MicroRNAs and Mammarenaviruses: Modulating Cellular Metabolism
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. MicroRNAs
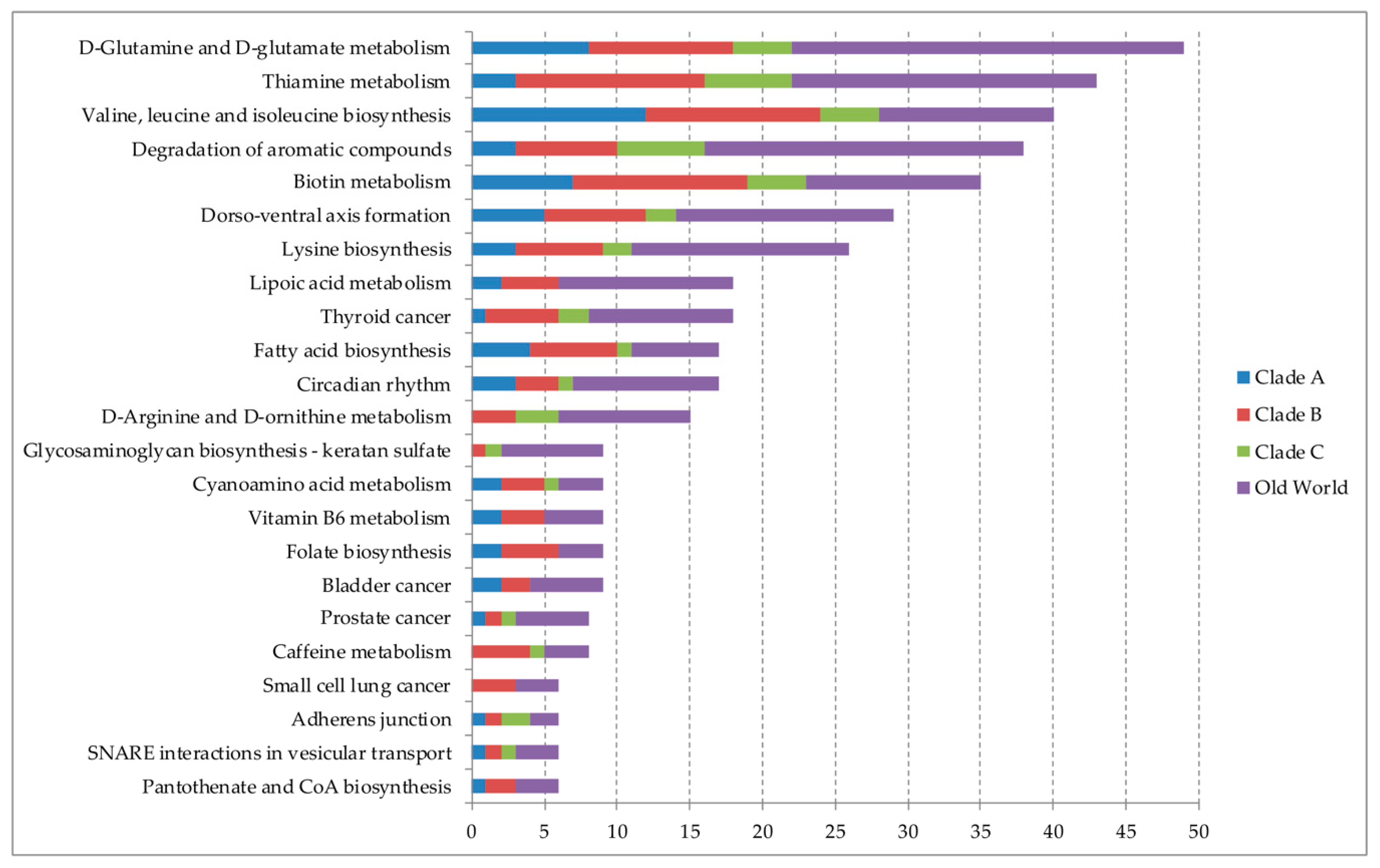
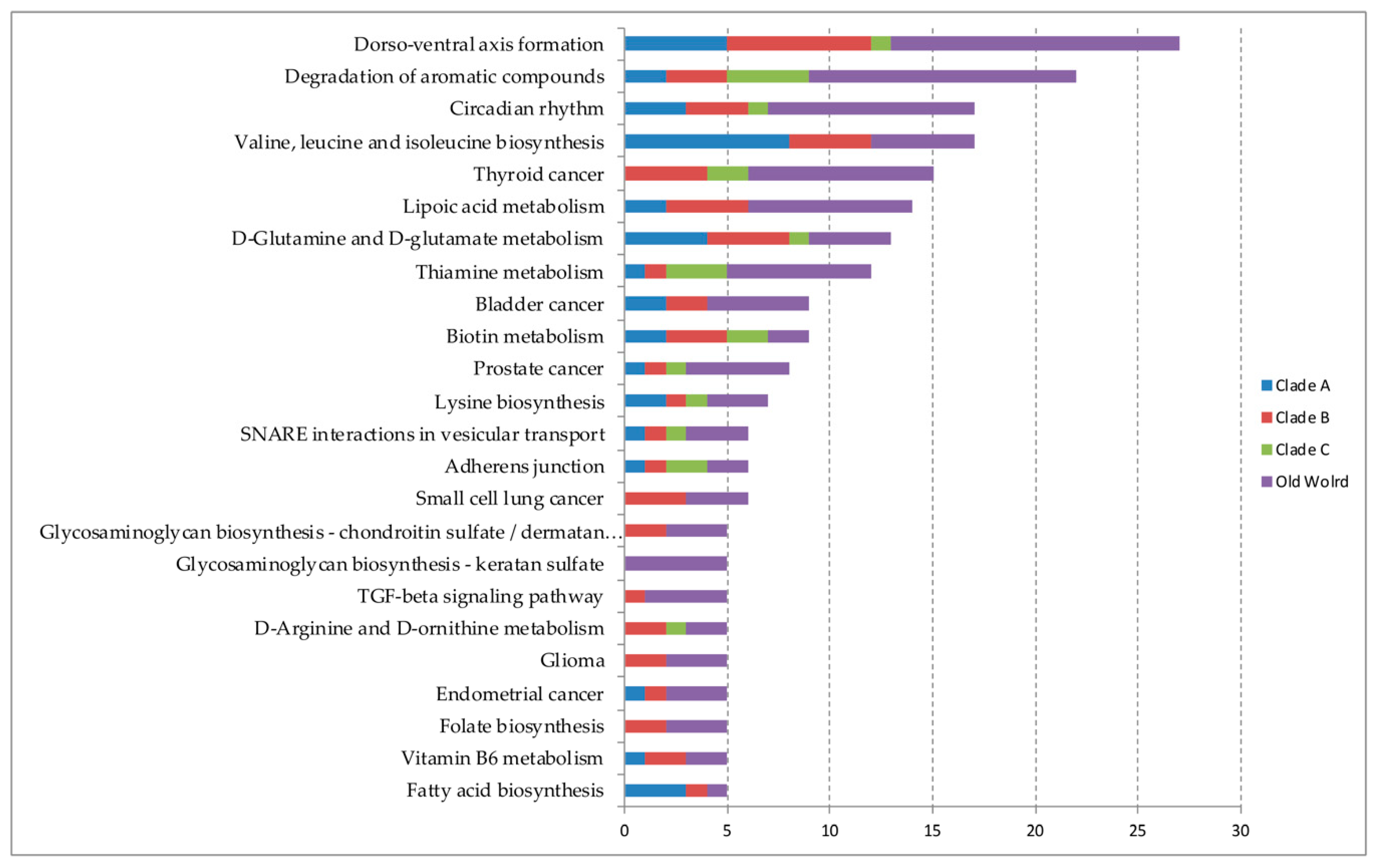
3.2. miRNAs and Target Metabolic/Cellular Pathways
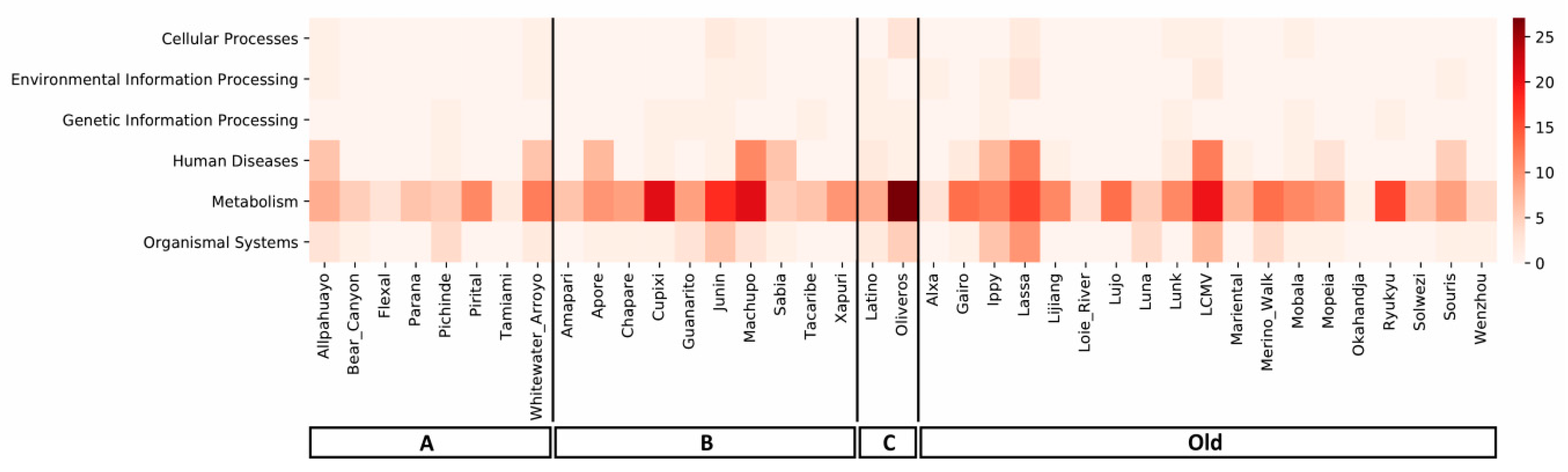
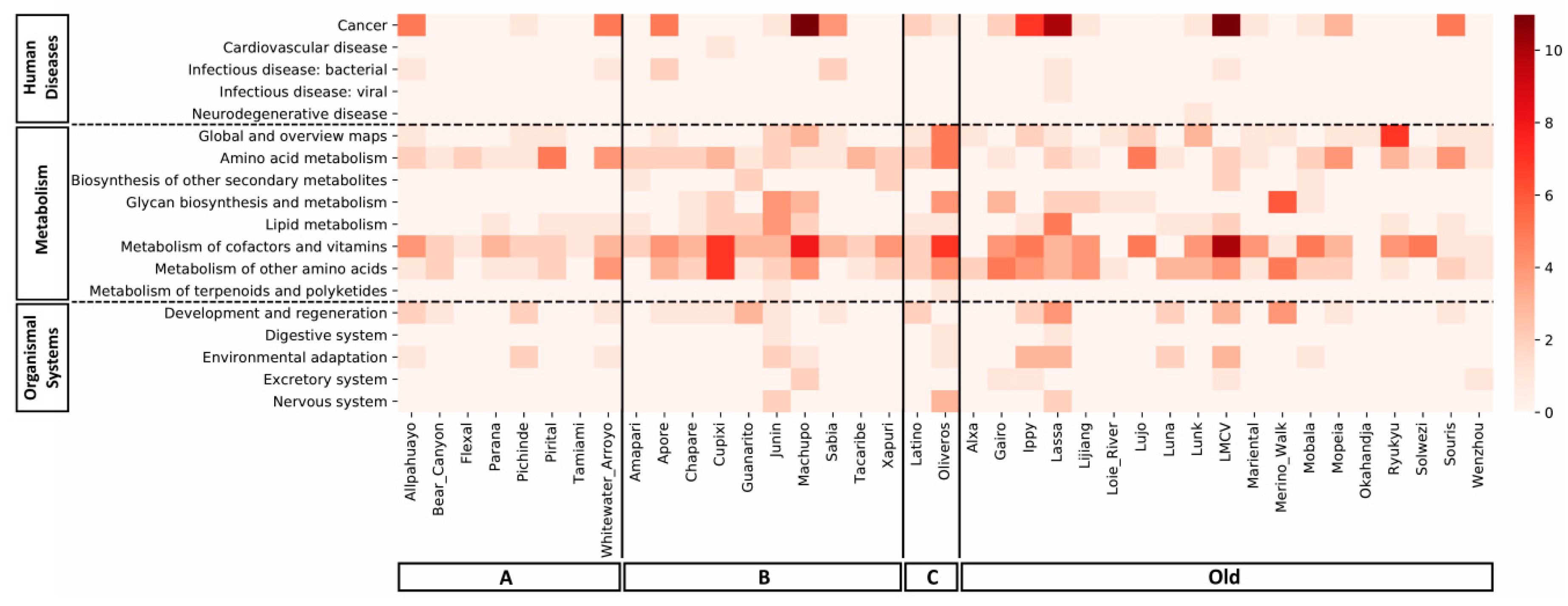
3.3. Mammarenavirus Species, miRNAs, and Target Metabolic/Cellular Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Radoshitzky, S.R.; Bào, Y.; Buchmeier, M.J.; Charrel, R.N.; Clawson, A.N.; Clegg, C.S.; DeRisi, J.L.; Emonet, S.; Gonzalez, J.-P.; Kuhn, J.H.; et al. Past, present, and future of arenavirus taxonomy. Arch. Virol. 2015, 160, 1851–1874. [Google Scholar] [CrossRef] [PubMed]
- Grande-Pérez, A.; Martin, V.; Moreno, H.; De La Torre, J.C. Arenavirus Quasispecies and Their Biological Implications. In Endogenous ADP-Ribosylation; Springer Science and Business Media LLC: Berlin, Switzerland, 2015; Volume 392, pp. 231–275. [Google Scholar]
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072. [Google Scholar] [CrossRef] [PubMed]
- Maes, P.; Alkhovsky, S.V.; Bao, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.; Choi, I.R.; et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: Update 2018. Arch. Virol. 2018, 163, 2295–2310. [Google Scholar] [CrossRef] [PubMed]
- Garnett, L.E.; Strong, J.E. Lassa fever: With 50 years of study, hundreds of thousands of patients and an extremely high disease burden, what have we learned? Curr. Opin. Virol. 2019, 37, 123–131. [Google Scholar] [CrossRef]
- Ambrosio, A.; Saavedra, M.; Mariani, M.; Gamboa, G.; Maiza, A. Argentine hemorrhagic fever vaccines. Hum. Vaccines 2011, 7, 694–700. [Google Scholar] [CrossRef]
- Delaine, M.; Weingertner, A.-S.; Nougairede, A.; Lepiller, Q.; Fafi-Kremer, S.; Favre, R.; Charrel, R. Microcephaly Caused by Lymphocytic Choriomeningitis Virus. Emerg. Infect. Dis. 2017, 23, 1548–1550. [Google Scholar] [CrossRef]
- Brisse, M.; Ly, H. Hemorrhagic Fever-Causing Arenaviruses: Lethal Pathogens and Potent Immune Suppressors. Front. Immunol. 2019, 10, 372. [Google Scholar] [CrossRef]
- Ly, H. Differential Immune Responses to New World and Old World Mammalian Arenaviruses. Int. J. Mol. Sci. 2017, 18, 1040. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ang, C.E.; Trevino, A.E.; Chang, H.Y. Diverse lncRNA mechanisms in brain development and disease. Curr. Opin. Genet. Dev. 2020, 65, 42–46. [Google Scholar] [CrossRef]
- Girardi, E.; López, P.; Pfeffer, S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh, D.W.; Gardner, C.L.; Sun, C.; Haddow, A.D.; Wang, E.; Chapnik, E.; Mildner, A.; Weaver, S.C.; Ryman, K.D.; Klimstra, W.B. RNA viruses can hijack vertebrate microRNAs to suppress innate immunity. Nat. Cell Biol. 2014, 506, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ke, X.; Wang, M.; He, S.; Li, Q.; Zheng, C.; Zhang, Z.; Liu, Y.; Wang, H. Human MicroRNA hsa-miR-296-5p Suppresses Enterovirus 71 Replication by Targeting the Viral Genome. J. Virol. 2013, 87, 5645–5656. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.; Lima, C.H.D.A.; Miranda, R.L.; Gadelha, M.R. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infect. Genet. Evol. 2020, 85, 104417. [Google Scholar] [CrossRef]
- Quillet, A.; Saad, C.; Ferry, G.; Anouar, Y.; Vergne, N.; Lecroq, T.; Dubessy, C. Improving Bioinformatics Prediction of microRNA Targets by Ranks Aggregation. Front. Genet. 2020, 10, 1330. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C.S. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Liu, H.; Yue, D.; Chen, Y.; Gao, S.-J.; Huang, Y. Improving performance of mammalian microRNA target prediction. BMC Bioinform. 2010, 11, 476. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Hsu, S.-D.; Lin, F.-M.; Wu, W.-Y.; Liang, C.; Huang, W.-C.; Chan, W.-L.; Tsai, W.-T.; Chen, G.-Z.; Lee, C.-J.; Chiu, C.-M.; et al. miRTarBase: A database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2011, 39, D163–D169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zuo, Z.; Cai, G.; Kang, S.; Gao, X.; Li, T. miRecords: An integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Gretz, N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; Van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34, D140–D144. [Google Scholar] [CrossRef]
- McKinney, W. Pandas: A Foundational Python Library for Data Analysis and Statistics. Python High Perform. Sci. Comput. 2011, 14, 9. [Google Scholar]
- Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2016, 219, 346–361. [Google Scholar] [CrossRef]
- Bruscella, P.; Bottini, S.; Baudesson, C.; Pawlotsky, J.-M.; Feray, C.; Trabucchi, M. Viruses and miRNAs: More Friends than Foes. Front. Microbiol. 2017, 8, 824. [Google Scholar] [CrossRef]
- Cullen, B.R. Viruses and microRNAs. Nat. Genet. 2006, 38, S25–S30. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Arend, K.C.; Li, Y.; Yamane, D.; McGivern, D.R.; Kato, T.; Wakita, T.; Moorman, N.J.; Lemon, S.M. miR-122 Stimulates Hepatitis C Virus RNA Synthesis by Altering the Balance of Viral RNAs Engaged in Replication versus Translation. Cell Host Microbe 2015, 17, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Masaki, T.; Yamane, D.; McGivern, D.R.; Lemon, S.M. Competing and noncompeting activities of miR-122 and the 5′ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc. Natl. Acad. Sci. USA 2013, 110, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Hsu, S.-D.; Hsu, C.-S.; Lai, T.-C.; Chen, S.-J.; Shen, R.; Huang, Y.; Chen, H.-C.; Lee, C.-H.; Tsai, T.-F.; et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef]
- Gualdoni, G.A.; Mayer, K.A.; Kapsch, A.-M.; Kreuzberg, K.; Puck, A.; Kienzl, P.; Oberndorfer, F.; Frühwirth, K.; Winkler, S.; Blaas, D.; et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc. Natl. Acad. Sci. USA 2018, 115, E7158–E7165. [Google Scholar] [CrossRef]
- Nagy, P.D.; Strating, J.R.; Van Kuppeveld, F.J. Building Viral Replication Organelles: Close Encounters of the Membrane Types. PLoS Pathog. 2016, 12, e1005912. [Google Scholar] [CrossRef]
- Hsu, N.-Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.-H.; Takvorian, P.M.; Pau, C.; Van Der Schaar, H.; Kaushik-Basu, N.; Balla, T.; et al. Viral Reorganization of the Secretory Pathway Generates Distinct Organelles for RNA Replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef]
- Diamond, D.L.; Syder, A.J.; Jacobs, J.M.; Sorensen, C.M.; Walters, K.-A.; Proll, S.C.; McDermott, J.E.; Gritsenko, M.A.; Zhang, Q.; Zhao, R.; et al. Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics. PLoS Pathog. 2010, 6, e1000719. [Google Scholar] [CrossRef]
- Manchester, M.; Anand, A. Metabolomics: Strategies to Define the Role of Metabolism in Virus Infection and Pathogenesis. Adv. Virus Res. 2017, 98, 57–81. [Google Scholar] [PubMed]
- Noto, A.; Dessì, A.; Puddu, M.; Mussap, M.; Fanos, V. Metabolomics technology and their application to the study of the viral infection. J. Matern. Neonatal Med. 2014, 27, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the Cellular Metabolome during Human Cytomegalovirus Infection. PLoS Pathog. 2006, 2, e132. [Google Scholar] [CrossRef] [PubMed]
- Yangfang, Y.; Ye, Y.; Chenghua, L.; Shao, Y.; Xie, X.; Zhang, W.; Liu, H.-P.; Li, C. Metabolic product response profiles of Cherax quadricarinatus towards white spot syndrome virus infection. Dev. Comp. Immunol. 2016, 61, 236–241. [Google Scholar] [CrossRef]
- Suhyuen, L.; Huang, Y.-T.; Chen, I.-T.; Lee, D.-Y.; Hsieh, Y.-C.; Li, C.-Y.; Geendong, C.; Liang, S.-Y.; Lin, S.-Y.; Huang, S.-W.; et al. An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway. PLoS Pathog. 2014, 10, e1004196. [Google Scholar] [CrossRef]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.-J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef]
- Delgado, T.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Global Metabolic Profiling of Infection by an Oncogenic Virus: KSHV Induces and Requires Lipogenesis for Survival of Latent Infection. PLoS Pathog. 2012, 8, e1002866. [Google Scholar] [CrossRef]
- Wang, L.; Swevers, L.; Rombouts, C.; Meeus, I.; Van Meulebroek, L.; Vanhaecke, L.; Smagghe, G. A Metabolomics Approach to Unravel Cricket Paralysis Virus Infection in Silkworm Bm5 Cells. Viruses 2019, 11, 861. [Google Scholar] [CrossRef]
- Cruzat, V.F.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Yelamanchi, S.D.; Jayaram, S.; Thomas, J.K.; Gundimeda, S.; Khan, A.A.; Singhal, A.; Prasad, T.S.K.; Pandey, A.; Somani, B.L.; Gowda, H. A pathway map of glutamate metabolism. J. Cell Commun. Signal. 2016, 10, 69–75. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Carroll, P.A.; Thalhofer, A.B.; Lagunoff, M. Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival. PLoS Pathog. 2015, 11, e1005052. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Kalisak, E.; Trauger, S.A.; Manchester, M.; Siuzdak, G. Response and Recovery in the Plasma Metabolome Tracks the Acute LCMV-Induced Immune Response. J. Proteome Res. 2009, 8, 3578–3587. [Google Scholar] [CrossRef]
- Yu, Y.; Clippinger, A.J.; Alwine, J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011, 19, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, A.; Williamson, M.K.; Khan, M.B.; Zeidler, J.D.; Da Poian, A.T.; El-Bacha, T.; Struys, E.A.; Huthoff, H. Evidence for Altered Glutamine Metabolism in Human Immunodeficiency Virus Type 1 Infected Primary Human CD4+ T Cells. AIDS Res. Hum. Retroviruses 2017, 33, 1236–1247. [Google Scholar] [CrossRef]
- Sanchez, M.D.; Ochoa, A.C.; Foster, T.P. Development and evaluation of a host-targeted antiviral that abrogates herpes simplex virus replication through modulation of arginine-associated metabolic pathways. Antivir. Res. 2016, 132, 13–25. [Google Scholar] [CrossRef]
- Archard, L.C.; Williamson, J.D. The Effect of Arginine Deprivation on the Replication of Vaccinia Virus. J. Gen. Virol. 1971, 12, 249–258. [Google Scholar] [CrossRef]
- Butorov, E.V. Influence of L-lysine amino acid on the HIV-1 RNA replicationin vitro. Antivir. Chem. Chemother. 2015, 24, 39–46. [Google Scholar] [CrossRef]
- Butorov, E.V. Plasma L-Carnitine and L-Lysine Concentrations in HIV-Infected Patients. Open Biochem. J. 2017, 11, 119–131. [Google Scholar] [CrossRef]
- Butorov, E.V. Relationship between plasma l -lysine concentrations and levels of HIV-1 RNA. Virulence 2013, 4, 646–653. [Google Scholar] [CrossRef][Green Version]
- Keshavarz, M.; Solaymani-Mohammadi, F.; Namdari, H.; Arjeini, Y.; Mousavi, M.J.; Rezaei, F. Metabolic host response and therapeutic approaches to influenza infection. Cell. Mol. Biol. Lett. 2020, 25, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, H.S.; Duan, S.; Morfouace, M.; Rezinciuc, S.; Shulkin, B.L.; Shelat, A.; Zink, E.E.; Milasta, S.; Bajracharya, R.; Oluwaseum, A.J.; et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017, 19, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L.; McDonald, N.J.; Sheng, J.; Shaw, M.W.; Hodge, T.W.; Rubin, D.H.; O’Brien, W.A.; Smee, D.F. Inhibition of Influenza a Virus Replication by Antagonism of a PI3K-AKT-mTOR Pathway Member Identified by Gene-Trap Insertional Mutagenesis. Antivir. Chem. Chemother. 2012, 22, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Magaway, C.; Kim, E.; Jacinto, E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells 2019, 8, 1584. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Wang, H.-Y.; Chang, A.; Zheng, X.F.S. Emerging Role of MicroRNAs in mTOR Signaling. Cell. Mol. Life Sci. 2017, 74, 2613–2625. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.-M.; Ding, L.; Zhang, X.-J.; Ma, Z. mTOR signaling-related MicroRNAs and Cancer involvement. J. Cancer 2018, 9, 667–673. [Google Scholar] [CrossRef]
- Ray, L.B. miRNAs mediate mTORC1 effects. Science 2020, 367, 753–754. [Google Scholar] [CrossRef][Green Version]
- El-Bacha, T.; Midlej, V.; Da Silva, A.P.P.; Da Costa, L.S.; Benchimol, M.; Galina, A.; Da Poian, A.T. Mitochondrial and bioenergetic dysfunction in human hepatic cells infected with dengue 2 virus. Biochim. Biophys. Acta 2007, 1772, 1158–1166. [Google Scholar] [CrossRef]
- Landini, M.P. Early Enhanced Glucose Uptake in Human Cytomegalovirus-infected Cells. J. Gen. Virol. 1984, 65, 1229–1232. [Google Scholar] [CrossRef]
- Bardeletti, G. Respiration and ATP Level in BHK21/13S Cells during the Earliest Stages of Rubella Virus Replication. Intervirology 1977, 8, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-L.; Chien, K.-Y.; Lai, C.-H.; Li, G.-J.; Lin, J.-F.; Ho, H.-Y. Metabolic Reprogramming of Host Cells in Response to Enteroviral Infection. Cells 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Gale, T.V.; Horton, T.M.; Grant, D.S.; Garry, R.F. Metabolomics analyses identify platelet activating factors and heme breakdown products as Lassa fever biomarkers. PLoS Negl. Trop. Dis. 2017, 11, e0005943. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.; MacKenzie, C.G.; Talmage, D.W. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell. Immunol. 1979, 48, 100–110. [Google Scholar] [CrossRef]
- McMahon, R.J. Biotin in metabolism and molecular biology. Annu. Rev. Nutr. 2002, 22, 221–239. [Google Scholar] [CrossRef]
- Watanabe, T. Teratogenic Effects of Biotin Deficiency in Mice. J. Nutr. 1983, 113, 574–581. [Google Scholar] [CrossRef]
- Watanabe, T.; Dakshinamurti, K.; Persaud, T.V.N. Biotin Influences Palatal Development of Mouse Embryos in Organ Culture. J. Nutr. 1995, 125, 2114–2121. [Google Scholar] [CrossRef]
- Báez-Saldaña, A.; Díaz, G.; Espinoza, B.; Ortega, E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am. J. Clin. Nutr. 1998, 67, 431–437. [Google Scholar] [CrossRef]
- Kinori, M.; Schwartzstein, H.; Zeid, J.L.; Kurup, S.P.; Mets, M.B. Congenital lymphocytic choriomeningitis virus—an underdiagnosed fetal teratogen. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 79–81. [Google Scholar] [CrossRef]
- Anderson, J.L.; Levy, P.T.; Leonard, K.B.; Smyser, C.D.; Tychsen, L.; Cole, F.S. Congenital lymphocytic choriomeningitis virus: When to consider the diagnosis. J. Child Neurol. 2014, 29, 837–842. [Google Scholar] [CrossRef]
- Okabe, N.; Urabe, K.; Fujita, K.; Yamamoto, T.; Yao, T.; Doi, S. Biotin effects in Chrohn’s disease. Dig. Dis. Sci. 1988, 33, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Agrawal, A.; Said, H.M. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am. J. Physiol. Physiol. 2016, 311, C386–C391. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Vinciguerra, M.; Carbone, A.; Relógio, A. The Circadian Clock, the Immune System, and Viral Infections: The Intricate Relationship Between Biological Time and Host-Virus Interaction. Pathogens 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New Insights into the Circadian Rhythm and Its Related Diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Spencer, R.L.; Pearce, B.D.; Pisell, T.L.; Tanapat, P.; Leung, J.J.; Dhabhar, F.S.; McEwen, B.S.; Biron, C.A. Effects of viral infection on corticosterone secretion and glucocorticoid receptor binding in immune tissues. Psychoneuroendocrinology 1997, 22, 455–474. [Google Scholar] [CrossRef]
- Edgar, R.S.; Stangherlin, A.; Nagy, A.D.; Nicoll, M.P.; Efstathiou, S.; O’Neill, J.S.; Reddy, A. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc. Natl. Acad. Sci. USA 2016, 113, 10085–10090. [Google Scholar] [CrossRef] [PubMed]
- Long, J.E.; Drayson, M.T.; Taylor, A.E.; Toellner, K.M.; Lord, J.M.; Phillips, A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 2016, 34, 2679–2685. [Google Scholar] [CrossRef]
- Moreno, H.; Möller, R.; Fedeli, C.; Gerold, G.; Kunz, S. Comparison of the Innate Immune Responses to Pathogenic and Nonpathogenic Clade B New World Arenaviruses. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- Fernandes, J.; Guterres, A.; De Oliveira, R.C.; Chamberlain, J.; Lewandowski, K.; Teixeira, B.R.; Coelho, T.A.; Crisóstomo, C.F.; Bonvicino, C.R.; D’Andrea, P.S.; et al. Xapuri virus, a novel mammarenavirus: Natural reassortment and increased diversity between New World viruses. Emerg. Microbes Infect. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Abraham, J.; Kwong, J.A.; Albariño, C.G.; Lu, J.G.; Radoshitzky, S.R.; Salazar-Bravo, J.; Farzan, M.; Spiropoulou, C.F.; Choe, H. Host-Species Transferrin Receptor 1 Orthologs Are Cellular Receptors for Nonpathogenic New World Clade B Arenaviruses. PLoS Pathog. 2009, 5, e1000358. [Google Scholar] [CrossRef]
- Ferrer, M.F.; Thomas, P.; Ortiz, A.O.L.; Errasti, A.E.; Charo, N.; Romanowski, V.; Gorgojo, J.; Rodriguez, M.E.; Silva, E.A.C.; Gómez, R.M. Junin Virus Triggers Macrophage Activation and Modulates Polarization According to Viral Strain Pathogenicity. Front. Immunol. 2019, 10, 2499. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.; Reynard, S.; Carnec, X.; Pietrosemoli, N.; Dillies, M.-A.; Baize, S. Non-Pathogenic Mopeia Virus Induces More Robust Activation of Plasmacytoid Dendritic Cells than Lassa Virus. Viruses 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Finkelstein, J.L.; Stewart, A.M.; Kenneth, J.; Polhemus, M.E.; Endy, T.P.; Cardenas, W.; Mehta, S. Review article: Micronutrients and dengue. Am. J. Trop. Med. Hyg. 2014, 91, 1049–1056. [Google Scholar] [CrossRef]
| New World Mammarenaviruses | |||||
| Clade A Viruses | Total miRNAs (Binding-Sites) | Clade B Viruses | Total miRNAs (Binding-Sites) | ||
| L Segment | S Segment | L Segment | S Segment | ||
| Allpahuayo virus | 12 | 04 | Amapari virus | 14 | 03 |
| Bear Canyon virus | 10 | 05 | Aporé virus | 15(16) | 06 |
| Flexal virus | 11 | 03 | Chapare virus | 15 | 04 |
| Paraná virus | 09 | 07 | Cupixi virus | 23(25) | 05 |
| Pichindé virus | 14 | 04 | Guanarito virus | 12 | 07 |
| Pirital virus | 13 | 09 | Junín virus | 13 | 05 |
| Tamiami virus | 12(13) | 03(04) | Machupo virus | 17 | 08 |
| Whitewater Arroyo virus | 22(23) | 06 | Sabiá virus | 09 | 06(07) |
| Total binding-sites | 105 | 42 | Tacaribe virus | 14 | 02 |
| Clade C viruses | Total microRNAs | Xapuri virus * | 07 | 05 | |
| L segment | S segment | Total binding-sites | 140 | 54 | |
| Latino virus | 16 | 03 | * Didactically classified here as belonging to Clade B NW mammarenaviruses. | ||
| Oliveros virus | 16 | 10 | |||
| Total | 32 | 13 | |||
| Old World Mammarenaviruses | |||||
| Viruses | Total miRNAs (Binding-Sites) | Viruses | Total miRNAs (Binding-Sites) | ||
| L Segment | S Segment | L Segment | S Segment | ||
| Alxa virus | 08 | 12 | Mariental virus | 12 | 03 |
| Gairo virus | 11 | 12 | Merino Walk virus | 14 | 04 |
| Ippy virus | 17 | 05 | Mobala virus | 12 | 08 |
| Lassa virus | 16 | 08 | Mopeia virus | 14 | 04 |
| Lijiang virus | 12(13) | 07 | Okahandja virus | 09 | 09(10) |
| Loie River virus | 07 | 01 | Ryukyu virus | 19(21) | 03 |
| Lujo virus | 20 | 06 | Solwezi virus | 010 | 04 |
| Luna virus | 13 | 08 | Souris virus | 05 | 09 |
| Lunk virus | 18(22) | 10 | Wenzhou virus | 09 | 06 |
| Lymphocytic choriomeningitis virus | 11 | 05 | Total binding-sites | 244 | 125 |
| miRNAs | Mammarenaviruses (Genomic Position) |
|---|---|
| Clade A New World Viruses (L Segment) | |
| hsa-miR-122b-3p | Flexal (2745–2755: RdRp)/Paraná (2814–2824: RdRp) |
| Clade A New World Viruses (S Segment) | |
| hsa-miR-2052 | Pirital (954–944: GPC)/Bear Canyon (2422–2412: NP)/Pichindé (948–938: GPC)/Allpahuayo (945–955: GPC) |
| Clade B New World Viruses (L Segment) | |
| hsa-miR-122b-3p | Aporé (2977–2967: RdRp/7195–7185: 3′UTR)/Machupo (2920–2910: RdRp) |
| hsa-miR-147b-5p | Aporé (6826–6816: RdRp)/Machupo (1424–1434: RdRp)/Tacaribe (6771–6761: RdRp) |
| hsa-miR-3149 | Machupo (3195–3185: RdRp)/Tacaribe (3173–3163: RdRp)/Xapuri (4629–4639: RdRp) |
| hsa-miR-12122 | Amapari (3524–3534: RdRp)/Chapare (3552–3562: RdRp)/Tacaribe (3553–3563: RdRp) |
| Old World Viruses (L Segment) | |
| hsa-miR-3120-3p | Lujo (3497–3487: RdRp)/Lunk (3599–3589: RdRp)/Merino Walk (5697–5687: RdRp)/Mopeia (3591–3581: RdRp) |
| hsa-miR-3134 | Alxa (5200–5190: RdRp)/Wenzhou (2378–2388: RdRp)/Lymphocytic choriomeningitis (1975–1985: RdRp) |
| Old World Viruses (S Segment) | |
| hsa-miR-4327 | Lunk (2716–2706: NP)/Merino Walk (2721–2711: NP)/Mariental (2721–2711: NP)/Souris (2777–2767: NP) |
| hsa-miR-6516-3p | Lujo (1548–1538: NP)/Lijiang (1655–1645: NP)/Mariental (1673–1663: NP) |
| hsa-miR-6740-5p | Alxa (2834–2824: NP)/Gairo (2788–2778: NP)/Lassa (2784–2774: NP)/Lymphocytic choriomeningitis (2787–2777: S-IGR) |
| miRNAs | Species (Genomic Position) | |
|---|---|---|
| L Segment | S Segment | |
| hsa-miR-122b-3p | Aporé (2977–2967: RdRp/7195–7185: 3′UTR) | Alxa (1328–1338: GPC) |
| Flexal (2745–2755: RdRp) | ||
| Machupo (2920–2910: RdRp) | ||
| Paraná (2814–2824: RdRp) | ||
| hsa-miR-9-5p | Pichindé (4923–4933: RdRp) | Tacaribe (2639–2649: NP) |
| Ryukyu (4996–5006: RdRp) | ||
| Mobala (5050–5060: RdRp) | ||
| hsa-miR-3611 | Aporé (3887–3897: RdRp) | Xapuri (1849–1859: NP) |
| Flexal (3774–3784: RdRp) | ||
| hsa-miR-3617-5p | Pichindé (7003–6993: RdRp) | Mopeia (2392–2402: NP) |
| Paraná (1198–1208: GPC)) | ||
| hsa-miR-1229-3p | Cupixi (4426–4436: RdRp) | Lassa (2783–2793: NP) |
| Oliveros (6625–6635: RdRp) | ||
| hsa-miR-3085-5p | Amapari (3334–3324: RdRp) | Lujo (1316–1306: GPC) |
| Lunk (1709–1699: RdRp) | ||
| hsa-miR-4256 | Tamiami (1844–1834: RdRp) | Chapare (856–866: GPC) |
| Xapuri (2518–2528: RdRp) | ||
| hsa-miR-3913-5p | Chapare (2959–2969: RdRp) | Luna (2453–2463: NP) |
| Lujo (2961–2971: RdRp) | ||
| hsa-miR-4735-3p | Lassa (2553–2563: RdRp) | Whitewater Arroyo (1734–1744: NP) |
| Merino Walk (2369–2359: RdRp) | ||
| hsa-miR-4762-5p | Chapare (1305–1315: RdRp) | Paraná (2680–2870: NP) |
| Latino (3939–3949: RdRp) | ||
| microRNAs | Mammarenaviruses | Genomic Position |
|---|---|---|
| Clade A (L Segment) | ||
| hsa-miR-6083 | Whitewater Arroyo | 1597–1607: RdRp/5470–5480: RdRp |
| hsa-miR-7856-5p | Tamiami | 3946–3956: RdRp/4552–4562: RdRp |
| Clade B (L Segment) | ||
| hsa-miR-122b-3p | Aporé | 2977–2967: RdRp/7195–7185: 3′UTR |
| hsa-miR-376a-3p | Cupixi | 1543–1553: RdRp/3120–3130: RdRp |
| hsa-miR-376b-3p | Cupixi | 1543–1553: RdRp/3120–3130: RdRp |
| Clade B (S Segment) | ||
| hsa-miR-5700 | Sabiá | 1039–1029: GPC/1030–1040: GPC |
| Old World (L Segment) | ||
| hsa-miR-4460 | Lijiang | 209–199: Z/4747–4737: RdRp |
| hsa-miR-8485 | Ryukyu | 354–364/356–366/388–398 (L-IGR) |
| hsa-miR-8485 | Lunk | 418–428/420–430/422–432/424–434/426–436 (L-IGR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.; Miranda, R.L.; de Lemos, E.R.S.; Guterres, A. MicroRNAs and Mammarenaviruses: Modulating Cellular Metabolism. Cells 2020, 9, 2525. https://doi.org/10.3390/cells9112525
Fernandes J, Miranda RL, de Lemos ERS, Guterres A. MicroRNAs and Mammarenaviruses: Modulating Cellular Metabolism. Cells. 2020; 9(11):2525. https://doi.org/10.3390/cells9112525
Chicago/Turabian StyleFernandes, Jorlan, Renan Lyra Miranda, Elba Regina Sampaio de Lemos, and Alexandro Guterres. 2020. "MicroRNAs and Mammarenaviruses: Modulating Cellular Metabolism" Cells 9, no. 11: 2525. https://doi.org/10.3390/cells9112525
APA StyleFernandes, J., Miranda, R. L., de Lemos, E. R. S., & Guterres, A. (2020). MicroRNAs and Mammarenaviruses: Modulating Cellular Metabolism. Cells, 9(11), 2525. https://doi.org/10.3390/cells9112525





