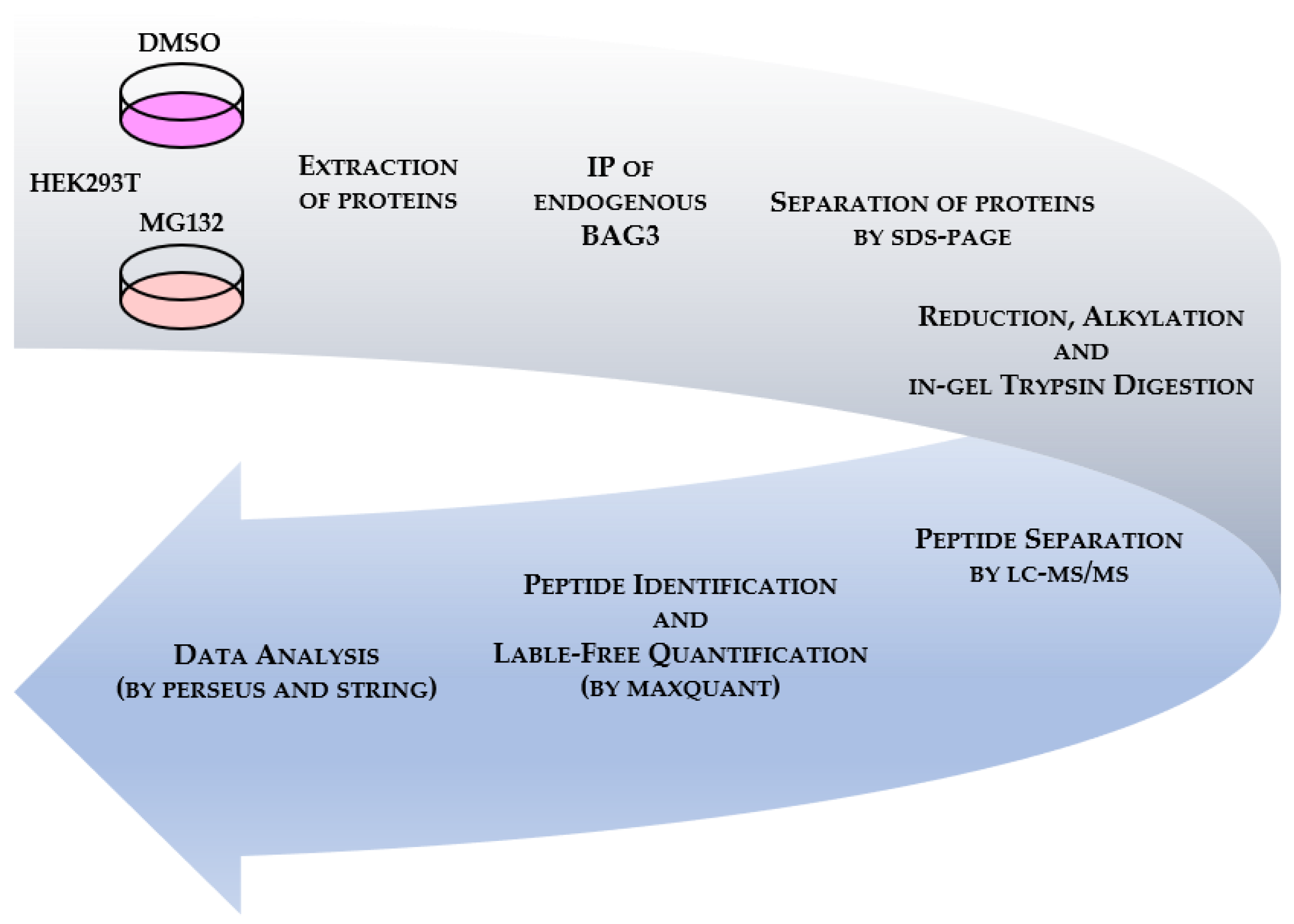
3.2. BAG3 Interaction Profiling under Basal and Proteostasis Stress Conditions
Processing of the proteomic interaction data set by Perseus resulted in the identification of 1335 proteins in total (
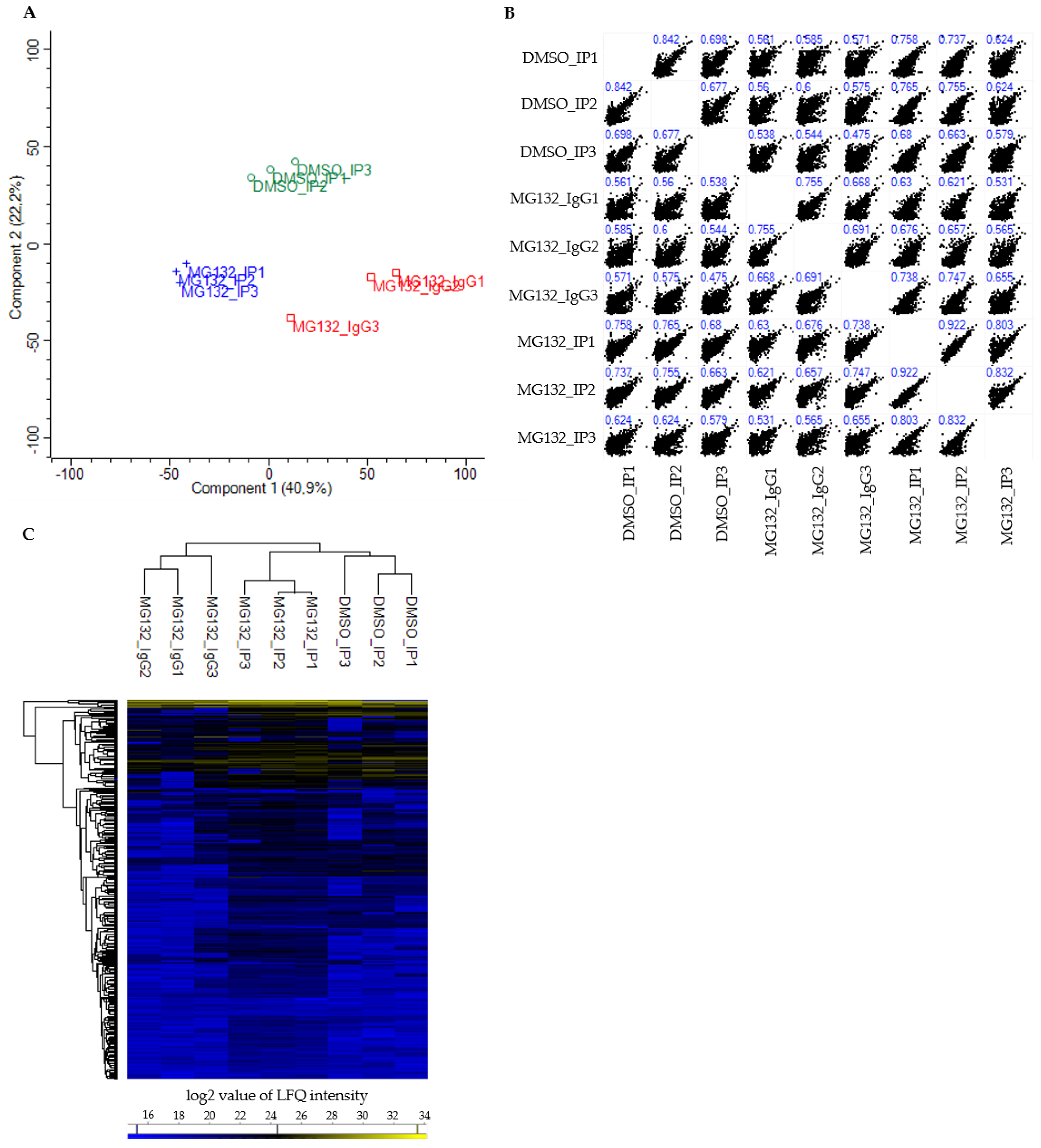
Supplementary Table S1). To evaluate the quality of the generated BAG3 interactome data set, we performed a two-dimensional principal component analysis (PCA) as well as a hierarchical clustering, and created a multi scatter plot of the LFQ intensities of all proteins detected in the corresponding samples (
Figure 2).
The PCA score plot showed that the DMSO_IP replicates (DMSO_IP1-DMSO_IP3) as well as the MG132_IP replicates (MG132_IP1-MG132_IP3) clustered tightly together, the replicates of the IP control MG132_IgG (MG132_IgG1-MG132_IgG3) formed a third more loose cluster (
Figure 2A). Nevertheless, all three analyzed groups (DMSO_IP, MG132_IP and MG132_IgG) were clearly separated from each other with a variance of component 1 of 40.9% and a variance of component 2 of 22.2% (
Figure 2A). The multi scatter plot of the respective LFQ intensities and the corresponding Pearson’s correlation coefficients illustrated a moderate to strong correlation between the three different groups (DMSO_IP, MG132_IP and MG132_IgG) and within the replicates of one group (
Figure 2B). To visualize the protein abundance in all replicates, we carried out a hierarchical clustering and a heat map of the LFQ intensities of all detected proteins (
Figure 2C). As already revealed by PCA, the three independent biological replicates of each group clustered strongly together. Here, the replicates of DMSO_IP and MG132_IP correlated more than the replicates of DMSO_IP and MG132_IgG or the replicates of MG132_IP and MG132_IgG. A majority of the identified proteins was detected in all three groups equally and at low abundance. However, the heat map additionally revealed that the LFQ intensities of a portion of proteins were altered in DMSO_IP and MG132_IP compared to the IP control MG132_IgG and also differed in DMSO_IP and MG132_IP (
Figure 2C). Taken together, these data point to enriched interaction partners of BAG3 in DMSO_IP and MG132_IP in comparison to control MG132_IgG and a changed quantitative binding of specific proteins to BAG3 upon proteasome inhibition with MG132.
To distinguish between specific interactors of BAG3 and background binders, a statistical analysis of the LFQ intensities of proteins detected in DMSO_IP and MG132_IP compared to their LFQ intensities detected in the IP control MG132_IgG was conducted by a two-sample analysis (Student’s t-test,
p-value = 0.05, S0 = 0). First, identified proteins with a
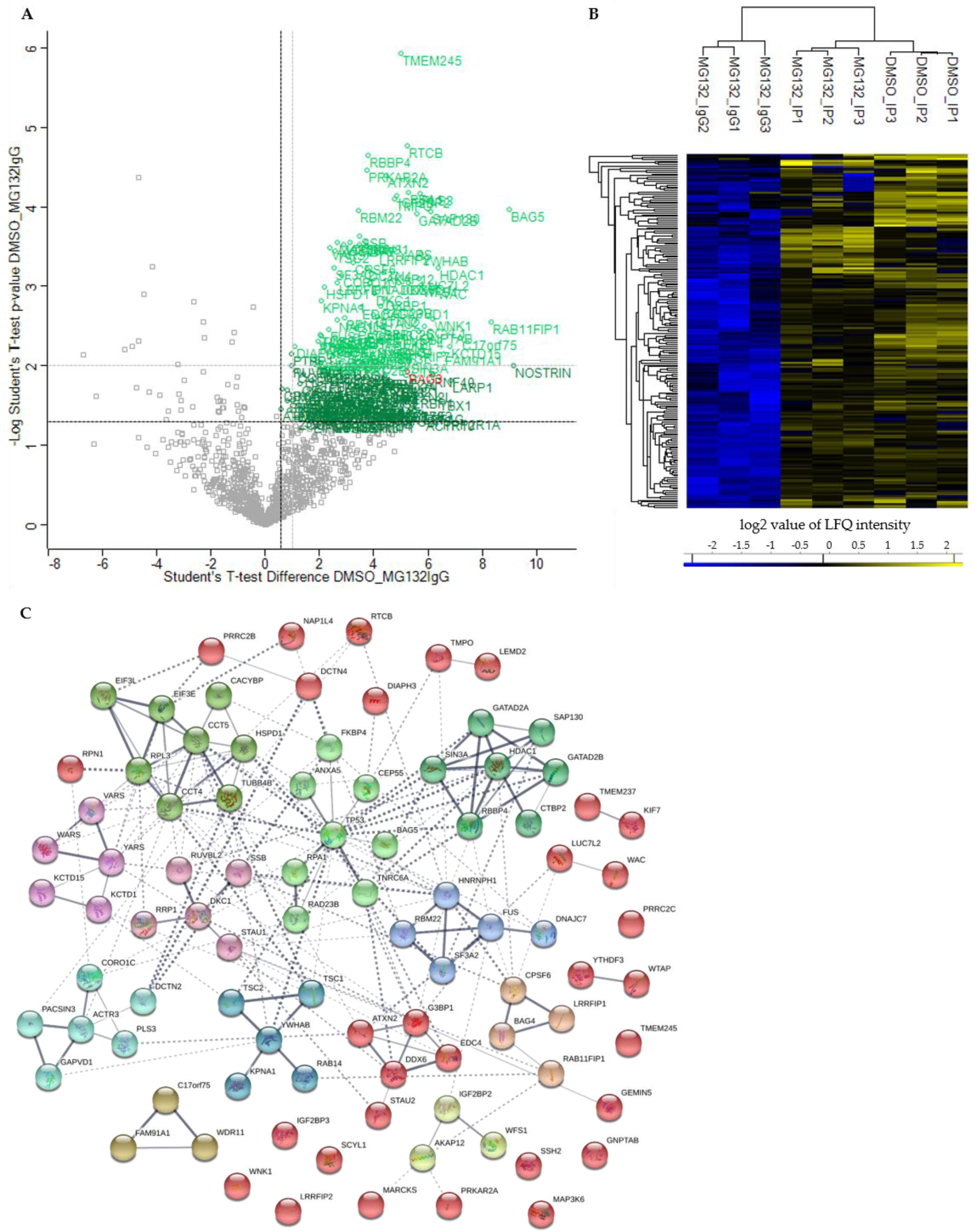
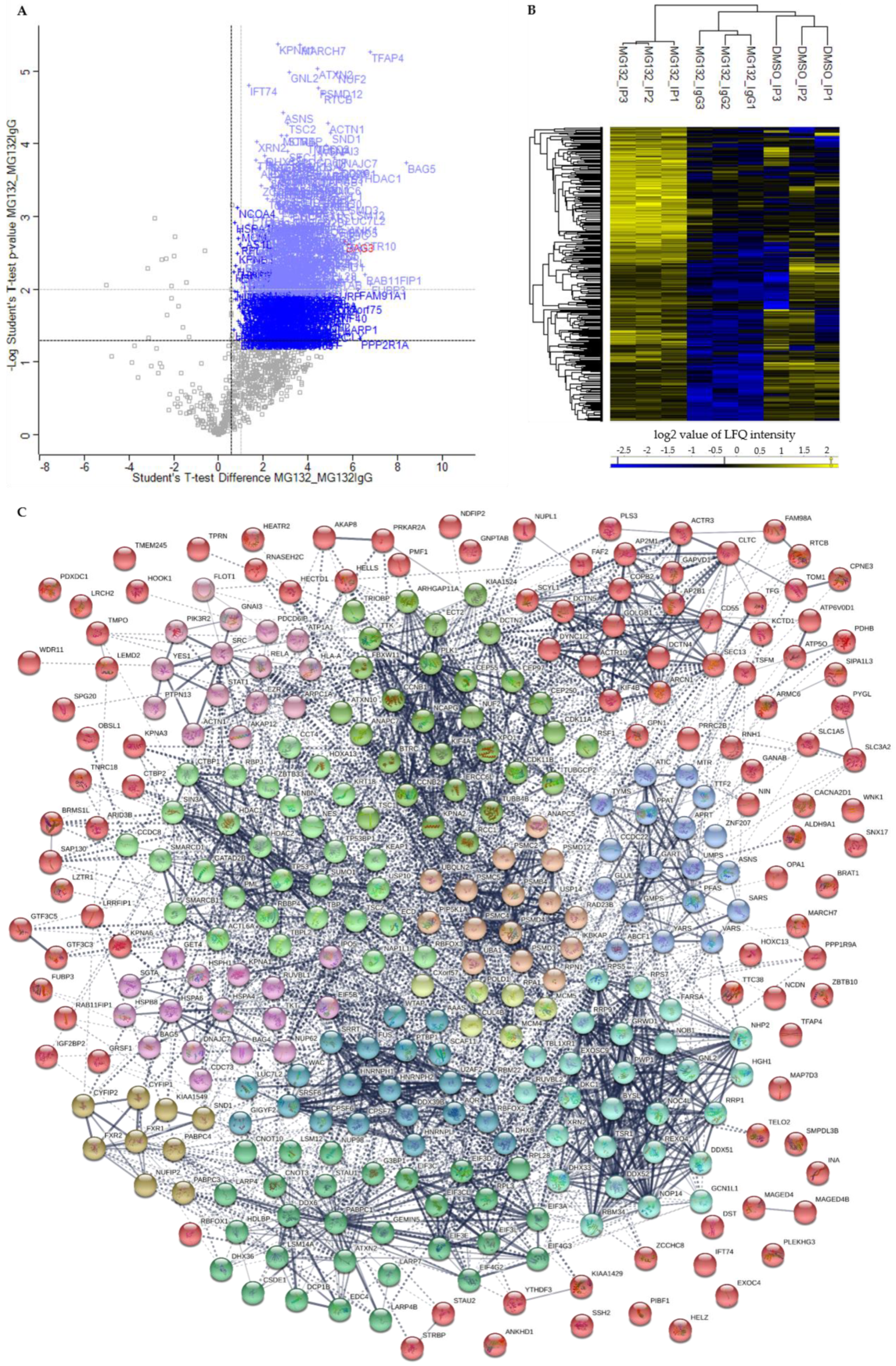
p-value ≤ 0.05 and a ratio ≥ 1.5 were considered as significant. In comparison to control MG132_IgG, we could identify in total 191 significant potential BAG3 interactors under basal conditions (DMSO_IP) and the immense quantity of 561 significant potential BAG3 interactors upon proteasome inhibition (MG132_IP) (
Figure 3A, green labelled proteins;
Figure 4A, blue labelled proteins;
Supplementary Tables S2 and S3). In both cases, BAG3 was found among the significantly enriched binders (
Figure 3A and
Figure 4A, red labelled), indicating for the specificity and a good quality of the performed BAG3 immunoprecipitation. The higher protein abundance of the identified specific BAG3 interactors in DMSO_IP and MG132_IP compared to MG132_IgG was additionally visualized by heat maps and hierarchical clustering (
Figure 3B and
Figure 4B). To cut down the list of significant interactors and to determine the top 25 significantly enriched BAG3 interactors under basal and proteostasis stress conditions, we strengthened the significance criteria and filtered for proteins with a
p-value ≤ 0.01 and a ratio ≥ 2 (class A interactors). Thus, 91 proteins were defined as class A BAG3 interactors under basal conditions (DMSO_IP) and 309 proteins were defined as class A BAG3 interactors under proteostasis stress conditions (MG132_IP) (
Figure 3A, light green labelled proteins;
Figure 4A, light blue labelled proteins;
Supplementary Tables S4 and S5).
Among the top 25 enriched class A BAG3 interactors detected under basal conditions (DMSO_IP), the BAG protein family member BAG5, the transmembrane protein TMEM245, the RNA-binding protein LUC7L2, the adaptor proteins YWHAB, the enzyme GlcNAc-1-phosphotransferase GNPTAB, the two components of the Sin3/HDAC complex HDAC1 and SIN3A, the Sin3/HDAC complex-associated protein SAP130, the tRNA-splicing ligase subunit RTCB, the cytoskeleton-linked proteins KIF7 and PLS3 as well as transcriptional repressor proteins such as KCTD1, KCTD15, WAC and GATAD2B were found (
Table 1). The enriched proteins RAB11FIP1, SCYL1 and the components of the WDR11 complex NJMU-R1 and FAM91A1 are supposed to be involved in cellular transport processes. The listed proteins WNK1 and WFS1 are both described to be implicated in regulating electrolyte homeostasis. Moreover, the TriC (
T-complex protein ring complex) complex subunit CCT5, the RNA helicase DDX6, the isomerase FKBP4 and RPA1, a part of the replication protein A complex, were detected to be highly enriched potential BAG3 interactors under basal conditions (
Table 1). Interestingly, BAG5, HDAC1, RAB11FIP1, LUC7L2, DDX6, PLS3, WNK1, RPA1 and SCYL1 also belong to the top 25 hits of enriched class A BAG3 interactors identified under proteostasis stress conditions (MG132_IP) (
Table 2). Beside the DNAJ/HSP40 family member DNAJC7 and the ribosome subunit RPS7, the protein LSM12, the endonuclease SND1, the glutamine synthetase GLUL, the helicase G3PB1, the transcription regulating proteins TFAP4 and FUBP3 as well as the cytoskeleton-associated proteins ACTR10 and ACTN1 represented significantly enriched potential BAG3 interactors detected in MG132_IP (
Table 2). Additionally, the components EIF3L and EIF3C of the eIF-3 (
eukaryotic translation initiation factor) complex, the subunit Nuf2 of the kinetochore-essential NDC80 complex, the subunit GNAI3 of the G protein (
guanine nucleotide-binding protein), as well as the subunits PMSC5 and PMSD3 of the 26S proteasome complex, were among the top 25 hits of potential BAG3 interactors found upon proteasome inhibition (MG132_IP) (
Table 2).
To get more global insights into the cellular function and localization of the class A BAG3 interactors (
p-value ≤ 0.01 and ratio ≥ 2) identified under basal conditions and upon proteasome inhibition, a list of these proteins was subjected to the STRING database for Gene Ontology (GO) functional annotation and enrichment analysis as well as for creating PPI networks (DMSO_IP: 90 proteins; MG132_IP: 319 proteins;
Supplementary Tables S4 and S5). Beside the category Biological Process, the categories Molecular Function and Cellular Component were analyzed in detail.
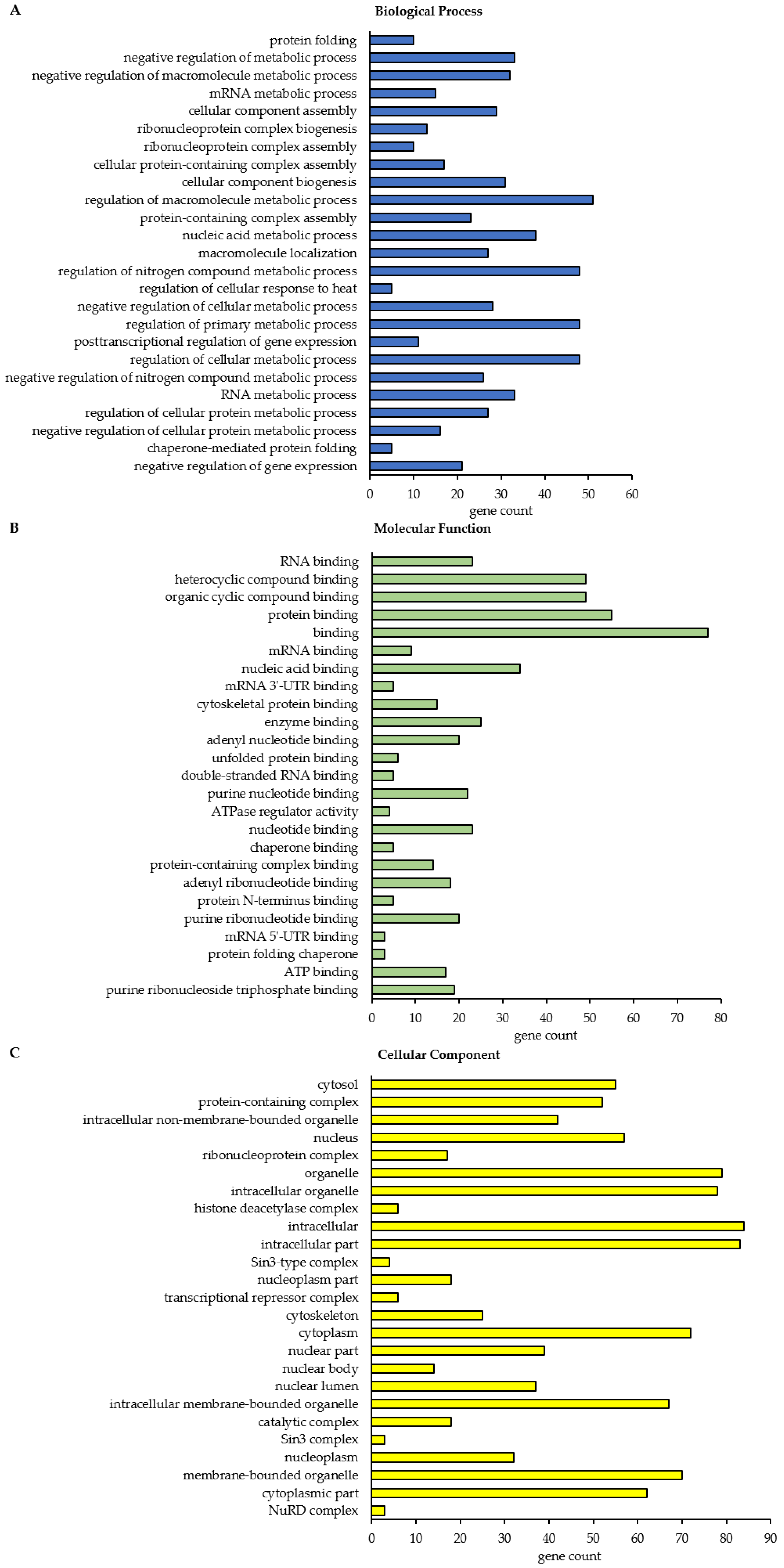
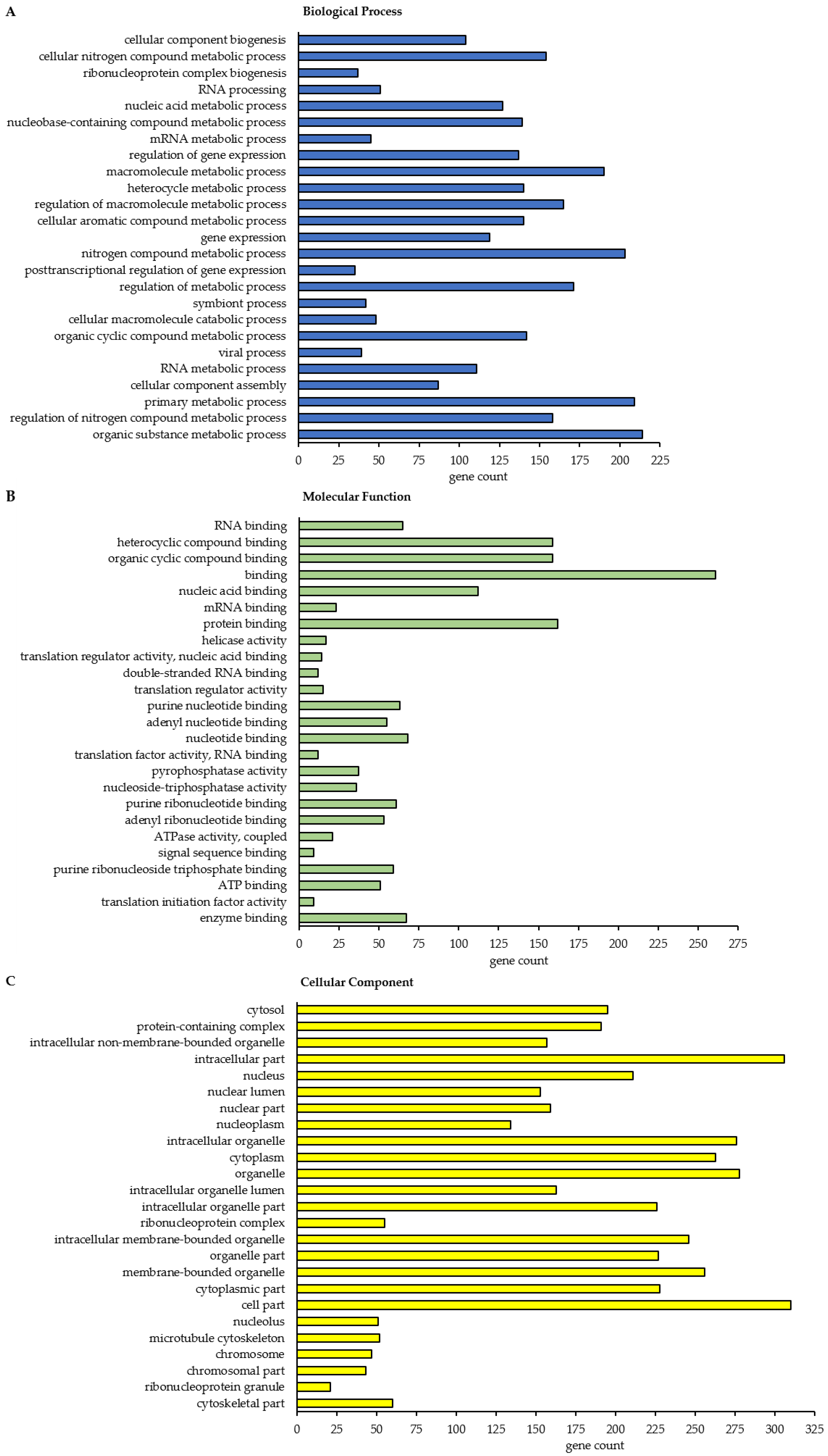
In total, this STRING analysis revealed 114 different enriched GO terms related to Biological Process for class A BAG3 interactors detected under basal conditions and 404 distinct enriched GO terms for class A BAG3 interactors detected upon proteasome inhibition by MG132 (
Supplementary Tables S6 and S7). Among the top 25 most enriched FDR-ranked Biological Process GO terms that were determined for potential BAG3 interactors detected under basal conditions, we found various GO terms linked to metabolic processes and their regulation, in most cases it was a negative regulation (
Figure 5A;
Table A1). In particular, RNA/mRNA metabolic processes, the regulation of gene expression as well as the cellular process protein folding were numbered among these 25 top hits of GO terms. GO terms associated with cellular component biogenesis, including cellular component or protein-containing complex assembly (especially of ribonucleoprotein complexes), and with macromolecule localization were also functionally annotated for the potential BAG3 interactors identified in DMSO_IP. Moreover, the GO term regulation of cellular response to heat stress was significantly enriched among the determined top 25 Biological Process GO terms under basal conditions. As for BAG3 binding proteins detected under basal conditions, GO terms related to component/complex biogenesis and assembly were high FDR-ranked among the top 25 enriched Biological Process GO terms that were defined for BAG3 interactors detected under proteostasis stress conditions (MG132_IP) (
Figure 6A;
Table A2). Various metabolic processes, including RNA metabolic processes (e.g., RNA processing) and gene expression, were also found among the 25 top hits of GO terms that were determined for BAG3 interactors identified in MG132_IP. Additionally, catabolic macromolecule processes were highly significantly enriched among the determined top 25 Biological Process GO terms upon proteasome inhibition. In contrast to the STRING analysis of the BAG3 interactors identified in DMSO_IP, the corresponding functional enrichment analysis of the BAG3 interactors detected in MG132_IP assigned the GO term symbiont process and in detail the GO term viral process to the 25 top hits of Biological Process GO terms. Regarding the category Molecular Function, we identified 49 enriched GO terms for the potential BAG3 interactors detected under basal conditions (DMSO_IP) and 111 enriched GO terms for the potential BAG3 interactors detected under proteostasis stress conditions (MG132_IP) (
Supplementary Tables S8 and S9). In both cases, the list of the top 25 FDR-ranked enriched Molecular Function GO terms mostly contained GO terms linked to binding functions (
Figure 5B and
Figure 6B;
Table A3 and
Table A4). Beside nucleic acid binding (mainly RNA/mRNA-binding) and nucleotide binding, protein binding, including enzyme binding, unfolded protein binding, cytoskeletal protein binding and protein-containing complex binding, was significantly enriched, especially in the top 25 list of GO terms found for class A BAG3 interactors under basal conditions. While the functional enrichment analysis ranked activities such as ATPase regulator activity and protein folding chaperone among the top 25 enriched Molecular function GO terms that were defined for BAG3 interactors identified in DMSO_IP, GO terms concerning activities such as helicase activity, ATPase activity, phosphatase activity, translation regulator activity, translation factor activity or translation initiation factor activity were allocated to the top 25 hits of GO terms that were found for BAG3 interactors detected in MG132_IP (
Figure 6B;
Table A4). In the category Cellular Component, the STRING analysis resulted in the identification of 58 enriched GO terms for the class A BAG3 interactors detected under basal conditions and 118 enriched GO terms for the class A BAG3 interactors detected upon proteasome inhibition with MG132 (
Supplementary Tables S10 and S11). Noteworthy, almost all identified class A BAG3 interactors under basal and proteostasis stress conditions were functionally annotated as intracellular/intracellular part. Under both conditions, Cellular Component GO terms associated with nuclear components (e.g., nucleus, nuclear part, nuclear lumen, nucleoplasm or nucleolus) and cytoplasmic components were the GO terms with the highest significance (
Figure 5C and
Figure 6C;
Table A5 and
Table A6).
Moreover, the GO term protein-containing complex, in particular ribonucleoprotein complex, was revealed as highly enriched. Protein-containing complexes such as histone deacetylase complexes (Sin3-type complex) or transcriptional repressor complexes (NuRD complex) were dedicated to the top 25 list of Cellular Component GO terms that were determined for BAG3 interactors identified in DMSO_IP. Both top 25 hit lists of Cellular Component GO terms additionally comprised GO terms grouped under the term organelle (membrane bounded or non-membrane bounded). Cytoskeleton, chromosome as well as ribonucleoprotein granule have to be highlighted as significantly enriched non-membrane bounded organelles in the top 25 enriched Cellular Component GO terms that were defined for BAG3 interactors detected in MG132_IP.
The PPI networks generated by the STRING database encompassed 90 nodes (equivalent to proteins) and 203 edges (equivalent to interactions) for the BAG3 interactome (class A interactors;
p-value ≤ 0.01 and ratio ≥ 2) under basal conditions (DMSO_IP) and 319 nodes and 2160 edges for the BAG3 interactome upon proteasome inhibition (MG132_IP) (
Figure 3C and
Figure 4C). The enrichment analysis of interactions with a PPI enrichment
p-value of < 1.0e-16 displayed a significantly higher number of interactions (edges) than expected (DMSO_IP: 89 edges; MG132_IP: 1216 edges). To elucidate interacting proteins sharing a related function or involved in the same biological pathway, the PPI networks were subdivided into 12 clusters using the clustering method k-means clustering provided by the STRING database (
Figure 3C and
Figure 4C;
Supplementary Tables S12 and S13). Most of the densely connected proteins in the clusters were also predicted to interact with proteins of other clusters, indicating a multiple role of the BAG3 interactors in different pathways. In addition to clustered proteins, a substantial number of identified potential BAG3 interactors were not associated to any cluster of the networks, illustrating a diverse spectrum of BAG3 interactors with various functions in different pathways.
The 12 clusters found in the BAG3 PPI network under basal conditions (DMSO_IP) refer to following protein interactions, protein complexes or cellular processes (
Figure 3C;
Supplementary Table S12): In Cluster 1 (red nodes), proteins with no predicted interaction with another protein of the network or with only one interactor were collected. Additionally, Cluster 1 contains proteins that could not be assigned to one of the 12 generated k-means clusters, but are predicted to interact with proteins of the clusters. A small subcluster of Cluster 1 consists of the five proteins ATXN2, G3BP1, ECD4, DDX6 and STAU1, all involved in mRNA metabolism. Besides the calcyclin-binding protein CACYBP, the chaperonin HSPD1 and the tubulin family protein TUBB4B, Cluster 2 (lime green nodes) comprises the TriC (
T-complex protein ring complex) complex proteins CCT4 and CCT5, the ribosomal protein RPL3 as well as the components EIF3E and EIF3L of the eIF-3 complex, all involved in translation and protein folding. Cluster 3 (light green nodes) was formed around the multifunctional tumor suppressor protein p53 (TP53) and contains proteins linked to the p53 pathway, such as RPA1, BAG5, TNRC6A or ANXA5. In Cluster 4 (green nodes), two components of the Sin3/HDAC complex, namely HDAC1 and SIN3A, were associated with the Sin3- or HDAC1-associated proteins SAP130 and RBBP4 as well as the transcriptional repressor proteins GATAD2A/B and corepressor CTBP2; this cluster is functionally linked to the regulation of chromatin accessibility and remodeling and thereby linked to transcriptional regulation in particular pathways. The ARP2-ARP3 complex component ACTR3 as well as proteins such as PACSIN3, GAPVD1, PLS3, CORO1C, and DCTN2 of Cluster 5 (cyan nodes) play a role in biological processes requiring actin cytoskeleton (re-)organization (e.g., endocytosis). The center protein of Cluster 6 (dark cyan) is the adaptor protein 14-3-3 beta/alpha (YWHAB) regulating a broad spectrum of signaling pathways and processes. YWHAB is connected to the importin subunit KPNA1, the GTPase RAB14 and the TSC1–TSC2 (hamartin–tuberin) complex, a negative regulator of mTORC1 (
mammalian target of rapamycin complex 1).
In Cluster 7 (blue nodes), the ribonucleoproteins HNRNPH1 and FUS, both components of the hnRNP (heterogeneous nuclear ribonucleoprotein) complex, were linked to the splicing factor subunit SF3A2 and the splicing factor RBM22, all functioning in pre-mRNA processing. FUS recruited the co-chaperone DNAJC7 to this cluster. Cluster 8 (purple nodes) associates the t-RNA ligases VARS, YARS and WARS with the BTB/POZ domain-containing proteins KCTD1 and KCTD15, both potentially repressing AP-2 transcriptional activity. The core protein of Cluster 9 (pink nodes) is DKC1, a subunit of the H/ACA snoRNP (small nucleolar ribonucleoprotein) complex, which is connected to proteins such as SSB1, RRP1, STAU1 or the ATPase RUVBL2, all related to RNA processing. CPSF6, a component of the CFIm (cleavage factor Im) complex, the transcriptional repressor LRRFIP1 and the BAG family member BAG4 were dedicated to Cluster 10 (sandy brown nodes). Cluster 11 (brown nodes) represents the components of the WDR11 complex involved in endosome-to-trans-Golgi network trafficking. Cluster 12 (yellow nodes) reflects the predicted interaction of the RNA-binding factor IGF2BP2 with WFS1 and the PKA (protein kinase A) and PKC (protein kinase C) anchoring protein AKAP12, maybe based on their proposed function in insulin signaling.
Based on the relatively larger number of identified potential interactors, the PPI network of BAG3 upon proteasome inhibition is more extensive and complex; thus, the 12 constructed k-means clusters represent subnetworks of more or less densely connected proteins and of different sizes (
Figure 4C;
Supplementary Table S13). Therefore, only representative proteins of the respective clusters were discussed in the following, revealing the overall biological function of the corresponding cluster (for detailed function of the proteins see
Supplementary Table S13). As in the BAG3 network under basal conditions, Cluster 1 (red nodes) of the BAG3 network under proteostasis stress unified a variety of proteins that either associate only with one other protein, are not connected to any protein of the network at all or are not allocated to one of the 12 constructed k-means clusters, but are supposed to interact with members of these clusters. Nevertheless, Cluster 1 comprises a larger subcluster containing potential BAG3 interactors that are implicated in cytoskeleton dynamics and organization as well as in cellular transport processes. The cytoskeletal proteins DCTN4, DCTN5 and ACTR10 (all linked to the multiprotein complex dynactin), the ATP-binding component ACTR3 of the ARP2/3 complex, the dynein intermediate chain DYNC1I2 (subunit of dynein 1 complex), the motor protein KIF4B, the coatomer subunits ARCN1/COPD and COPB2, the coatomer-binding protein SCYL1, the AP-2 (
adaptor protein) complex subunits AP2M1 and AP2B1 as well as the CLTC protein, the heavy chain of clathrin, were found in this subcluster. Potential BAG3 interactors related to cell cycle were assembled in Cluster 2 (lime green nodes). In addition to the centrosome-associated proteins CEP55, CEP97, CEP250 and TUBGCP2, the cyclin-B proteins CCNB1 and CCNB2, the kinases PLK1, TTK and CDK11A/B, the APC/C component ANAPC7, the kinetochore protein NUF2, the kinesin KIF4A, the cytoskeletal proteins DCTN2 and TUBB4B as well as the regulatory subunit NCAPG of the condensin-2 complex are members of Cluster 2. Cluster 3 (light green nodes) contains proteins linked to the p53 pathway and proteins fulfilling a function in chromatin organization and transcriptional regulation by remodeling and providing accessibility of chromatin. Core proteins of this cluster are the cellular tumor suppressor protein p53 (TP53) and components of the Sin3/HDAC complex, namely the scaffolding protein SIN3A and the catalytic subunits HDAC1 and HDAC2. In addition to other proteins, the BAF (hSWI/SNF) complex subunits SMARCB1, SMARCD1, and ACTL6A, the histone-binding proteins RBBP4, the transcriptional repressor protein GATAD2B, and the corepressor of transcription regulators CTBP1 were numbered among this cluster. Proteins of Cluster 4 (green nodes) are involved in mRNA metabolic processes including processing, degradation and translation of mRNAs. Besides proteins regulating mRNA translation such as LARP4, LARP4B, DHX36, ATXN2 or STAU1, the multifunctional key player in mRNA metabolism PABPC1, the core stress granule protein G3BP1 and factors involved in mRNA processing or decapping/degradation, such as the components CNOT3 and CNOT10 of the CCR4-NOT complex, the RNA helicase DDX6 or the decapping proteins EDC4 and DCP1B, are part of Cluster 4. Additionally, translation initiations factors such as subunits of the eIF-3 complex (EIF3A, EIF3C, EIF3CL, EIF3D, EIF3E, EIF3L) or of the eIF-4G complex (EIF4G2, EIF4G3) as well as the ribosome subunits RPL3 and RPL28 were assigned to this cluster. Cluster 5 (cyan nodes) exhibits proteins functioning in (pre-) ribosomal RNA processing and transport, thereby also implicated in ribosome biogenesis. Among others, the RNA helicases DDX51, DDX52 and DHX33, the H/ACA snoRNP complex subunits DKC1 and NHP2, the ribosomal biogenesis factor NOC4L, the ribosome assembly factor NOB1, the exonuclease REXO4, the ribosomal RNA-processing proteins RRP1, RRP9, NOP14, BYSL, TSR1 and RBM34 as well as the ribosomal subunits RPS5 and RPS7 were dedicated to Cluster 5. Proteins of Cluster 6 (dark cyan nodes) play a role in pre-mRNA processing, especially in splicing (alternative splicing), 3′end processing, polyadenylation, and stabilization of pre-mRNA, therefore functionally related to proteins of Cluster 4. Key proteins of this cluster are the hnRNPs HNRNPH1, HNRNPH2, HNRNPL, PTBP1 and FUS that were connected to proteins such as the splicing factors U2AF2, SRSF6 and RBM22, the CFIm (
cleavage factor I) 3′ end processing complex subunits CPSF6 and CPSF7, the RNA-binding proteins RBFOX2 and LUC7L2 as well as the intron-binding spliceosomal protein AQR. The members of Cluster 7 (blue nodes) are mainly enzymes involved in biosynthetic processes, including nucleotide biosynthesis as well as biosynthesis of amino acids and proteins. Beside the transferases GART, PPAT, APRT and ATIC, the synthetases GMPS, UMPS, TYMS, GLUL, MTR and PFAS as well as the t-RNA ligases VARS, YARS and SARS were connected to each other in Cluster 7. Potential BAG3 interactors of Cluster 8 (purple nodes) can be grouped under the broader term protein quality control. The HSP70 chaperones HSPA4 and HSPA6, the small HSP HSPB8, the nucleotide-exchange factor HSPH1 as well as the co-chaperones DNAJC7, BAG4, BAG5, and SGTA were assigned to this cluster. Cluster 9 (pink nodes) encompasses proteins linked to cellular signaling pathways, including targets, anchoring/adaptor or recruiting proteins. The tyrosine kinases SRC and YES1, the regulatory subunit PIK3R2 of the PIK3 kinase, the nuclear factor NFκB subunit p65 (RELA) or the signal transducer and transcription activator STAT1 represent members of this cluster. In Cluster 10 (sandy brown nodes), potential BAG3 interactors associated with proteasomal degradation were combined. In addition to the 26S proteasome subunits PSMB4, PSMC2, PSMC4, PSMC5, PSMD3, PSMD4 and PSMD12, the E1 enzyme UBA1, the ubiquilin UBQLN2, the proteasome-associated deubiquitinase USP14, the multiubiquitin chain receptor RAD23B and the APC/C component ANAPC5 (an ubiquitin ligase) were found in Cluster 10. Among other proteins, Cluster 11 (brown nodes) accommodates the FMRP-related proteins FXR1 and FXR2, the cytoplasmic and nuclear FMR1-interacting proteins CYFIP1, CYFIP2, and NUFIP2, as well as the RNA-binding proteins PABPC3 and PABPC4. Core protein of Cluster 12 (yellow nodes) is the subunit RPA1 of the heterotrimeric RPA (
replication protein A) complex, recruiting the components MCM4 and MCM5 of the MCM (
minichromosome maintenance) complex, the catalytic component POLD1 of the DNA polymerase delta complex, the core component CUL4B of the cullin-RING-based E3 ligase complex as well as the RPA-related protein RADX (CXorf57) to the cluster. All proteins can be linked to DNA replication.
In conclusion, the functional annotation and enrichment analysis as well as the PPI enrichment analysis revealed that the class A BAG3 interactomes do not fundamentally differ under basal conditions and upon proteasome inhibition. Consistently, the functional annotation and enrichment analyses of potential BAG3 interactors identified under basal (DMSO_IP) and stress conditions (MG132_IP) share a vast number of enriched GO terms in the categories Biological Process, Molecular Function and Cellular Component; herein, enriched Biological Process GO terms perfectly match with the corresponding enriched Molecular Function and Cellular Component GO terms. Furthermore, the PPI enrichment analyses and clustering of the BAG3 interactomes under basal conditions and upon proteasome inhibition are largely similar. In summary, potential BAG3 interactors identified in this study have been shown to be linked to diverse key cellular processes, including RNA metabolic processes (particularly mRNA and rRNA processing), gene expression (regulation of transcription by chromatin (re-) organization, translation), protein folding and degradation, cytoskeleton dynamics and organization, transport/trafficking, or cell cycle, and to signaling pathways, such as the p53 or SRC signaling pathways. A majority of these potential BAG3 interactors are RNA-, nucleotide- or protein-binding proteins and part of larger protein-containing complexes. In line with the already described cellular functions of BAG3, these results emphasize the multifunctionality of BAG3 and its adaptor protein function, enabling it to recruit different complexes to their site of action. Nevertheless, a more detailed examination of the interactome data set and the performed STRING analysis suggest that there are differences between the BAG3 interactomes under basal conditions and upon proteasome inhibition. Notably, while some potential BAG3 interactors were detected only under basal conditions, a much higher number of potential BAG3 binding proteins were only found upon proteasome inhibition by MG132. Under proteostasis stress, BAG3 seems to be either more or less involved in specific biological processes or even to be implicated in more diverse pathways, not least demonstrated by the larger number of significant interactors upon inhibition of proteasomal degradation. In response to cell stress, BAG3 may change its localization to specific cellular components and thus bind more or less to specific proteins. In this manner, BAG3 can actively respond to cellular proteostasis stress and is able to assist in adapting cellular homeostasis to stress.
3.3. Alteration in BAG3 Protein-Protein Interactions under Proteostasis Stress
To determine those proteins whose interaction with BAG3 was significantly modulated upon proteasome inhibition and to quantify this modulation, three Student’s t-test (
p-value = 0.05, S0 = 0) were performed in succession with the DMSO_IP and MG132_IP data sets. Thus, only proteins found with a
p-value ≤ 0.05 and a ratio ≥ 1.5 in DMSO_IP as well as in MG132_IP compared to IP control MG132_IgG were considered as significant hits and further analyzed. Proteins exhibiting a
p-value ≤ 0.05 and a ratio ≤ 0.75 or ≥ 1.5 in the two-sample analysis of MG132_IP compared to DMSO_IP were finally declared as bona fide interactors whose interaction with BAG3 was altered upon proteasome inhibition (
Figure 7A, purple labelled;
Supplementary Table S14). In total, we found 39 proteins whose interaction with BAG3 was significantly changed upon proteasome inhibition by MG132. In detail, 16 proteins showed an increased interaction with BAG3 (ratio ≥ 1.5) and 23 proteins a reduced interaction (ratio ≤ 0.75) upon proteasome inhibition (
Table 3 and
Table 4). Heat map and hierarchical clustering of the LFQ intensities of these bona fide interactors reflected their higher or lower protein abundance in DMSO_IP or in MG132_IP, respectively (
Figure 7B).
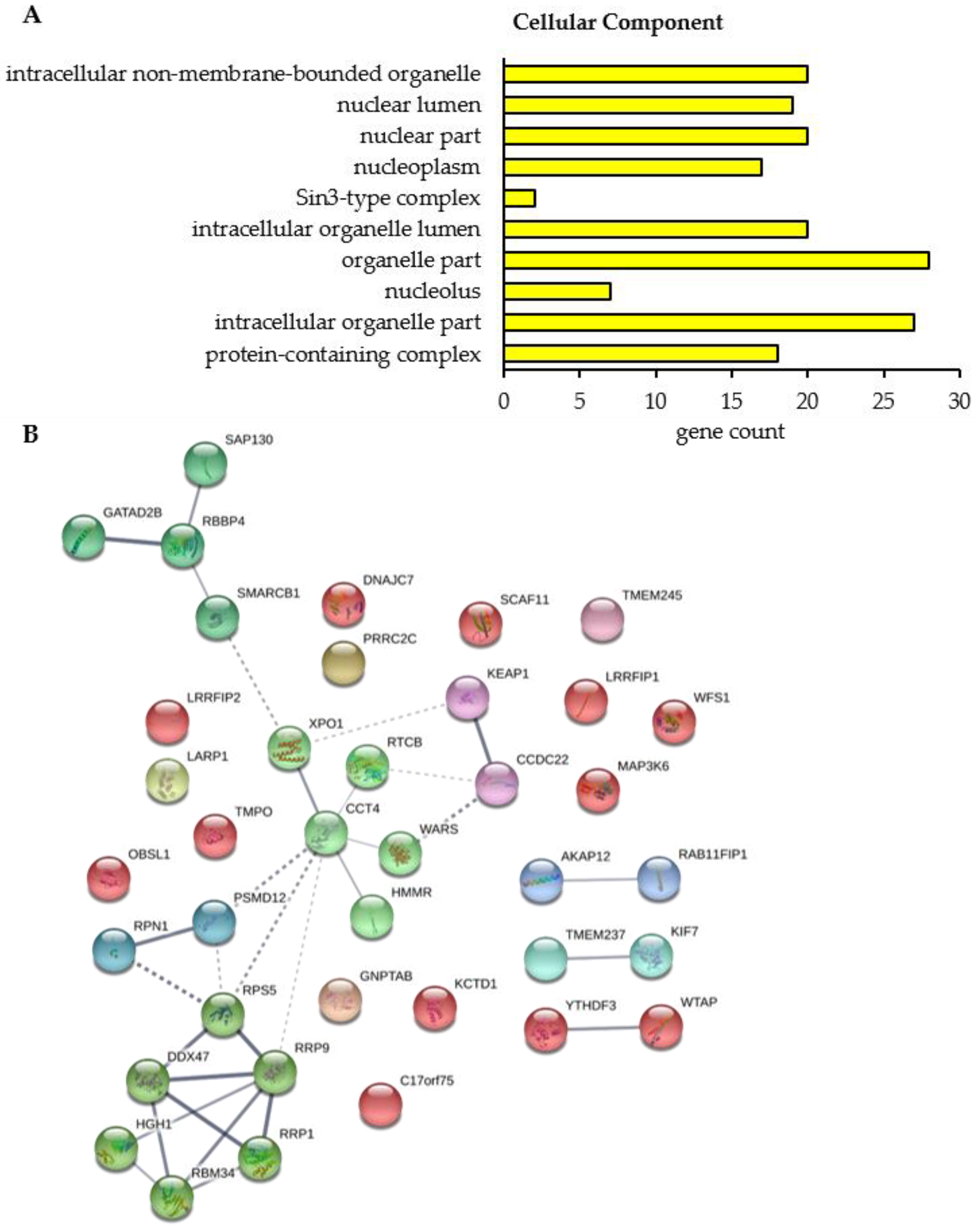
To figure out the cellular function and a potential interaction between the proteins whose binding to BAG3 was modulated upon proteasome inhibition, we performed a GO functional annotation and enrichment analysis and constructed a PPI network by the STRING database. Only in the category Cellular Component, the GO functional annotation and enrichment analysis revealed 10 significantly enriched GO terms for the 39 proteins whose interaction with BAG3 was altered upon proteasome inhibition (
Figure 8A;
Table A7 and
Supplementary Table S15). The majority of enriched Cellular Component GO terms was dedicated to the parent term nucleus, including nuclear lumen, nuclear part, nucleoplasm and nucleolus. Half of the identified proteins were functionally annotated to protein-containing complexes and thus to catalytic complexes (e.g., Sin3-type complex), in particular (
Table A7).
The PPI network of the proteins whose interaction with BAG3 was changed upon proteasome inhibition is made up of 39 nodes (corresponding to proteins) and 31 edges (corresponding to interactions) (
Figure 8B). The construction of this network with a PPI enrichment
p-value of 2.39e-03 resulted in more edges/interactions than expected (12 edges). To reveal proteins possessing similar functions or participating in the same cellular processes, the PPI network was clustered into 12 subnetworks using the k-means clustering method of STRING database (
Figure 8B;
Supplementary Table S16). Cluster 1 (red nodes) represents a slacker cluster and encompasses a total of 12 proteins, almost all of them are not connected with another member of the network. A small subcluster was formed by the proteins WTAP and YTHDF3, both linked to N6-methyladenosine (m6A) methylation of mRNAs and thereby involved in mRNA splicing, stability and translation. WTAP as a regulatory subunit of the WMM (WTAP-METTL3-METTL14) N6-methyltransferase complex is a so called m6A writer and catalyzes the formation of m6A methylation. In contrast, the m6A reader protein YTHDF3 specifically binds to m6A-containing mRNAs and promotes their translation. In addition, the HSP70 and HSP90 co-chaperone DNAJC7, the component NJMU-R1 (C17orf75) of the WDR11 complex, the kinase MAP3K6 of the JNK signaling pathway, the transmembrane protein WFS1, participating in the regulation of cellular Ca
2+ homeostasis, and SCAF11, regulating spliceosome assembly, were dedicated to Cluster 1. Moreover, Cluster 1 contains the lamina-associated protein TMPO, a potential player in structural nucleus organization, and the OBSL1 protein, the core component of the 3M complex regulating microtubule dynamics and genome integrity. The protein KCTD1 present in Cluster 1 has been described to repress transcriptional activity of AP-2 family members and to inhibit Wnt signal transduction pathway by β-catenin degradation. Furthermore, Cluster 1 comprises the related proteins LRRFIP1 and LRRFIP2. LRRFIP1 is implicated in diverse cellular processes in the nucleus and cytoplasm, including immune response to microorganisms and auto-immunity, remodeling of the cytoskeletal system, signal transduction pathways and transcriptional regulation of genes. The poorly studied protein LRRFIP2 has been reported to regulate TLR (
Toll-like receptor) signaling and to potentially modulate the canonical Wnt signaling. Within Cluster 1, BAG3 binding to the proteins DNAJC7, OBSL1, SCAF11, and WTAP was detected to be enhanced upon proteasome inhibition, however the proteins MAP3K6, WFS1, NJMU-R1 (C17orf75), TMPO, KCTD1, LRRFIP1, and LRRFIP2 were found to interact less with BAG3 under proteostasis stress conditions (
Table 3 and
Table 4).
In Cluster 2 (lime green nodes), the DEAD box RNA helicase DDX47 is associated with the proteins RRP1, RRP9, HGH1 and RBM34 as well as with the ribosomal protein RPS5; all proteins of this cluster are linked to rRNA processing and thereby to ribosome biogenesis. The interaction of BAG3 with all these proteins was intensified upon MG132 treatment (
Table 3). The core protein of Cluster 3 (light green nodes) is the molecular chaperone CCT4, a component of the chaperonin multiprotein TriC complex, which is connected to the nuclear export protein XPO1 and the t-RNA ligase WARS on one side and to the hyaluronic acid receptor HMMR and the catalytic subunit RTCB of the tRNA-splicing ligase complex on the other side. While the interaction of XPO1 and HMMR with BAG3 was increased upon proteasome inhibition, a diminished binding of BAG3 to CCT4, RTCB and WARS was determined under these conditions (
Table 3 and
Table 4). Cluster 4 (green nodes) was formed around the core histone-binding subunit RBBP4 that interacts with the histone deacetylase complex-associated protein SAP130, the transcriptional repressor GATAD2B and the core component of the BAF (hSWI/SNF) complex SMARCB1; all proteins are associated with chromatin remodeling and transcriptional regulation. While BAG3 associated less with SMARCB1 under proteostasis stress conditions, RBBP4, GATAD2B and SAP130 showed an enhanced binding to BAG3 upon proteasome inhibition (
Table 3 and
Table 4). The Clusters 5–8 each consist of two proteins. Both proteins of Cluster 5 (cyan nodes) are functionally related to ciliogenesis. The transmembrane protein TMEM237 is localized at the transition zone of cilia. The kinesin-4 family protein KIF7 represents a cilia-associated motor protein that translocates in response to activation of the Shh (
sonic hedgehog) pathway from basal body to tip in cilia and is thereby required for ciliary formation and microtubule stability. Upon inhibition of proteasomal degradation, BAG3 bound to these proteins in a reduced manner (
Table 4). Cluster 6 (dark cyan nodes) is composed of the non-ATPase regulatory subunit PSMD12 of the 26S proteasome complex and RPN1, a subunit of the membrane OST complex. The mentioned proteins are involved in protein degradation and protein modification (N-linked glycosylation), respectively. The binding of PSMD12 to BAG3 was enhanced upon MG132 treatment, however RPN1 interacted less with BAG3 upon proteasome inhibition (
Table 3 and
Table 4). The anchoring protein AKAP12 and the RAB11 effector protein RAB11FIP1 were unified in Cluster 7 (blue nodes). AKAP12 mediates the subcellular compartmentation of PKA (
protein kinase A) and PKC (
protein kinase C). RAB11FIP1 has been described to act in endosomal recycling and trafficking processes. The functional connection of both proteins might be based on their link to PKA/PKC and RAB11, involved in cellular trafficking processes such as the endosomal recycling process. A diminished interaction of both proteins with BAG3 was detected upon proteasome inhibition (
Table 4). In contrast, the binding of BAG3 to the proteins KEAP1 and CCDC22 of Cluster 8 (purple nodes) was shown to be enhanced under proteostasis stress (
Table 3). Both proteins function in major cellular signaling pathways. The CCDC22 protein is implicated in the regulation of NF-κB signaling by mediating the degradation of inhibitory IκB proteins and thereby activating the NF-κB signaling pathway. Additionally, it is a component of the CCC (COMMD/CCDC22/CCDC93) complex that regulates the endosomal recycling of surface proteins. KEAP1 represents a cysteine-based key sensor of oxidative and electrophilic stress. As a substrate-specific E3 ligase adaptor protein, KEAP1 ubiquitinates the transcription factor NRF2 and targets it to proteasomal degradation, thereby negatively regulating the expression of cytoprotective genes (NRF2 pathway). In response to oxidative stress, KEAP1 is inactivated and NRF2 can mediate gene expression. Moreover, KEAP1 has been reported to be sequestered by p62 (SQSTM1) under stress conditions, also leading to NRF2 activation. Clusters 9 to 12 exhibit only one protein each. Cluster 9 (pink node) contains the mostly uncharacterized transmembrane protein TMEM245 whose interaction with BAG3 was reduced under proteostasis stress (
Table 4). GNPTAB of Cluster 10 was observed to bind less to BAG3 upon proteasome inhibition (
Table 4). The gene
GNPTAB encodes two subunits of the enzyme GlcNAc-1-phosphotransferase that catalyzes the transfer of GlcNAc-1-phosphate to mannose residues in the oligosaccharides of newly synthesized lysosomal hydrolases in M6P synthesis and is thereby involved in the targeting of hydrolases to lysosomes. Cluster 11 (brown nodes) consists of the protein C-terminus-binding and RNA-binding protein PRRC2C involved in formation of stress granules. Its interaction with BAG3 was reduced upon proteasome failure (
Table 4). The conserved RNA-binding protein LARP1 in Cluster 12 (yellow nodes) was determined to interact to a lesser extent with BAG3 upon proteasome inhibition (
Table 4). LARP1 associates in an mTOR-dependent manner with the 5′cap of mRNAs (5′ terminal oligopyrimidine (5′TOP) motif), thereby regulating the stability and/or translation of mRNAs that are essential for ribosome biogenesis, cell growth and proliferation.
Taken together, the performed quantitative BAG3 interaction profilings under basal conditions and upon proteasome inhibition by MG132 revealed that BAG3 indeed alters its interaction with specific proteins involved in diverse signaling pathways and biological processes. These processes include mRNA and rRNA metabolism, chromatin remodeling and transcriptional regulation, protein folding and quality control, cytoskeletal dynamics as well as trafficking. BAG3 showed either a significantly diminished or increased interaction with proteins implicated in these pathways. Thus, the reduced or enhanced interaction of BAG3 with these proteins could result in an inhibiting or activating effect on the protein function and therefore on the respective signaling pathway or cellular process.