ROS Overproduction Sensitises Myeloma Cells to Bortezomib-Induced Apoptosis and Alleviates Tumour Microenvironment-Mediated Cell Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cell Lines and Culture Models
2.3. Primary Samples, Treatments, and CD138 Expression Analyses
2.4. Cell Viability Assay
2.5. Assessment of Apoptosis
2.6. ROS Production Measurement
2.7. Immunoblotting
2.8. Semi-Quantitative RT-PCR Analyses
2.9. Statistical Analyses
3. Results
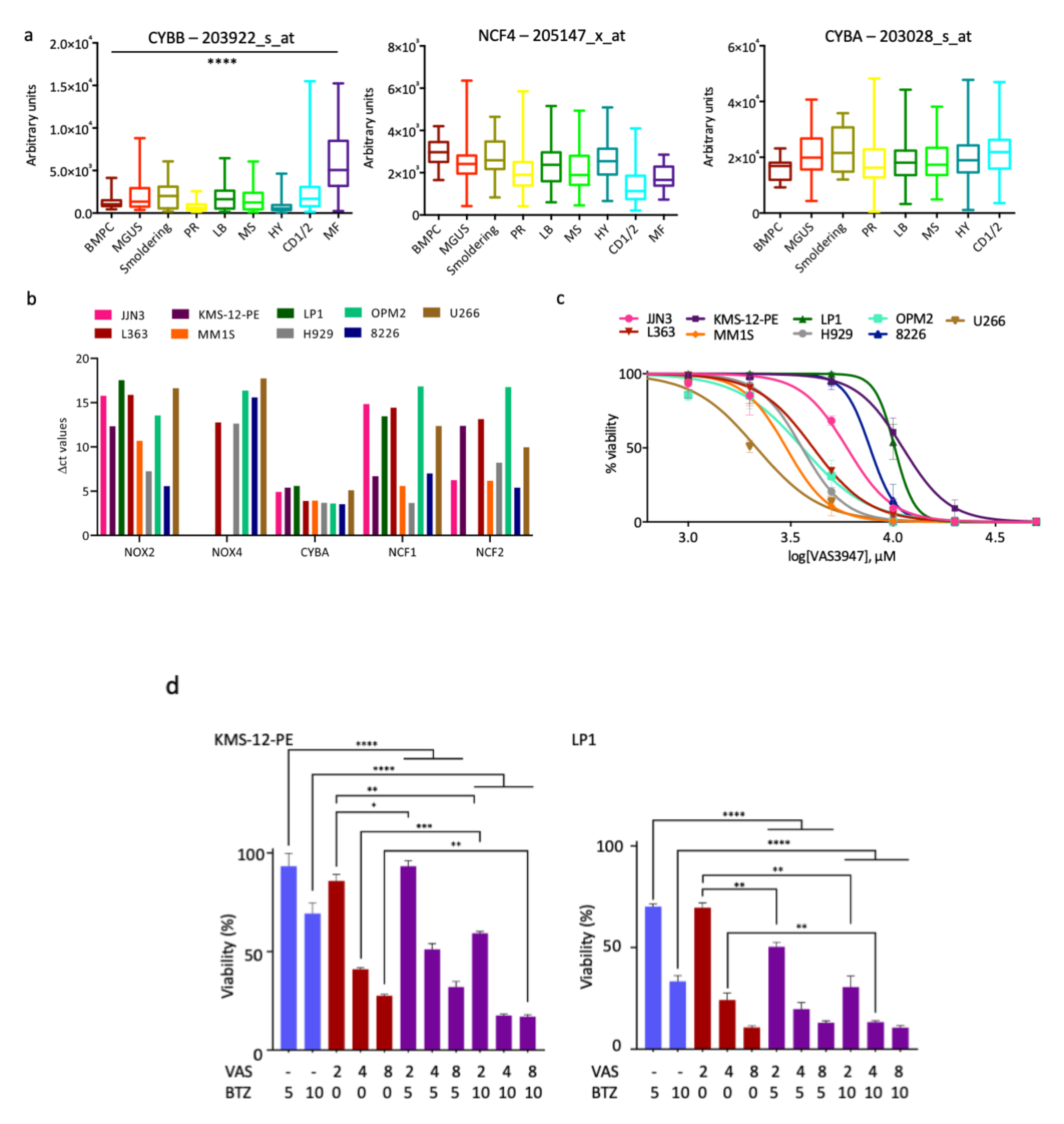
3.1. NOX Subunits Are Expressed in MM Patients and Cell Lines
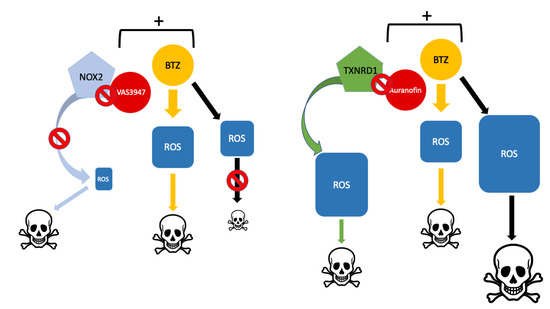
3.2. A Pan-NOX Inhibitor Is Cytotoxic on MM Cell Lines but Has Adverse Effects When Combined with Bortezomib
3.3. Antioxidant Proteins Are Overexpressed in Myeloma Patients and MM Cell Lines
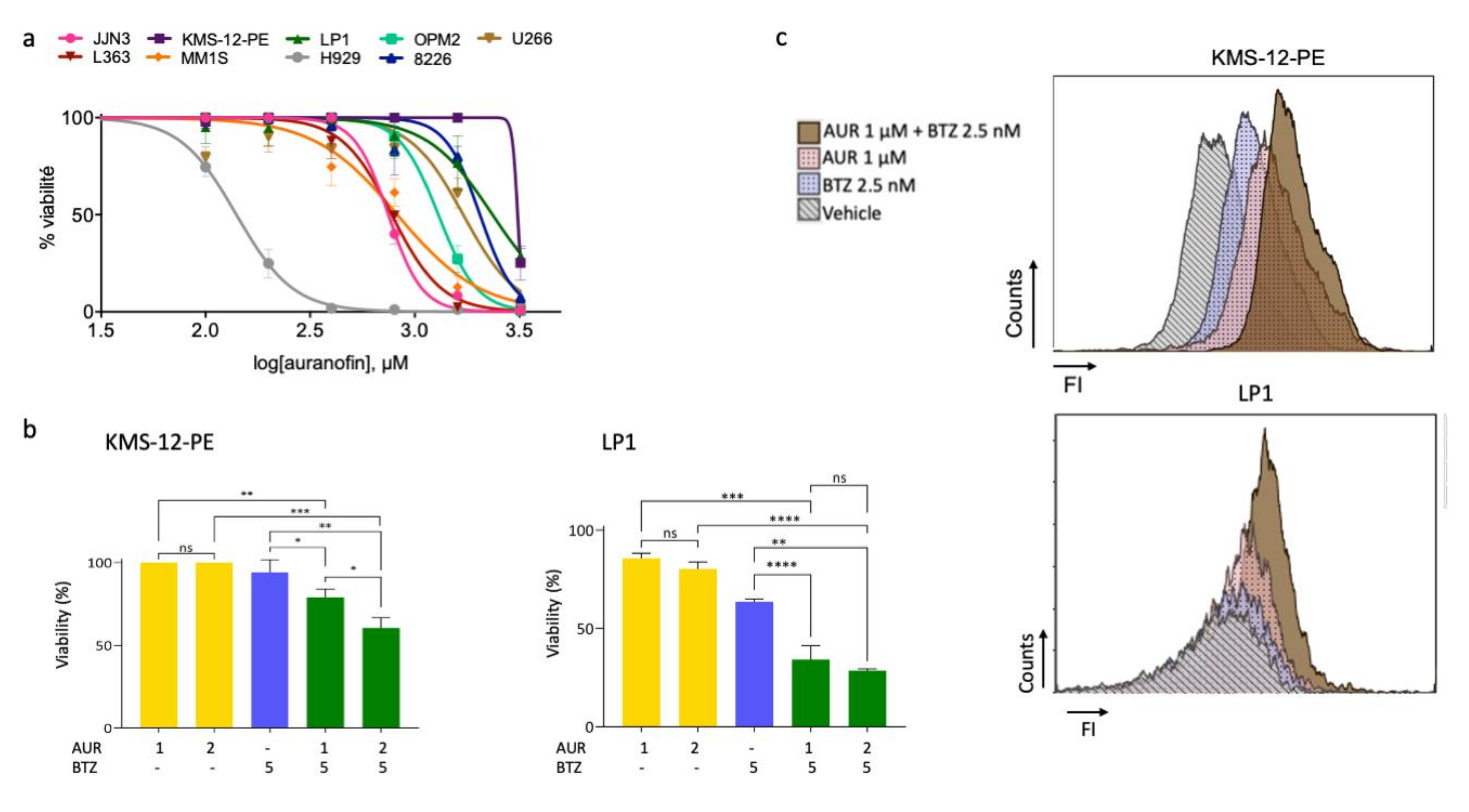
3.4. A Thioredoxine Reductase Inhibitor Is Cytotoxic on MM Cell Lines and Has Synergistic Effects When Combined with Bortezomib
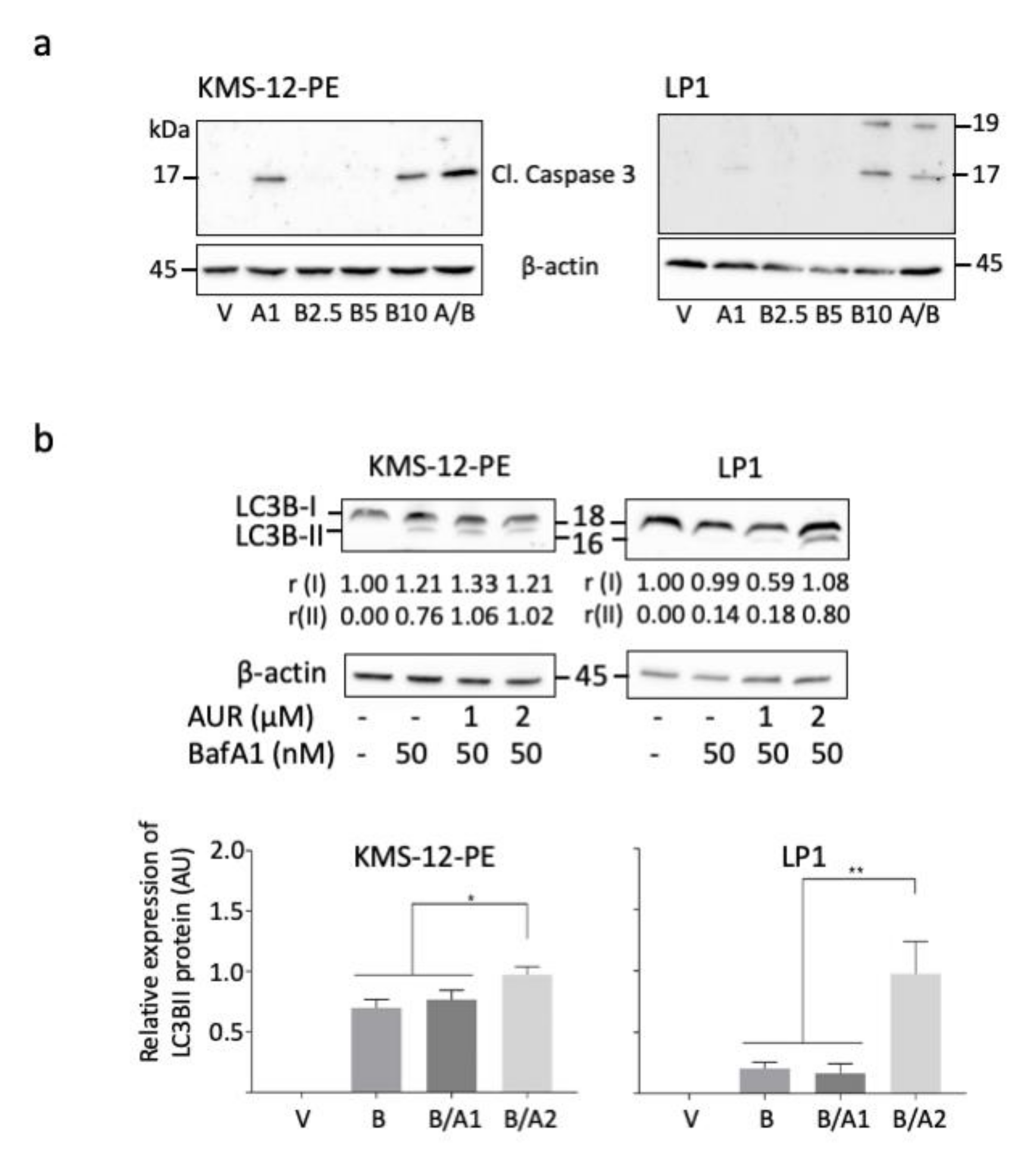
3.5. Bortezomib/Auranofin Combined Treatment Triggers Apoptosis and Auranofin Activates Autophagy
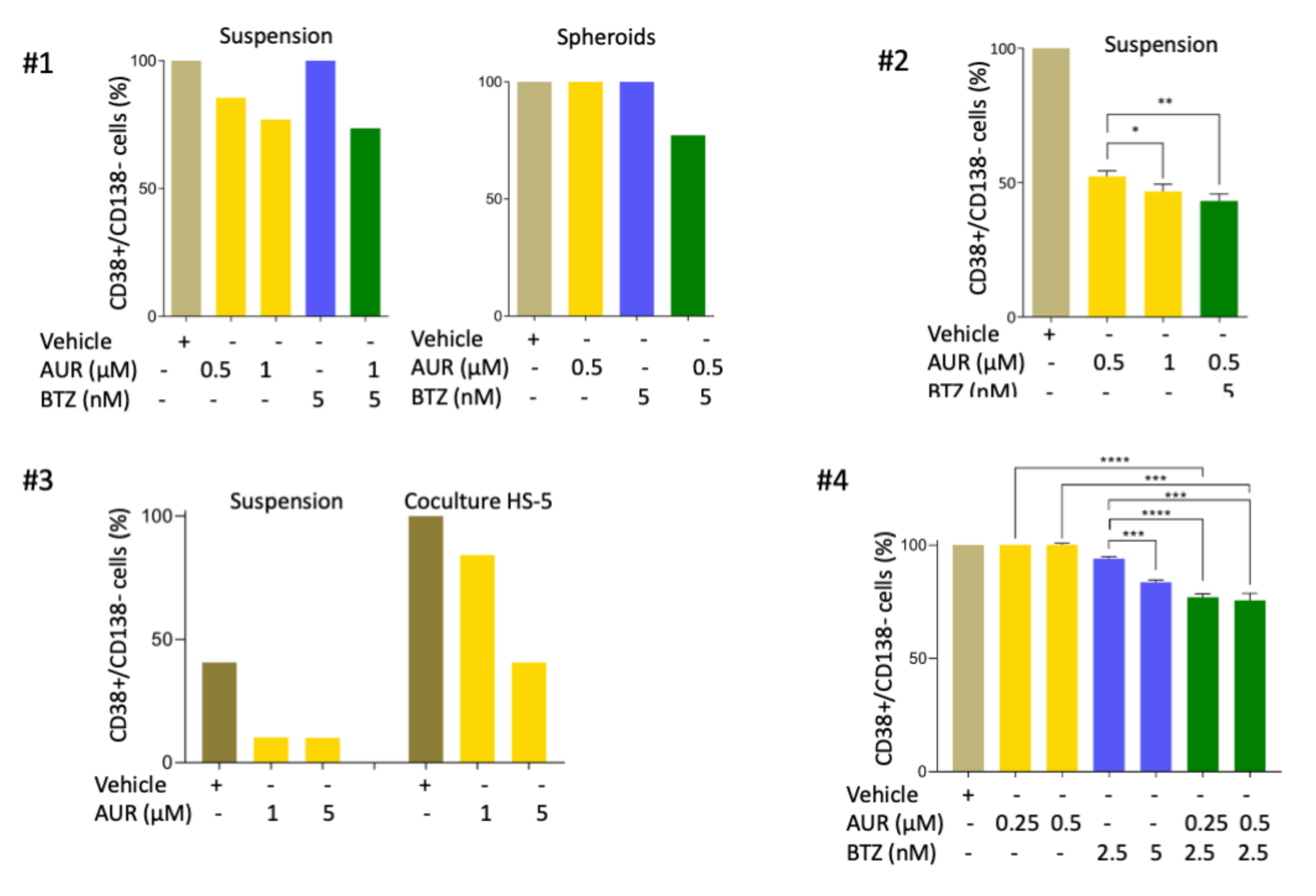
3.6. Bortezomib/Auranofin Combined Treatment Alleviates TME-Mediated Cell Resistance
3.7. Auranofin Triggers Apoptosis of Primary MM Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- Grigoreva, T.A.; Tribulovich, V.G.; Garabadzhiu, A.V.; Melino, G.; Barlev, N.A. The 26S proteasome is a multifaceted target for anti-cancer therapies. Oncotarget 2015, 6, 24733–24749. [Google Scholar] [CrossRef]
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Wallington-Beddoe, C.T.; Sobieraj-Teague, M.; Kuss, B.J.; Pitson, S.M. Resistance to proteasome inhibitors and other targeted therapies in myeloma. Br. J. Haematol. 2018, 182, 11–28. [Google Scholar] [CrossRef]
- Barrio, S.; Stühmer, T.; Da-Viá, M.; Barrio-Garcia, C.; Lehners, N.; Besse, A.; Cuenca, I.; Garitano-Trojaola, A.; Fink, S.; Leich, E.; et al. Spectrum and functional validation of PSMB5 mutations in multiple myeloma. Leukemia 2018, 33, 447–456. [Google Scholar] [CrossRef]
- Acosta-Alvear, D.; Cho, M.Y.; Wild, T.; Buchholz, T.J.; Lerner, A.G.; Simakova, O.; Hahn, J.; Korde, N.; Landgren, O.; Maric, I.; et al. Paradoxical resistance of multiple myeloma to proteasome inhibitors by decreased levels of 19S proteasomal subunits. eLife 2015, 4, e08153. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Mendillo, M.L.; Zhao, J.; Carette, J.E.; Merrill, P.H.; Cikes, D.; Varadarajan, M.; van Diemen, F.R.; Penninger, J.M.; Goldberg, A.L.; et al. Compromising the 19S proteasome complex protects cells from reduced flux through the proteasome. eLife 2015, 4, e08467. [Google Scholar] [CrossRef]
- Catley, L.; Weisberg, E.; Kiziltepe, T.; Tai, Y.T.; Hideshima, T.; Neri, P.; Tassone, P.; Atadja, P.; Chauhan, D.; Munshi, N.C.; et al. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood 2006, 108, 3441–3449. [Google Scholar] [CrossRef]
- Nikesitch, N.; Lee, J.M.; Ling, S.; Roberts, T.L. Endoplasmic reticulum stress in the development of multiple myeloma and drug resistance. Clin. Transl. Immunol. 2018, 7, e1007. [Google Scholar] [CrossRef]
- Hawley, T.S.; Riz, I.; Yang, W.; Wakabayashi, Y.; DePalma, L.; Chang, Y.T.; Peng, W.; Zhu, J.; Hawley, R.G. Identification of an ABCB1 (P-glycoprotein)-positive carfilzomib-resistant myeloma subpopulation by the pluripotent stem cell fluorescent dye CDy1. Am. J. Hematol. 2013, 88, 265–272. [Google Scholar] [CrossRef]
- Dytfeld, D.; Luczak, M.; Wrobel, T.; Usnarska- Zubkiewicz, L.; Brzezniakiewicz, K.; Jamroziak, K.; Giannopoulos, K.; Przybylowicz-Chalecka, A.; Ratajczak, B.; Czerwinska-Rybak, J.; et al. Comparative proteomic profiling of refractory/relapsed multiple myeloma reveals biomarkers involved in resistance to bortezomib-based therapy. Oncotarget 2016, 7, 56726–56736. [Google Scholar] [CrossRef]
- Bustany, S.; Bourgeais, J.; Tchakarska, G.; Body, S.; Hérault, O.; Gouilleux, F.; Sola, B. Cyclin D1 unbalances the redox status controlling cell adhesion, migration, and drug resistance in myeloma cells. Oncotarget 2016, 7, 45214–45224. [Google Scholar] [CrossRef]
- Bustany, B.; Cahu, J.; Guardiola, P.; Sola, B. Cyclin D1 sensitizes myeloma cells to endoplasmic reticulum stress-mediated apoptosis by activating the unfolded protein response pathway. BMC Cancer 2015, 15, 262. [Google Scholar] [CrossRef]
- Goel, A.; Spitz, D.R.; Weiner, G.J. Manipulation of cellular redox parameters for improving therapeutic responses in B-cell lymphoma and multiple myeloma. J. Cell Biochem. 2012, 113, 419–425. [Google Scholar] [CrossRef]
- Huang, Y.H.; Molavi, O.; Alshareef, A.; Haque, M.; Wang, Q.; Chu, M.P.; Venner, C.P.; Sandhu, I.; Peters, A.C.; Lavasanifar, A.; et al. Constitutive activation of STAT3 in myeloma cells cultured in a three-dimensional, reconstructed bone marrow model. Cancers 2018, 10, 206. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2016 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2016, 91, 719–734. [Google Scholar] [CrossRef]
- Surget, S.; Chiron, D.; Gomez-Bougie, P.; Descamps, G.; Ménoret, E.; Bataille, R.; Moreau, P.; Le Gouill, S.; Amiot, M.; Pellat-Deceunynck, C. Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells. Cancer Res. 2012, 72, 4562–4573. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Body, S.; Esteve-Arenys, A.; Miloudi, H.; Recasens-Zorzo, C.; Tchakarska, G.; Moros, A.; Bustany, S.; Vidal-Crespo, A.; Rodriguez, V.; Lavigne, R.; et al. Cytoplasmic cyclin D1 controls the migration and invasiveness of mantle lymphoma cells. Sci. Rep. 2017, 7, 13946. [Google Scholar] [CrossRef]
- Zhan, F.; Huang, Y.; Colla, S.; Stewart, J.P.; Hanamura, I.; Gupta, S.; Epstein, J.; Yaccoby, S.; Sawyer, J.; Burington, B.; et al. The molecular classification of multiple myeloma. Blood 2006, 108, 2020–2028. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Bhatia, M.; Tonissen, K.F. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget 2015, 6, 15410–15424. [Google Scholar] [CrossRef]
- Zheng, Z.; Fan, S.; Zheng, J.; Huang, W.; Gasparetto, C.; Chao, N.J.; Hu, J.; Kang, Y. Inhibition of thioredoxin activates mitophagy and overcomes adaptive bortezomib resistance in multiple myeloma. J. Hematol. Oncol. 2018, 11, 29. [Google Scholar] [CrossRef]
- Fiskus, W.; Saba, N.; Shen, M.; Ghias, M.; Liu, J.; Gupta, S.D.; Chauhan, L.; Rao, R.; Gunewardena, S.; Schorno, K.; et al. Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res. 2014, 74, 2520–2532. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Lopez, E.; Guo, H.; Zhang, H.; Liu, Y.; Chen, Z.; Huang, S.; Zhou, S.; Leeming, A.; et al. Repurposing auranofin to treat TP53-mutated or PTEN-deleted refractory B-cell lymphoma. Blood Cancer J. 2019, 9, 95. [Google Scholar] [CrossRef]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Tonissen, K.F. TrxR1 inhibition overcomes both hypoxia-induced and acquired bortezomib resistance in multiple myeloma through NF-κβ inhibition. Cell Cycle 2016, 5, 559–572. [Google Scholar] [CrossRef]
- Tessoulin, B.; Descamps, G.; Dousset, C.; Amiot, M.; Pellat-Deceunynck, C. Targeting oxidative stress with auranofin or Prima-1Met to circumvent p53 or Bax/Bak deficiency in myeloma cells. Front. Oncol. 2019, 9, 128. [Google Scholar] [CrossRef]
- Nair, R.R.; Emmons, M.F.; Cress, A.E.; Argilagos, R.F.; Lam, K.; Kerr, W.T.; Wang, H.G.; Dalton, W.S.; Hazlehurst, L.A. HYD1-induced increase in reactive oxygen species leads to autophagy and necrotic cell death in multiple myeloma cells. Mol. Cancer Ther. 2009, 8, 2441–2451. [Google Scholar] [CrossRef]
- Michallet, A.S.; Mondiere, P.; Taillardet, M.; Leverrier, Y.; Genestier, L.; Defrance, T. Compromising the unfolded protein response induces autophagy-mediated cell death in multiple myeloma cells. PLoS ONE 2011, 6, e25820. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2020 updtate on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef]
- Nerini-Molteni, S.; Ferrarini, M.; Cozza, S.; Caligaris-Cappio, F.; Sitia, R. Redox homeostasis modulates the sensitivity of myeloma cells to bortezomib. Br. J. Haematol. 2008, 141, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Ann. Rev. Pathol. 2014, 9, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Shain, K.H.; Dalton, W.S.; Tao, J. The tumor microenvironment shapes hallmarks of mature B-cell malignancies. Oncogene 2015, 34, 4673–4682. [Google Scholar] [CrossRef] [PubMed]
- Tessoulin, B.; Eveillard, M.; Lok, A.; Chiron, D.; Moreau, P.; Amiot, M.; Moreau-Aubry, A.; Le Gouill, S.; Pellat-Deceunynck, C. p53 dysregulation in B-cell malignancies: More than a single gene in the pathway to hell. Blood Rev. 2017, 31, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.; McCormick, M.L.; Wendlandt, E.; Zhan, F.; Goel, A. Copper–zinc superoxide dismutase-mediated redox regulation of bortezomib resistance in multiple myeloma. Redox Biol. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, S.J.; Kim, J.T.; Kim, S.J.; Min, J.K.; Bae, K.H.; Jung, H.; Kim, B.Y.; Lim, J.S.; Yang, Y.; et al. Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget 2016, 7, 85332–85348. [Google Scholar] [CrossRef]
- Oh, B.M.; Lee, S.J.; Cho, H.J.; Park, Y.S.; Kim, J.T.; Yoon, S.R.; Lee, S.C.; Lim, J.S.; Kim, B.Y.; Choe, Y.K.; et al. Cystatin SN inhibits auranofin-induced cell death by autophagic induction and ROS regulation via glutathione reductase activity in colorectal cancer. Cell Death Dis. 2017, 8, e2682. [Google Scholar] [CrossRef]
- Radenkovic, F.; Holland, O.; Vanderlelie, J.J.; Perkins, A.V. Selective inhibition of endogenous antioxidants with Auranofin causes mitochondrial oxidative stress which can be countered by selenium supplementation. Biochem. Pharmacol. 2017, 146, 42–52. [Google Scholar] [CrossRef]
- Gourzones, C.; Bellanger, C.; Lamure, S.; Gadacha, O.G.; Garcia De Paco, E.; Vincent, L.; Cartron, G.; Klein, B.; Moreaux, J. Antioxidant defenses confer resistance to high dose melphalan in multiple myeloma cells. Cancers 2019, 11, 439. [Google Scholar] [CrossRef]
- Gonzalez-Santamarta, M.; Quinet, G.; Reyes-Garau, D.; Sola, B.; Roué, G.; Rodriguez, M.S. Resistance to the proteasome inhibitors: Lessons from multiple myeloma and mantle cell lymphoma. In Proteostasis and Disease. Advances in Experimental Medicine and Biology; Barrio, R., Sutherland, J.D., Rodriguez, M.S., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 1233, pp. 153–174. [Google Scholar] [CrossRef]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol sensitizes carfilzomib-induced apoptosis via promoting oxidative stress in multiple myeloma cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]




| Cell Lines | IC50 VAS (μM) | IC50 AUR (μM) | IC50 BTZ (nM) |
|---|---|---|---|
| JJN3 | >4.3 | 0.8 | 4.6 |
| KMS-12-PE | 7.8 | >3.5 | 19.2 |
| LP1 | 8.0 | >3.0 | 9.8 |
| L363 | 3.7 | 0.9 | 4.3 |
| MM.1S | 1.5 | 0.4 | nd |
| H929 | 2.7 | 0.09 | 3.7 |
| OPM2 | 3.2 | 1.2 | 2.7 |
| 8226 | 6.4 | >4.3 | 9.1 |
| U266 | 2.6 | 2.2 | 4.8 |
| Cell Lines | VAS (μM) | BTZ (nM) | CI | Effects |
|---|---|---|---|---|
| LP1 | 2 | 2 | 1.382 ± 0.135 | Antagonistic |
| 2 | 5 | 2.290 ± 0.788 | Antagonistic | |
| 2 | 10 | 1.289 ± 0.419 | Antagonistic | |
| 3 | 3 | 1.646 ± 0.063 | Antagonistic | |
| 4 | 4 | 1.516 ± 0.625 | Antagonistic | |
| KMS-12-PE | 2 | 10 | 11.001 ± 5.607 | Antagonistic |
| 4 | 5 | 1.074 ± 0.435 | Additive | |
| 4 | 10 | 1.007 ± 0.136 | Additive |
| Cell Lines | AUR (μM) | BTZ (nM) | CI | Effects |
|---|---|---|---|---|
| OPM2 | 1 | 3.5 | 0.806 ± 0.216 | Synergistic |
| 1 | 5 | 0.003 ± 0.001 | Synergistic | |
| 2 | 3.5 | 0.205 ± 0.067 | Synergistic | |
| H929 | 0.5 | 3.5 | 0.027 ± 0.002 | Synergistic |
| L363 | 0.5 | 5 | 2.28 × 10−6 ± 5.00 × 10−5 | Synergistic |
| 1 | 3.5 | 2.23 × 10−5 ± 1.29 × 10−5 | Synergistic | |
| 1 | 5 | 1.56 × 10−6 ± 3.89 × 10−7 | Synergistic | |
| 1 | 10 | 1.68 × 10−9 ± 1.95 × 10−9 | Synergistic | |
| JJN3 | 0.1 | 5 | 0.808 ± 0.082 | Synergistic |
| 0.5 | 5 | 0.503. ± 0.109 | Synergistic | |
| LP1 | 2 | 5 | 0.068 ± 0.01 | Synergistic |
| 3 | 5 | 0.488 ± 0.094 | Synergistic | |
| 1 | 10 | 0.047 ± 0.02 | Synergistic | |
| KMS-12-PE | 1 | 5 | 1/∞ | Synergistic |
| 1 | 10 | 3.48 × 10−3 ± 5.40 × 10−4 | Synergistic | |
| 2 | 5 | 1/∞ | Synergistic | |
| 2 | 10 | 1.60 × 10−4 ± 1.54 × 10−4 | Synergistic |
| Cell Line | Culture Model | Treatment 1 | Treatment 2 | p-Value |
|---|---|---|---|---|
| H929 | Suspension | AUR 2.5 | AUR 5 | <0.0001 |
| BTZ 20 | BTZ 50 | 0.002 | ||
| BTZ 20 | AUR 2.5 + BTZ 20 | 0.0002 | ||
| BTZ 20 | AUR 5 + BTZ 20 | 0.0002 | ||
| AUR 2.5 | AUR 2.5 + BTZ 20 | 0.2648 | ||
| AUR 5 | AUR 5 + BTZ 20 | 0.0008 | ||
| AUR 2.5 + BTZ 20 | AUR 5 + BTZ 20 | 0.0048 | ||
| Coculture | BTZ 20 | BTZ 50 | 0.0006 | |
| BTZ 20 | AUR 2.5 + BTZ 20 | <0.0001 | ||
| BTZ 20 | AUR 5 + BTZ 20 | <0.0001 | ||
| AUR 2.5 | AUR 2.5 + BTZ 20 | 0.0034 | ||
| AUR 5 | AUR 5 + BTZ 20 | <0.0001 | ||
| L363 | Suspension | AUR 2.5 | AUR 5 | 0.6863 |
| BTZ 20 | BTZ 50 | <0.0001 | ||
| BTZ 20 | AUR 5 + BTZ 20 | <0.0001 | ||
| AUR 5 | AUR 5 + BTZ 20 | 0.1071 | ||
| Coculture | BTZ 20 | BTZ 50 | <0.0001 | |
| BTZ 20 | AUR 2.5 + BTZ 20 | <0.0001 | ||
| AUR 5 | AUR 5 + BTZ 20 | 0.0764 |
| Cell Line | Treatment | Model 1 | Model 2 | p-Value |
|---|---|---|---|---|
| H929 | AUR 2.5 | Suspension | Coculture | <0.0001 |
| AUR 5 | Suspension | Coculture | <0.0001 | |
| BTZ 20 | Suspension | Coculture | 0.0033 | |
| BTZ 50 | Suspension | Coculture | <0.0001 | |
| AUR 2.5 + BTZ 20 | Suspension | Coculture | <0.0001 | |
| AUR 5 + BTZ 20 | Suspension | Coculture | <0.0001 | |
| L363 | AUR 2.5 | Suspension | Coculture | <0.0001 |
| AUR 5 | Suspension | Coculture | <0.0001 | |
| BTZ 20 | Suspension | Coculture | <0.0001 | |
| BTZ 50 | Suspension | Coculture | 0.0061 | |
| AUR 5 + BTZ 20 | Suspension | Coculture | <0.0001 |
| Cell Line | Culture Model | AUR (μM) | BTZ (nM) | CI | Effects |
|---|---|---|---|---|---|
| H929 | HS-5 | 2.5 | 20 | 0.139 ± 0.214 | Synergistic |
| 5 | 20 | 0.061 ± 0.006 | Synergistic | ||
| 3-D | 5 | 50 | 0.728 ± 0.077 | Synergistic | |
| L363 | HS-5 | 5 | 20 | 0.895 ± 0.028 | Synergistic |
| 3-D | 5 | 50 | 0.908 ± 0.036 | Synergistic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caillot, M.; Zylbersztejn, F.; Maitre, E.; Bourgeais, J.; Hérault, O.; Sola, B. ROS Overproduction Sensitises Myeloma Cells to Bortezomib-Induced Apoptosis and Alleviates Tumour Microenvironment-Mediated Cell Resistance. Cells 2020, 9, 2357. https://doi.org/10.3390/cells9112357
Caillot M, Zylbersztejn F, Maitre E, Bourgeais J, Hérault O, Sola B. ROS Overproduction Sensitises Myeloma Cells to Bortezomib-Induced Apoptosis and Alleviates Tumour Microenvironment-Mediated Cell Resistance. Cells. 2020; 9(11):2357. https://doi.org/10.3390/cells9112357
Chicago/Turabian StyleCaillot, Mélody, Florence Zylbersztejn, Elsa Maitre, Jérôme Bourgeais, Olivier Hérault, and Brigitte Sola. 2020. "ROS Overproduction Sensitises Myeloma Cells to Bortezomib-Induced Apoptosis and Alleviates Tumour Microenvironment-Mediated Cell Resistance" Cells 9, no. 11: 2357. https://doi.org/10.3390/cells9112357
APA StyleCaillot, M., Zylbersztejn, F., Maitre, E., Bourgeais, J., Hérault, O., & Sola, B. (2020). ROS Overproduction Sensitises Myeloma Cells to Bortezomib-Induced Apoptosis and Alleviates Tumour Microenvironment-Mediated Cell Resistance. Cells, 9(11), 2357. https://doi.org/10.3390/cells9112357