, A.; Ballesteros-Martinez, C.; Laguna, J.J.; et al. The TNF-like weak inducer of the apoptosis/fibroblast growth factor-inducible molecule 14 axis mediates histamine and platelet-activating factor-induced subcutaneous vascular leakage and anaphylactic shock. J. Allergy Clin. Immunol. 2020, 145, 583–596.e6. [Google Scholar] [CrossRef] [PubMed]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 6725. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; de Castellarnau, C.; Ferrer, L.L.; Puigdemont, A.; Santamaría, L.F.; de Mora, F. Mast cells induce upregulation of P-selectin and intercellular adhesion molecule 1 on carotid endothelial cells in a new in vitro model of mast cell to endothelial cell communication. Immunol. Cell Biol. 2002, 80, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thorlacius, H. Mast cell-derived tumour necrosis factor-alpha mediates macrophage inflammatory protein-2-induced recruitment of neutrophils in mice. Br. J. Pharmacol. 2005, 145, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Tonnesen, M.G.; Marchese, M.J.; Clark, R.A.; Bahou, W.F.; Gruber, B.L. Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. J. Cell Physiol. 1995, 165, 40–53. [Google Scholar] [CrossRef]
- Huang, C.; Friend, D.S.; Qiu, W.T.; Wong, G.W.; Morales, G.; Hunt, J.; Stevens, R.L. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 1998, 160, 1910–1919. [Google Scholar]
- Jehle, A.B.; Li, Y.; Stechschulte, A.C.; Stechschulte, D.J.; Dileepan, K.N. Endotoxin and mast cell granule proteases synergistically activate human coronary artery endothelial cells to generate interleukin-6 and interleukin-8. J. Interferon Cytokine Res. 2000, 20, 361–368. [Google Scholar] [CrossRef]
- Kinoshita, M.; Okada, M.; Hara, M.; Furukawa, Y.; Matsumori, A. Mast cell tryptase in mast cell granules enhances MCP-1 and interleukin-8 production in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1858–1863. [Google Scholar] [CrossRef]
- Chi, L.; Stehno-Bittel, L.; Smirnova, I.; Stechschulte, D.J.; Dileepan, K.N. Signal transduction pathways in mast cell granule-mediated endothelial cell activation. Mediators Inflamm. 2003, 12, 79–87. [Google Scholar] [CrossRef]
- Kondeti, V.; Al-Azzam, N.; Duah, E.; Thodeti, C.K.; Boyce, J.A.; Paruchuri, S. Leukotriene D4 and prostaglandin E2 signals synergize and potentiate vascular inflammation in a mast cell-dependent manner through cysteinyl leukotriene receptor 1 and E-prostanoid receptor 3. J. Allergy Clin. Immunol. 2016, 137, 289–298. [Google Scholar] [CrossRef]
- Hart, P.H. Regulation of the inflammatory response in asthma by mast cell products. Immunol. Cell Biol. 2001, 79, 149–153. [Google Scholar] [CrossRef]
- Echtenacher, B.; Maennel, D.N.; Hueltner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 1996, 381, 75–77. [Google Scholar] [CrossRef]
- Malaviya, R.; Ikeda, T.; Ross, E.; Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996, 381, 77–80. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Hughes, E.L.; Becker, F.; Flower, R.J.; Buckingham, J.C.; Gavins, F.N.E. Mast cells mediate early neutrophil recruitment and exhibit anti-inflammatory properties via the formyl peptide receptor 2/lipoxin A4 receptor. Br. J. Pharm. 2017, 174, 2393–2408. [Google Scholar] [CrossRef]
- de Almeida, A.D.; Silva, I.S.; Fernandes-Braga, W.; Lima-Filho, A.C.M.; Florentino, R.O.M.; Barra, A.; de Oliveira Andrade, L.; Leite, M.F.; Cassali, G.D.; Klein, A. A role for mast cells and mast cell tryptase in driving neutrophil recruitment in LPS-induced lung inflammation via protease-activated receptor 2 in mice. Inflamm. Res. 2020, 69, 1059–1070. [Google Scholar] [CrossRef]
- Weber, F.C.; Nemeth, T.; Csepregi, J.Z.; Dudeck, A.; Roers, A.; Ozsvari, B.; Oswald, E.; Puskás, L.G.; Jakob, T.; Mócsai, A.; et al. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J. Exp. Med. 2015, 212, 15–22. [Google Scholar] [CrossRef]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hueltner, L.; Roecken, M. Mast cells control neutrophil recruitment during T cell-mediated delayed type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 2000, 192, 1441–1452. [Google Scholar] [CrossRef]
- Kneilling, M.; Mailhammer, R.; Hueltner, L.; Schoenberger, T.; Fuchs, K.; Schaller, M.; Bukala, D.; Massberg, S.; Sander, C.A.; Braumueller, H.; et al. Direct crosstalk between mast cell-TNF and TNFR1-expressing endothelia mediates local tissue inflammation. Blood 2009, 114, 1696–1706. [Google Scholar] [CrossRef]
- Kroner, J.; Kovtun, A.; Kemmler, J.; Messmann, J.J.; Strauss, G.; Seitz, S.; Schinke, T.; Amling, M.; Kotrba, J.; Froebel, J.; et al. Mast cells are critical regulators of bone fracture-induced inflammation and osteoclast formation and activity. J. Bone Min. Res. 2017, 32, 2431–2444. [Google Scholar] [CrossRef]
- Chillo, O.; Kleinert, E.C.; Lautz, T.; Lasch, M.; Pagel, J.I.; Heun, Y.; Troidl, K.; Fischer, S.; Caballero-Martinez, A.; Mauer, A.; et al. Perivascular Mast Cells Govern Shear Stress-Induced Arteriogenesis by Orchestrating Leukocyte Function. Cell Rep. 2016, 16, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Wezel, A.; Lagraauw, H.M.; van der Velden, D.; de Jager, S.C.; Quax, P.H.; Kuiper, J.; Bot, I. Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression. Atherosclerosis 2015, 241, 289–296. [Google Scholar] [CrossRef]
- Bot, I.; de Jager, S.C.; Zernecke, A.; Lindstedt, K.A.; van Berkel, T.J.; Weber, C.; Biessen, E.A.L. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein E-deficient mice. Circulation 2007, 115, 2516–2525. [Google Scholar] [CrossRef]
- Ngo Nyekel, F.; Pacreau, E.; Benadda, S.; Msallam, R.; Åbrink, M.; Pejler, G.; Davoust, J.; Benhamou, M.; Charles, N.; Launay, P.; et al. Mast Cell Degranulation Exacerbates Skin Rejection by Enhancing Neutrophil Recruitment. Front. Immunol. 2018, 9, 2690. [Google Scholar] [CrossRef]
- Schramm, R.; Schaefer, T.; Menger, M.D.; Thorlacius, H. Acute mast cell-dependent neutrophil recruitment in the skin is mediated by KC and LFA-1: Inhibitory mechanisms of dexamethasone. J. Leukoc. Biol. 2002, 72, 1122–1132. [Google Scholar] [PubMed]
- Dudeck, J.; Kotrba, J.; Immler, R.; Hoffmann, A.; Voss, M.; Alexaki, V.I.; Morton, L.; Jahn, S.R.; Winzer, S.; Kollias, G.; et al. Directional Mast Cell Degranulation of TNF into Blood Vessels Primes Neutrophil Extravasation. Available online: https://ssrn.com/abstract=3651507 (accessed on 30 October 2020).
- Sutherland, R.E.; Olsen, J.S.; McKinstry, A.; Villalta, S.A.; Wolters, P.J. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J. Immunol. 2008, 181, 5598–5605. [Google Scholar] [CrossRef]
- Doener, F.; Michel, A.; Reuter, S.; Friedrich, P.; Bohm, L.; Relle, M.; Codarri, L.; Tenzer, S.; Klein, M.; Bopp, T.; et al. Mast cell derived mediators promote murine neutrophil effector functions. Int. Immunol. 2013, 25, 553–561. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Ying, S.; Phipps, S.; Kay, A.B. Interactions between eotaxin, histamine and mast cells in early microvascular events associated with eosinophil recruitment to the site of allergic skin reactions in humans. Clin. Exp. Allergy. 2004, 34, 1276–1282. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Levi-Schaffer, F. Eosinophils interaction with mast cells: The allergic effector unit. Methods Mol. Biol. 2014, 1178, 231–249. [Google Scholar]
- Lampinen, M.; Carlson, M.; Håkansson, L.D.; Venge, P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 2004, 59, 793–805. [Google Scholar] [CrossRef]
- Yang, D.; de la Rosa, G.; Tewary, P.; Oppenheim, J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009, 30, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.; Bignon, A.; Gueguen, C.; de Chaisemartin, L.; Gorges, R.; Sandré, C.; Mascarell, L.; Balabanian, K.; Kerdine-Roemer, S.; Pallardy, M.; et al. Neutrophil extracellular traps downregulate lipopolysaccharide-induced activation of monocyte-derived dendritic cells. J. Immunol. 2014, 193, 5689–5698. [Google Scholar] [CrossRef] [PubMed]
- Kandler, K.; Shaykhiev, R.; Kleemann, P.; Klescz, F.; Lohoff, M.; Vogelmeier, C.; Bals, R. The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int. Immunol. 2006, 18, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Morioka, Y.; Yamasaki, K.; Leung, D.; Gallo, R.L. Cathelicidin antimicrobial peptides inhibit hyaluronan-induced cytokine release and modulate chronic allergic dermatitis. J. Immunol. 2008, 181, 3915–3922. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- McLachlan, J.B.; Hart, J.P.; Pizzo, S.V.; Shelburne, C.P.; Staats, H.F.; Gunn, M.D.; Abraham, S.N. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 2003, 4, 1199–1205. [Google Scholar] [CrossRef]
- Jawdat, D.M.; Rowden, G.; Marshall, J.S. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J. Immunol. 2006, 177, 1755–1762. [Google Scholar] [CrossRef]
- Demeure, C.E.; Brahimi, K.; Hacini, F.; Marchand, F.; Péronet, R.; Huerre, M.; St-Mezard, P.; Nicolas, J.F.; Brey, P.; Delespesse, G.; et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J. Immunol. 2005, 174, 3932–3940. [Google Scholar] [CrossRef]
- Chatterjee, V.; Gashev, A.A. Mast cell-directed recruitment of MHC class II positive cells and eosinophils towards mesenteric lymphatic vessels in adulthood and elderly. Lymphat. Res. Biol. 2014, 12, 37–47. [Google Scholar] [CrossRef]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Dusio, G.F.; Gasheva, O.Y.; Skoog, H.; Tobin, R.; Peddaboina, C.; Meininger, C.J.; Zawieja, D.C.; Newell-Rogers, M.K.; Gashev, A.A. Mast cells and histamine are triggering the NF-κB-mediated reactions of adult and aged perilymphatic mesenteric tissues to acute inflammation. Aging 2016, 8, 3065–3090. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Maejima, D.; Nagai, T.; Bridenbaugh, E.; Thangaswamy, S.; Chatterjee, V.; Meiniger, C.J.; Gashev, A.A. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 2014, 21, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Gasheva, O.Y.; Zawieja, D.C.; Meininger, C.J.; Gashev, A.A. Histamine-mediated autocrine signaling in mesenteric perilymphatic mast cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R590–R604. [Google Scholar] [CrossRef]
- Rampart, M.; De Smet, W.; Fiers, W.; Herman, A.G. Inflammatory properties of recombinant tumor necrosis factor in rabbit skin in vivo. J. Exp. Med. 1989, 169, 2227–2232. [Google Scholar] [CrossRef]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of mast cell secretory granules on skin inflammation boosts dendritic cell migration and priming efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864.e4. [Google Scholar] [CrossRef]
- Kunder, C.A.; St John, A.L.; Li, G.; Leong, K.W.; Berwin, B.; Staats, H.F.; Abraham, S.N. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009, 206, 2455–2467. [Google Scholar] [CrossRef]
- Schwartz, L.B.; Riedel, C.; Caulfield, J.P.; Wasserman, S.I.; Austen, K.F. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J. Immunol. 1981, 126, 2071–2078. [Google Scholar]
- Kunder, C.A.; St John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- St John, A.L.; Chan, C.Y.; Staats, H.F.; Leong, K.W.; Abraham, S.N. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nat. Mater. 2012, 11, 250–257. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Surviladze, Z.; Cambi, A.; Lidke, D.S.; Wilson, B.S. Mast cell synapses and exosomes: Membrane contacts for information exchange. Front. Immunol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, M.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast cell: An emerging partner in immune interaction. Front. Immunol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.P.; Nakano, H.; St John, A.L.; Chan, C.; McLachlan, J.B.; Gunn, M.D.; Staats, H.F.; Abraham, S.N. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 2009, 6, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bryce, P.J.; Miller, M.L.; Miyajima, I.; Tsai, M.; Galli, S.J.; Oettgen, H.C. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity 2004, 20, 381–392. [Google Scholar] [CrossRef]
- Jawdat, D.M.; Albert, E.J.; Rowden, G.; Haidl, I.D.; Marshall, J.S. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 2004, 173, 5275–5282. [Google Scholar] [CrossRef]
- Dawicki, W.; Jawdat, D.W.; Xu, N.; Marshall, J.S. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J. Immunol. 2010, 184, 2116–2123. [Google Scholar] [CrossRef]
- Suto, H.; Nakae, S.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cell associated TNF promotes dendritic cell migration. J. Immunol. 2006, 176, 4102–4112. [Google Scholar] [CrossRef]
- Dudeck, J.; Ghouse, S.M.; Lehmann, C.H.K.; Hoppe, A.; Schubert, N.; Nedospasov, S.A.; Dudziak, D.; Dudeck, A. Mast-cell-derived TNF amplifies CD81 dendritic cell functionality and CD81 T cell priming. Cell Rep. 2015, 13, 399–411. [Google Scholar] [CrossRef]
- Caron, G.; Delneste, Y.; Roelandts, E.; Duez, C.; Herbault, N.; Magistrelli, G.; Bonnefoy, J.Y.; Pestel, J.; Jeannin, P. Histamine induces CD86 expression and chemokine production by human immature dendritic cells. J. Immunol. 2001, 166, 6000–6006. [Google Scholar] [CrossRef]
- Amaral, M.M.; Davio, C.; Ceballos, A.; Salamone, G.; Canones, C.; Geffner, J.; Vermeulen, M. Histamine improves antigen uptake and cross-presentation by dendritic cells. J. Immunol. 2007, 179, 3425–3433. [Google Scholar] [CrossRef]
- Mazzoni, A.; Young, H.A.; Spitzer, J.H.; Visintin, A.; Segal, D.M. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J. Clin. Investig. 2001, 108, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Kitawaki, T.; Kadowaki, N.; Sugimoto, N.; Kambe, N.; Hori, T.; Miyachi, Y.; Nakahata, T.; Uchiyama, T. IgEactivated mast cells in combination with pro-inflammatory factors induce Th2-promoting dendritic cells. Int. Immunol. 2006, 18, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Siraganian, R.P.; Leifer, C.A.; Segal, D.M. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J. Immunol. 2006, 177, 3577–3581. [Google Scholar] [CrossRef] [PubMed]
- Stassen, M.; Hartmann, A.K.; Delgado, S.J.; Dehmel, S.; Braun, A. Mast cells within cellular networks. J. Allergy Clin. Immunol. 2019, 144, S46–S54. [Google Scholar] [CrossRef]
- Skokos, D.; Botros, H.G.; Demeure, C.; Morin, J.; Peronet, R.; Birkenmeier, G.; Boudaly, S.; Mécheri, S. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 2003, 170, 3037–3045. [Google Scholar] [CrossRef]
- Admyre, C.; Telemo, E.; Almqvist, N.; Lötvall, J.; Lahesmaa, R.; Scheynius, A.; Gabrielsson, S. Exosomes-nanovesicles with possible roles in allergic inflammation. Allergy 2008, 63, 404–408. [Google Scholar] [CrossRef]
- Choi, H.W.; Suwanpradid, J.; Kim, I.H.; Staats, H.F.; Haniffa, M.; MacLeod, A.S.; Abraham, S.N. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science 2018, 362, eaao0666. [Google Scholar] [CrossRef]
- Otsuka, A.; Kubo, M.; Honda, T.; Egawa, G.; Nakajima, S.; Tanizaki, H.; Kim, B.; Matsuoka, S.; Watanabe, T.; Nakae, S.; et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 2011, 6, e25538. [Google Scholar] [CrossRef]
- Dudeck, A.; Suender, C.A.; Lopez Kostka, S.; von Stebut, E.; Maurer, M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur. J. Immunol. 2011, 41, 1883–1893. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Dudeck, J.; Medyukhina, A.; Fröbel, J.; Svensson, C.M.; Kotrba, J.; Gerlach, M.; Gradtke, A.C.; Schroeder, B.; Speier, S.; Figge, M.T.; et al. Mast cells acquire MHCII from dendritic cells during skin inflammation. J. Exp. Med. 2017, 214, 3791–3811. [Google Scholar] [CrossRef]
- Frandji, P.; Tkaczyk, C.; Oskeritzian, C.; David, B.; Desaymard, C.; Mécheri, S. Exogenous and endogenous antigens are differentially presented by mast cells to CD4+ T lymphocytes. Eur. J. Immunol. 1996, 26, 2517–2528. [Google Scholar] [CrossRef]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Espagnolle, N.; Mars, L.T.; Liblau, R.; Valitutti, S.; Espinosa, E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood 2009, 114, 4979–4988. [Google Scholar] [CrossRef]
- Nakano, N.; Nishiyama, C.; Yagita, H.; Koyanagi, A.; Akiba, H.; Chiba, S.; Ogawa, H.; Okumura, K. Notch signaling confers antigen-presenting cell functions on mast cells. J. Allergy Clin. Immunol. 2009, 123, 74–81.e1. [Google Scholar] [CrossRef]
- Nakano, N.; Nishiyama, C.; Yagita, H.; Koyanagi, A.; Ogawa, H.; Okumura, K. Notch1-mediated signaling induces MHC class II expression through activation of class II transactivator promoter III in mast cells. J. Biol. Chem. 2011, 286, 12042–12048. [Google Scholar] [CrossRef]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef]
- Suurmond, J.; van Heemst, J.; van Heiningen, J.; Dorjée, A.L.; Schilham, M.W.; van der Beek, F.B.; Huizinga, T.W.J.; Schuerwegh, A.J.M.; Toes, R.E.M. Communication between human mast cells and CD4(+) T cells through antigen-dependent interactions. Eur. J. Immunol. 2013, 43, 1758–1768. [Google Scholar] [CrossRef]
- Kashiwakura, J.; Yokoi, H.; Saito, H.; Okayama, Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: Comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J. Immunol. 2004, 173, 5247–5257. [Google Scholar] [CrossRef]
- Schubert, N.; Dudeck, J.; Liu, P.; Karutz, A.; Speier, S.; Maurer, M.; Tuckermann, J.; Dudeck, A. Mast cell promotion of T cell-driven antigen-induced arthritis despite being dispensable for antibody-induced arthritis in which T cells are bypassed. Arthritis Rheumatol. 2015, 67, 903–913. [Google Scholar] [CrossRef]
- van der Velden, D.; Lagraauw, H.M.; Wezel, A.; Launay, P.; Kuiper, J.; Huizinga, T.W.; Toes, R.E.M.; Bot, I.; Stoop, J.N. Mast cell depletion in the preclinical phase of collagen-induced arthritis reduces clinical outcome by lowering the inflammatory cytokine profile. Arthritis Res. 2016, 18, 138. [Google Scholar] [CrossRef]
- Kritikou, E.; van der Heijden, T.; Swart, M.; van Duijn, J.; Slütter, B.; Wezel, A.; Smeets, H.J.; Maffia, P.; Kuiper, J.; Bot, I. Hypercholesterolemia Induces a Mast Cell-CD4+ T Cell Interaction in Atherosclerosis. J. Immunol. 2019, 202, 1531–1539. [Google Scholar] [CrossRef]
- Mantri, C.K.; St John, A.L. Immune synapses between mast cells and γδ T cells limit viral infection. J. Clin. Investig. 2019, 129, 1094–1108. [Google Scholar] [CrossRef]
- Stelekati, E.; Bahri, R.; D’Orlando, O.; Orinska, Z.; Mittrücker, H.W.; Langenhaun, R.; Glatzel, M.; Bollinger, A.; Paus, R.; Bulfone-Paus, S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity 2009, 31, 665–676. [Google Scholar] [CrossRef]
- Huels, C.; Germann, T.; Goedert, S.; Hoehn, P.; Koelsch, S.; Hültner, L.; Palm, N.; Ruede, E.; Schmitt, E. Co-activation of naive CD4+ T cells and bone marrow-derived mast cells results in the development of Th2 cells. Int. Immunol. 1995, 7, 525–532. [Google Scholar] [CrossRef]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Song, J.P.; Liu, X.; Jiang, J.; Chen, X.; Yang, L.; Hu, T.; Zheng, P.Y.; Liu, Z.G.; Yang, P.C. Mast cell-derived serine proteinase regulates T helper 2 polarization. Sci. Rep. 2014, 4, 4649. [Google Scholar] [CrossRef]
- Suurmond, J.; Habets, K.L.; Dorjée, A.L.; Huizinga, T.W.; Toes, R.E. Expansion of Th17 Cells by Human Mast Cells Is Driven by Inflammasome-Independent IL-1β. J. Immunol. 2016, 197, 4473–4481. [Google Scholar] [CrossRef]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef]
- Salamon, P.; Shefler, I.; Moshkovits, I.; Munitz, A.; Horwitz Klotzman, D.; Mekori, Y.A.; Hershko, A.Y. IL-33 and IgE stimulate mast cell production of IL-2 and regulatory T cell expansion in allergic dermatitis. Clin. Exp. Allergy 2017, 47, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; Pan, Y.; Pierini, A.; Galli, S.J.; Negrin, R.S. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood 2013, 122, 3659–3665. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Breitling, S.; Hui, Z.; Zabini, D.; Hu, Y.; Hoffmann, J.; Goldenberg, N.M.; Tabuchi, A.; Buelow, R.; Dos Santos, C.; Kuebler, W.M. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L710–L721. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.B.; Shelburne, C.P.; Hart, J.P.; Pizzo, S.V.; Goyal, R.; Brooking-Dixon, R.; Staats, H.F.; Abraham, S.N. Mast cell activators: A new class of highly effective vaccine adjuvants. Nat. Med. 2008, 14, 536–541. [Google Scholar] [CrossRef]
- McGowen, A.L.; Hale, L.P.; Shelburne, C.P.; Abraham, S.N.; Staats, H.F. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine 2009, 27, 3544–3552. [Google Scholar] [CrossRef]
- Schubert, N.; Lisenko, K.; Auerbach, C.; Weitzmann, A.; Ghouse, S.M.; Muhandes, L.; Haase, C.; Haering, T.; Schulze, L.; Voehringer, D.; et al. Unimpaired Responses to Vaccination with Protein Antigen Plus Adjuvant in Mice with Kit-Independent Mast Cell Deficiency. Front. Immunol. 2018, 9, 1870. [Google Scholar] [CrossRef]
- Tkaczyk, C.; Frandji, P.; Botros, H.G.; Poncet, P.; Lapeyre, J.; Peronet, R.; David, B.; Mécheri, S. Mouse bone marrow-derived mast cells and mast cell lines constitutively produce B cell growth and differentiation activities. J. Immunol. 1996, 157, 1720–1728. [Google Scholar]
- Merluzzi, S.; Frossi, B.; Gri, G.; Parusso, S.; Tripodo, C.; Pucillo, C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 2010, 115, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.K.; Garcia-Faroldi, G.; Lundberg, M.; Pejler, G.; Kleinau, S. Activated mast cells promote differentiation of B cells into effector cells. Sci. Rep. 2016, 6, 20531. [Google Scholar] [CrossRef]
- Valeri, V.; Tonon, S.; Vibhushan, S.; Gulino, A.; Belmonte, B.; Adori, M.; Hedestam, G.B.K.; Gautier, G.; Tripodo, C.; Blank, U.; et al. Mast cells crosstalk with B cells in the gut and sustain IgA response in the inflamed intestine. Eur. J. Immunol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, S.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I.; Feoktistov, I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: An A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J. Immunol. 2004, 172, 7726–7733. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, M.B.; Lee, D.; Min, K.Y.; Koo, J.; Kim, H.W.; Park, Y.H.; Kim, S.J.; Ikutani, M.; Takaki, S.; et al. The regulatory B cell-mediated peripheral tolerance maintained by mast cell IL-5 suppresses oxazolone-induced contact hypersensitivity. Sci. Adv. 2019, 5, eaav8152. [Google Scholar] [CrossRef] [PubMed]
- Dahdah, A.; Gautier, G.; Attout, T.; Fiore, F.; Lebourdais, E.; Msallam, R.; Daeron, M.; Monteiro, R.C.; Charles, N.; Davoust, J.; et al. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J. Clin. Investig. 2014, 124, 4577–4589. [Google Scholar] [CrossRef] [PubMed]
- Bawazeer, M.A.; Theoharides, T.C. IL-33 stimulates human mast cell release of CCL5 and CCL2 via MAPK and NF-κB, inhibited by methoxyluteolin. Eur. J. Pharm. 2019, 865, 172760. [Google Scholar] [CrossRef]
- Katsanos, G.S.; Anogeianaki, A.; Orso, C.; Tetè, S.; Salini, V.; Antinolfi, P.L.; Sabatino, G. Mast cells and chemokines. J. Biol. Regul. Homeost. Agents 2008, 22, 145–151. [Google Scholar]
- Mori, Y.; Hirose, K.; Suzuki, K.; Nakajima, H.; Seto, Y.; Ikeda, K.; Shimoda, K.; Nakayama, K.; Saito, Y.; Iwamoto, I. Tyk2 is essential for IFN-alpha-induced gene expression in mast cells. Int. Arch. Allergy Immunol. 2004, 134, 25–29. [Google Scholar] [CrossRef]
- Castellani, M.L.; Vecchiet, J.; Salini, V.; Conti, P.; Theoharides, T.C.; Caraffa, A.; Antinolfi, P.; Teté, S.; Ciampoli, C.; Cuccurullo, C.; et al. Stimulation of CCL2 (MCP-1) and CCL2 mRNA by substance P in LAD2 human mast cells. Transl. Res. 2009, 154, 27–33. [Google Scholar] [CrossRef]
- Ott, V.L.; Cambier, J.C.; Kappler, J.; Marrack, P.; Swanson, B.J. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat. Immunol. 2003, 4, 974–981. [Google Scholar] [CrossRef]
- Ebert, S.; Becker, M.; Lemmermann, N.A.; Büttner, J.K.; Michel, A.; Taube, C.; Podlech, J.; Boehm, V.; Freitag, K.; Thomas, D.; et al. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog. 2014, 10, e1004100. [Google Scholar] [CrossRef]
- Staats, H.F.; Fielhauer, J.R.; Thompson, A.L.; Tripp, A.A.; Sobel, A.E.; Maddaloni, M.; Abraham, S.N.; Pascual, D.W. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS ONE 2011, 6, e16532. [Google Scholar] [CrossRef]
- Bento, D.; Staats, H.F.; Borges, O. Effect of particulate adjuvant on the anthrax protective antigen dose required for effective nasal vaccination. Vaccine 2015, 33, 3609–3613. [Google Scholar] [CrossRef]
- Meng, S.; Liu, Z.; Xu, L.; Li, L.; Mei, S.; Bao, L.; Deng, W.; Li, L.; Lei, R.; Xie, L.; et al. Intranasal Immunization with Recombinant HA and Mast Cell Activator C48/80 Elicits Protective Immunity against 2009 Pandemic H1N1 Influenza in Mice. PLoS ONE 2011, 6, e19863. [Google Scholar] [CrossRef] [PubMed]
- Bento, D.; Jesus, S.; Lebre, F.; Gonçalves, T.; Borges, O. Chitosan Plus Compound 48/80: Formulation and Preliminary Evaluation as a Hepatitis B Vaccine Adjuvant. Pharmaceutics 2019, 11, 72. [Google Scholar] [CrossRef]
- Wang, S.H.; Kirwan, S.M.; Abraham, S.N.; Staats, H.F.; Hickey, A.J. Stable dry powder formulation for nasal delivery of anthrax vaccine. J. Pharm. Sci. 2012, 101, 31–47. [Google Scholar] [CrossRef]
- Bento, D.; Staats, H.F.; Gonçalves, T.; Borges, O. Development of a novel adjuvanted nasal vaccine: C48/80 associated with chitosan nanoparticles as a path to enhance mucosal immunity. Eur. J. Pharm. Biopharm. 2015, 93, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Matsui, N.; Ito, D.; Takabatake, Y.; Nashioka, E.; Tada, S.; Kanagawa, M.; Fukuishi, N.; Akagi, M. Compound 48/80, a mast cell stimulator, enhances synthesis of IgE and IgG induced by intranasal application of ovalbumin in mice. Biol. Pharm. Bull. 2015, 38, 1954–1959. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Huang, S.; Lu, F. Mast cell activator compound 48/40 is not an effective adjuvant for UV-attenuated Toxoplasma gondii vaccine. Parasitol. Res. 2017, 116, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A. Mast cell-MrgprB2: Sensing secretagogues or a means to overreact? Immunol. Cell Biol. 2015, 93, 221–223. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef] [PubMed]
- Sample, C.J.; Hudak, K.E.; Barefoot, B.E.; Koci, M.D.; Wanyonyi, M.S.; Abraham, S.; Staats, H.F.; Ramsburg, E.A. A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 2013, 48, 96–105. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Choi, H.W.; Walker, Q.D.; Blough, B.; Kuhn, C.M.; Abraham, S.N.; Staats, H.F. Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy. NPJ Vaccines 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhang, Y.; Lai, Y.; Chen, W.; Xiao, Z.; Zhang, W.; Jin, M.; Yu, B. LL-37-induced human mast cell activation through G protein-coupled receptor MrgX2. Int. Immunopharm. 2017, 49, 6–12. [Google Scholar] [CrossRef]
- Kim, S.H.; Yang, I.Y.; Kim, J.; Lee, K.Y.; Jang, Y.S. Antimicrobial peptide LL-37 promotes antigen-specific immune responses in mice by enhancing Th17-skewed mucosal and systemic immunities. Eur. J. Immunol. 2015, 45, 1402–1413. [Google Scholar] [CrossRef]
- Bramwell, V.W.; Somavarapu, S.; Outschoorn, I.; Alpar, H.O. Adjuvant action of melittin following intranasal immunisation with tetanus and diphtheria toxoids. J. Drug Target. 2003, 11, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Kitani, S. Gangliosides inhibit bee venom melittin cytotoxicity but not phospholipase A2-induced degranulation in mast cells. Toxicol. Appl. Pharm. 2011, 252, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, N.; Endo, M.; Kanno, H.; Matsukawa, N.; Tsutsumi, R.; Takeshita, R.; Sato, S. Polymyxins as Novel and Safe Mucosal Adjuvants to Induce Humoral Immune Responses in Mice. PLoS ONE 2013, 8, e61643. [Google Scholar] [CrossRef] [PubMed]
- Peachell, P.T.; Pearce, F.L. Some studies on the release of histamine from mast cells treated with polymyxin. Agents Actions 1984, 14, 379–385. [Google Scholar] [CrossRef]
- Yoshino, N.; Takeshita, R.; Kawamura, H.; Sasaki, Y.; Kagabu, M.; Sugiyama, T.; Muraki, Y.; Sato, S. Mast cells partially contribute to mucosal adjuvanticity of surfactin in mice. Immun. Inflamm. Dis. 2018, 6, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Kayamuro, H.; Yoshioka, Y.; Abe, Y.; Arita, S.; Katayama, K.; Nomura, T.; Yoshikawa, T.; Kubota-Koketsu, R.; Ikuta, K.; Okamoto, S.; et al. Interleukin-1 Family Cytokines as Mucosal Vaccine Adjuvants for Induction of Protective Immunity against Influenza Virus. J. Virol. 2010, 84, 12703–12712. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells 2020, 9, 2538. https://doi.org/10.3390/cells9122538
Katsoulis-Dimitriou K, Kotrba J, Voss M, Dudeck J, Dudeck A. Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells. 2020; 9(12):2538. https://doi.org/10.3390/cells9122538
Chicago/Turabian StyleKatsoulis-Dimitriou, Konstantinos, Johanna Kotrba, Martin Voss, Jan Dudeck, and Anne Dudeck. 2020. "Mast Cell Functions Linking Innate Sensing to Adaptive Immunity" Cells 9, no. 12: 2538. https://doi.org/10.3390/cells9122538
APA StyleKatsoulis-Dimitriou, K., Kotrba, J., Voss, M., Dudeck, J., & Dudeck, A. (2020). Mast Cell Functions Linking Innate Sensing to Adaptive Immunity. Cells, 9(12), 2538. https://doi.org/10.3390/cells9122538





