1. Introduction
The nuclear receptor constitutive androstane receptor (CAR, NR1I3) is primarily expressed in the liver and acts as an essential xenobiotic and endobiotic sensor. After xenobiotic stimulation, CAR drives the cellular response by modulating the expression of a wide set of drug metabolizing enzymes and transporters [
1,
2]. Besides xenobiotic detoxification, activation of CAR is also involved in the regulation of other hepatic functions such as gluconeogenesis, lipid synthesis, fatty acid oxidation, and clearance of steroid hormones and bilirubin [
2,
3].
CAR activation is a multi-step process with the nuclear accumulation of CAR monomer being the first key event. The interaction of the CAR/RXRα heterodimer with the regulatory elements of target genes subsequently triggers a cellular response. CAR is activated either by ligand interaction with the CAR ligand-binding domain (CAR-LBD) or indirectly by dephosphorylation via the signaling of the epidermal growth factor (EGF) [
4,
5,
6].
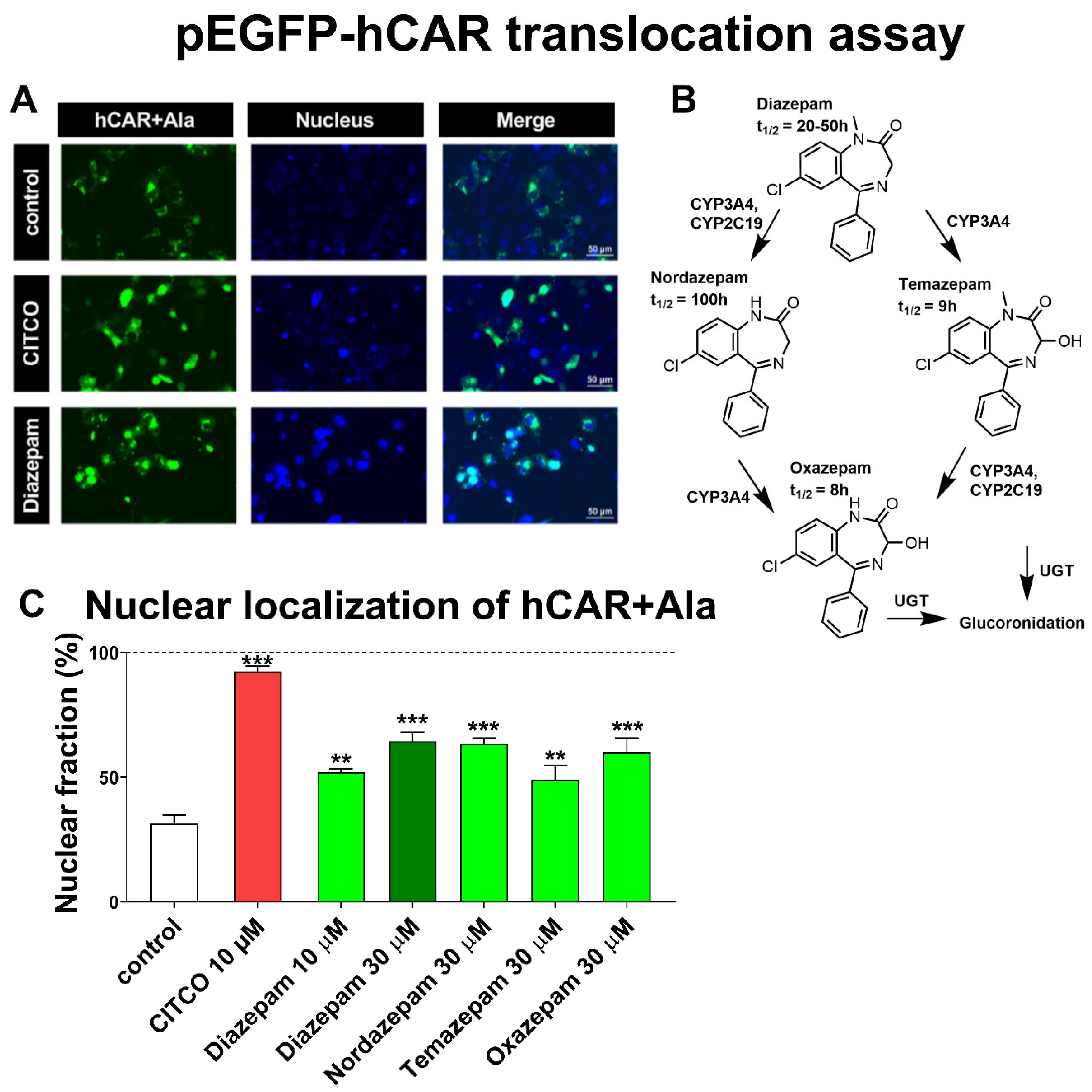
Diazepam was identified as an indirect activator of human CAR (hCAR) in a translocation assay in primary human hepatocytes [
7]. In rodents, diazepam is a CYP2B inducer and promotes liver tumorigenesis as a non-genotoxic carcinogen [
8]. However, the exact mechanism of CAR activation mediated by diazepam remains unrevealed. In addition, neither the significance of diazepam effects mediated via hCAR in human hepatocytes nor the correlation of its effects between rodent and human models have been examined. In the human liver, diazepam is mainly metabolized by the CYP3A4 and CYP2C19 enzymes, during which both genes are targets of activated CAR [
9,
10,
11]. It has also been reported that inhibition of CYP2B6, which is the most typical CAR inducible cytochrome P450 enzyme, decreases metabolism of diazepam [
12]. Therefore, diazepam-mediated hCAR activation should be also considered to elucidate its own feedback metabolism regulation.
Today, we have no efficient hCAR ligand that might help us define the value of CAR in human therapy. For instance, 6-(4-chlorophenyl) imidazo [2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzy-l)oxime (CITCO) is the only specific human ligand available for experimental purposes [
13]. The discovery of a hCAR activator suitable for clinical studies or one already used in pharmacotherapy would be a milestone in the characterization of hCAR as a molecular target in pharmacotherapy. Among the variety of compounds characterized as hCAR activators using in vitro high-throughput screenings, only a few compounds are on the market [
7,
14,
15]. For instance, the routinely used pharmaceuticals diazepam and phenytoin have been described as hCAR activators in vitro, but only one solitary drug-to-drug interaction possibly connected with hCAR activation has been reported [
16].
Phenobarbital is an indirect and nonspecific activator of both hCAR and rodent CAR, but also the pregnane X receptor (PXR). Studies with phenobarbital indicate that the mechanism of liver tumor promotion connected with CAR activation is relevant only for rodent models and not for humans [
17]. Consequently, it is not always possible to extrapolate data obtained in animal models to hCAR and clinical situations in humans.
hCAR displays a high degree of constitutive transcriptional activity. Thus, numerous hCAR assays have not been sensitive, or they have provided biased data in the identification of novel hCAR ligands. In the past 10 years, new efficient methods for CAR activation testing have been developed, including translocation assays and gene reporter assays with different hCAR variants or mutants [
7,
18,
19]. In addition, advanced tools and methods in the study of specific gene expression after hCAR activation have been developed such as HepaRG CAR-knockout cells and mice with humanized CAR [
20].
In this report, we used different molecular biology methods to characterize hCAR activation by diazepam and its metabolites, its mechanism, and the significance of these events in vivo. Employing a translocation assay, gene reporter assays, in silico docking, a TR-FRET assay, as well as induction studies in primary human hepatocytes (PHHs), HepaRG cell lines, and in vivo in CAR humanized mice, we characterized diazepam as a novel direct hCAR in vitro ligand, but with no significant in vivo effects in CAR humanized mice in therapeutically relevant doses.
2. Materials and Methods
2.1. Chemicals
Diazepam (D0899), oxazepam (O5254), temazepam (T8275), and desmethyldiazepam (D7282) were obtained from Sigma-Aldrich (St. Louis, MO, USA), which is now known as Merck (Darmstadt, Germany). Control compounds CITCO (6-(4-chlorophenyl) imidazo [2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzy-l)oxime), rifampicin (RIF), and PK11195 were also purchased from Sigma-Aldrich. Phenobarbital (Luminal® 200 mg/mL injection) was manufactured by Desitin Pharma spol.s.r.o. (Prague, Czech Republic).
2.2. Cell Culture
COS-1 (SV40 transformed African green monkey kidney) and human hepatocellular carcinoma HepG2 were purchased from the European Collection of Authenticated Cell Cultures (Salisbury, UK) and cultured at 37 °C in a 5% CO2 atmosphere in antibiotic-free Dulbecco’s Modified Eagle Medium(DMEM medium (Invitrogen, Carlsbad, CA, USA) supplemented 10% fetal bovine serum (FBS, Sigma-Aldrich) and sodium-pyruvate (Sigma-Aldrich).
2.3. Translocation Assay
COS-1 cells were seeded on 48-well plates (20,000 cells/well) and, 24 h after seeding, were transfected (Lipofectamine™ 3000) with 100 ng/well pEGFP-hCAR+Ala construct. The cells were treated with CITCO (10 μM), diazepam, nordazepam, temazepam and oxazepam (10 μM and 30 μM), or control (DMSO 0.1%) for 24 h. For microscopy, living cells were stained with Hoechst 33342 (0.2 µM, 5 min at 37 °C) (Sigma-Aldrich). Confocal microscopy was performed with a Nicon Ti ECLIPSE microscope and Nikon A1 plus camera (Nikon, Tokyo, Japan) using 405 and 488 nm lasers. Microphotographs were taken using the NIS Elements AR 4.20 software (Laboratory Imaging, Czech Republic). Four microphotographs of every treatment were taken and cells with green fluorescence protein (GFP) cytosolic or nuclear localization was counted in more than 150 cells in each picture. Each experiment was performed in biological triplicates (n = 3).
pEGFP-hCAR construct kindly donated by Dr. Y. Kanno [
21] was used for site directed mutagenesis (using GeneArtTM Site-Directed Mutagenesis System, Thermo Fisher Scientific, Waltham, MA, USA). To insert extra alanine at position 271 of the CAR-LBD, the primers were used as previously described (50-CCCTCTTCTCTCCTGCTGACCGACCTGGAGTTAC-30 and 50-GTAACTCCAGGTCGGTCAGCAGGAGAGAAGAGGG-30) [
18].
2.4. Docking
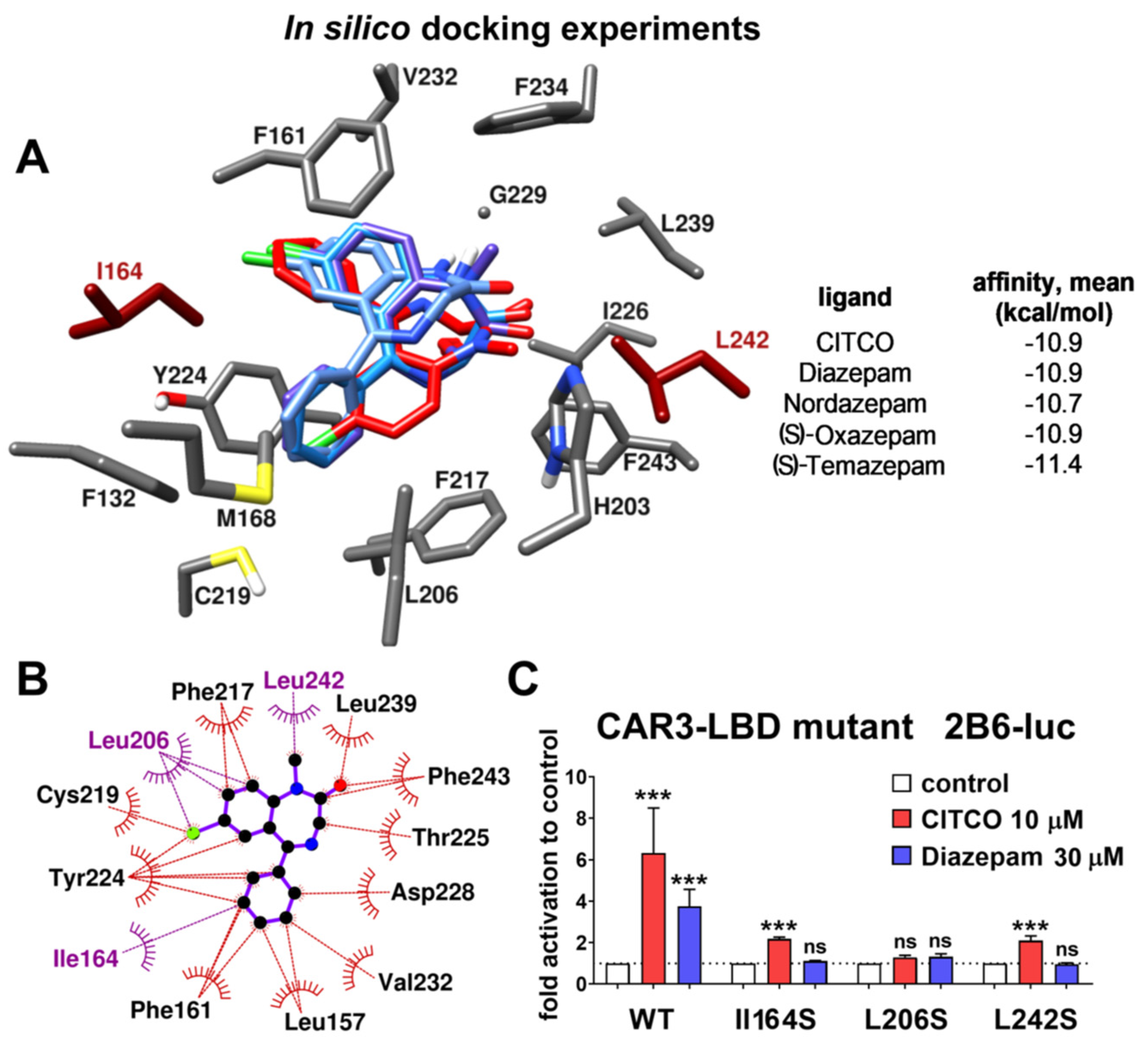
2.4.1. Receptor and Ligands Preparation
The crystal structure of the hCAR model was retrieved from the RCSB Protein Data Bank (
www.rcsb.org) (PDB code: 1XVP) [
22]. The model was limited to chains D (containing the ligand binding domain) and H (nuclear receptor coactivator). The binding domain was checked for possible errors and clashes and then prepared by standard procedure employing AutoDock Tools 1.5.6 [
23]. Water molecules were deleted, polar hydrogens were added, Kollman charges were assigned, and the prepared model was converted to a PDBQT format.
Structures of ligand molecules were drawn in PerkinElmer Chem3D (version 19.0.1.28, Waltham, MA, USA), energetically minimized utilizing the inbuilt MM2 force filed and exported as PDB files. Preparation of ligands was done by AutoDock Tools 1.5.6. Gasteiger charges were computed, non-polar hydrogens were merged, the torsion tree was built, and, finally, the model was exported as a PDBQT file. The target grid box defining the CARs active site was defined by a box with side lengths of 22 × 25 × 22 grid points (1 Å spacing) and centered at (24, 53, 30).
2.4.2. Molecular Docking
Docking experiments were performed with diazepam, its three metabolites (nordazepam, oxazepam, and temazepam) and originally bound ligand CITCO employing AutoDock Vina 1.1.2. [
24]. The exhaustiveness was increased to 16, the number of binding modes was limited to 5, and, otherwise, the default values were kept for the rest of the parameters. Five independent runs with random seed were performed for each ligand. Average affinity for corresponding poses is presented as the final affinity value.
2.4.3. Figures
Figures representing the docking results were generated in Chimera 1.13 [
25]. Only residues in proximity to ligands are displayed. All other residues and all non-polar hydrogens are omitted for clarity. The 2D ligand-protein interaction diagram was generated in LigPlot+ [
26].
Numbering in figures and in text is fully in accordance with the numbering used in the 1XVP model. Nevertheless, if this sequence is compared with the canonical sequence from Uniprot.org databank (Q14994-1), residues 232–235 are missing and, therefore, numbering differs by 4 from this point.
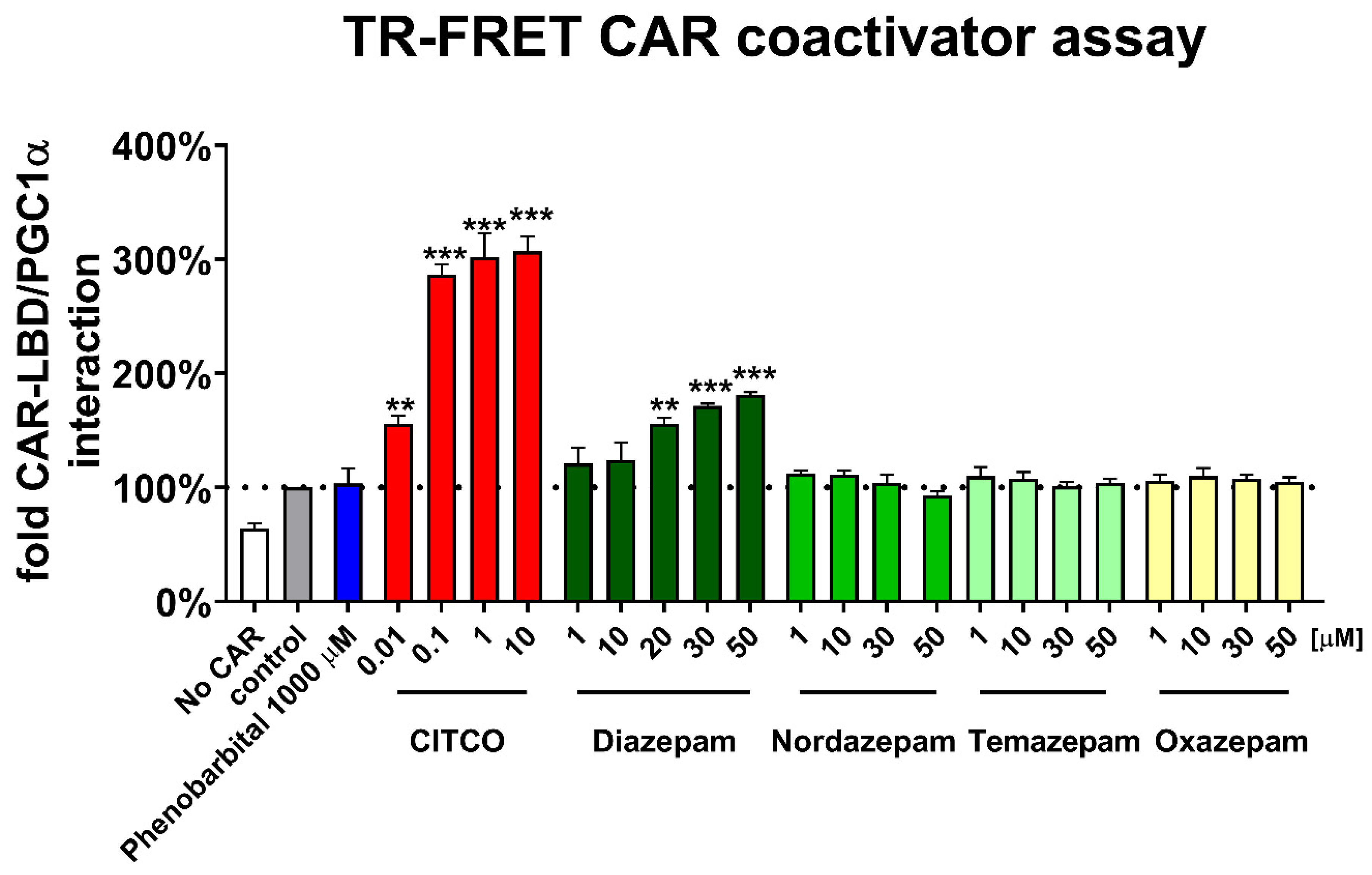
2.5. Time-Resolved Fluorescence Energy Transfer (TR-FRET CAR) Coactivator Binding Assay
The LanthaScreen
® TR-FRET CAR Coactivator Binding Assay (Thermo Fisher Scientific, Waltham, MA, USA) was used with some modifications to the manufacturers’ protocol reported in our previous paper [
27]. In order to allow better interaction of benzodiazepines with the CAR LBD, incubation time was extended to 3 h. For fluorescence measurement, the Synergy Biotek plate reader was used with appropriate filters (BioTek Instruments Inc., Winooski, VT, USA). Results were calculated as an interaction of the CAR-LBD with the coactivator fragment relative to the control (DMSO 0.1%). Data are presented as the means and SD of a technical quadruplicate (
n = 4).
2.6. Plasmids
CYP2B6-luc reporter plasmid (originally entitled as B-1.6k/PB/XREM) was kindly donated by Dr. Hongbing Wang (University of Maryland School of Pharmacy, Baltimore, MD, USA). The expression construct for a ligand-activated CAR receptor transcription variant 3 (pTracer-CMV2-CAR3) was a kind gift from Dr. C. J. Omiecinski (Pennsylvania State University, State College, PA, USA). Another expression vector for CAR variant 3 (pcDNA3.1-CAR3, Clone ID: OHu09315D, Accession No. NM_005122.4) used for data presented in Figure 2B was obtained from Genscript (NJ, USA). Mutant variants Il164S, L206S, and L242S of the CAR3 were synthetized by Genscript from the pcDNA3.1-CAR3 vector. The expression plasmid pSG5-hRXRα was a generous gift from Dr. C. Carlberg (University of Kuopio, Kuopio, Finland).
CAR-LBD assembly assays were performed with constructs encoding C (151–349 aa, helices 3–12) and N (103–150 aa, helix 1) terminal parts of hCAR using the protocol described in our previous report [
27]. pGL5-luc and pRL-TK were obtained from Promega (Hercules, CA, USA).
pGAL4-CAR+AAA construct encoding for human CAR-LBD with three extra alanine residues was a gift from Dr. Albert Braeuning (BfR Institute, Berlin, Germany).
2.7. Transient Transfection and Luciferase Gene Reporter Assays
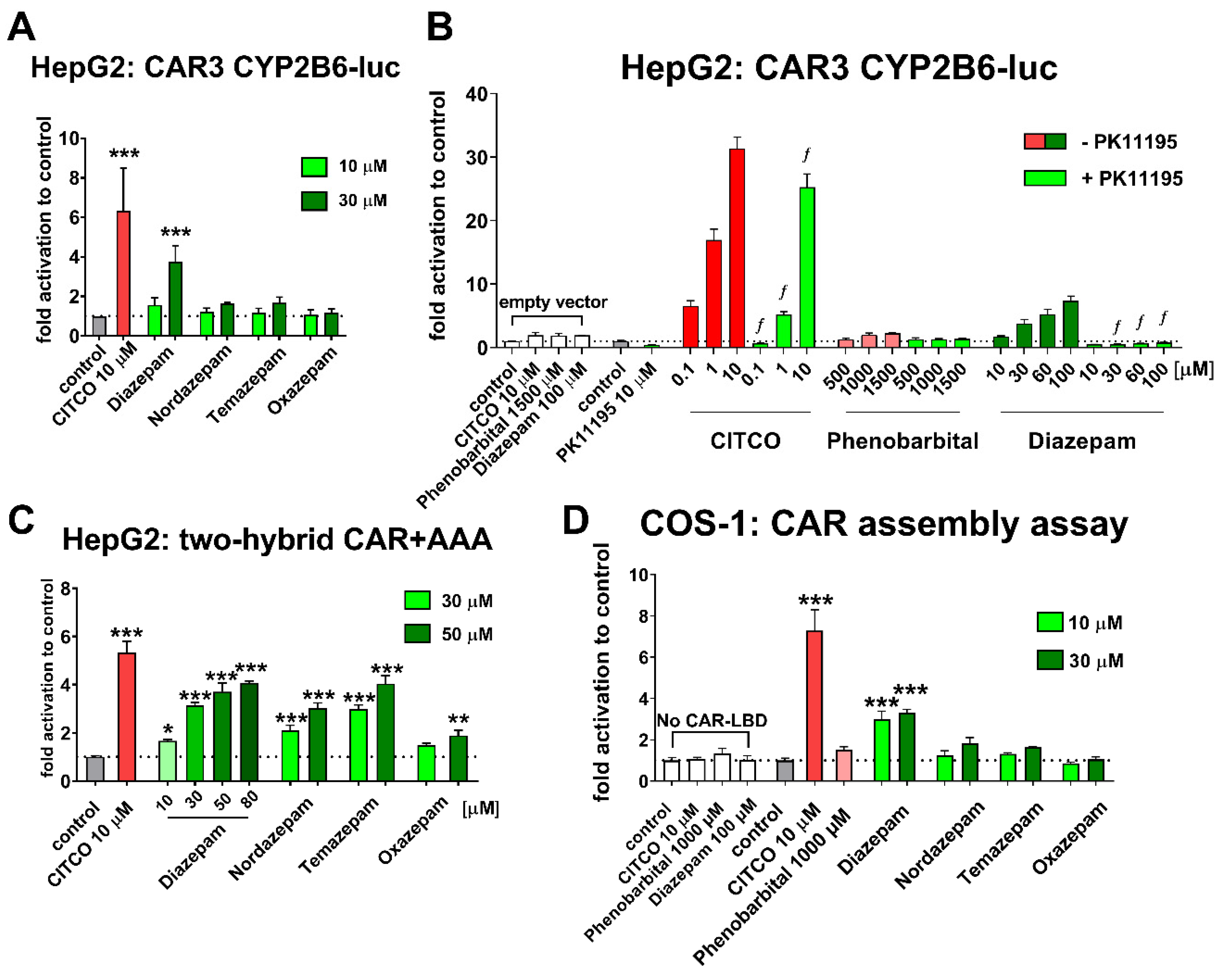
All transient transfection gene reporter assays were carried out using Lipofectamine™ 3000 (Thermo Fischer Scientific, Waltham, MA, USA), according to the manufacturer’s protocol. The HepG2 or COS-1 cells were seeded at density 40,000 cells/cm2 on 48-well plates and were transfected after 24 h with either CAR3 variant expression vector and heterodimerization partner RXRα expression vectors (100 ng/well) and the p2B6-luc luciferase reporter construct (150 ng/well). In CAR3-LBD mutant assays CAR3 vectors were used with mutated amino acids in ligand binding cavity, such as Il164S, L206S, and L242S. In the CAR assembly assay, cells were transfected with two constructs encoding two parts of the CAR ligand binding domain, C (151–349 aa, helices 3–12) and N (103–150 aa, helix 1) (100 ng/well of each) together with the pGL5-luc (150 ng/well) luciferase reporter plasmid (Promega, Hercules, CA, USA) containing an upstream activation domain (UAS) binding domain. In the mammalian two-hybrid assay, the fusion plasmid GAL4-CAR+AAA (100 ng/well) was co-transfected together with the VP16-SRC-1 receptor interacting domain (100 ng/well), heterodimerization partner RXRα (50 ng/well), and pGL5-luc (150 ng/well) luciferase vector. All transient transfection assays were normalized with the Renilla reniformis luciferase transfection control plasmid (pRL-TK, 30 ng/well). After 24 h from transfection cells, they were treated with tested compounds for the following 24 h. Luminescence activity was measured using the Dual luciferase detection kit (Promega) and fold-activity was expressed relative to the vehicle-treated samples (0.1% DMSO). In all experiments, results are presented as means and SD from at least three independent experiments performed in triplicates, except for COS-1 CAR assembly assay, where representative experiments are depicted.
2.8. qRT-PCR
Cryopreserved differentiated HepaRG™ cells (HPRGC10, ThermoFisher Scientific, Waltham, MA, USA) were seeded according to the manufacturer’s protocol and cells were treated by studied compounds after 72 h of stabilization (control (DMSO 0.1%), CITCO 10 µM, diazepam 30 and 50 µM, nordazepam, temazepam, and oxazepam 50 µM, respectively) for 48 h. Total RNA was isolated using TRIZOL® and reverse transcription was performed with the RevertAid RT Reverse Transcription Kit. qPCR was performed using TaqMan™ Fast Advanced Master Mix with TaqMan probes for CYP2B6 (Hs04183483_g1), GAPDH (Hs02786624_g1), and B2M (Hs00187842_m1) genes (all reagents for qRT-PCR were obtained from Thermo Fischer Scientific Catalog number: 4331182, Waltham, MA, USA). The delta-delta method was used for gene expression quantification normalized to GAPDH and B2M gene expression average. Three technical replicates were used for each reaction.
2.9. Primary Human Hepatocyte Isolation and Culture
Primary human hepatocytes (PHHs) were isolated as described previously [
28] from liver resections performed in adult patients for medical reasons unrelated to our research program. Liver samples were obtained from the Biologic Resource Center of Montpellier University Hospital (CRB-CHUM,
http://www.chumontpellier.fr, Biobank ID: BB-0033-00031), and this study benefitted from the expertise of Dr. Benjamin Rivière (hepatogastroenterology sample collection) and Dr. Edouard Tuaillon (CRB-CHUM manager). The patients’ clinical characteristics are the following: Liv1—female, 28 years, focal nodular hyperplasia, Liv2—female, 45 years, adenoma. The procedure was approved by the French Ethics Committee and written or oral consent was obtained from the patients.
PHHs were seeded at confluency (2.1 × 105 cell/cm2) in ISOM medium supplemented with 2% fetal bovine serum and cultured in 5% CO2 humidified atmosphere at 37 °C. ISOM medium was changed to hepatocyte growth medium (HGM: WME medium supplemented with 5 µg/mL insulin, 0.1 µM hydrocortisone, 10 µg/mL transferrin, 250 µg/mL ascorbic acid, 3.75 mg/mL fatty acid-free bovine serum albumin, 2 mM glutamine, penicillin, and streptomycin) at day 1 post-seeding. One day after, the cells were treated with tested compounds for 24 h.
2.9.1. siRNA Transfection
Adherent PHHs were transfected with 20 nM non-targeting siRNA (scrambled, siSC) or siRNAs specific for CAR or Pregnane-X receptor (PXR, siCAR or siPXR, Dharmacon, Lafayette, CO, USA) at day 1 and day 3 after seeding using Lipofectamine RNAiMAX reagent (Life Technologies, Carlsbad, CA, USA). At day 4 post-seeding, PHHs were treated with tested molecules for 24 h.
2.9.2. RNA Isolation and RT-PCR Assays of PHHs
After extraction with Trizol reagent (Life Technologies), 500 ng of total RNA was reverse-transcribed using a random hexaprimer and the MMLV Reverse Transcriptase Kit (Life Technologies). Quantitative polymerase chain reactions were performed using the Roche SYBER Green reagent and a LightCycler 480 apparatus (Roche Diagnostic, Meylan, France). The amplification specificity was evaluated by determining the product melting curve. Results are expressed as indicated in the figure legends. The primer sequences summarized are the following: CYP2B6, ATGGGGCACTGAAAAAGACTGA, AGAGGCGGGGACACTGAATGAC, GADPH, GTCTCCTCTGACTTCAACAGCG, ACCACCCTGTTGCTGTAGCCAA, respectively. The following program was used: one step at 95 °C for 10 min and then 50 cycles of denaturation at 95 °C for 10 s, annealing at 62 °C for 15 s and elongation at 72 °C for 15 s.
2.10. Animal Experiments
All animal studies were performed in accordance with European Directive 86/609/EEC and they were approved by the Czech Central Commission for Animal Welfare. Humanized PXR-CAR-CYP3A4/3A7 mice (model 11585) were obtained from Taconic (Rensselaer, NY, USA) and kept in a temperature-controlled and light-controlled facility with a 12-h light-dark cycling. All animals had free access to a commercially available laboratory chow diet (Velaz, Prague, Czech Republic). Male 9–17 weeks old animals were randomized into three groups (control (vehicle, PEG300 and H2O 1:1, n = 4), CITCO (10 mg/kg, n = 3), diazepam (1 mg/kg, n = 6)), and formulations were administered in two following days intraperitoneally. Animals were sacrificed 24 h after the second administration and livers were removed and snap frozen in liquid nitrogen for further total RNA isolation. To detect mRNA levels, the same procedure was performed as in cellular experiments and TaqMan specific probes (from Thermo Fischer Scientific Catalog number: 4331182) for Cyp2b10 (Mm00456588_mH), Cyp2c29 (Mm00725580_s1), CYP3A4 (Hs00604506_m1), Fasn (Mm00662319_m1), G6pc (Mm00839363_m1), Mki67 (Mm01278617_m1), Foxm1 (Mm00514924_m1), Gadd45b (Mm00435123_m1), Pcna (Mm00448100_g1), Gapdh (Mm99999915_g1), and B2m (Mm00437762_m1) genes were used.
2.11. Statistics
Data are presented as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) with a Dunnett’s post hoc test or Bonferroni test were applied. GraphPad Prism 8.4.3 Software (GraphPad Software, Inc., San Diego, CA, USA) was used to perform statistical analysis. A p-value of < 0.05 was considered to be statistically significant.
4. Discussion
In this report, we characterized diazepam and its metabolites nordazepam, temazepam, and oxazepam as stimulators of hCAR nuclear translocation, which is the first key step in CAR activation. We also utilized in silico docking experiments and found that these benzodiazepines fit the hCAR LBD cavity and their lipophilic moieties form non-polar interactions with the cavity. However, in luciferase gene reporter and TR-FRET assays, diazepam proved as a significant direct hCAR activator, but its metabolites display much lower activities toward hCAR. Furthermore, gene expression studies in primary human hepatocytes have shown that diazepam activates CAR, but not PXR, in the regulation of CYP2B6 mRNA. To elucidate clinical importance of this interaction, we also performed in vivo experiments with humanized CAR/PXR/CYP3A4 mice with a therapeutic-relevant dose of diazepam, and we observed no effects of diazepam on several key genes involved in xenobiotic and endobiotic metabolism and liver proliferation, which are also sensitive target genes to CAR activation.
Diazepam was identified as the translocator of wild-type EYFP-tagged hCAR in primary human hepatocytes [
7]. However, most inducible hCAR target gene CYP2B6 mRNA up-regulation in primary human hepatocytes depends on treatment concentration, as Li, Chen, Cottrell and Wang [
7] described diazepam as an inductor of both CYP3A4 and CYP2B6 mRNA at 50 µM, whereas Vrzal, Kubesova, Pavek and Dvorak [
30] observed no significant induction of CYP3A4 mRNA in human hepatocytes at 18 µM. Consistently, we observed the diazepam-mediated stimulation of hCAR+Ala mutant translocation in the immortalized kidney cell line COS-1 at 10 μM and a similar stimulation by the diazepam metabolites temazepam, oxazepam, and nordazepam at 30 μM (
Figure 1). Similarly, in a two-hybrid CAR+AAA LBD-SRC1 coactivator assay, diazepam, nordazepam, and temazepam were significantly active at 30 µM, while the final metabolite oxazepam had significant activity toward CAR+AAA LBD activation only at 50 µM (
Figure 2C). However, in luciferase gene reporter assays with either CAR3 variant expression vector as well as in a CAR-LBD assembly assay, only diazepam significantly activated hCAR (
Figure 2A,D). The CAR3 transcription variant (wild type hCAR with five extra amino acids, APYLT) and CAR+Ala differs in their activities after ligand activation, when artificial CAR+Ala shows higher capacity for activation when compared to the CAR3 variant [
18]. Thus, the activation of EGFP-CAR+Ala and pGAL4-CAR+AAA by all tested benzodiazepines, but only diazepam-mediated activation of CAR3 gene reporter and the CAR assembly assay is the consequence of differently inducible CAR variants.
In contrast to primary human hepatocytes, both COS-1 and HepG2 cell lines lack any reasonable metabolic activity to convert diazepam into its metabolites. These data, therefore, suggest that the significant translocation of hCAR and induction of CYP3A4 and CYP2B6 mRNA in primary human hepatocytes reported by Li, Chen, Cottrell and Wang [
7] is not a consequence of a diazepam metabolite that can activate hCAR.
Diazepam has been proposed as a phenobarbital-like indirect CAR activator, as diazepam was not able to reverse the inhibition of the wild-type hCAR mediated by the PK11195 inhibitor in luciferase reporter assays. In our experiments with the highly ligand-activable variant CAR3 and CAR inhibitor PK11195, however, a significant effect of the inhibitor was shown on the diazepam-mediated activation of CAR3 (
Figure 2B). In agreement with this finding, we observed that diazepam activates the luciferase reporter in the CAR-LBD assembly assay (
Figure 2D). In neither CAR3 reporter nor assembly luciferase assays, the indirect CAR activator phenobarbital proved any interaction with CAR constructs, which confirms ability of these assays to distinguish direct CAR activation. Diazepam also promotes interaction of CAR-LBD with a coactivator peptide in the TR-FRET assay, suggesting that diazepam directly activates hCAR and its CAR3 variant (
Figure 2B,D and
Figure 3). Based on in silico docking experiments and experiments with CAR3-LBD mutants in a gene reporter assay, we suggest that residues Ile164 and Leu242 might be important for the positioning of diazepam in the hCAR ligand binding pocket (
Figure 4).
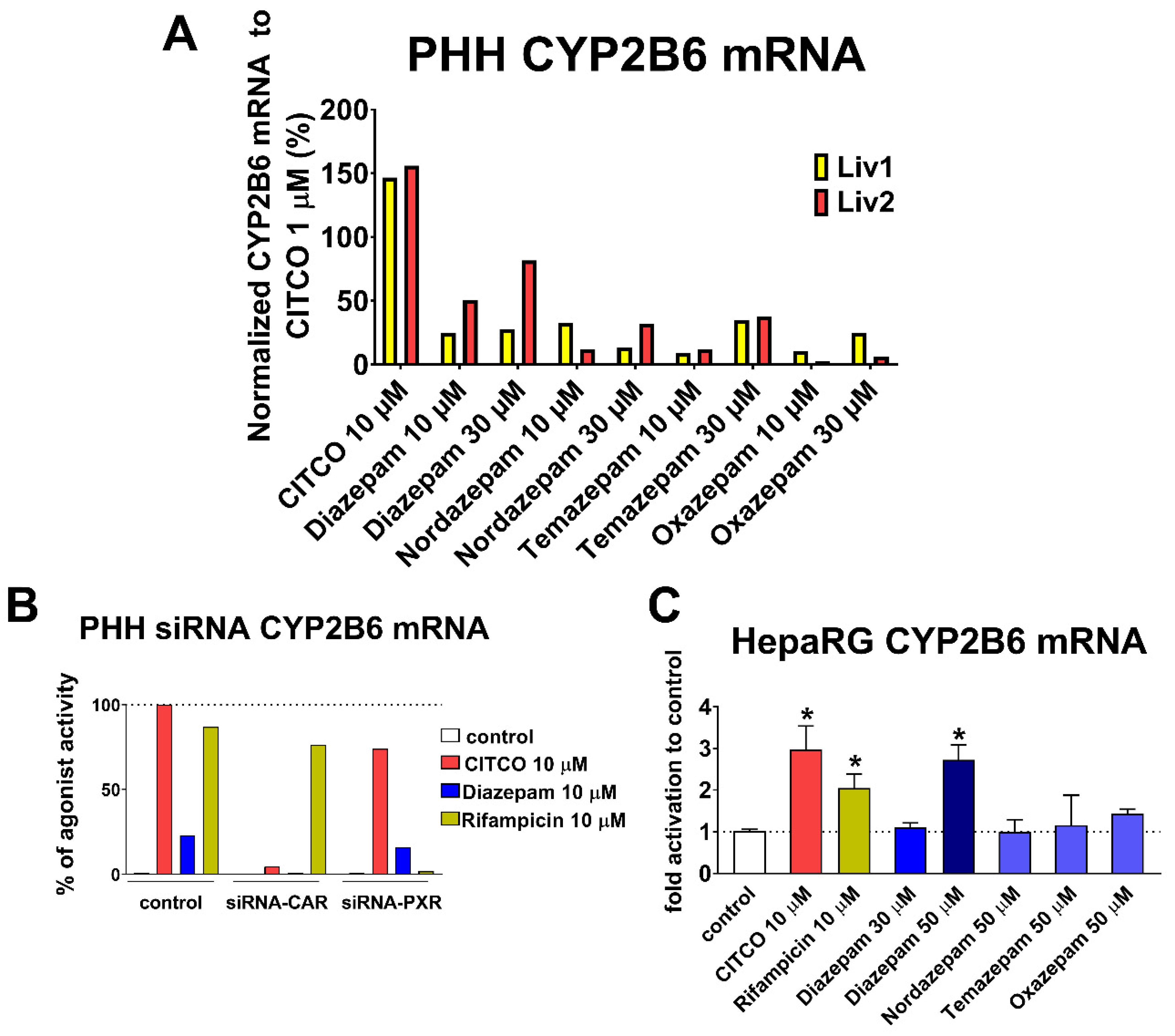
In our subsequent RT-qPCR experiments, CYP2B6 mRNA induction by benzodiazepines was observed in primary human hepatocytes, where metabolites lost activity toward almost inactive oxazepam (
Figure 5A). Diazepam showed weak to moderate CYP2B6 mRNA induction at 10 µM and 30 µM. Therefore, lower affinity and activity characterize diazepam as an hCAR low-affinity partial agonist. Diazepam also induced CYP2B6 mRNA in differentiated HepaRG cells only at the concentration of 50 µM (
Figure 5C), where CAR transcriptional activity is lower compared to PPHs.
In rodent models, diazepam stimulates the induction of Cyp2b enzymes via CAR and even promotes hepatic proliferation in mice receiving high doses of diazepam [
8]. Cyp2b induction and liver proliferation were for a long time considered as connected events in CAR activation, but recent studies have shown that a common hallmark of CAR activation is the induction of CYP2B6/Cyp2b10 enzymes. Liver proliferation, however, is restricted to rodent CAR [
31,
32]. The hCAR/hPXR/hCYP3A4 mice strain is, so far, one of the most realistic in vivo models of hCAR, responding to specific hCAR ligands and reflecting the hCAR specific transcriptional regulation limited to the mouse genome. This model was recently used not only to study xenobiotic metabolism induction [
33], but even for liver proliferation studies [
32].
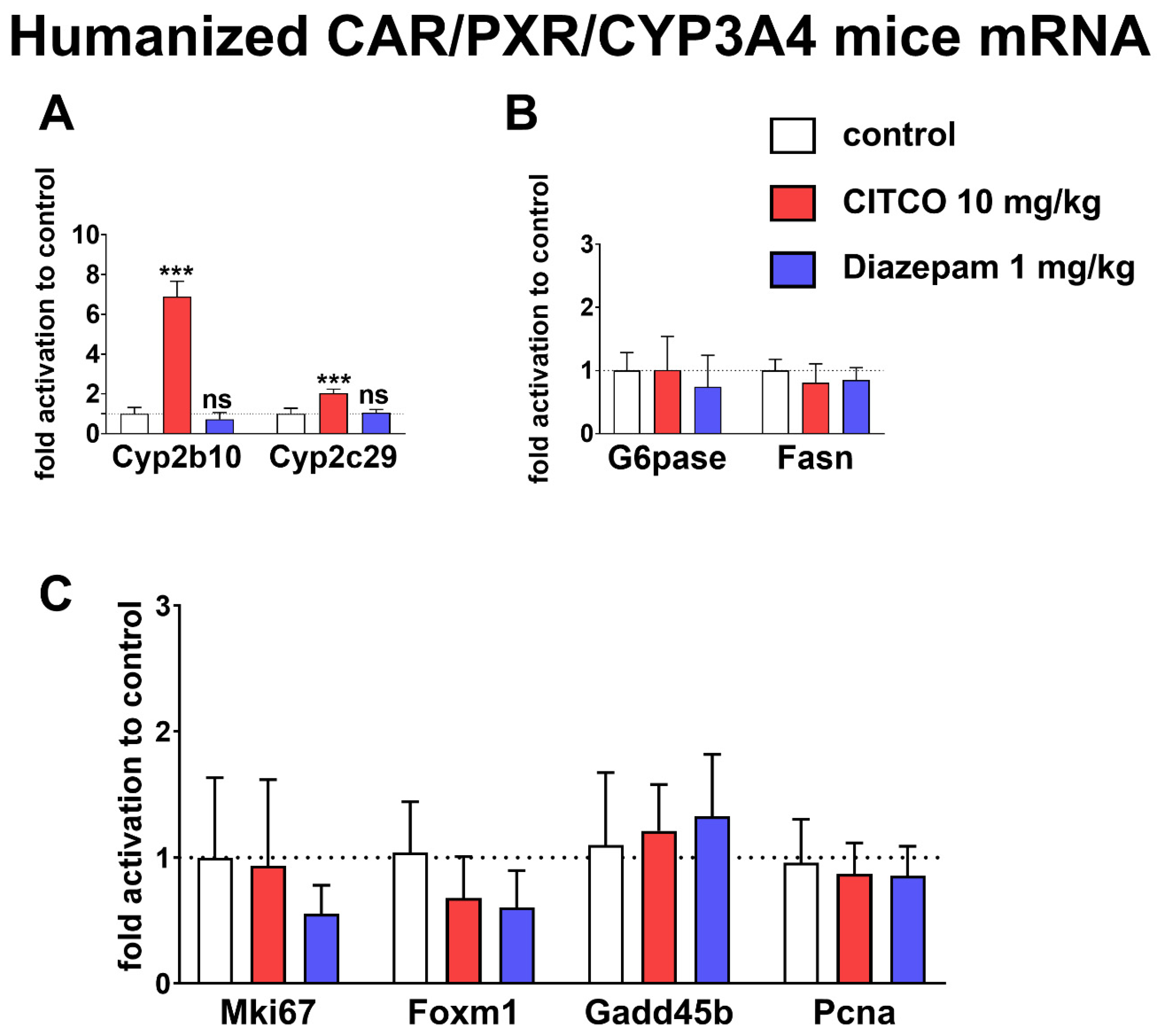
In humanized CAR/PXR/CYP3A4 mice, CITCO treated mice (10 mg/kg) exhibited
Cyp2b10 and
Cyp2c29 genes induction. However, we observed no Cyp2b10 mRNA (the murine CYP2B6 analog) induction after 1 mg/kg diazepam administration (
Figure 6A). The endogenous metabolism genes
Fasn and
G6pase revealed non-significant down-regulation trends. However, these genes might be co-regulated by diazepam through a translocator protein [
34] (
Figure 6B). Furthermore, the proliferation and apoptosis-related genes
Mki67,
Foxm1,
Gadd45b, and
Pcna were regulated neither by diazepam nor CITCO (
Figure 6C). The formerly described CAR-mediated liver proliferation genes upregulation promoted by the both diazepam and CITCO were not detected in our work, as these compounds had been used in much higher doses in previous rodent studies (diazepam up to 10,000 ppm in the powdered diet or CITCO at 50 mg/kg dose, respectively) [
8,
35].
After chronic administration of diazepam in human medicine, plasmatic concentrations of diazepam are in the range of 0.1–1.0 µM [
36]. Matsuda, et al. [
37] measured portal and systemic concentration of benzodiazepine midazolam after administration of the same dose as in our in vivo experiment (1 mg/kg). They observed that midazolam concentration immediately after oral administration was at about 3.07 µM. However, after 15 to 30 min, the drug was almost completely distributed with portal concentration under 0.3 µM. Thus, we do not expect that diazepam and its metabolites activate CAR in human medicine relevant doses.
In this work, we have comprehensively described the relationship between hCAR and diazepam, which was already postulated as an indirect hCAR activator. We found a correlation in all protein and vector-based methods as well as in silico docking experiments, and we describe diazepam as a direct hCAR ligand. However, significant CYP2B6/Cyp2B10 mRNA induction was shown only in primary human hepatocytes, but not in vivo in a humanized mice model when a therapeutically relevant dose was used. This is most likely due to the low affinity of diazepam to hCAR.