Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate
Abstract
1. Introduction
2. Materials and Methods
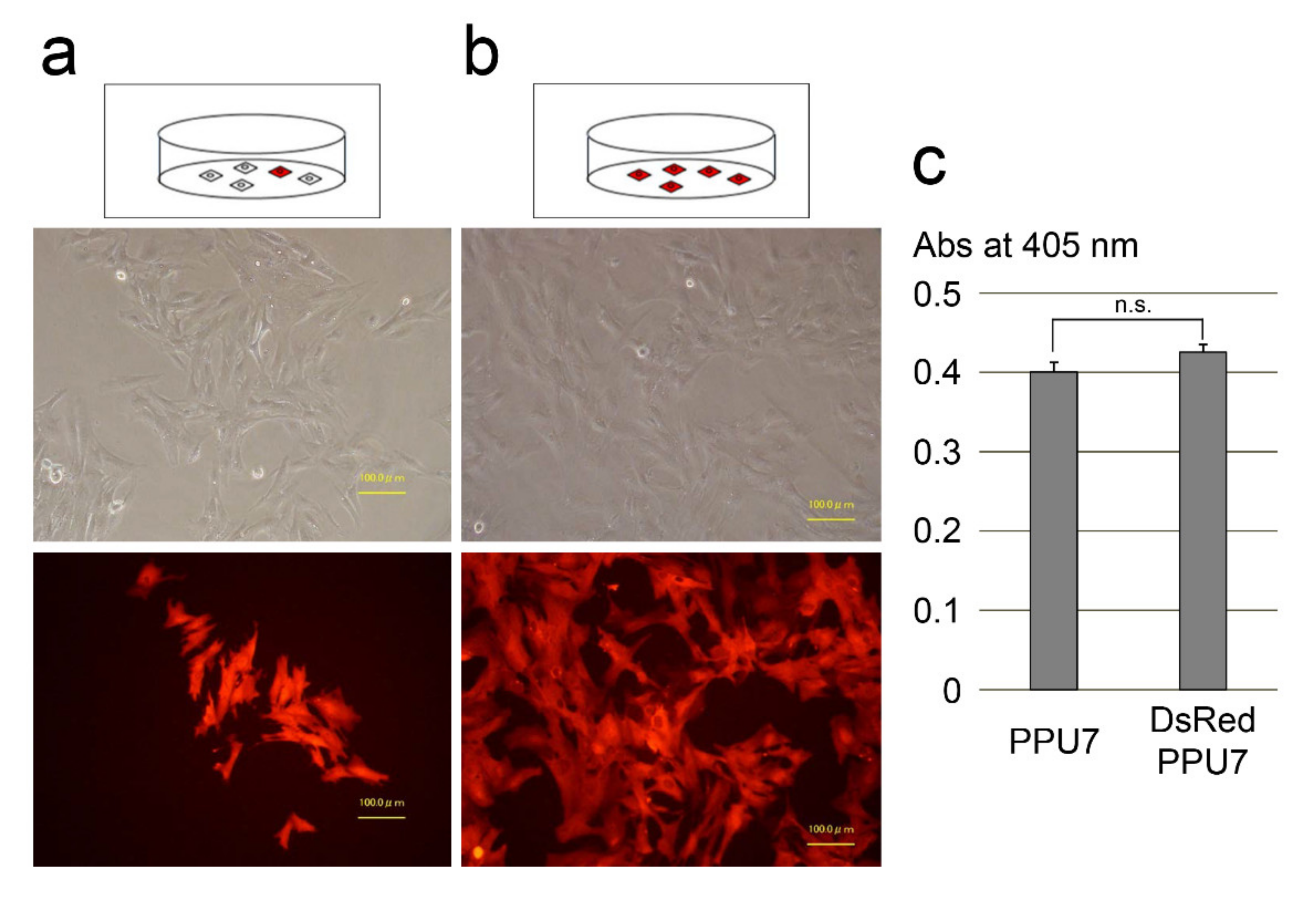
2.1. Gene Transfection of Discosoma Species Red Fluorescent Protein (DsRed) into a Porcine Dental-Pulp Cell Line (PPU7)
2.2. Alkaline Phosphatase (ALP) Activity Assay of the PPU7 Cell Line
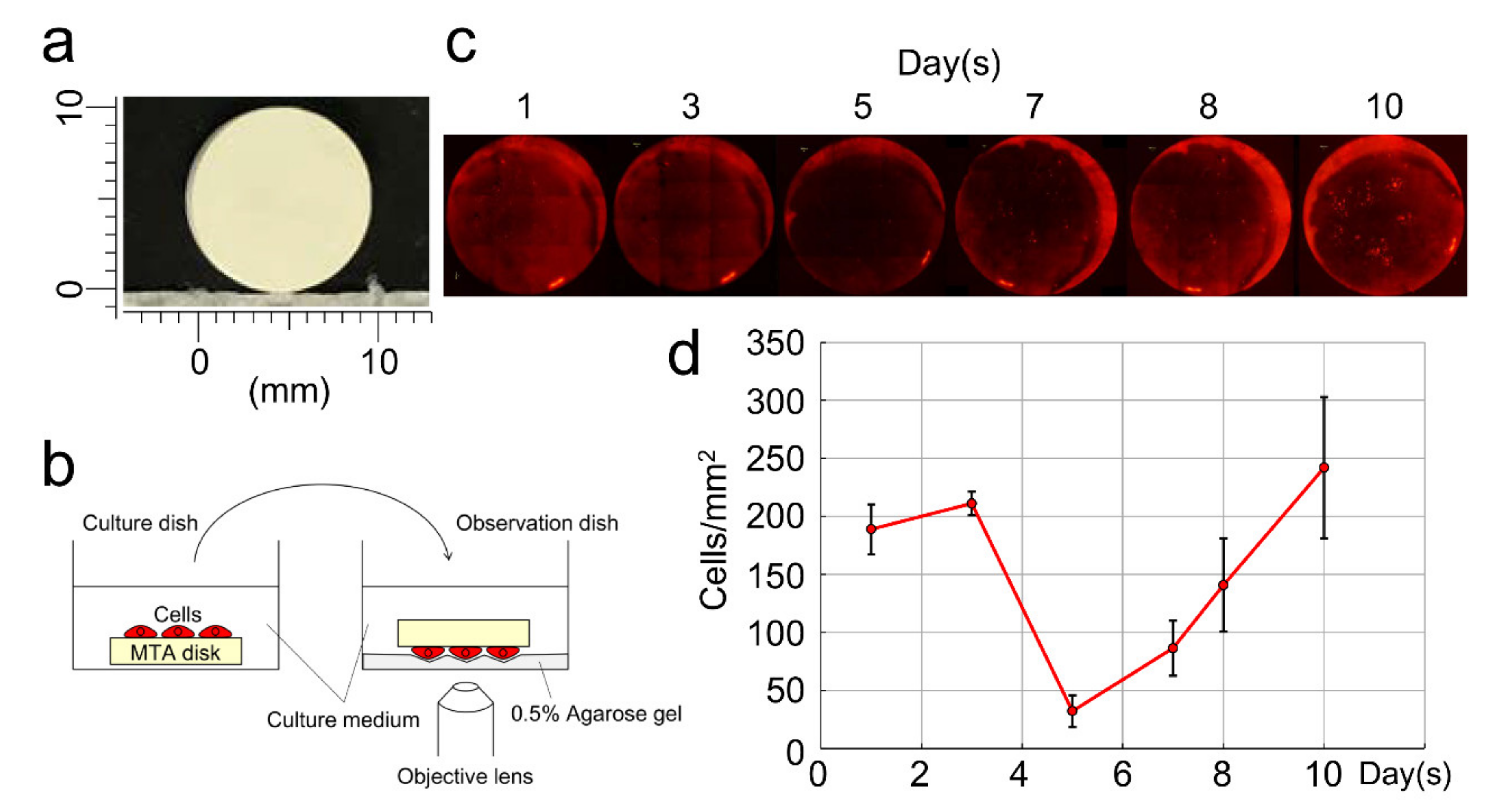
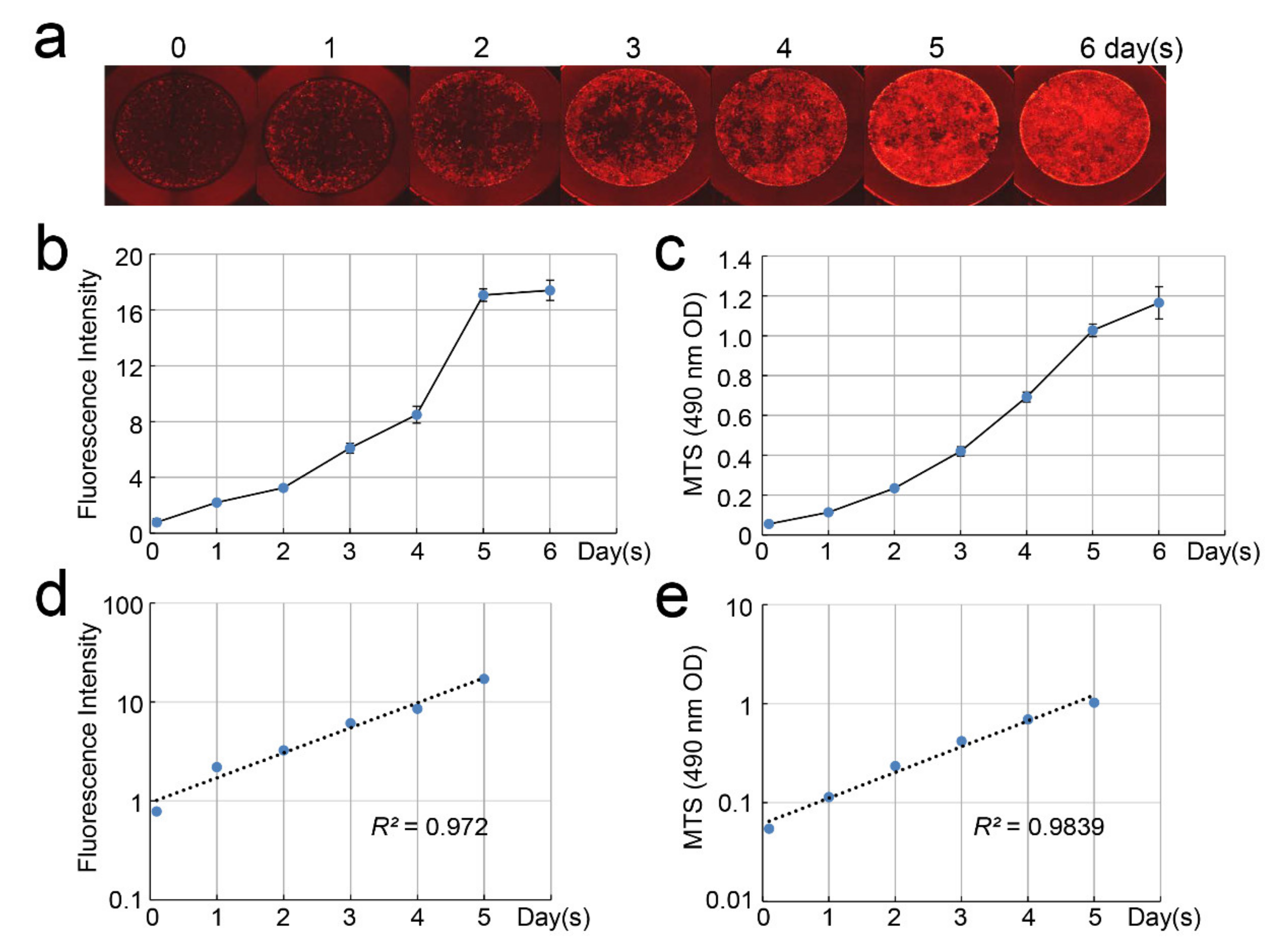
2.3. Changes in the Number of DsRed-PPU7 Cells on the Mineral Trioxide Aggregate (MTA) Disk
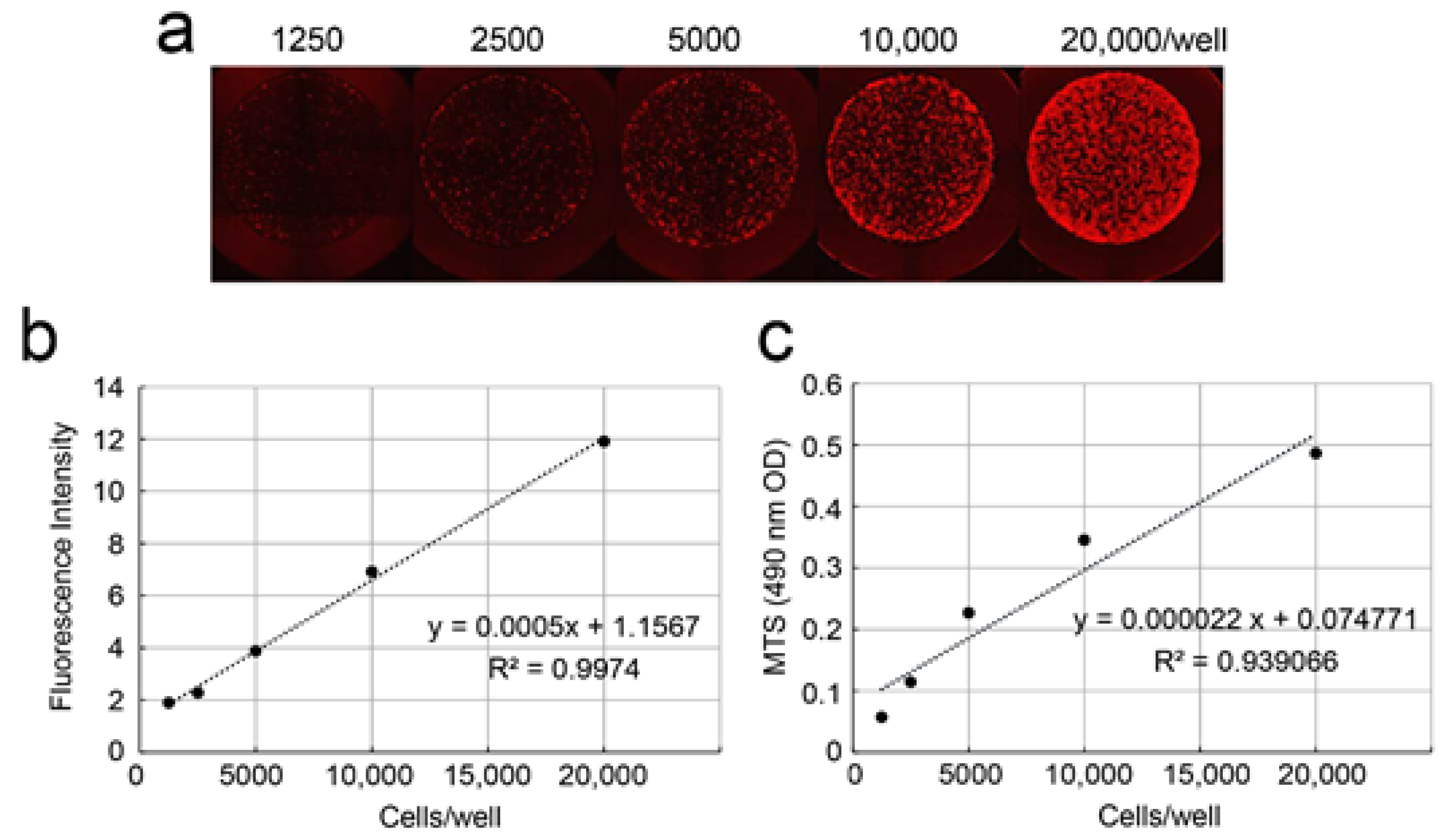
2.4. Correlation of Fluorescence Intensity and Cell-Proliferation Rate of DsRed-PPU7 Cells
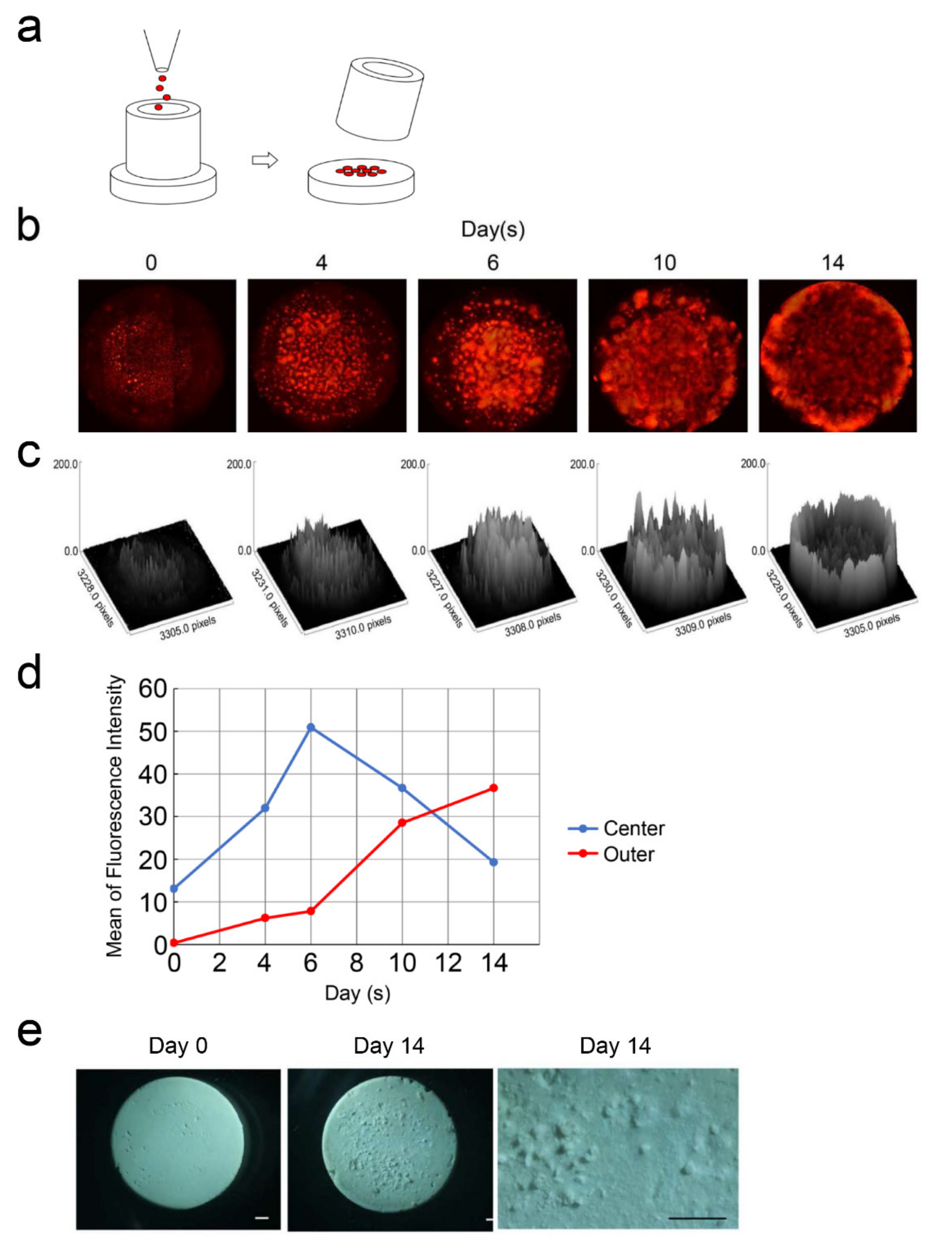
2.5. Characterization of DsRed-PPU7 Cells on MTA Disks
2.6. Quantitative Polymerase Chain Reaction (qPCR) Analysis
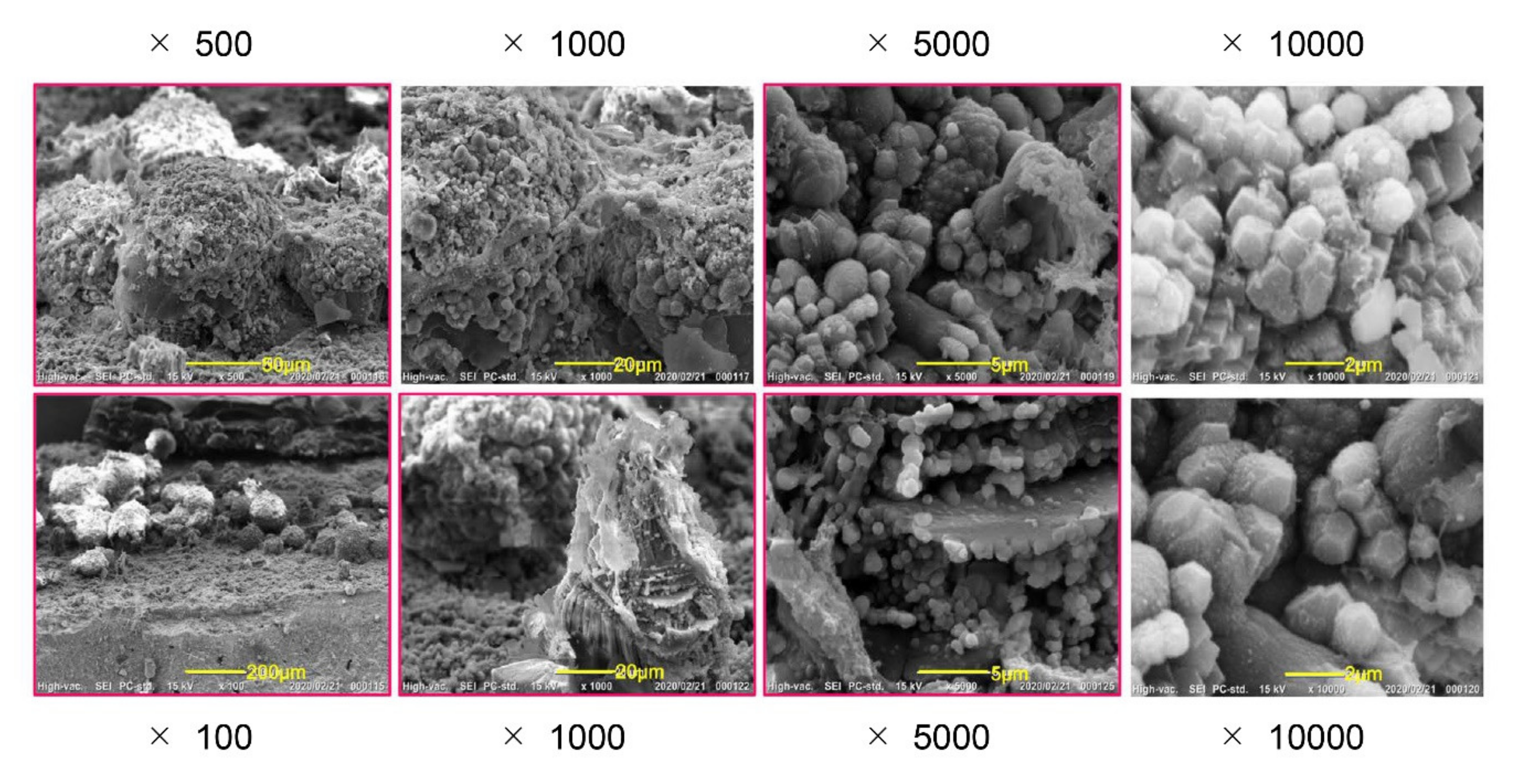
2.7. Scanning Electron Microscopy (SEM) and Electron-Probe Microanalysis (EPMA)
2.8. Statistical Analysis
3. Results
3.1. Comparison of PPU7 and DsRed-PPU7 Cells
3.2. Changes in the Number of DsRed-PPU7 Cells on the MTA Disk
3.3. Correlation between Fluorescence Intensity and Cell-Proliferation Rate of DsRed-PPU7 Cells
3.4. DsRed-PPU7 Cells on the MTA Disk
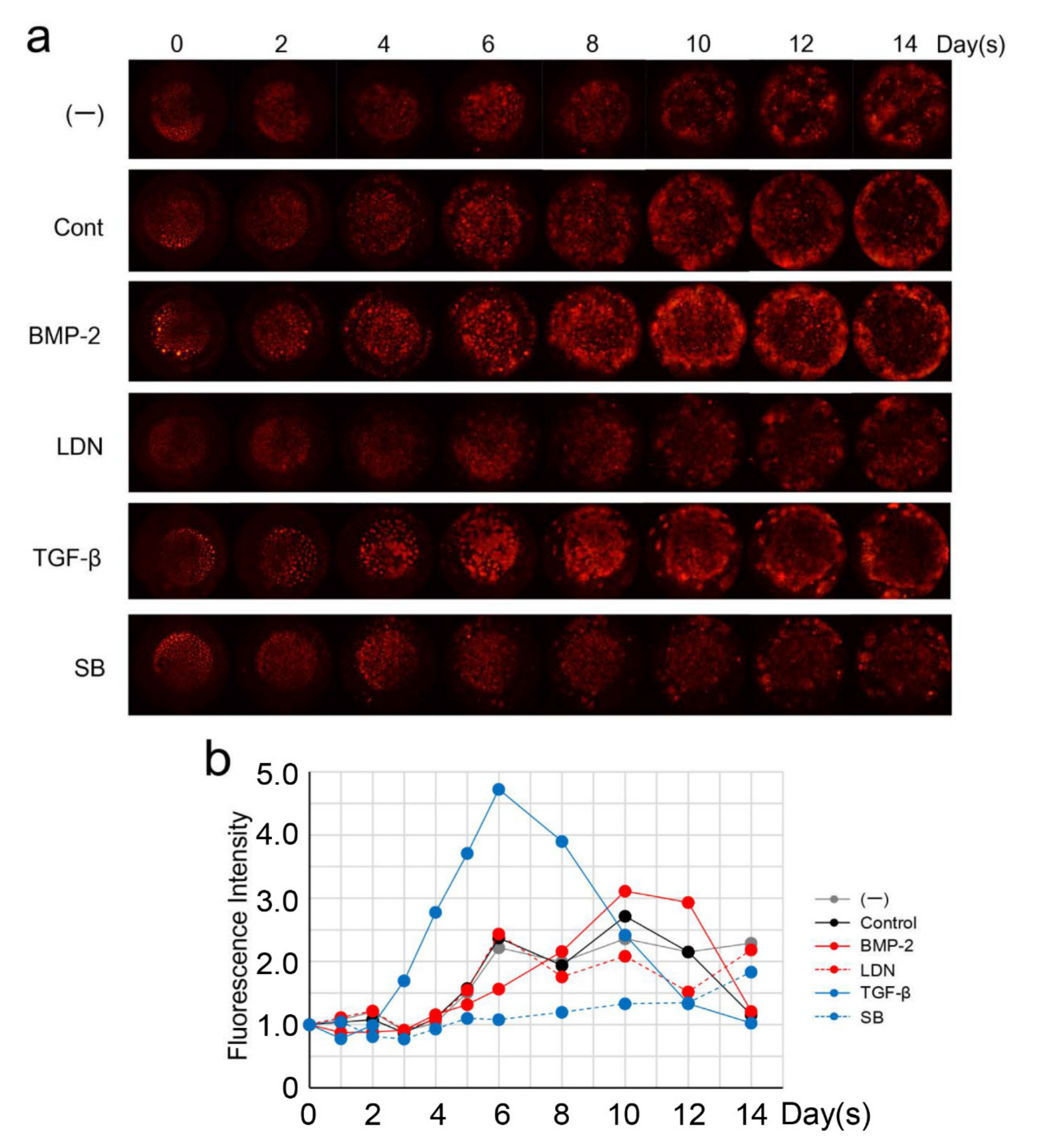
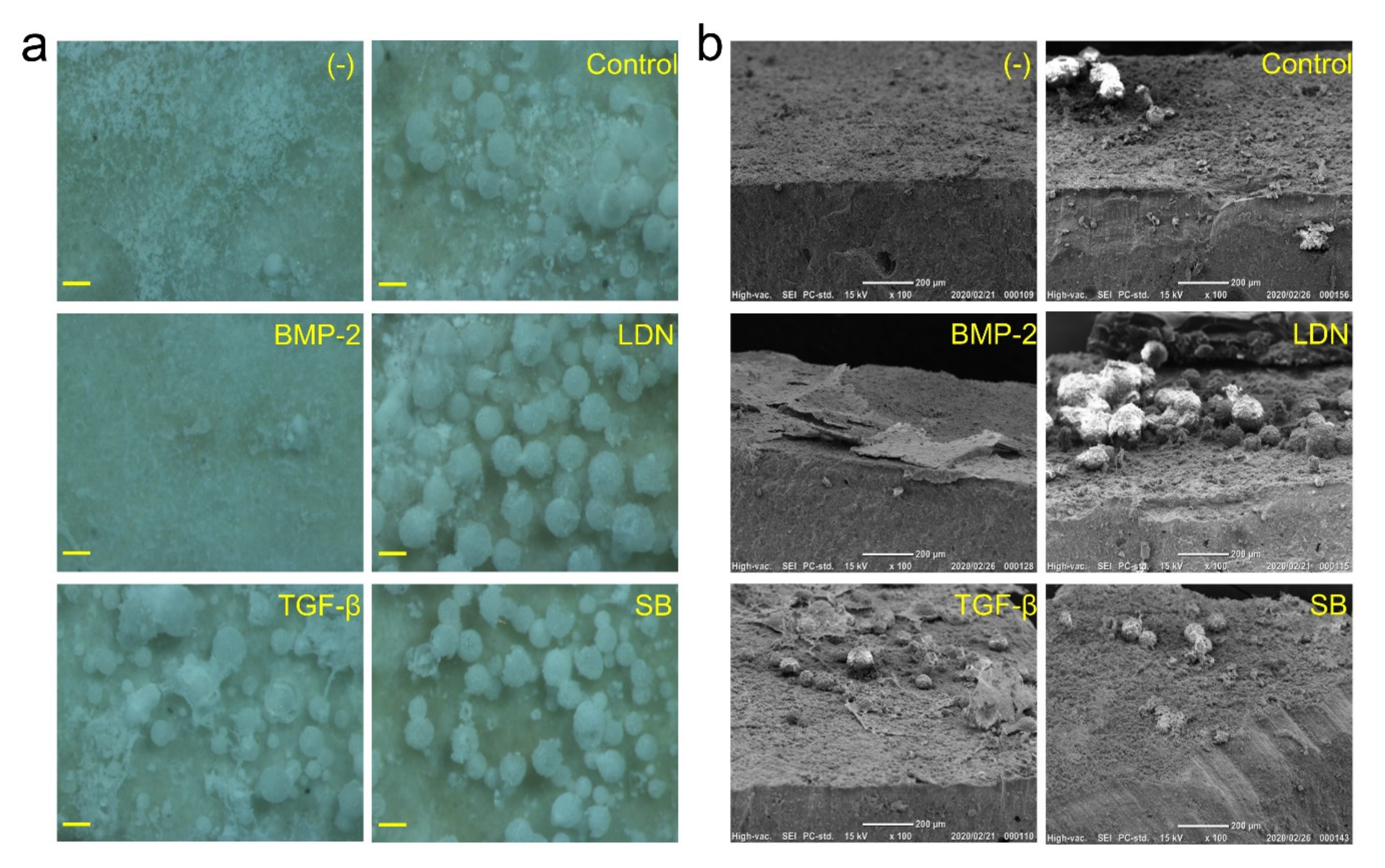
3.5. Effect of BMP and TGF-β on DsRed-PPU7 Cells on the MTA Disk
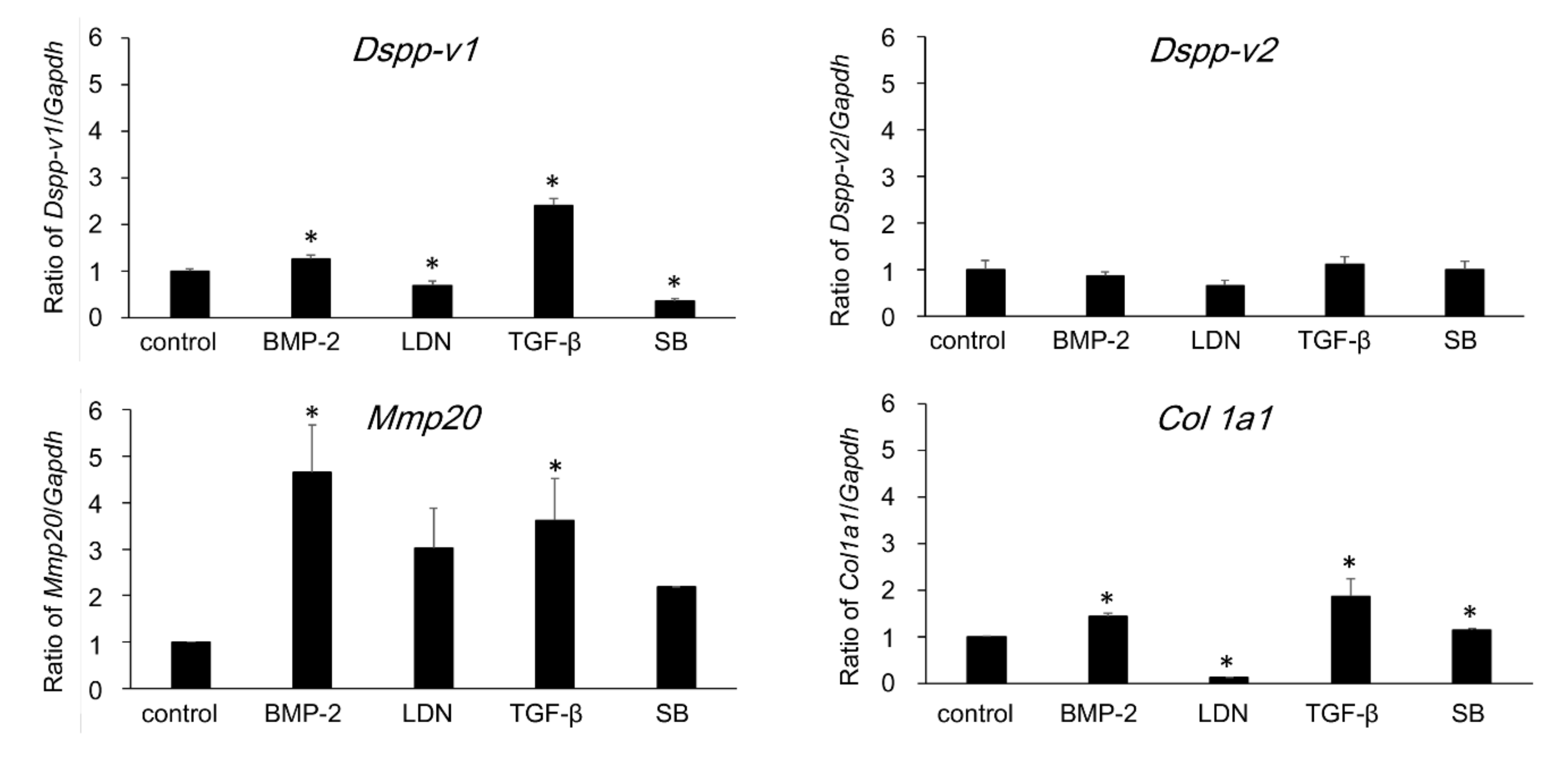
3.6. Direction of Differentiation of DsRed-PPU7 Cells on the MTA Disk
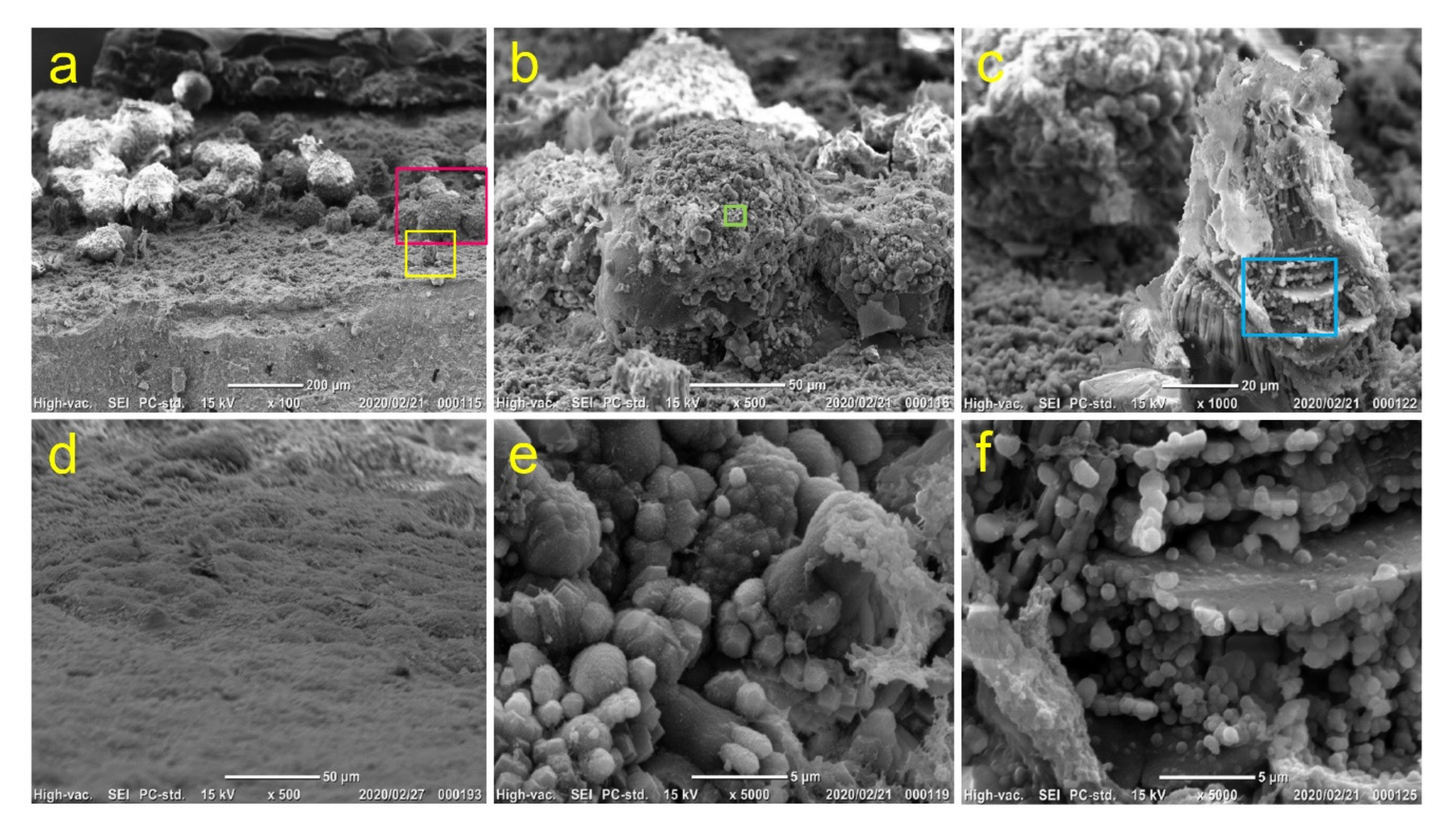
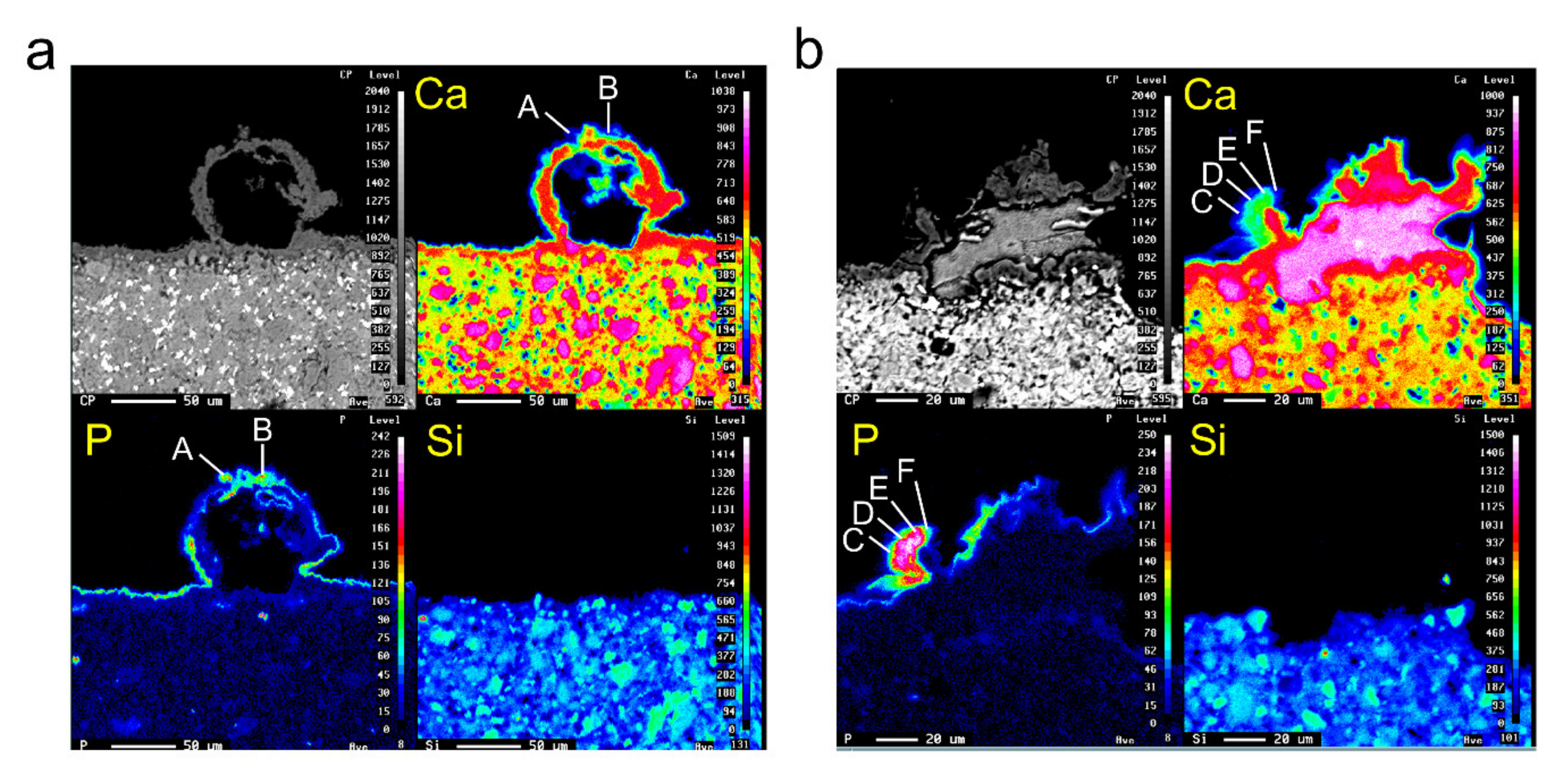
3.7. Observation of Mineralized Precipitates on the MTA Disk
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MTS: | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphentl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt). |
| LDN | 4-[6-[4-(1-piperazinyl)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinolinehydrochloride. |
| RNA | ribonucleic acid |
| mRNA | messenger ribonucleic acid |
| PBS | phosphate buffer saline |
| NaOH | sodium hydroxide |
| MgCl2 | magnesium chloride. |
| HCl | hydrochloric acid |
| CO2 | carbon dioxide |
| Ca | calcium |
| P | phosphorus |
| Si | silicon |
| Smad | homologs of Caenorhabditis elegans (Sma) and Drosophila (Mad) proteins |
Appendix A
Appendix A.1. Fluorescence Microscope Observation of DeRed-PPU7 Cells on MTA Disk
Appendix A.2. Experimental Animal Model
Appendix A.3. Preparation of DsRed-PPU7 Cells
Appendix A.4. Splice Variants of Dentin Sialophosphoprotein

| Gene | Sequence (5′→3′) | Size (bp) | qPCR Protocol (45 Cycles) | ||
|---|---|---|---|---|---|
| Dspp-v1 | F | CCCAGAAACCCAATCAGAGA | 300 | Denaturation Annealing Extension | 95 °C, 10 s 60 °C, 10 s 72 °C, 15 s |
| R | TATGTGTTTTGCTGGGTCCA | ||||
| Dspp-v2 | F | CCCAGAAACCCAATCAGAGA | 149 | ||
| R | GGGAAGGAAGGGGAGAATTT | ||||
| Mmp20 | F | ATGCAGCTTACGAAGTGGCT | 95 | ||
| R | GGGAGGACCTTGCATTTGGA | ||||
| Col1a1 | F | TGCGACGAAATCAAGAACTG | 204 | ||
| R | TCCAGGAAGTCCAGGTTGTC | ||||
| Gapdh | F | CCATCACCATCTTCCAGGAG | 346 | ||
| R | ACAGTCTTCTGGGTGGCAGT | ||||
References
- Roberts, H.W.; Toth, J.M.; Berzins, D.W.; Charlton, D.G. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent. Mater. 2008, 24, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Aapplications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Pitt Ford, T.R. Mineral trioxide aggregate: A review of the constituents and biological properties of the material. Int. Endod. J. 2006, 39, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Darvell, B.W.; Wu, R.C. “MTA”-An Hydraulic Silicate Cement: Review update and setting reaction. Dent. Mater. 2011, 27, 407–422. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Brady, K.; Sweeney, R.; Curtis, R.V.; Ford, T.R. The constitution of mineral trioxide aggregate. Dent. Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Dammaschke, T.; Gerth, H.U.; Züchner, H.; Schäfer, E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent. Mater. 2005, 21, 731–738. [Google Scholar] [CrossRef]
- Tomás-Catalá, C.J.; Collado-González, M.; García-Bernal, D.; Oñate-Sánchez, R.E.; Forner, L.; Llena, C.; Lozano, A.; Moraleda, J.M.; Rodríguez-Lozano, F.J. Biocompatibility of New Pulp-capping Materials NeoMTA Plus, MTA Repair HP, and Biodentine on Human Dental Pulp Stem Cells. J. Endod. 2018, 44, 126–132. [Google Scholar] [CrossRef]
- Okiji, T.; Yoshiba, K. Reparative Dentinogenesis Induced by Mineral Trioxide Aggregate: A Review from the Biological and Physicochemical Points of View. Int J. Dent. 2009, 2009, 464280. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Hadimli, H.H.; Ataoglu, H.; Orstavik, D. Antibacterial Effect of Selected Root-End Filling Materials. J. Endod. 2006, 32, 345–349. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Naghshbandi, J.; Oglesby, S.; Simon, J.H.; Rotstein, I. Comparison of Antifungal Activity of White-Colored and Gray-Colored Mineral Trioxide Aggregate (MTA) at Similar Concentrations Against Candida albicans. J. Endod. 2006, 32, 365–367. [Google Scholar] [CrossRef]
- Kaneko, T.; Gu, B.; Sone, P.P.; Zaw, S.Y.M.; Murano, H.; Zaw, Z.C.T.; Okiji, T. Dental Pulp Tissue Engineering Using Mesenchymal Stem Cells: A Review with a Protocol. Stem Cell Rev. Rep. 2018, 14, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.K.; Caicedo, R.; Ritwik, P.; Moiseyeva, R.; Kawashima, I. Physicochemical Basis of the Biologic Properties of Mineral Trioxide Aggregate. J. Endod. 2005, 31, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Bozeman, T.B.; Lemon, R.R.; Eleazer, P.D. Elemental Analysis of Crystal Precipitate from Gray and White MTA. J. Endod. 2006, 32, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Rueggeberg, F.A.; Loushine, R.J.; Weller, R.N. Calcium Phosphate Phase Transformation Produced by the Interaction of the Portland Cement Component of White Mineral Trioxide Aggregate with a Phosphate-containing Fluid. J. Endod. 2007, 33, 1347–1351. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Felippe, M.S.; Felippe, W.T. Biomineralization Ability and Interaction of Mineral Trioxide Aggregate and White Portland Cement with Dentin in a Phosphate-containing Fluid. J. Endod. 2009, 35, 731–736. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T.; Okawa, S. Morphological and chemical analysis of different precipitates on mineral trioxide aggregate immersed in different fluids. Dent. Mater. J. 2010, 29, 512–517. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Tinti, A.; Prati, C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int. Endod. J. 2010, 43, 917–929. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Cornélio, A.L.G.; Mestieri, L.B.; Fuentes, A.S.C.; Salles, L.P.; Rossa-Junior, C.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Human dental pulp cells response to mineral trioxide aggregate (MTA) and MTA Plus: Cytotoxicity and gene expression analysis. Int. Endod. J. 2017, 50, 780–789. [Google Scholar] [CrossRef]
- Eid, A.A.; Gosier, J.L.; Primus, C.M.; Hammond, B.D.; Susin, L.F.; Pashley, D.H.; Tay, F.R. In Vitro Biocompatibility and Oxidative Stress Profiles of Different Hydraulic Calcium Silicate Cements. J. Endod. 2014, 40, 255–260. [Google Scholar] [CrossRef]
- Schneider, R.; Holland, G.R.; Chiego, D., Jr.; Hu, J.C.; Nör, J.E.; Botero, T.M. White Mineral Trioxide Aggregate Induces Migration and Proliferation of Stem Cells from the Apical Papilla. J. Endod. 2014, 40, 931–936. [Google Scholar] [CrossRef]
- Park, S.J.; Heo, S.M.; Hong, S.O.; Hwang, Y.C.; Lee, K.W.; Min, K.S. Odontogenic Effect of a Fast-setting Pozzolan-based Pulp Capping Material. J. Endod. 2014, 40, 1124–1131. [Google Scholar] [CrossRef]
- Minamikawa, H.; Yamada, M.; Deyama, Y.; Suzuki, K.; Kaga, M.; Yawaka, Y.; Ogawa, T. Effect of N-acetylcysteine on Rat Dental Pulp Cells Cultured on Mineral Trioxide Aggregate. J. Endod. 2011, 37, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Niu, L.N.; Primus, C.M.; Opperman, L.A.; Pashley, D.H.; Watanabe, I.; Tay, F.R. In Vitro Osteogenic/Dentinogenic Potential of an Experimental Calcium Aluminosilicate Cement. J. Endod. 2013, 39, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Lee, K.N.; Koh, J.T.; Min, K.S.; Chang, H.S.; Hwang, I.N.; Hwang, Y.C.; Oh, W.M. Effects of 3 Endodontic Bioactive Cements on Osteogenic Differentiation in Mesenchymal Stem Cells. J. Endod. 2014, 40, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Ke, M.C.; Chen, Y.H.; Kuo, H.K.; Yu, H.J.; Chen, C.T.; Tseng, Y.C.; Chuang, P.C.; Wu, P.C. Mineral trioxide aggregate affects cell viability and induces apoptosis of stem cells from human exfoliated deciduous teeth. BMC Pharmacol. Toxicol. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Karakida, T.; Onuma, K.; Saito, M.M.; Yamamoto, R.; Chiba, T.; Chiba, R.; Hidaka, Y.; Fujii-Abe, K.; Kawahara, H.; Yamakoshi, Y. Potential for Drug Repositioning of Midazolam for Dentin Regeneration. Int. J. Mol. Sci. 2019, 20, 670. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Niwa, T.; Karakida, T.; Kobayashi, K.; Yamamoto, R.; Chiba, R.; Yamakoshi, Y.; Hosoya, N. Effects of Er:YAG and Diode Laser Irradiation on Dental Pulp Cells and Tissues. Int. J. Mol. Sci. 2018, 19, 2429. [Google Scholar] [CrossRef]
- Niwa, T.; Yamakoshi, Y.; Yamazaki, H.; Karakida, T.; Chiba, R.; Hu, J.C.; Nagano, T.; Yamamoto, R.; Simmer, J.P.; Margolis, H.C.; et al. The dynamics of TGF-β in dental pulp, odontoblasts and dentin. Sci. Rep. 2018, 8, 4450. [Google Scholar] [CrossRef]
- Nagano, T.; Oida, S.; Suzuki, S.; Iwata, T.; Yamakoshi, Y.; Ogata, Y.; Gomi, K.; Arai, T.; Fukae, M. Porcine Enamel Protein Fractions Contain Transforming Growth Factor-β1. J. Periodontol. 2006, 77, 1688–1694. [Google Scholar] [CrossRef]
- Maruoka, Y.; Oida, S.; Iimura, T.; Takeda, K.; Asahina, I.; Enomoto, S.; Sasaki, S. Production of functional human bone morphogenetic protein-2 using a baculovirus/Sf-9 insect cell system. Biochem. Mol. Biol. Int. 1995, 35, 957–963. [Google Scholar]
- Yamamoto, R.; Oida, S.; Yamakoshi, Y. Dentin Sialophosphoprotein-derived Proteins in the Dental Pulp. J. Dent. Res. 2015, 94, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A review of the literature on the efficacy of mineral trioxide aggregate in conservative dentistry. Dent. Mater. J. 2019, 38, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Takita, T.; Hayashi, M.; Takeichi, O.; Ogiso, B.; Suzuki, N.; Otsuka, K.; Ito, K. Effect of mineral trioxide aggregate on proliferation of cultured human dental pulp cells. Int. Endod. J. 2006, 39, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.V.; Fradkov, A.F.; Labas, Y.A.; Savitsky, A.P.; Zaraisky, A.G.; Markelov, M.L.; Lukyanov, S.A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999, 17, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Strack, R.L.; Strongin, D.E.; Bhattacharyya, D.; Tao, W.; Berman, A.; Broxmeyer, H.E.; Keenan, R.J.; Glick, B.S. A noncytotoxic DsRed variant for whole-cell labeling. Nat. Methods 2008, 5, 955–957. [Google Scholar] [CrossRef]
- Torabinejad, M.; Hong, C.U.; McDonald, F.; Pitt Ford, T.R. Physical and Chemical Properties of a New Root-End Filling Material. J. Endod. 1995, 21, 349–353. [Google Scholar] [CrossRef]
- Fridland, M.; Rosado, R. Mineral Trioxide Aggregate (MTA) Solubility and Porosity with Different Water-to-Powder Ratios. J. Endod. 2003, 29, 814–817. [Google Scholar] [CrossRef]
- Fridland, M.; Rosado, R. MTA Solubility: A Long Term Study. J. Endod. 2005, 31, 376–379. [Google Scholar] [CrossRef]
- Saidon, J.; He, J.; Zhu, Q.; Safavi, K.; Spångberg, L.S. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2003, 95, 483–489. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Harrington, H.A.; Ho, K.L.; Ghosh, S.; Tung, K.C. Construction and analysis of a modular model of caspase activation in apoptosis. Theor. Biol. Med. Model. 2008, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Ohsawa, S.; Hamada, S.; Asou, H.; Kuida, K.; Uchiyama, Y.; Yoshida, H.; Miura, M. Caspase-9 Activation Revealed by Semaphorin 7A Cleavage Is Independent of Apoptosis in the Aged Olfactory Bulb. J. Neurosci. 2009, 29, 11385–11392. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Motani, R.; Sakuta, T.; Yamaguchi, N.; Koga, T.; Matsuo, K.; Nagaoka, S.; Abeyama, K.; Maruyama, I.; Torii, M. The Role of Vascular Endothelial Growth Factor in Human Dental Pulp Cells: Induction of Chemotaxis, Proliferation, and Differentiation and Activation of the AP-1-dependent Signaling Pathway. J. Dent. Res. 2000, 79, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp. Cell Res. 2012, 318, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Kim, J.M.; Choi, Y.; Park, K. MTA promotes chemotaxis and chemokinesis of immune cells through distinct calcium-sensing receptor signaling pathways. Biomaterials 2018, 150, 14–24. [Google Scholar] [CrossRef]
- Yang, X.; van der Kraan, P.M.; van den Dolder, J.; Walboomers, X.F.; Bian, Z.; Fan, M.; Jansen, J.A. STRO-1 Selected Rat Dental Pulp Stem Cells Transfected with Adenoviral-Mediated Human Bone Morphogenetic Protein 2 Gene Show Enhanced Odontogenic Differentiation. Tissue Engi. 2007, 13, 2803–2812. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Tucker, A.; Sharpe, P.T. Organized Tooth-specific Cellular Differentiation Stimulated by BMP4. J. Dent. Res. 2005, 84, 603–606. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Koivumäki, S.; Pääkkönen, V.; Ilvesaro, J.; Soini, Y.; Salo, T.; Metsikkö, K.; Tuukkanen, J. Polarity of Mature Human Odontoblasts. J. Dent. Res. 2013, 92, 1011–1016. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Sun, X.; Bai, S.; Li, S.; Shi, J. Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch. Oral Biol. 2011, 56, 1221–1229. [Google Scholar] [CrossRef]
- Tomson, P.L.; Grover, L.M.; Lumley, P.J.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 2007, 35, 636–642. [Google Scholar] [CrossRef]
- Da Rosa, W.L.O.; Piva, E.; da Silva, A.F. Disclosing the physiology of pulp tissue for vital pulp therapy. Int. Endod. J. 2018, 51, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, L.; Li, K.; Xie, N.; Xi, Y.; Wang, Y.; Zheng, X.; Chen, X.; Wang, M.; Ye, X. Zinc-modified Calcium Silicate Coatings Promote Osteogenic Differentiation through TGF-β/Smad Pathway and Osseointegration in Osteopenic Rabbits. Sci. Rep. 2017, 7, 3440. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, J.; Li, Y.; Li, Y.; Jing, L.; Yang, M.; Wang, J.; Sun, Z. Silica nanoparticles induce liver fibrosis via TGF-β1/Smad3 pathway in ICR mice. Int. J. Nanomed. 2017, 12, 6045–6057. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Kuratate, M.; Shigetani, Y.; Han, L.; Okiji, T. Compositional Change of Mineral Trioxide Aggregate Immersed in Water: Alteration of Elemental Distribution in the Surface Layer. Jpn. J. Coservative Dent. 2009, 52, 348–354. [Google Scholar]
- Sloan, A.J.; Rutherford, R.B.; Smith, A.J. Stimulation of the rat dentine–pulp complex by bone morphogenetic protein-7 in vitro. Arch. Oral Biol. 2000, 45, 173–177. [Google Scholar] [CrossRef]
- Chen, S.; Gu, T.T.; Sreenath, T.; Kulkarni, A.B.; Karsenty, G.; MacDougall, M. Spatial Expression of Cbfa1/Runx2 Isoforms in Teeth and Characterization of Binding Sites in the DSPP Gene. Connect. Tissue Res. 2002, 43, 338–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori-Sanuki, T.; Karakida, T.; Chiba-Ohkuma, R.; Miake, Y.; Yamamoto, R.; Yamakoshi, Y.; Hosoya, N. Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate. Cells 2020, 9, 2336. https://doi.org/10.3390/cells9102336
Hattori-Sanuki T, Karakida T, Chiba-Ohkuma R, Miake Y, Yamamoto R, Yamakoshi Y, Hosoya N. Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate. Cells. 2020; 9(10):2336. https://doi.org/10.3390/cells9102336
Chicago/Turabian StyleHattori-Sanuki, Tamaki, Takeo Karakida, Risako Chiba-Ohkuma, Yasuo Miake, Ryuji Yamamoto, Yasuo Yamakoshi, and Noriyasu Hosoya. 2020. "Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate" Cells 9, no. 10: 2336. https://doi.org/10.3390/cells9102336
APA StyleHattori-Sanuki, T., Karakida, T., Chiba-Ohkuma, R., Miake, Y., Yamamoto, R., Yamakoshi, Y., & Hosoya, N. (2020). Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate. Cells, 9(10), 2336. https://doi.org/10.3390/cells9102336







