Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential
Abstract
1. Introduction
2. Categorization and Structure of lncRNAs
3. LncRNA Molecular Functions
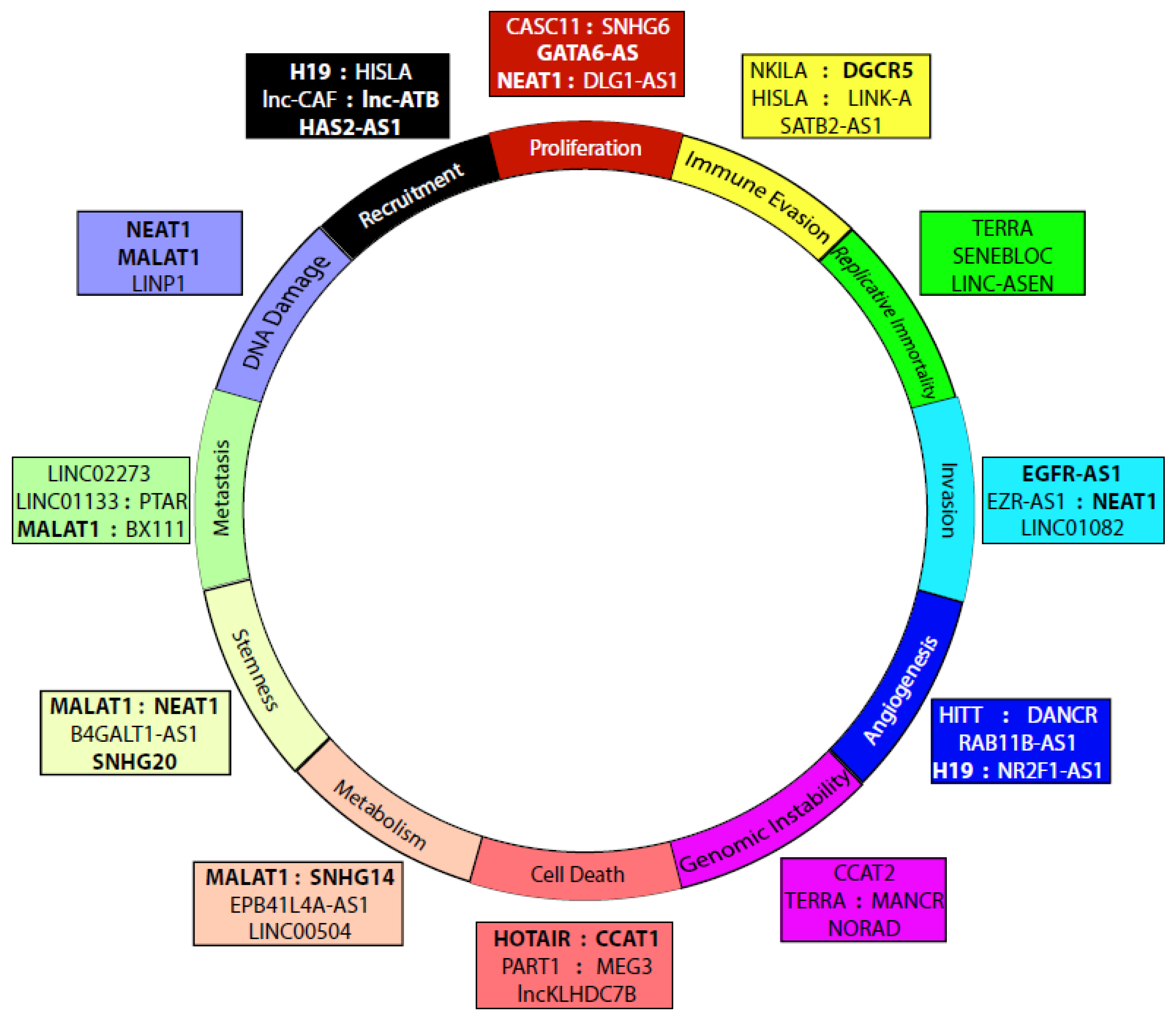
4. LncRNAs in Cancer
4.1. Proliferation
4.2. Immune Evasion
4.3. Metastasis/Tumorigenesis
4.4. Replicative Immortality
4.5. Invasion/Migration
4.6. Angiogenesis
4.7. Genome Instability and Mutation
4.8. Resisting Cell Death
4.9. Altered Metabolism
4.10. Stemness/Multipotency
4.11. DNA Damage Response
4.12. Recruitment of Stromal Cells
5. LncRNAs in Glioma and GBM
6. LncRNAs as Biomarkers
7. LncRNAs as Therapeutic Targets
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, R.E.; Lim, D.A. Forging our understanding of lncRNAs in the brain. Cell Tissue Res. 2017, 371, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Isin, M.; Dalay, N. LncRNAs and neoplasia. Clin. Chim. Acta 2015, 444, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yangyang, Z.; Lu, J.; Song, G.; Zhu, Y.; Li, Z.; Zhaohui, L.; Shen, B.; Huang, X.; Zhu, H.; et al. Opposing Roles for the lncRNA Haunt and Its Genomic Locus in Regulating HOXA Gene Activation during Embryonic Stem Cell Differentiation. Cell Stem Cell 2015, 16, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Blokhin, I.; Khorkova, O.; Hsiao, J.; Wahlestedt, C. Developments in lncRNA drug discovery: Where are we heading? Expert Opin. Drug Discov. 2018, 13, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef]
- Von Rosenstiel, C.; Wiestler, B.; Haller, B.; Schmidt-Graf, F.; Gempt, J.; Bettstetter, M.; Rihani, L.; Wu, W.; Meyer, B.; Schlegel, J.; et al. Correlation of the quantitative level of MGMT promoter methylation and overall survival in primary diagnosed glioblastomas using the quantitative MethyQESD method. J. Clin. Pathol. 2019, 73, 112–115. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Guo, B.; Zhang, S.; Wu, R.; Zhang, Z.; et al. LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J. Exp. Med. 2019, 217, e20190950. [Google Scholar] [CrossRef]
- Heskett, M.B.; Smith, L.G.; Spellman, P.; Thayer, M.J. Reciprocal monoallelic expression of ASAR lncRNA genes controls replication timing of human chromosome 6. RNA 2020, 26, 724–738. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Li, L.; He, Y.; Zheng, D.; Yu, P.; Yu, L.; Tang, L.; Wang, Y.; Wang, Z. EZH2 RIP-seq Identifies Tissue-specific Long Non-coding RNAs. Curr. Gene Ther. 2018, 18, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Kadakkuzha, B.M.; Liu, X.-A.; Narváez, M.; Kaye, A.; Akhmedov, K.; Puthanveettil, S.V. Asymmetric localization of natural antisense RNA of neuropeptide sensorin in Aplysia sensory neurons during aging and activity. Front. Genet. 2014, 5, 84. [Google Scholar] [CrossRef][Green Version]
- Lin, X.; Spindler, T.J.; Fonseca, M.A.D.S.; Corona, R.I.; Seo, J.-H.; Dezem, F.S.; Li, L.; Lee, J.M.; Long, H.W.; Sellers, T.A.; et al. Super-Enhancer-Associated LncRNA UCA1 Interacts Directly with AMOT to Activate YAP Target Genes in Epithelial Ovarian Cancer. iScience 2019, 17, 242–255. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, B.; Yuan, X.; Qiu, Y.; Peng, J.; Huang, Y.; Zhang, C.; Zhang, Y.; Lin, Z.; Li, J.; et al. Super-Enhancer–Associated Long Noncoding RNA HCCL5 Is Activated by ZEB1 and Promotes the Malignancy of Hepatocellular Carcinoma. Cancer Res. 2018, 79, 572–584. [Google Scholar] [CrossRef]
- Gendrel, A.-V.; Heard, E. Fifty years of X-inactivation research. Development 2011, 138, 5049–5055. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.-S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xu, C.; Le, W.; Ge, B.; Wang, T. lncRNA CASC11 promotes cancer cell proliferation in bladder cancer through miRNA-150. J. Cell. Biochem. 2019, 120, 13487–13493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, W.; Lin, J.; Xiao, J.; Tian, Y. lncRNA CCAT1 promotes bladder cancer cell proliferation, migration and invasion. Int. Braz. J. Urol. 2019, 45, 549–559. [Google Scholar] [CrossRef]
- Zhuang, C.; Ma, Q.; Zhuang, C.; Ye, J.; Zhang, F.; Gui, Y. LncRNA GClnc1 promotes proliferation and invasion of bladder cancer through activation of MYC. FASEB J. 2019, 33, 11045–11059. [Google Scholar] [CrossRef]
- Rui, X.; Xu, Y.; Huang, Y.; Ji, L.; Jiang, X. lncRNA DLG1-AS1 Promotes Cell Proliferation by Competitively Binding with miR-107 and Up-Regulating ZHX1 Expression in Cervical Cancer. Cell. Physiol. Biochem. 2018, 49, 1792–1803. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.-B.; Wang, Z.-W.; Wang, X.-H. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1020–1026. [Google Scholar] [PubMed]
- Li, K.; Ma, Y.-B.; Tian, Y.-H.; Xu, X.-L.; Gao, Y.; He, Y.-Q.; Pan, W.-T.; Zhang, J.-W.; He, C.-J.; Wei, L. Silencing lncRNA SNHG6 suppresses proliferation and invasion of breast cancer cells through miR-26a/VASP axis. Pathol. Res. Pr. 2019, 215, 152575. [Google Scholar] [CrossRef]
- Cui, Y.; Fan, Y.; Zhao, G.; Zhang, Q.; Bao, Y.; Cui, Y.; Ye, Z.; Chen, G.; Piao, X.; Guo, F.; et al. Novel lncRNA PSMG3-AS1 functions as a miR-143-3p sponge to increase the proliferation and migration of breast cancer cells. Oncol. Rep. 2020, 43, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, B.; Zhang, T.; Zhang, Y.; Wang, F. LncRNA GATA6-AS Promotes Cancer Cell Proliferation and Inhibits Apoptosis in Glioma by Downregulating lncRNA TUG1. Cancer Biotherapy Radiopharm. 2019, 34, 660–665. [Google Scholar] [CrossRef]
- Xu, M.; Xu, X.; Pan, B.; Chen, X.; Lin, K.; Zeng, K.; Liu, X.; Xu, T.; Sun, L.; Qin, J.; et al. LncRNA SATB2-AS1 inhibits tumor metastasis and affects the tumor immune cell microenvironment in colorectal cancer by regulating SATB2. Mol. Cancer 2019, 18, 1–16. [Google Scholar] [CrossRef]
- Hu, Q.; Ye, Y.; Chan, L.-C.; Li, Y.; Liang, K.; Lin, A.; Egranov, S.D.; Zhang, Y.; Xia, W.; Gong, J.; et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 2019, 20, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, J.; Yang, L.; Ouyang, Q.; Li, J.; Lao, L.; Zhao, J.; Liu, J.; Lu, Y.; Xing, Y.; et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 2018, 19, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Wang, X.; Li, H. LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int. J. Biol. Macromol. 2018, 118, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.-P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Wu, X.; Hou, P.; Qiu, Y.; Wang, Q.; Lu, X. Large-Scale Analysis Reveals the Specific Clinical and Immune Features of DGCR5 in Glioma. OncoTargets Ther. 2020, 13, 7531–7543. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Wang, Y. LncRNA XLOC_006390 facilitates cervical cancer tumorigenesis and metastasis as a ceRNA against miR-331-3p and miR-338-3p. J. Gynecol. Oncol. 2018, 29, e95. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-J.; Chen, H.-Y.; Ye, Z.; Zhu, S.; Zeng, Z.; He, C.; Liu, M.-L.; Huang, K.; Zhong, J.-X.; Xu, F.-Y.; et al. Hypoxia-induced LncRNA-BX111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription. Oncogene 2018, 37, 5811–5828. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, T.; Han, Y.; Jiang, H.; Wang, C.; You, T.; Zhao, X.; Shan, H.; Yang, R.; Yang, L.; et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, L.; Wang, Y.; Jiang, X.; Xia, H.; Zhuang, Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J. Neuro Oncol. 2014, 121, 101–108. [Google Scholar] [CrossRef]
- Gutschner, T.; Hämmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells. Cancer Res. 2012, 73, 1180–1189. [Google Scholar] [CrossRef]
- Kim, J.; Piao, H.-L.; Kim, B.-J.; Yao, F.; Han, Z.; Wang, Y.; Xiao, Z.; Siverly, A.N.; Lawhon, S.E.; Ton, B.N.; et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat. Genet. 2018, 50, 1705–1715. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Lin, Y.; Wei, Y.; Liang, S.; Dong, C. LINC01133 inhibits breast cancer invasion and metastasis by negatively regulating SOX4 expression through EZH2. J. Cell. Mol. Med. 2019, 23, 7554–7565. [Google Scholar] [CrossRef]
- Xiu, B.; Chi, Y.; Liu, L.; Chi, W.; Zhang, Q.; Chen, J.; Guo, R.; Si, J.; Li, L.; Xue, J.; et al. LINC02273 drives breast cancer metastasis by epigenetically increasing AGR2 transcription. Mol. Cancer 2019, 18, 1–20. [Google Scholar] [CrossRef]
- Sieverling, L.; PCAWG-Structural Variation Working Group; Hong, C.; Koser, S.D.; Ginsbach, P.; Kleinheinz, K.; Hutter, B.; Braun, D.M.; Cortés-Ciriano, I.; Xi, R.; et al. Genomic footprints of activated telomere maintenance mechanisms in cancer. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Fernandes, S.G.; Dsouza, R.; Pandya, G.; Kirtonia, A.; Tergaonkar, V.; Lee, S.Y.; Garg, M.; Khattar, E. Role of Telomeres and Telomeric Proteins in Human Malignancies and Their Therapeutic Potential. Cancers 2020, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Napier, C.E.; Vryer, R.; Dimitriadis, E.; Manuelpillai, U.; Sharkey, A.; Craig, J.M.; Reddel, R.R.; Saffery, R. DNA methylation mediated up-regulation ofTERRAnon-coding RNA is coincident with elongated telomeres in the human placenta. Mol. Hum. Reprod. 2016, 22, 791–799. [Google Scholar] [CrossRef]
- Feretzaki, M.; Nunes, P.R.; Lingner, J. Expression and differential regulation of human TERRA at several chromosome ends. RNA 2019, 25, 1470–1480. [Google Scholar] [CrossRef]
- Xu, C.L.; Sang, B.; Liu, G.Z.; Li, J.M.; Zhang, X.D.; Liu, L.X.; Thorne, R.F.; Wu, M. SENEBLOC, a long non-coding RNA suppresses senescence via p53-dependent and independent mechanisms. Nucleic Acids Res. 2020, 48, 3089–3102. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Kang, D.; Han, N.; Lee, Y.; Hwang, H.J.; Lee, S.-B.; You, J.S.; Min, B.S.; Park, H.J.; Ko, Y.-G.; et al. A novel long noncoding RNA Linc-ASEN represses cellular senescence through multileveled reduction of p21 expression. Cell Death Differ. 2019, 27, 1844–1861. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Cho, W.H.; Kang, D.W.; Cha, S.H. Extraneural Metastasis of Glioblastoma Multiforme Presenting as an Unusual Neck Mass. J. Korean Neurosurg. Soc. 2012, 51, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, X.; Chen, Y.; Wang, Y.; Li, Z. LncRNA RHPN1-AS1 promoted cell proliferation, invasion and migration in cervical cancer via the modulation of miR-299–3p/FGF2 axis. Life Sci. 2019, 239, 116856. [Google Scholar] [CrossRef]
- Tong, R.; Zhang, J.; Wang, C.; Li, X.; Yu, T.; Wang, L. LncRNA PTCSC3 inhibits the proliferation, invasion and migration of cervical cancer cells via sponging miR-574-5p. Clin. Exp. Pharmacol. Physiol. 2019, 47, 439–448. [Google Scholar] [CrossRef]
- Wang, W.; Ge, L.; Xu, X.-J.; Yang, T.; Yuan, Y.; Ma, X.-L.; Zhang, X.-H. LncRNA NEAT1 promotes endometrial cancer cell proliferation, migration and invasion by regulating the miR-144-3p/EZH2 axis. Radiol. Oncol. 2019, 53, 434–442. [Google Scholar] [CrossRef]
- Zhuang, S.T.; Cai, Y.; Liu, H.; Qin, Y.; Wen, J.-F. LncRNA NEAT1/miR-185-5p/IGF2 axis regulates the invasion and migration of colon cancer. Mol. Genet. Genom. Med. 2020, 8, e1125. [Google Scholar] [CrossRef]
- Xiong, W.; Qin, J.; Cai, X.; Liu, Q.; Li, C.; Ju, Y.; Wang, Q.; Li, Y.; Yang, Y. Overexpression LINC01082 suppresses the proliferation, migration and invasion of colon cancer. Mol. Cell. Biochem. 2019, 462, 33–40. [Google Scholar] [CrossRef]
- Yan, D.; Liu, W.; Liu, Y.; Luo, M. LINC00261 suppresses human colon cancer progression via sponging miR-324-3p and inactivating the Wnt/β-catenin pathway. J. Cell. Physiol. 2019, 234, 22648–22656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Wang, F.; Zhang, S.; Ding, J. Silencing of lncRNA EZR-AS1 inhibits proliferation, invasion, and migration of colorectal cancer cells through blocking transforming growth factor β signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Q.; Guo, Z.-Y.; Xie, J. The lncRNA EGFR-AS1 is linked to migration, invasion and apoptosis in glioma cells by targeting miR-133b/RACK1. Biomed. Pharmacother. 2019, 118, 109292. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, F.; Harris, A.L.; Tavassoli, M.; Gatter, K.C. Blood vessels and cancer much more than just angiogenesis. Cell Death Discov. 2015, 1, 15064. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhao, K.; Lin, Q.; Li, H.; Xue, X.; Ge, W.; He, H.; Liu, D.; Xie, H.; et al. A novel LncRNA HITT forms a regulatory loop with HIF-1α to modulate angiogenesis and tumor growth. Cell Death Differ. 2019, 27, 1431–1446. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, L.; Xue, X.; Xie, H.; Shi, H.; Hu, Y. A lncRNA coordinates with Ezh2 to inhibit HIF-1α transcription and suppress cancer cell adaption to hypoxia. Oncogene 2019, 39, 1860–1874. [Google Scholar] [CrossRef]
- Niu, Y.; Bao, L.; Chen, Y.; Wang, C.; Luo, M.; Zhang, B.; Zhou, M.; Wang, J.E.; Fang, Y.V.; Kumar, A.; et al. HIF2-Induced Long Noncoding RNA RAB11B-AS1 Promotes Hypoxia-Mediated Angiogenesis and Breast Cancer Metastasis. Cancer Res. 2020, 80, 964–975. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Wang, Z.; Kuang, X.; Shao, N.; Lin, Y. lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through activating IGF-1/IGF-1R/ERK pathway. J. Cell Mol. Med. 2020, 24, 8236–8247. [Google Scholar] [CrossRef]
- Luo, L.-H.; Rao, L.; Luo, L.-F.; Chen, K.; Ran, R.-Z.; Liu, X.-L. Long non-coding RNA NKILA inhibited angiogenesis of breast cancer through NF-κB/IL-6 signaling pathway. Microvasc. Res. 2020, 129, 103968. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhao, D. Long intergenic noncoding RNA LINC00284 knockdown reduces angiogenesis in ovarian cancer cells via up-regulation of MEST through NF-κB1. FASEB J. 2019, 33, 12047–12059. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; Qi, X.; Li, Q.; Wang, D.; Yi, T.; Yin, R.; Zhao, X.; Zhong, X.; Bian, C. LncRNA DANCR promotes tumor growth and angiogenesis in ovarian cancer through direct targeting of miR-145. Mol. Carcinog. 2019, 58, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Tian, Y.F.; Wu, H.; Ouyang, S.Y.; Kuang, W.L. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1α/VEGF axis. Neoplasma 2020, 67, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tracy, K.; Tye, C.E.; Ghule, P.N.; Malaby, H.L.H.; Stumpff, J.; Stein, J.L.; Stein, J.L.; Lian, J.B. Mitotically-Associated lncRNA (MANCR) Affects Genomic Stability and Cell Division in Aggressive Breast Cancer. Mol. Cancer Res. 2018, 16, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Munschauer, M.; Nguyen, C.T.; Sirokman, K.; Hartigan, C.R.; Hogstrom, L.; Engreitz, J.M.; Ulirsch, J.C.; Fulco, C.P.; Subramanian, V.; Chen, J.; et al. The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nat. Cell Biol. 2018, 561, 132–136. [Google Scholar] [CrossRef]
- Lee, S.; Kopp, F.; Chang, T.-C.; Sataluri, A.; Chen, B.; Sivakumar, S.; Yu, H.; Xie, Y.; Mendell, J.T. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 2016, 164, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dragomir, M.P.; Fabris, L.; Bayraktar, R.; Knutsen, E.; Liu, X.; Tang, C.; Li, Y.; Shimura, T.; Ivkovic, T.C.; et al. The Long Noncoding RNA CCAT2 induces chromosomal instability through BOP1—AURKB signaling. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Zhao, W.; Geng, D.; Li, S.; Chen, Z.; Sun, M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018, 7, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Geng, D.; Li, S.; Chen, Z.; Zhao, W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol. Chem. 2018, 399, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, X.; Liu, Y. LncRNA-ATB promotes apoptosis of non-small cell lung cancer cells through MiR-200a/β-Catenin. J. Buon 2020, 24, 2280–2286. [Google Scholar]
- Wu, M.; Huang, Y.; Chen, T.; Wang, W.; Yang, S.; Ye, Z.; Xi, X. LncRNA MEG3 inhibits the progression of prostate cancer by modulating miR-9-5p/QKI-5 axis. J. Cell. Mol. Med. 2018, 23, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Anaya, F.O.; Romero-Córdoba, S.; Rebollar-Vega, R.; Arrieta, O.; Bautista-Piña, V.; Dominguez-Reyes, C.; Villegas-Carlos, F.; Tenorio-Torres, A.; Alfaro-Riuz, L.; Jiménez-Morales, S.; et al. Expression of long non-coding RNA ENSG 00000226738 (Lnc KLHDC 7B) is enriched in the immunomodulatory triple-negative breast cancer subtype and its alteration promotes cell migration, invasion, and resistance to cell death. Mol. Oncol. 2019, 13, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Li, B.; Liu, Q.; Cui, Y. lncRNA CCAT1 Promotes Glioma Tumorigenesis by Sponging miR-181b. J. Cell. Biochem. 2017, 118, 4548–4557. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. THE METABOLISM OF TUMORS IN THE BODY. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Chen, Y.; Cao, D. Long non-coding RNA LINC00504 regulates the Warburg effect in ovarian cancer through inhibition of miR-1244. Mol. Cell. Biochem. 2019, 464, 39–50. [Google Scholar] [CrossRef]
- Malakar, P.; Stein, I.; Saragovi, A.; Winkler, R.; Stern-Ginossar, N.; Berger, M.; Pikarsky, E.; Karni, R. Long Noncoding RNA MALAT1 Regulates Cancer Glucose Metabolism by Enhancing mTOR-Mediated Translation of TCF7L2. Cancer Res. 2019, 79, 2480–2493. [Google Scholar] [CrossRef]
- Liao, M.; Liao, W.; Xu, N.; Li, B.; Liu, F.; Zhang, S.; Wang, Y.; Wang, S.; Zhu, Y.; Chen, D.; et al. LncRNA EPB41L4A-AS1 regulates glycolysis and glutaminolysis by mediating nucleolar translocation of HDAC2. EBioMedicine 2019, 41, 200–213. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Zheng, J.; Song, J.; Liu, Y.; Ruan, X.; Shen, S.; Shao, L.; Yang, C.; Wang, D.; et al. Lin28A promotes IRF6-regulated aerobic glycolysis in glioma cells by stabilizing SNHG14. Cell Death Dis. 2020, 11, 447. [Google Scholar] [CrossRef]
- Du, P.; Liao, Y.; Zhao, H.; Zhang, J.; Muyiti; Keremu; Mu, K. ANXA2P2/miR-9/LDHA axis regulates Warburg effect and affects glioblastoma proliferation and apoptosis. Cell Signal 2020, 74, 109718. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, J.; Geng, Q.; Wang, G. Lnc RNA MALAT 1 increases the stemness of gastric cancer cells via enhancing SOX 2 mRNA stability. FEBS Open Biol. 2019, 9, 1212–1222. [Google Scholar] [CrossRef]
- Jen, J.; Tang, Y.-A.; Lu, Y.-H.; Lin, C.-C.; Lai, W.-W.; Wang, Y.-C. Oct4 transcriptionally regulates the expression of long non-coding RNAs NEAT1 and MALAT1 to promote lung cancer progression. Mol. Cancer 2017, 16, 1–12. [Google Scholar] [CrossRef]
- Shin, V.Y.; Chen, J.; Cheuk, I.W.-Y.; Siu, M.-T.; Ho, C.-W.; Wang, X.; Jin, H.; Kwong, A. Long non-coding RNA NEAT1 confers oncogenic role in triple-negative breast cancer through modulating chemoresistance and cancer stemness. Cell Death Dis. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Chen, A.; Wu, X.; Zhou, M.; Haq, I.U.; Mariyam, Z.; Feng, Q. NEAT1 acts as an inducer of cancer stem cell-like phenotypes in NSCLC by inhibiting EGCG-upregulated CTR1. J. Cell. Physiol. 2018, 233, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zheng, M.; Cao, X.; Zhang, Y.; Zhang, Y.; Gao, X.; Cao, Y.; Shi, M.; Han, H.; Liang, L. Adenovirus infection promotes the formation of glioma stem cells from glioblastoma cells through the TLR9/NEAT1/STAT3 pathway. Cell Commun. Signal. 2020, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.-X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 2006, 103, 12405–12410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, Z.; Guo, X.; Dong, H.; Zhou, K.; Huang, Z.; Xiao, Z. lncRNA B4GALT1-AS1 promotes colon cancer cell stemness and migration by recruiting YAP to the nucleus and enhancing YAP transcriptional activity. J. Cell. Physiol. 2019, 234, 18524–18534. [Google Scholar] [CrossRef]
- Gao, X.F.; He, H.Q.; Zhu, X.B.; Xie, S.L.; Cao, Y. LncRNA SNHG20 promotes tumorigenesis and cancer stemness in glioblastoma via activating PI3K/Akt/mTOR signaling pathway. Neoplasma 2019, 66, 532–542. [Google Scholar] [CrossRef]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Cantafio, M.E.G.; et al. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia 2019, 34, 234–244. [Google Scholar] [CrossRef]
- Taiana, E.; Ronchetti, D.; Todoerti, K.; Nobili, L.; Tassone, P.; Amodio, N.; Neri, A. LncRNA NEAT1 in Paraspeckles: A Structural Scaffold for Cellular DNA Damage Response Systems? Non-Coding RNA 2020, 6, 26. [Google Scholar] [CrossRef]
- De Silva, H.C.; Lin, M.Z.; Phillips, L.; Martin, J.L.; Baxter, R.C. IGFBP-3 interacts with NONO and SFPQ in PARP-dependent DNA damage repair in triple-negative breast cancer. Cell. Mol. Life Sci. 2019, 76, 2015–2030. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, J.; Fang, H.; Fang, J.; Li, C.; Chen, W.; Liu, L.; Ondrejka, S.; Gong, Z.; Reu, F.; et al. Targeting the MALAT1/PARP1/LIG3 complex induces DNA damage and apoptosis in multiple myeloma. Leukemia 2018, 32, 2250–2262. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, Y.; Xiao, J. Long noncoding RNA MALAT1 knockdown reverses chemoresistance to temozolomide via promoting microRNA-101 in glioblastoma. Cancer Med. 2018, 7, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.-G.; Wang, H.; Song, Y.; Ni, Y.; Ni, Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Passi, A.; Vigetti, D.; Buraschi, S.; Iozzo, R.V. Dissecting the role of hyaluronan synthases in the tumor microenvironment. FEBS J. 2019, 286, 2937–2949. [Google Scholar] [CrossRef]
- Tong, L.; Wang, Y.; Ao, Y.; Sun, X. CREB1 induced lncRNA HAS2-AS1 promotes epithelial ovarian cancer proliferation and invasion via the miR-466/RUNX2 axis. Biomed. Pharmacother. 2019, 115, 108891. [Google Scholar] [CrossRef]
- Bian, E.; Chen, E.; Xu, Y.; Yang, Z.; Tang, F.; Ma, C.; Wang, H.; Zhao, B. Exosomal lncRNA-ATB activates astrocytes that promote glioma cell invasion. Int. J. Oncol. 2018, 54, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bayona, S.; Aldaz, P.; Auzmendi-Iriarte, J.; Saenz-Antoñanzas, A.; Garcia, I.; Arrazola, M.; Gerovska, D.; Undabeitia, J.; Querejeta, A.; Egaña, L.; et al. PR-LncRNA signature regulates glioma cell activity through expression of SOX factors. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liao, X.; Huang, M. LncRNA CASC7 inhibits the progression of glioma via regulating Wnt/β-catenin signaling pathway. Pathol. Res. Pr. 2019, 215, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, C.; Yang, J.; Sun, Y.; Zhang, S.; Yang, J.; Yang, Y.; Wang, Y.; Jiao, B. Long noncoding RNA CASC9/miR-519d/STAT3 positive feedback loop facilitate the glioma tumourigenesis. J. Cell Mol. Med. 2018, 22, 6338–6344. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lu, S.; Xu, Y.; Zheng, J. Long non-coding RNA AGAP2-AS1 promotes the proliferation of glioma cells by sponging miR-15a/b-5p to upregulate the expression of HDGF and activating Wnt/β-catenin signaling pathway. Int. J. Biol. Macromol. 2019, 128, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cai, J.; Wang, Q.; Wang, Y.; Liu, M.; Yang, J.; Zhou, J.; Kang, C.-S.; Li, M.; Jiang, C. Long Noncoding RNANEAT1, Regulated by the EGFR Pathway, Contributes to Glioblastoma Progression Through the WNT/β-Catenin Pathway by Scaffolding EZH2. Clin. Cancer Res. 2017, 24, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Wang, H.; Zhao, C.-D.; Li, R. Long noncoding RNA LINC01426 promotes glioma progression through PI3K/AKT signaling pathway and serves as a prognostic biomarker. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6358–6368. [Google Scholar] [PubMed]
- Jin, Z.; Piao, L.; Sun, G.; Lv, C.; Jing, Y.; Jin, R. Long Non-Coding RNA PART1 Exerts Tumor Suppressive Functions in Glioma via Sponging miR-190a-3p and Inactivation of PTEN/AKT Pathway. OncoTargets Ther. 2020, 13, 1073–1086. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, F.; Zhang, X.; Tang, Y.; Wang, S. LncRNA LINC01446 promotes glioblastoma progression by modulating miR-489-3p/TPT1 axis. Biochem. Biophys. Res. Commun. 2018, 503, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Wang, J.; Yang, X.; Wen, L.; Feng, J. lncRNA MNX1-AS1 Promotes Glioblastoma Progression Through Inhibition of miR-4443. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Yao, Y.; Hu, X.; Zhu, Y. LncRNA DCST1-AS1 downregulates miR-29b through methylation in glioblastoma (GBM) to promote cancer cell proliferation. Clin. Transl. Oncol. 2020, 22, 2230–2235. [Google Scholar] [CrossRef]
- Ren, S.; Xu, Y. AC016405.3, a novel long noncoding RNA, acts as a tumor suppressor through modulation of TET2 by microRNA-19a-5p sponging in glioblastoma. Cancer Sci. 2019, 110, 1621–1632. [Google Scholar] [CrossRef]
- Shi, T.; Guo, D.; Xu, H.; Su, G.; Chen, J.; Zhao, Z.; Shi, J.; Wedemeyer, M.; Attenello, F.; Zhang, L.; et al. HOTAIRM1, an enhancer lncRNA, promotes glioma proliferation by regulating long-range chromatin interactions within HOXA cluster genes. Mol. Biol. Rep. 2020, 47, 2723–2733. [Google Scholar] [CrossRef]
- Li, Q.; Dong, C.; Cui, J.; Wang, Y.; Hong, X. Over-expressed lncRNA HOTAIRM1 promotes tumor growth and invasion through up-regulating HOXA1 and sequestering G9a/EZH2/Dnmts away from the HOXA1 gene in glioblastoma multiforme. J. Exp. Clin. Cancer Res. 2018, 37, 265. [Google Scholar] [CrossRef]
- Xiong, Y.; Kuang, W.; Lu, S.; Guo, H.; Wu, M.; Ye, M.; Wu, L. Long noncoding RNA HOXB 13- AS 1 regulates HOXB 13 gene methylation by interacting with EZH 2 in glioma. Cancer Med. 2018, 7, 4718–4728. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, X.; Wu, Z.; Hu, D.; Jia, J.; Guo, J.; Tang, T.; Yao, J.; Liu, H.; Tang, H. Long Noncoding RNA LINC00467 Promotes Glioma Progression through Inhibiting P53 Expression via Binding to DNMT1. J. Cancer 2020, 11, 2935–2944. [Google Scholar] [CrossRef]
- Hu, W.; Wang, T.; Yang, Y.; Zheng, S. Tumor heterogeneity uncovered by dynamic expression of long noncoding RNA at single-cell resolution. Cancer Genet. 2015, 208, 581–586. [Google Scholar] [CrossRef]
- Lv, D.; Wang, X.; Dong, J.; Zhuang, Y.; Huang, S.; Ma, B.; Chen, P.; Li, X.; Zhang, B.; Li, Z.; et al. Systematic characterization of lncRNAs’ cell-to-cell expression heterogeneity in glioblastoma cells. Oncotarget 2016, 7, 18403–18414. [Google Scholar] [CrossRef] [PubMed]
- Mineo, M.; Ricklefs, F.; Rooj, A.K.; Lyons, S.M.; Ivanov, P.; Ansari, K.I.; Nakano, I.; Chiocca, E.A.; Godlewski, J.; Bronisz, A. The Long Non-coding RNA HIF1A-AS2 Facilitates the Maintenance of Mesenchymal Glioblastoma Stem-like Cells in Hypoxic Niches. Cell Rep. 2016, 15, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, F.; Liao, Y.; Yuan, L.; Zhang, B. LncRNA AWPPH promotes the invasion and migration of glioma cells through the upregulation of HIF1α. Oncol. Lett. 2019, 18, 6781–6786. [Google Scholar] [CrossRef]
- Li, C.; Hu, G.; Wei, B.; Wang, L.; Liu, N. lncRNA LINC01494 Promotes Proliferation, Migration And Invasion In Glioma Through miR-122-5p/CCNG1 Axis. OncoTargets Ther. 2019, 12, 7655–7662. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, C.; Yao, H.; Zhang, X.; Zhou, Y.; Che, Y.; Huang, Y. Knockdown of long non-coding RNA NEAT1 inhibits glioma cell migration and invasion via modulation of SOX2 targeted by miR-132. Mol. Cancer 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Wang, H.; Chen, E.; Bian, E.; Xu, Y.; Ji, X.; Yang, Z.; Hua, X.; Zhang, Y.; Zhao, B. LncRNA-ATB promotes TGF-β-induced glioma cells invasion through NF-κB and P38/MAPK pathway. J. Cell Physiol. 2019, 234, 23302–23314. [Google Scholar] [CrossRef]
- Li, G.; Cai, Y.; Wang, C.; Huang, M.; Chen, J. LncRNA GAS5 regulates the proliferation, migration, invasion and apoptosis of brain glioma cells through targeting GSTM3 expression. The effect of LncRNA GAS5 on glioma cells. J. Neuro Oncol. 2019, 143, 525–536. [Google Scholar] [CrossRef]
- Wu, P.; Cai, J.; Chen, Q.; Han, B.; Meng, X.; Li, Y.; Li, Z.; Wang, R.; Lin, L.; Duan, C.; et al. Lnc-TALC promotes O6-methylguanine-DNA methyltransferase expression via regulating the c-Met pathway by competitively binding with miR-20b-3p. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Voce, D.J.; Bernal, G.M.; Wu, L.; Crawley, C.D.; Zhang, W.; Mansour, N.M.; Cahill, K.E.; Szymura, S.J.; Uppal, A.; Raleigh, D.R.; et al. Temozolomide Treatment Induces lncRNA MALAT1 in an NF-κB and p53 Codependent Manner in Glioblastoma. Cancer Res. 2019, 79, 2536–2548. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, Z.; Chen, X.; Wang, X.; Zeng, S.; Zhao, Z.; Qian, L.; Li, Z.; Wei, J.; Huo, L.; et al. Novel Function of lncRNA ADAMTS9-AS2 in Promoting Temozolomide Resistance in Glioblastoma via Upregulating the FUS/MDM2 Ubiquitination Axis. Front. Cell Dev. Biol. 2019, 7, 217. [Google Scholar] [CrossRef]
- Mazor, G.; Levin, L.; Picard, D.; Ahmadov, U.; Carén, H.; Borkhardt, A.; Reifenberger, G.; Leprivier, G.; Remke, M.; Rotblat, B. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Chen, M.; Cheng, Y.; Yuan, Z.; Wang, F.; Yang, L.; Zhao, H. NCK1-AS1 Increases Drug Resistance of Glioma Cells to Temozolomide by Modulating miR-137/TRIM24. Cancer Biother. Radiopharm. 2020, 35, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Z.; Xu, Y.; Chen, X.; Chen, T.; Ye, Y.; Ding, J.; Chen, Z.; Chen, L.; Qiu, X.; et al. A three-lncRNA signature predicts clinical outcomes in low-grade glioma patients after radiotherapy. Aging 2020, 12, 9188–9204. [Google Scholar] [CrossRef]
- Li, J.; Ji, X.; Wang, H. Targeting Long Noncoding RNA HMMR-AS1 Suppresses and Radiosensitizes Glioblastoma. Neoplasia 2018, 20, 456–466. [Google Scholar] [CrossRef]
- Brodie, S.; Lee, H.K.; Jiang, W.; Cazacu, S.; Xiang, C.; Poisson, L.M.; Datta, I.; Kalkanis, S.; Ginsberg, D.; Brodie, C. The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget 2017, 8, 31785–31801. [Google Scholar] [CrossRef]
- Dai, X.; Liao, K.; Zhuang, Z.; Chen, B.; Zhou, Z.; Zhou, S.; Lin, G.; Zhang, F.; Lin, Y.; Miao, Y.-F.; et al. AHIF promotes glioblastoma progression and radioresistance via exosomes. Int. J. Oncol. 2018, 54, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Fu, C.; Wang, C.; Duan, X.; Zou, W.; Zhao, T. Knockdown of long non-coding RNA PCAT1 in glioma stem cells promotes radiation sensitivity. Med. Mol. Morphol. 2018, 52, 114–122. [Google Scholar] [CrossRef]
- Jiang, N.; Pan, J.; Fang, S.; Zhou, C.; Han, Y.; Chen, J.; Meng, X.; Jin, X.; Gong, Z. Liquid biopsy: Circulating exosomal long noncoding RNAs in cancer. Clin. Chim. Acta 2019, 495, 331–337. [Google Scholar] [CrossRef]
- Wang, W.; Xie, Y.; Chen, F.; Liu, X.; Zhong, L.-L.; Wang, H.-Q.; Li, Q.-C. LncRNA MEG3 acts a biomarker and regulates cell functions by targeting ADAR1 in colorectal cancer. World J. Gastroenterol. 2019, 25, 3972–3984. [Google Scholar]
- Zeng, Y.-L.; Guo, Z.-Y.; Su, H.-Z.; Zhong, F.-D.; Jiang, K.-Q.; Yuan, G.-D. Diagnostic and prognostic value of lncRNA cancer susceptibility candidate 9 in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 6902–6915. [Google Scholar] [CrossRef] [PubMed]
- Guglas, K.; Kolenda, T.; Teresiak, A.; Kopczyńska, M.; Łasińska, I.; Mackiewicz, J.; Mackiewicz, A.; Lamperska, K. lncRNA Expression after Irradiation and Chemoexposure of HNSCC Cell Lines. Non-Coding RNA 2018, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Aryankalayil, M.J.; Chopra, S.; Levin, J.; Eke, I.; Makinde, A.; Das, S.; Shankavaram, U.; Vanpouille-Box, C.; DeMaria, S.; Coleman, C.N. Radiation-Induced Long Noncoding RNAs in a Mouse Model after Whole-Body Irradiation. Radiat. Res. 2018, 189, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Shi, L.; Yu, X.; Gu, Y.; Sun, X. LINP1 facilitates DNA damage repair through non-homologous end joining (NHEJ) pathway and subsequently decreases the sensitivity of cervical cancer cells to ionizing radiation. Cell Cycle 2018, 17, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Xu, X.-K.; Li, J.-L.; Kong, K.-K.; Li, H.; Chen, C.; He, J.; Wang, F.; Li, P.; Ge, X.-S.; et al. MALAT1 is a prognostic factor in glioblastoma multiforme and induces chemoresistance to temozolomide through suppressing miR-203 and promoting thymidylate synthase expression. Oncotarget 2017, 8, 22783–22799. [Google Scholar] [CrossRef]
- Li, X.; Meng, Y. Survival analysis of immune-related lncRNA in low-grade glioma. BMC Cancer 2019, 19, 813. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, Z.; Zhao, H.; Bao, S.; Cheng, L.; Sun, J. An Immune-Related Six-lncRNA Signature to Improve Prognosis Prediction of Glioblastoma Multiforme. Mol. Neurobiol. 2017, 55, 3684–3697. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Lv, W.; Li, Y.; Zhang, L.; Dong, C.; Zhang, J.; Cheng, G. LncRNA RPSAP52 Upregulates TGF-β1 to Increase Cancer Cell Stemness and Predict Postoperative Survival in Glioblastoma. Cancer Manag. Res. 2020, 12, 2541–2547. [Google Scholar] [CrossRef]
- Pan, X.; Zheng, G.; Gao, C. LncRNA PVT1: A Novel Therapeutic Target for Cancers. Clin. Lab. 2018, 64, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yang, L.; Zhang, X.; Zhou, Y.; Chen, R.; Yu, Y.; Dong, Z.; Zhang, M. LncRNA MATN1-AS1 prevents glioblastoma cell from proliferation and invasion via RELA regulation and MAPK signaling pathway. Ann. Transl. Med. 2019, 7, 784. [Google Scholar] [CrossRef]
- Kim, S.-S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2017, 46, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, Y.; Li, L.; Niu, Y.; Lin, W.; Lin, C.; Antonarakis, E.S.; Luo, J.; Yeh, S.; Chang, C. Preclinical Study using Malat1 Small Interfering RNA or Androgen Receptor Splicing Variant 7 Degradation Enhancer ASC-J9(®) to Suppress Enzalutamide-resistant Prostate Cancer Progression. Eur. Urol. 2017, 72, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-Y.; Wang, S.-H.; Yeh, C.-T.; Su, S.-C.; Ueng, S.-H.; Chuang, W.-Y.; Hsueh, C.; Wang, T.-H. Melatonin Sensitizes Hepatocellular Carcinoma Cells to Chemotherapy Through Long Non-Coding RNA RAD51-AS1-Mediated Suppression of DNA Repair. Cancers 2018, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, Y.; Wang, Y.; Tan, Y.; Wang, Q.; Cai, J.; Zhou, J.; Yang, C.; Zhao, K.; Yi, K.; et al. A Compound AC1Q3QWB Selectively Disrupts HOTAIR-Mediated Recruitment of PRC2 and Enhances Cancer Therapy of DZNep. Theranostics 2019, 9, 4608–4623. [Google Scholar] [CrossRef]
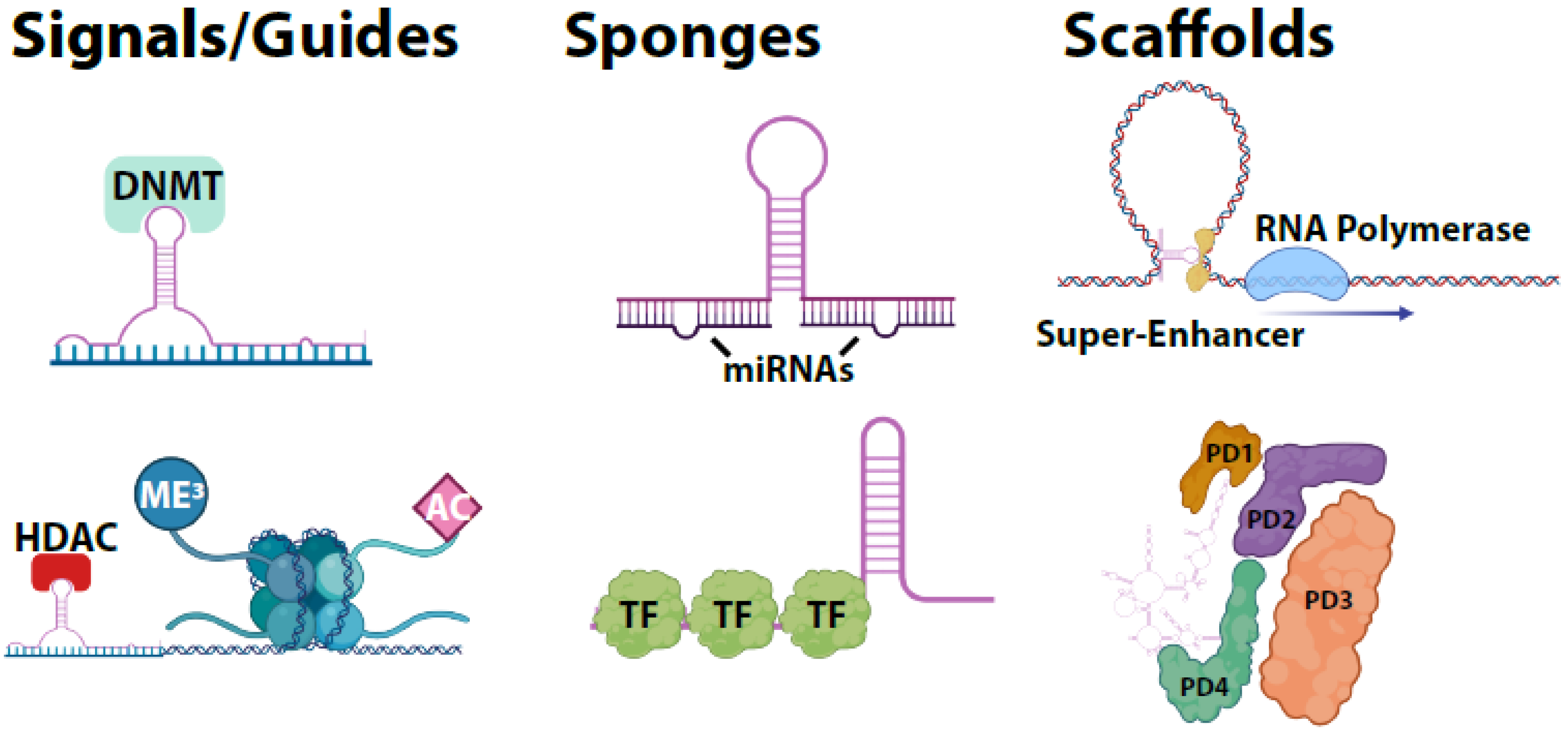
| lncRNA | Role | Level of Evidence | Therapeutic Strategy | Reference |
|---|---|---|---|---|
| CASC7 | Tumor suppression | GBM primary tissue and cell lines in vitro | Rescue, stabilize, or OE | [99] |
| CASC9 | Tumorigenesis | GBM cell lines in vitro and in vivo | Degrade, KD, or saturate with miRNA mimetic | [100] |
| AGAP2-AS1 | Proliferation and survival | GBM primary tissue, cell lines in vitro, and correlated with OS in TCGA patients | Degrade, KD, saturate with miRNA mimetic, or target downstream Wnt signaling | [101] |
| NEAT1 | Proliferation and invasion | GBM primary tissues, cell lines in vitro and in IC xenograft | Degrade, KD, saturate with miRNA-mimetic, or target SOX2, EGFR, or EZH2 | [102,118] |
| LINC01426 | Proliferation, invasion, and survival | TCGA clinical associations, GBM primary tissues, and cell lines in vitro | Degrade, KD, or target PI3K/Akt signaling | [103] |
| PART1 | Tumor suppression and growth inhibition | GBM tissues, cell lines in vitro, and TCGA clinical associations | Rescue, stabilize, OE, or target PI3K/Akt signaling | [104] |
| LINC01446 | Tumorigenesis and progression | Clinical associations, GBM cell lines in vitro and in xenograft | Degrade, KD, OE miR-489-3p, or target TPT1 | [105] |
| MNX1-AS1 | Proliferation, migration, and invasion | GBM tissues and cell lines in vitro | Degrade, KD, or OE miR-4443 | [106] |
| DCST1-AS1 | Proliferation | Clinical associations, GBM primary tissues and primary culture | Degrade, KD, OE miR-29b, saturate with miR-mimetic | [107] |
| AC016405.3 | Suppression of Proliferation and Invasion | Clinical associations, GBM primary tissues and cell lines in vitro | Rescue, stabilize, OE, sponging/KD miR-19a-5p, or OE of TET2 | [108] |
| HOTAIRM1 | Proliferation, invasion, and survival | TCGA clinical associations, GBM tissues, cell lines in vitro and in vivo | Degrade, KD, OE of G9a and EZH2, or KD of HOXA1 | [109,110] |
| HOXB13-AS1 | Proliferation/cell cycle progression | GBM tissues, cell lines in vitro and in vivo | Degrade, KD, KD of DNMT3B, OE of HOXB13 | [111] |
| LINC00467 | Proliferation and invasion | GBM cell lines (U87, LN229) in vitro | Degrade, KD, target DNMT1, rescue p53 activity/expression | [112] |
| HIFiA-AS2 | GSC maintenance | GBM cell lines in vitro and in vivo | Degrade, KD, target IGF2BP2, DHX9, or HMGA1 | [115] |
| H19 | Proliferation, invasion, and angiogenesis | GBM cell lines (HEB, U87, A172, U373) in vitro | Degrade, KD, OE miR-138, target HIF-1α and VEGF | [63] |
| LINC01494 | Proliferation and invasion | Clinical associations, GBM tissues, and cell lines in vitro | Degrade, KD, OE miR-122-5p, target CCNG1 | [117] |
| ATB | Invasion | GBM cell lines (LN-18, U251) in vitro | Degrade, KD, target TGF-β, NF-κB (pyrrolidinedi-thiocarbamate ammonium), and P38/MAPK (SB203580 | [119] |
| GAS5 | Inhibition of proliferation, invasion, survival | GBM cell lines (HEB, U251, U87) in vitro | Rescue, stabilize, OE, target GSTM3 | [120] |
| Lnc-TALC | Promotes TMZ resistance and tumor recurrence | TMZ-selected GBM cell lines (LN229, U251, 551W, HG7) in vitro | Degrade, KD, OE miR-20b-3p, target c-Met, AKT/FOXO3, and MGMT | [121] |
| MALAT1 | TMZ resistance and invasion | Clinical associations, GBM patient tissue and serum, GBM cell lines (U87) in vitro and in IC xenograft | Degrade, KD, ASC-J9®, target NF-κB, or restore p53 activity/expression | [122,138,144,145] |
| ADAMTs9-AS2 | TMZ resistance | Clinical associations, GBM cell lines (T98G-R, U118-R) in vitro | Degrade, KD, targeting FUS/MDM2 axis | [123] |
| TP73-AS1 | TMZ resistance and metabolism in GSCs | TCGA clinical associations, GSC lines (G26, G7) in vitro | Degrade, KD, targeting ALDH1A1 | [124] |
| NCK1-AS1 | TMZ resistance | GBM patient primary tissue, GBM cell lines (U251, A172) in vitro | Degrade, KD, OE of miR-137, targeting TRIM24 | [125] |
| HMMR-AS1 | Tumorigenesis, proliferation, invasion, radiation resistance | GBM cell lines (U87, U251, A172, U118) in vitro | Degrade, KD, target/disrupt HMMR interaction, target ATM, RAD51, BMI1 | [127] |
| TALNEC2 | Tumorigenesis and radiation resistance | TCGA clinical associations, GBM primary tissue, GBM cell lines (A172, U251, U87, T98G and LNZ308) in vitro | Degrade, KD, target E2F1 | [128] |
| AHIF | Invasion, survival, radiation resistance | GBM cell lines (U87, U251, A172, T98G) in vitro | Degrade, KD | [129] |
| PCAT1 | Stemness, survival, DNA repair | GBM cell lines in vitro | Degrade, KD, upregulate miR-129-5p, target HMGB1 | [130] |
| HOTAIR | Proliferation, invasion, therapy resistance, chromatin remodeling | Clinical associations, GBM patient tissue/serum, cell lines in vitro and in IC xenografts | Degrade, KD, AC1Q3QWB and DZNep combinational therapy, target EZH2 | [137,148] |
| RPSAP52 | Stemness and poor patient prognosis | GBM primary tissue, clinical associations, GBM cell lines (U-373 MG) in vitro | Degrade, KD, target TGF-β1 | [141] |
| MATN1-AS1 | Tumor suppressor, suppresses proliferation and invasion | GBM primary tissue and cell lines in vitro and in vivo | Rescue, stabilize, OE, target RELA, ERK1/2, Bcl-2, survivin, or MMP-9 | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stackhouse, C.T.; Gillespie, G.Y.; Willey, C.D. Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells 2020, 9, 2369. https://doi.org/10.3390/cells9112369
Stackhouse CT, Gillespie GY, Willey CD. Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells. 2020; 9(11):2369. https://doi.org/10.3390/cells9112369
Chicago/Turabian StyleStackhouse, Christian T., G. Yancey Gillespie, and Christopher D. Willey. 2020. "Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential" Cells 9, no. 11: 2369. https://doi.org/10.3390/cells9112369
APA StyleStackhouse, C. T., Gillespie, G. Y., & Willey, C. D. (2020). Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells, 9(11), 2369. https://doi.org/10.3390/cells9112369





