The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma
Abstract
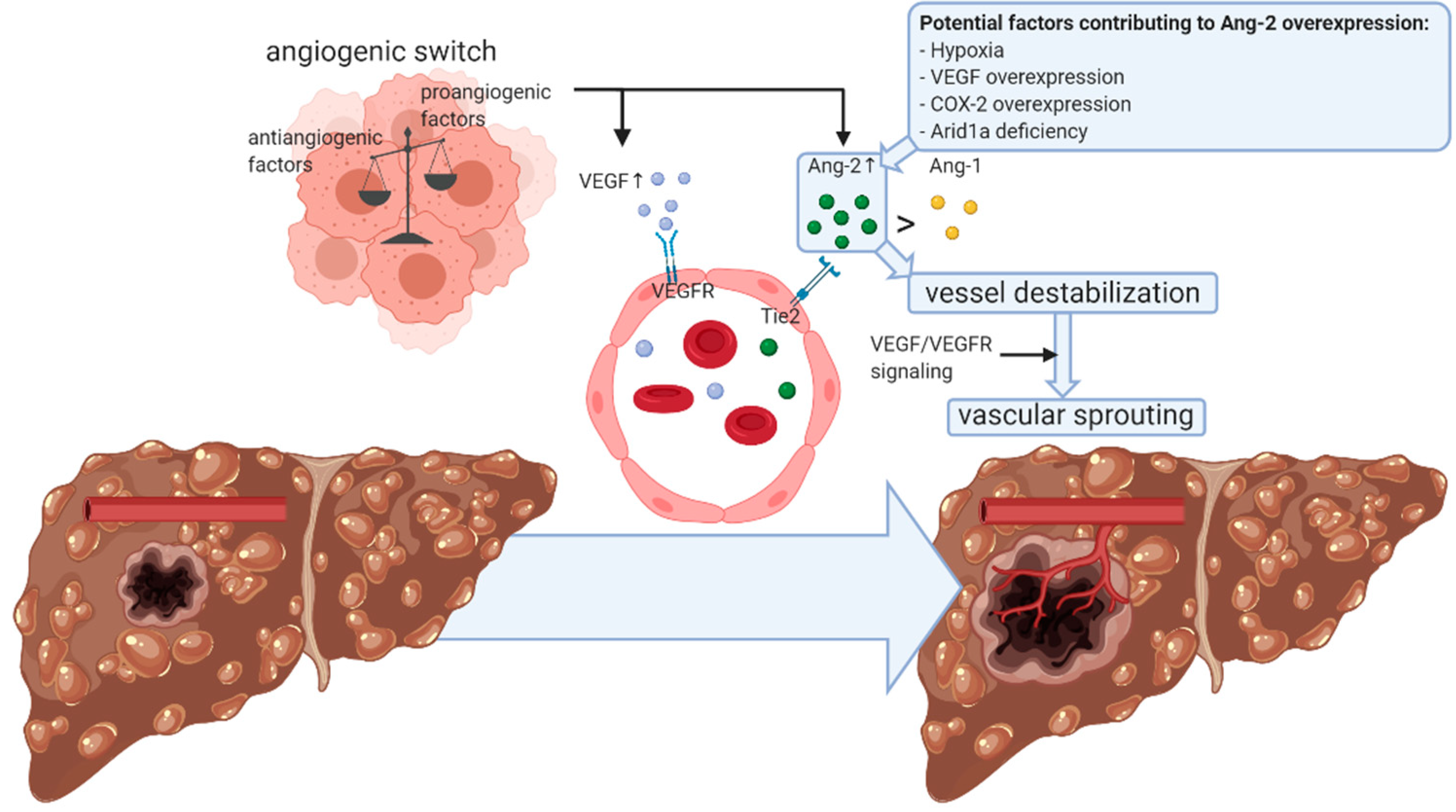
1. Hepatocellular Carcinoma
2. Angiogenic Sprouting in HCC
3. Role of Angiopoietins in Angiogenic Sprouting
4. Angiopoietin Expression and Signaling in HCC
4.1. Angiopoietin-2 Expression in HCC
4.2. Angiopoietin-1 Expression in HCC
5. Role of Angiopoetin-2 in Nonsprouting Vascular Remodeling in HCC
6. Angiopoietin-Targeting Therapeutic Strategies in HCC
6.1. Angiopoietin Targeting in Context of Chemotherapy
6.2. Direct Angiopoietin-Targeting Therapeutic Strategies
| Experimental HCC Animal Model | Ref. |
|---|---|
| Diethylnitrosamine-induced HCC rat model | [56] |
| Diethylnitrosamine-induced HCC mouse model | [31] |
| Nonalcoholic steatohepatitis-induced HCC mouse model | [38] |
| Subcutaneous syngeneic HCC mouse model (injection of MH134 cells in C3H mice) | [30] |
| Subcutaneous syngeneic HCC mouse model (injection of Hepa1-6 cells in C57BL/6 mice) | [48,49] |
| Orthotopic syngeneic HCC mouse model (injection of Hepa1-6 cells in C57BL/6 J mice) | [48,49] |
| Murine subcutaneous xenograft model of human HCC (injection of BEL-7404 cells in BALB/c nude mice) | [52] |
| Murine orthotopic xenograft model of human HCC (injection of VETC-2 cells in BALB/c nude mice) | [49] |
| Highly metastatic murine orthotopic xenograft model of human HCC (implantation of metastatic tumor tissue of murine subcutaneous xenograft model of human HCC in BALB/c nude mice) | [54] |
7. Angiopoietins as Diagnostic and Prognostic Biomarkers
7.1. Angiopoietins as Biomarker in Nonsystemic HCC Treatment
7.2. Angiopoietins as a Biomarker in Sorafenib Treatment
7.3. Angiopoietins as Biomarkers in Other Systemic Treatments
8. Alternative Angiopoietin-2-Related Targets and Biomarkers in HCC
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Muralidharan, P.; Raj, J.P. Update in global trends and aetiology of hepatocellular carcinoma. Contemp. Oncol. (Pozn.) 2018, 22, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.L.; Dempster, J.; Meler, J.D.; Orr, D.W.; Walberg, M.W.; Brown, B.; Berger, B.D.; O’Connor, J.K.; Goldstein, R.M. Hepatocellular carcinoma: Management of an increasingly common problem. Proc. (Bayl. Univ. Med. Cent.) 2008, 21, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Lu, S.C.; Le Marchand, L.; Noureddin, M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology 2016, 64, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [PubMed]
- Pocha, C.; Xie, C. Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease-one of a kind or two different enemies? Transl. Gastroenterol. Hepatol. 2019, 4, 72. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Neureiter, D.; Stintzing, S.; Kiesslich, T.; Ocker, M. Hepatocellular carcinoma: Therapeutic advances in signaling, epigenetic and immune targets. World J. Gastroenterol. 2019, 25, 3136–3150. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Piñero, F.; Dirchwolf, M.; Pessôa, M.G. Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and treatment response assessment. Cells 2020, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Poon, R.T. Vascular changes in hepatocellular carcinoma. Anat. Rec. (Hoboken) 2008, 291, 721–734. [Google Scholar] [CrossRef]
- Diaz-Sanchez, A.; Matilla, A.; Nunez, O.; Lorente, R.; Fernandez, A.; Rincon, D.; Campos, R.; Banares, R.; Clemente, G. Serum angiopoietin-2 level as a predictor of tumor invasiveness in patients with hepatocellular carcinoma. Scand. J. Gastroenterol. 2013, 48, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Duda, D.G.; Sahani, D.V.; Jain, R.K. Hcc and angiogenesis: Possible targets and future directions. Nat. Rev. Clin. Oncol. 2011, 8, 292–301. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Liu, Z.S.; Sun, Q. Expression of angiopoietins, tie2 and vascular endothelial growth factor in angiogenesis and progression of hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 4241–4245. [Google Scholar] [CrossRef] [PubMed]
- Sugimachi, K.; Tanaka, S.; Terashi, T.; Taguchi, K.; Rikimaru, T.; Sugimachi, K. The mechanisms of angiogenesis in hepatocellular carcinoma: Angiogenic switch during tumor progression. Surgery 2002, 131, S135–S141. [Google Scholar] [CrossRef] [PubMed]
- Sugimachi, K.; Tanaka, S.; Taguchi, K.; Aishima, S.; Shimada, M.; Tsuneyoshi, M. Angiopoietin switching regulates angiogenesis and progression of human hepatocellular carcinoma. J. Clin. Pathol. 2003, 56, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.C.; Tang, Z.Y. Angiogenesis in hepatocellular carcinoma: The retrospectives and perspectives. J. Cancer Res. Clin. Oncol. 2004, 130, 307–319. [Google Scholar] [CrossRef]
- Tanaka, S.; Sugimachi, K.; Yamashita Yi, Y.; Ohga, T.; Shirabe, K.; Shimada, M.; Wands, J.R.; Sugimachi, K. Tie2 vascular endothelial receptor expression and function in hepatocellular carcinoma. Hepatology 2002, 35, 861–867. [Google Scholar] [CrossRef]
- Moon, W.S.; Rhyu, K.H.; Kang, M.J.; Lee, D.G.; Yu, H.C.; Yeum, J.H.; Koh, G.Y.; Tarnawski, A.S. Overexpression of vegf and angiopoietin 2: A key to high vascularity of hepatocellular carcinoma? Mod. Pathol. 2003, 16, 552–557. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Shimizu, H.; Ohtsuka, M.; Wakabayashi, Y.; Ito, H.; Kimura, F.; Yoshidome, H.; Kato, A.; Nukui, Y.; Miyazaki, M. Angiopoietins and tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology 2003, 37, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Nagano, H.; Yamamoto, H.; Yang, Y.; Kondo, M.; Ota, H.; Nakamura, M.; Yoshioka, S.; Kato, H.; Damdinsuren, B.; et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: Importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int. 2006, 26, 414–423. [Google Scholar] [CrossRef]
- Meadows, K.L.; Hurwitz, H.I. Anti-vegf therapies in the clinic. Cold Spring Harb. Perspect. Med. 2012, 2, a006577. [Google Scholar] [CrossRef]
- Raybould, A.L.; Sanoff, H. Combination antiangiogenic and immunotherapy for advanced hepatocellular carcinoma: Evidence to date. J. Hepatocell. Carcinoma 2020, 7, 133–142. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-angiogenic agents in combination with immune checkpoint inhibitors: A promising strategy for cancer treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef]
- Wu, X.; Giobbie-Hurder, A.; Liao, X.; Connelly, C.; Connolly, E.M.; Li, J.; Manos, M.P.; Lawrence, D.; McDermott, D.; Severgnini, M.; et al. Angiopoietin-2 as a biomarker and target for immune checkpoint therapy. Cancer Immunol. Res. 2017, 5, 17–28. [Google Scholar] [CrossRef]
- Yoshiji, H.; Kuriyama, S.; Noguchi, R.; Yoshii, J.; Ikenaka, Y.; Yanase, K.; Namisaki, T.; Kitade, M.; Uemura, M.; Masaki, T.; et al. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut 2005, 54, 1768–1775. [Google Scholar] [CrossRef]
- Wang, F.; Dong, X.; Xiu, P.; Zhong, J.; Wei, H.; Xu, Z.; Li, T.; Liu, F.; Sun, X.; Li, J. T7 peptide inhibits angiogenesis via downregulation of angiopoietin-2 and autophagy. Oncol. Rep. 2015, 33, 675–684. [Google Scholar] [CrossRef][Green Version]
- Tait, C.R.; Jones, P.F. Angiopoietins in tumours: The angiogenic switch. J. Pathol. 2004, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Wands, J.R.; Arii, S. Induction of angiopoietin-2 gene expression by cox-2: A novel role for cox-2 inhibitors during hepatocarcinogenesis. J. Hepatol. 2006, 44, 233–235. [Google Scholar] [CrossRef]
- Hu, C.; Li, W.; Tian, F.; Jiang, K.; Liu, X.; Cen, J.; He, Q.; Qiu, Z.; Kienast, Y.; Wang, Z.; et al. Arid1a regulates response to anti-angiogenic therapy in advanced hepatocellular carcinoma. J. Hepatol. 2018, 68, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Mori, M.; Sakamoto, Y.; Makuuchi, M.; Sugimachi, K.; Wands, J.R. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J. Clin. Investig. 1999, 103, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Torimura, T.; Ueno, T.; Kin, M.; Harada, R.; Taniguchi, E.; Nakamura, T.; Sakata, R.; Hashimoto, O.; Sakamoto, M.; Kumashiro, R.; et al. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J. Hepatol. 2004, 40, 799–807. [Google Scholar] [CrossRef]
- Davis, S.; Aldrich, T.H.; Jones, P.F.; Acheson, A.; Compton, D.L.; Jain, V.; Ryan, T.E.; Bruno, J.; Radziejewski, C.; Maisonpierre, P.C.; et al. Isolation of angiopoietin-1, a ligand for the tie2 receptor, by secretion-trap expression cloning. Cell 1996, 87, 1161–1169. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Suri, C.; Jones, P.F.; Bartunkova, S.; Wiegand, S.J.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, T.H.; Papadopoulos, N.; et al. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science 1997, 277, 55–60. [Google Scholar] [CrossRef]
- Valenzuela, D.M.; Griffiths, J.A.; Rojas, J.; Aldrich, T.H.; Jones, P.F.; Zhou, H.; McClain, J.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; et al. Angiopoietins 3 and 4: Diverging gene counterparts in mice and humans. Proc. Natl. Acad. Sci. USA 1999, 96, 1904–1909. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, C.H.; Hwang, S.J.; Choi, H.H.; Kim, K.T.; Ahn, S.Y.; Kim, J.H.; Oh, J.L.; Lee, G.M.; Koh, G.Y. Biological characterization of angiopoietin-3 and angiopoietin-4. Faseb J. 2004, 18, 1200–1208. [Google Scholar] [CrossRef]
- Lefere, S.; Van de Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef]
- Kim, M.; Allen, B.; Korhonen, E.A.; Nitschké, M.; Yang, H.W.; Baluk, P.; Saharinen, P.; Alitalo, K.; Daly, C.; Thurston, G.; et al. Opposing actions of angiopoietin-2 on tie2 signaling and foxo1 activation. J. Clin. Investig. 2016, 126, 3511–3525. [Google Scholar] [CrossRef]
- Thurston, G.; Daly, C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012, 2, a006550. [Google Scholar] [CrossRef]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’Alessandro, R. Inflammatory mechanisms of hcc development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Z.; Wang, G.; Wang, C. Expression of angiopoietin-2 gene and its receptor tie2 in hepatocellular carcinoma. J. Tongji Med. Univ. 2001, 21, 228–230, 235. [Google Scholar]
- Sun, X.; Wang, S.C.; Wei, Y.; Luo, X.; Jia, Y.; Li, L.; Gopal, P.; Zhu, M.; Nassour, I.; Chuang, J.C.; et al. Arid1a has context-dependent oncogenic and tumor suppressor functions in liver cancer. Cancer Cell 2017, 32, 574–589.e576. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Meng, L.L.; Li, Y.Z.; Chen, S.X.; Xu, J.L.; Tang, Y.J.; Lin, N. Importance of activated hepatic stellate cells and angiopoietin-1 in the pathogenesis of hepatocellular carcinoma. Mol. Med. Rep. 2016, 14, 1721–1725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zeng, W.; Gouw, A.S.; van den Heuvel, M.C.; Zwiers, P.J.; Zondervan, P.E.; Poppema, S.; Zhang, N.; Platteel, I.; de Jong, K.P.; Molema, G. The angiogenic makeup of human hepatocellular carcinoma does not favor vascular endothelial growth factor/angiopoietin-driven sprouting neovascularization. Hepatology 2008, 48, 1517–1527. [Google Scholar] [CrossRef]
- Chen, J.A.; Shi, M.; Li, J.Q.; Qian, C.N. Angiogenesis: Multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol. Int. 2010, 4, 537–547. [Google Scholar] [CrossRef]
- Coelho, A.L.; Gomes, M.P.; Catarino, R.J.; Rolfo, C.; Lopes, A.M.; Medeiros, R.M.; Araújo, A.M. Angiogenesis in nsclc: Is vessel co-option the trunk that sustains the branches? Oncotarget 2017, 8, 39795–39804. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhou, H.C.; Zhang, C.; Shang, L.R.; Zhang, L.; Xu, J.; Zheng, L.; Yuan, Y.; Guo, R.P.; Jia, W.H.; et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial-mesenchymal transition-independent manner. Hepatology 2015, 62, 452–465. [Google Scholar] [CrossRef]
- Zhou, H.C.; Fang, J.H.; Shang, L.R.; Zhang, Z.J.; Sang, Y.; Xu, L.; Yuan, Y.; Chen, M.S.; Zheng, L.; Zhang, Y.; et al. Micrornas mir-125b and mir-100 suppress metastasis of hepatocellular carcinoma by disrupting the formation of vessels that encapsulate tumour clusters. J. Pathol. 2016, 240, 450–460. [Google Scholar] [CrossRef]
- Wada, H.; Nagano, H.; Yamamoto, H.; Arai, I.; Ota, H.; Nakamura, M.; Damdinsuren, B.; Noda, T.; Marubashi, S.; Miyamoto, A.; et al. Combination therapy of interferon-alpha and 5-fluorouracil inhibits tumor angiogenesis in human hepatocellular carcinoma cells by regulating vascular endothelial growth factor and angiopoietins. Oncol. Rep. 2007, 18, 801–809. [Google Scholar]
- Wada, H.; Nagano, H.; Yamamoto, H.; Noda, T.; Murakami, M.; Kobayashi, S.; Marubashi, S.; Eguchi, H.; Takeda, Y.; Tanemura, M.; et al. Combination of interferon-alpha and 5-fluorouracil inhibits endothelial cell growth directly and by regulation of angiogenic factors released by tumor cells. BMC Cancer 2009, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.K.; Cai, M.X.; Yang, J.; Lin, L.W.; Xue, E.S.; Huang, J.; Wei, H.F.; Zhang, X.J.; Ke, L.M. Chemotherapy with plga microspheres containing docetaxel decreases angiogenesis in human hepatoma xenograft. Med. Oncol. 2012, 29, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.; Jiang, K.; Ruan, Q. Angiopoietin2 enhances doxorubin resistance in hepg2 cells by upregulating survivin and ref-1 via msk1 activation. Cancer Lett. 2013, 337, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Zhang, J.F.; Yuan, Y.F.; He, Y.M.; Liu, Q.Y.; Mao, X.W.; Ai, Y.B.; Liu, Z.S. Suppression of angiogenesis and tumor growth in vitro and in vivo using an anti-angiopoietin-2 single-chain antibody. Exp. Ther. Med. 2014, 7, 543–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papadopoulos, K.P.; Kelley, R.K.; Tolcher, A.W.; Razak, A.R.; Van Loon, K.; Patnaik, A.; Bedard, P.L.; Alfaro, A.A.; Beeram, M.; Adriaens, L.; et al. A phase i first-in-human study of nesvacumab (regn910), a fully human anti-angiopoietin-2 (ang2) monoclonal antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2016, 22, 1348–1355. [Google Scholar] [CrossRef]
- Kuroda, H.; Ohtsuru, A.; Futakuchi, M.; Kawashita, Y.; Nagayama, Y.; Fukuda, E.; Namba, H.; Shirai, T.; Kanematsu, T.; Yamashita, S. Distinctive gene expression of receptor-type tyrosine kinase families during rat hepatocarcinogenesis. Int. J. Mol. Med. 2002, 9, 473–480. [Google Scholar] [CrossRef]
- Scholz, A.; Rehm, V.A.; Rieke, S.; Derkow, K.; Schulz, P.; Neumann, K.; Koch, I.; Pascu, M.; Wiedenmann, B.; Berg, T.; et al. Angiopoietin-2 serum levels are elevated in patients with liver cirrhosis and hepatocellular carcinoma. Am. J. Gastroenterol. 2007, 102, 2471–2481. [Google Scholar] [CrossRef]
- Kuboki, S.; Shimizu, H.; Mitsuhashi, N.; Kusashio, K.; Kimura, F.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Angiopoietin-2 levels in the hepatic vein as a useful predictor of tumor invasiveness and prognosis in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2008, 23, e157–e164. [Google Scholar] [CrossRef]
- Hsieh, M.Y.; Lin, Z.Y.; Chuang, W.L. Serial serum vegf-a, angiopoietin-2, and endostatin measurements in cirrhotic patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization. Kaohsiung J. Med. Sci. 2011, 27, 314–322. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pena, C.E.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef]
- Villa, E.; Critelli, R.; Lei, B.; Marzocchi, G.; Camma, C.; Giannelli, G.; Pontisso, P.; Cabibbo, G.; Enea, M.; Colopi, S.; et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut 2016, 65, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.C.; Brasil, I.R.C.; Torres, A.F.C.; Tavora, F. The evaluation of angiogenesis markers in hepatocellular carcinoma and precursor lesions in liver explants from a single institution. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 330–336. [Google Scholar] [CrossRef]
- Chen, Z.B.; Shen, S.Q.; Ding, Y.M.; Wang, W.X.; Tao, J.P.; Liang, L.J.; Hu, W.J. The angiogenic and prognostic implications of vegf, ang-1, ang-2, and mmp-9 for hepatocellular carcinoma with background of hepatitis b virus. Med. Oncol. 2009, 26, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, G.; Dino, K.; Schierle, K.; Dietel, C.; Aust, G.; Pratschke, J.; Seehofer, D.; Schmelzle, M.; Hau, H.M. Recipient hepatic tumor-associated immunologic infiltrates predict outcomes after liver transplantation for hepatocellular carcinoma. Ann. Transplant. 2020, 25, e919414. [Google Scholar] [CrossRef]
- Matsubara, T.; Kanto, T.; Kuroda, S.; Yoshio, S.; Higashitani, K.; Kakita, N.; Miyazaki, M.; Sakakibara, M.; Hiramatsu, N.; Kasahara, A.; et al. Tie2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology 2013, 57, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Pestana, R.C.; Hassan, M.M.; Abdel-Wahab, R.; Abugabal, Y.I.; Girard, L.M.; Li, D.; Chang, P.; Raghav, K.; Morris, J.; Wolff, R.A.; et al. Clinical and prognostic significance of circulating levels of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. Oncotarget 2018, 9, 37721–37732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inchingolo, R.; Posa, A.; Mariappan, M.; Spiliopoulos, S. Locoregional treatments for hepatocellular carcinoma: Current evidence and future directions. World J. Gastroenterol. 2019, 25, 4614–4628. [Google Scholar] [CrossRef]
- Carpizo, D.R.; Gensure, R.H.; Yu, X.; Gendel, V.M.; Greene, S.J.; Moore, D.F.; Jabbour, S.K.; Nosher, J.L. Pilot study of angiogenic response to yttrium-90 radioembolization with resin microspheres. J. Vasc. Interv. Radiol. 2014, 25, 297–306.e291. [Google Scholar] [CrossRef]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152–4163. [Google Scholar] [CrossRef]
- Miyahara, K.; Nouso, K.; Tomoda, T.; Kobayashi, S.; Hagihara, H.; Kuwaki, K.; Toshimori, J.; Onishi, H.; Ikeda, F.; Miyake, Y.; et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 1604–1611. [Google Scholar] [CrossRef]
- Marisi, G.; Petracci, E.; Raimondi, F.; Faloppi, L.; Foschi, F.G.; Lauletta, G.; Iavarone, M.; Canale, M.; Valgiusti, M.; Neri, L.M.; et al. Angpt2 and nos3 polymorphisms and clinical outcome in advanced hepatocellular carcinoma patients receiving sorafenib. Cancers 2019, 11, 1023. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Blanc, J.F.; Miles, S.; Ganten, T.; Trojan, J.; Cebon, J.; Liem, A.K.; Lipton, L.; Gupta, C.; Wu, B.; et al. Phase ii study of first-line trebananib plus sorafenib in patients with advanced hepatocellular carcinoma. Oncologist 2017, 22, 780–e65. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Scheiner, B.; Peck-Radosavljevic, M. Immunotherapy for advanced hepatocellular carcinoma: A focus on special subgroups. Gut 2020. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Seidel, H.; Kochert, K.; Meinhardt, G.; Finn, R.S.; Llovet, J.M.; Bruix, J. Biomarkers associated with response to regorafenib in patients with hepatocellular carcinoma. Gastroenterology 2019, 156, 1731–1741. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Garrett-Mayer, E.; Morris, J.S.; Xiao, L.; Lin, E.; Onicescu, G.; Hassan, M.M.; Hassabo, H.M.; Iwasaki, M.; Deaton, F.L.; et al. Efficacy of bevacizumab plus erlotinib for advanced hepatocellular carcinoma and predictors of outcome: Final results of a phase ii trial. Oncology 2012, 82, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Yau, T.; Park, J.W.; Lim, H.Y.; Lee, T.Y.; Obi, S.; Chan, S.L.; Qin, S.; Kim, R.D.; Casey, M.; et al. Randomized phase ii study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann. Oncol. 2015, 26, 2457–2463. [Google Scholar] [CrossRef]
- Sharma, B.K.; Srinivasan, R.; Kapil, S.; Singla, B.; Saini, N.; Chawla, Y.K.; Chakraborti, A.; Duseja, A.; Kalra, N.; Dhiman, R.K. Serum levels of angiogenic and anti-angiogenic factors: Their prognostic relevance in locally advanced hepatocellular carcinoma. Mol. Cell Biochem. 2013, 383, 103–112. [Google Scholar] [CrossRef]
- Xie, J.Y.; Wei, J.X.; Lv, L.H.; Han, Q.F.; Yang, W.B.; Li, G.L.; Wang, P.X.; Wu, S.B.; Duan, J.X.; Zhuo, W.F.; et al. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell. Commun. Signal. 2020, 18, 46. [Google Scholar] [CrossRef]
- Adachi, T.; Nouso, K.; Miyahara, K.; Oyama, A.; Wada, N.; Dohi, C.; Takeuchi, Y.; Yasunaka, T.; Onishi, H.; Ikeda, F.; et al. Monitoring serum proangiogenic cytokines from hepatocellular carcinoma patients treated with sorafenib. J. Gastroenterol. Hepatol. 2019, 34, 1081–1087. [Google Scholar] [CrossRef]
- Miyahara, K.; Nouso, K.; Morimoto, Y.; Takeuchi, Y.; Hagihara, H.; Kuwaki, K.; Onishi, H.; Ikeda, F.; Miyake, Y.; Nakamura, S.; et al. Pro-angiogenic cytokines for prediction of outcomes in patients with advanced hepatocellular carcinoma. Br. J. Cancer 2013, 109, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Wang, C.Q.; Yu, Y.; Qian, J.; Song, K.; Sun, Q.M.; Zhou, J. Tie2-expressing monocytes are associated with identification and prognoses of hepatitis b virus related hepatocellular carcinoma after resection. PLoS ONE 2015, 10, e0143657. [Google Scholar] [CrossRef]
- Shoji, H.; Yoshio, S.; Mano, Y.; Doi, H.; Sugiyama, M.; Osawa, Y.; Kimura, K.; Arai, T.; Itokawa, N.; Atsukawa, M.; et al. Pro-angiogenic tie-2-expressing monocytes/tems as a biomarker of the effect of sorafenib in patients with advanced hepatocellular carcinoma. Int. J. Cancer 2017, 141, 1011–1017. [Google Scholar] [CrossRef]
| Diagnosis/Treatment | Biomarker Potential | Ref. |
|---|---|---|
| Diagnosis | Ang-2 expression is higher in HCC tissue compared to adjacent noncancerous liver tissue. | [15,20,21,32,33,42,63] |
| Circulating Ang-2 levels are higher in HCC patients compared to cirrhosis patients. | [57,78] | |
| Ang-2 expression is higher in HCC tissue compared to benign liver disease tissue. | [79] | |
| Differential Ang-2 expression in HCC lesions, compared to non-neoplastic regenerative nodules. | [62] | |
| Surgical resection | Ang-2 expression in HCC tissue correlates with post-surgery recurrence. | [22,63] |
| Preoperative hepatic venous Ang-2 levels inversely correlate with post-surgery survival. | [58] | |
| Liver transplantation | Ang-2 expression in HCC tissue correlates with post-surgery graft rejection. | [64] |
| Transarterial radioembolization | Baseline circulating Ang-2 levels inversely correlate with post-treatment survival. | [68] |
| Surgical or locoregional treatment | Circulating Ang-2 levels inversely correlate with eligibility for surgical or locoregional treatment. | [13] |
| Sorafenib | Baseline circulating Ang-2 levels inversely correlate with response to sorafenib. | [70] |
| Baseline circulating Ang-2 levels inversely correlate with post-treatment time to progression and overall survival. | [60,70,80,81] | |
| Post-treatment increases of circulating Ang-2 levels inversely correlate with time to progression and overall survival. | [60] | |
| Regorafenib | Baseline circulating Ang-1 levels inversely correlate with post-treatment overall survival. | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanderborght, B.; Lefere, S.; Vlierberghe, H.V.; Devisscher, L. The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma. Cells 2020, 9, 2382. https://doi.org/10.3390/cells9112382
Vanderborght B, Lefere S, Vlierberghe HV, Devisscher L. The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma. Cells. 2020; 9(11):2382. https://doi.org/10.3390/cells9112382
Chicago/Turabian StyleVanderborght, Bart, Sander Lefere, Hans Van Vlierberghe, and Lindsey Devisscher. 2020. "The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma" Cells 9, no. 11: 2382. https://doi.org/10.3390/cells9112382
APA StyleVanderborght, B., Lefere, S., Vlierberghe, H. V., & Devisscher, L. (2020). The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma. Cells, 9(11), 2382. https://doi.org/10.3390/cells9112382







