ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca2+ and Energy Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Buffers, and Solutions
2.2. Cell Culture and Transfection
2.3. Measuring Subcellular ATP and Ca2+ Dynamics Using Genetically Encoded Fluorescent Biosensors
2.4. Live Cell Imaging of Endoplasmic Reticulum (ER)-to-Golgi Transport
2.5. Live Cell Imaging of Vesicle Movements
2.6. Imaging and Analysis of the ER and Microtubule Network
2.7. Calculation of Form Factor Changes in Fluorescent Structures
2.8. Statistical Analysis
3. Results
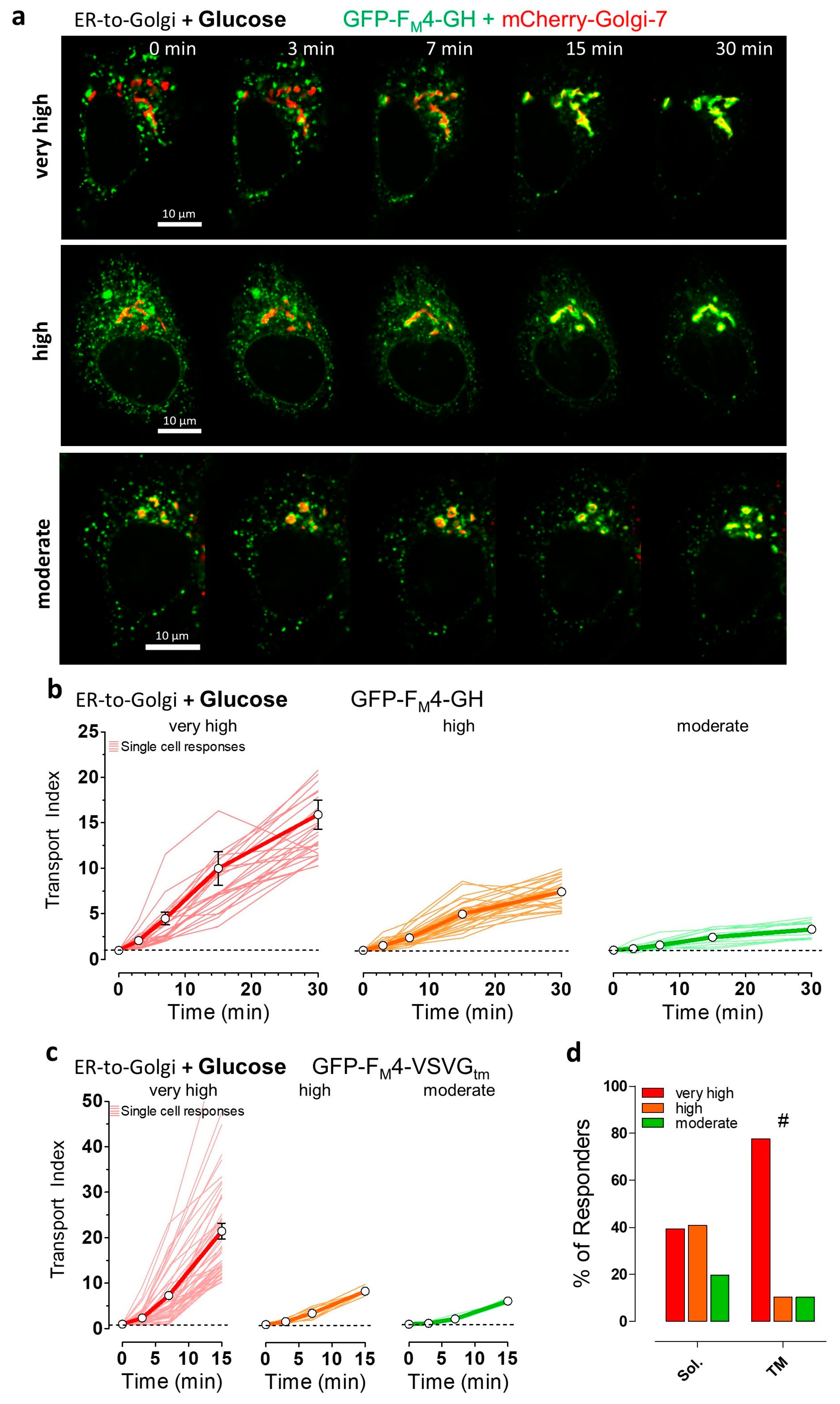
3.1. Single Cell Analysis Revealed Cargo-Dependent, Robust, and Efficient ER-to-Golgi Transport in HeLa Cells
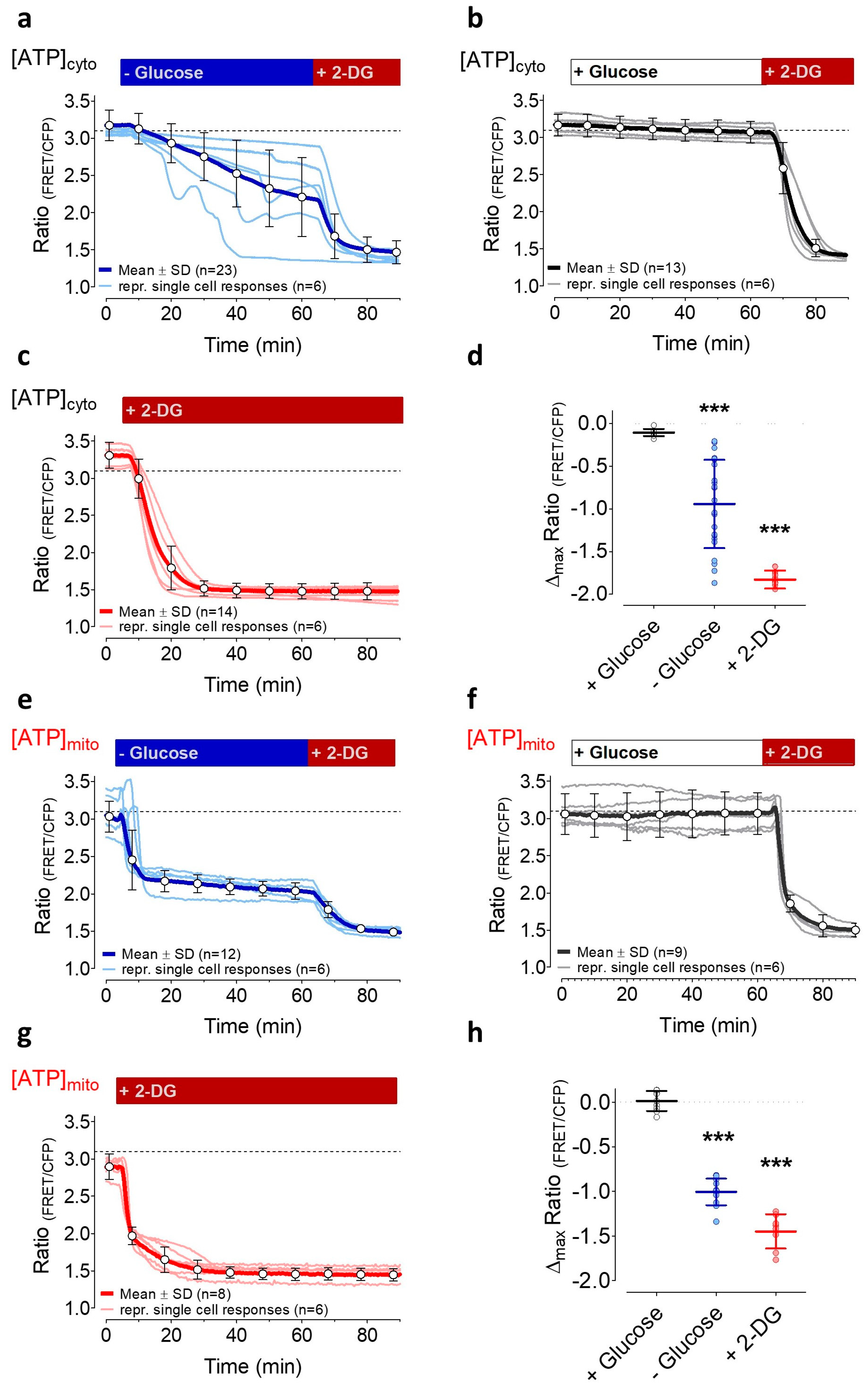
3.2. Induction of Energy Stresses Characteristically Lowers Subcellular ATP Levels
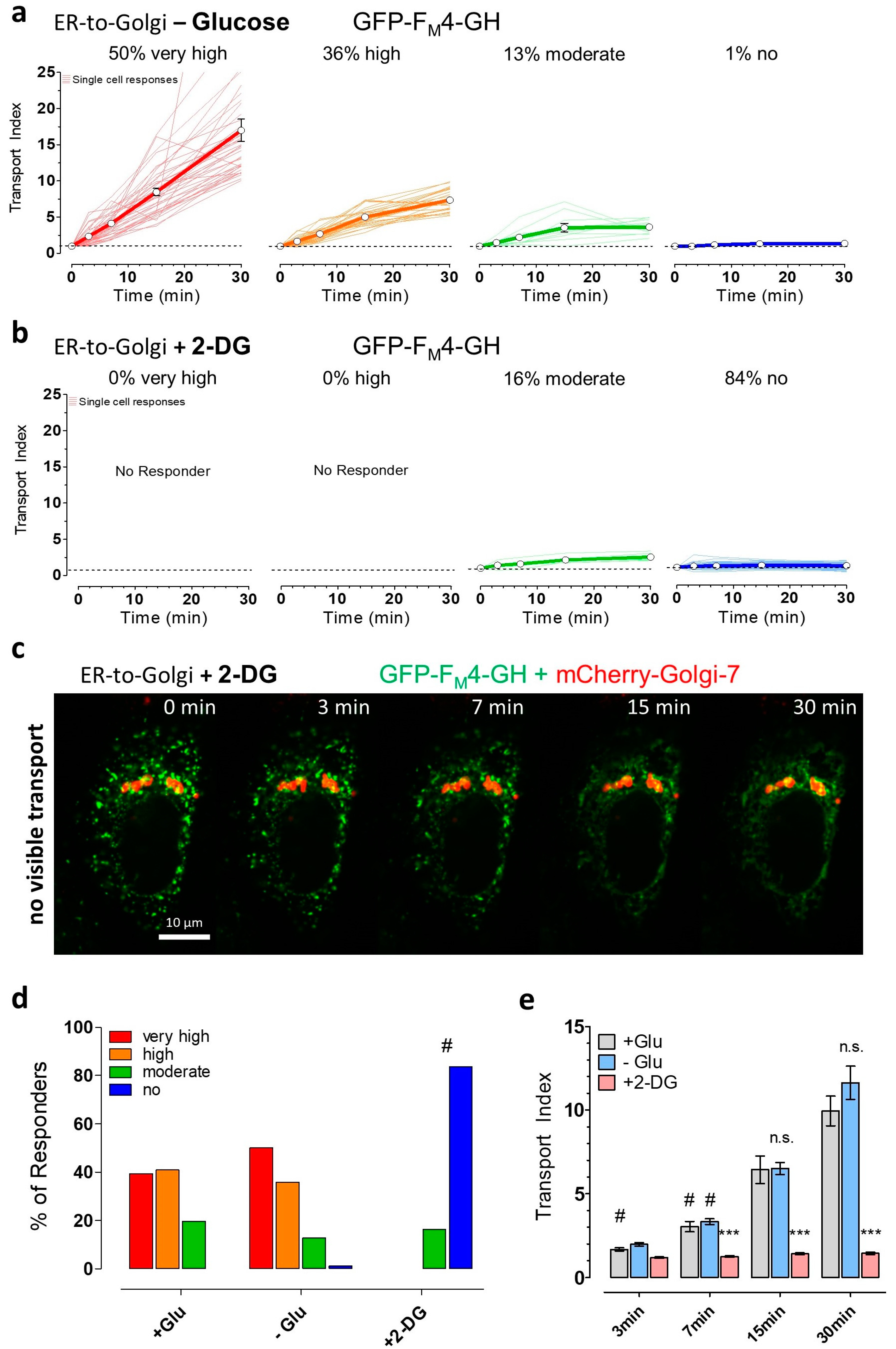
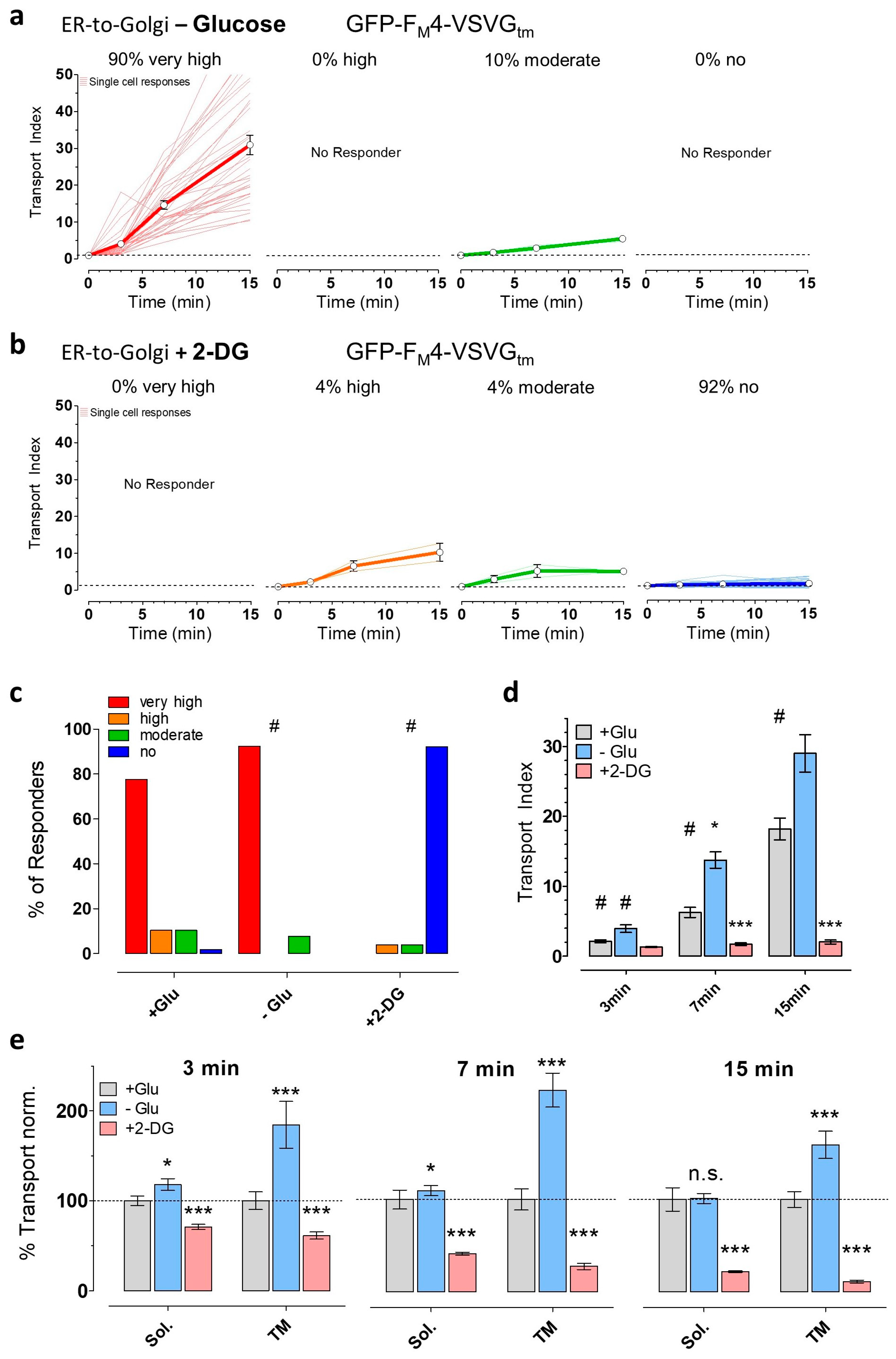
3.3. Partial ATP Depletion by Glucose Deprivation Enhances ER-to-Golgi Transport but Complete ATP Depletion Using 2-DG Prevents this Transport
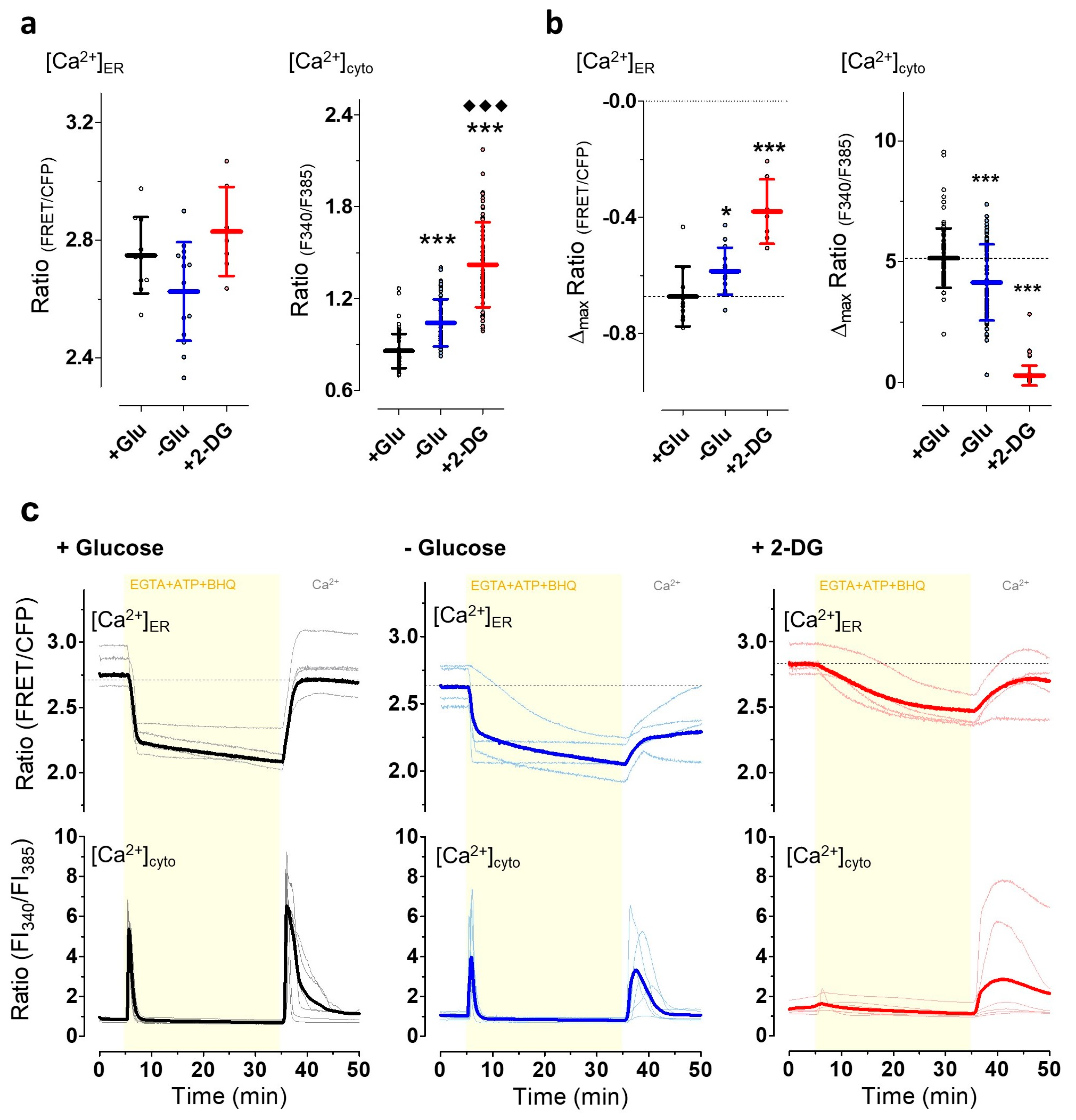
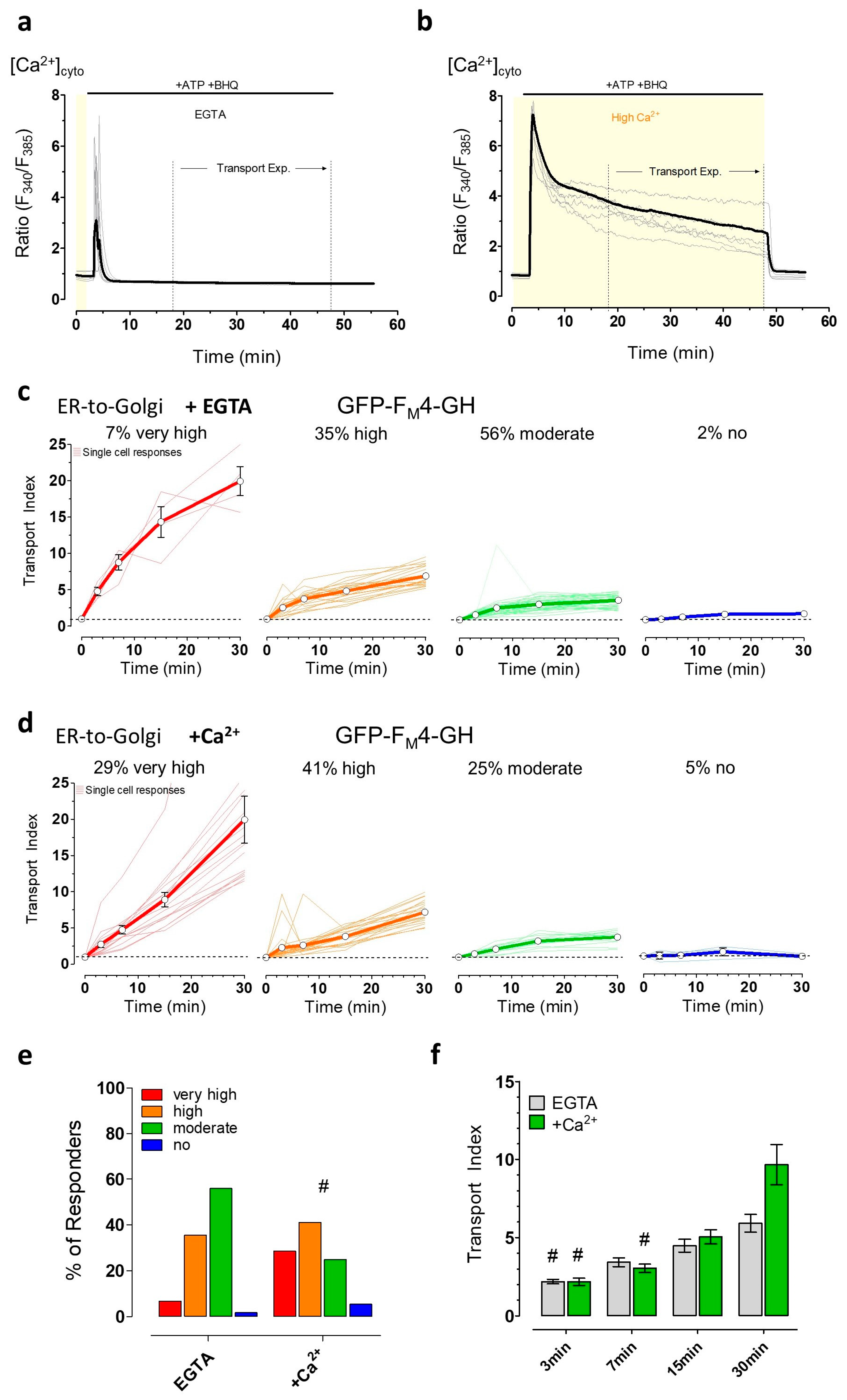
3.4. The Induction of Energy Stress Strikingly Alters Cellular Ca2+ Homeostasis and Mobilization, and Ca2+ Signals Differentially Impact ER-to-Golgi Transport as Compared with 2-DG
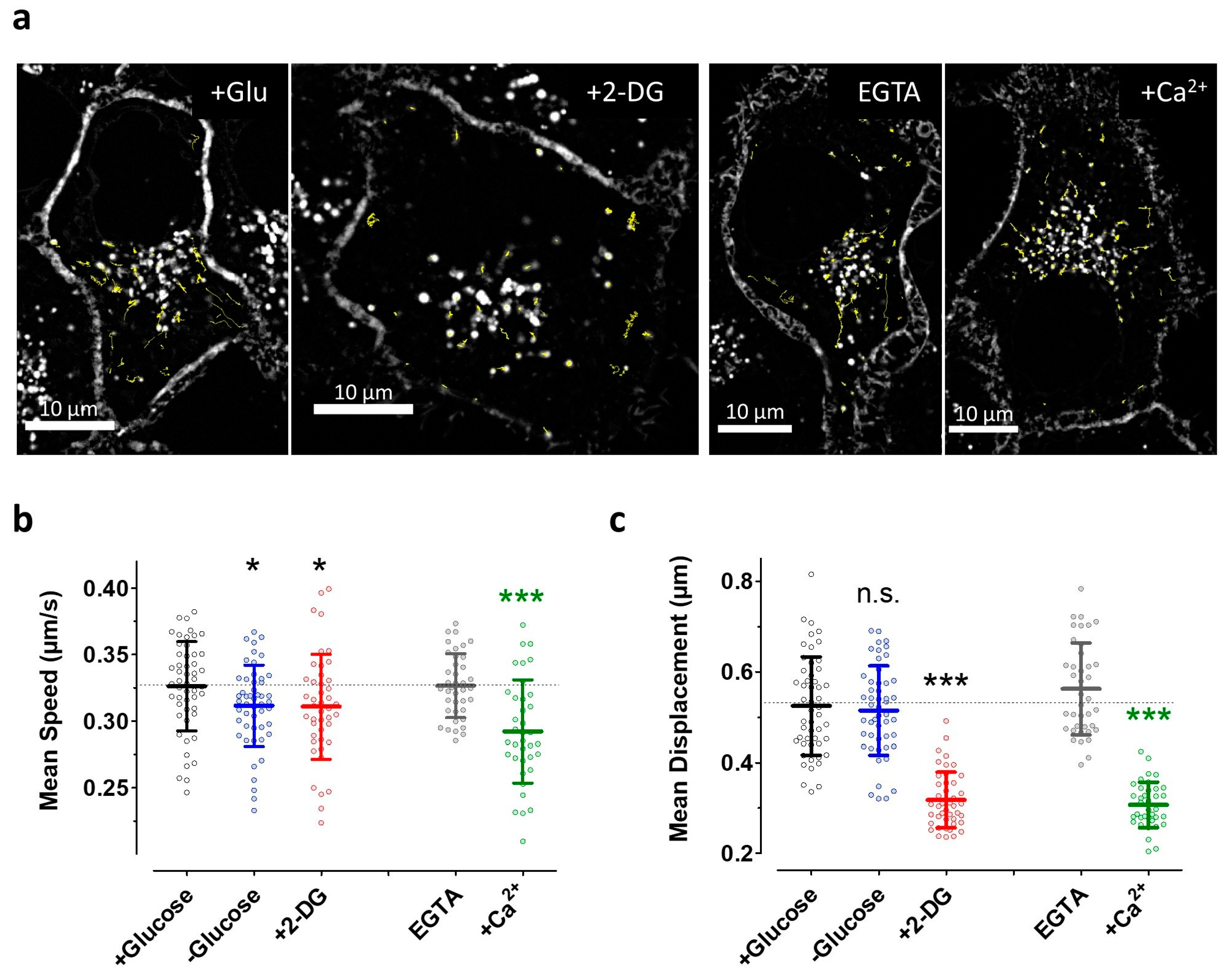
3.5. The Induction of Energy Stress by 2-DG and Cytosolic Ca2+ Elevations both Efficiently Impair Vesicle Movements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yarwood, R.; Hellicar, J.; Woodman, P.G.; Lowe, M. Membrane trafficking in health and disease. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.J.; Cliff, J.M.; Thomas, J.A.; Ward, T.H. Biogenesis of secretory organelles during B cell differentiation. J. Leukocyte Biol. 2010, 87, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Dejeans, N.; Manié, S.; Hetz, C.; Bard, F.; Hupp, T.; Agostinis, P.; Samali, A.; Chevet, E. Addicted to secrete – novel concepts and targets in cancer therapy. Trends Mol. Med. 2014, 20, 242–250. [Google Scholar] [CrossRef]
- Levine, C.G.; Mitra, D.; Sharma, A.; Smith, C.L.; Hegde, R.S. The Efficiency of Protein Compartmentalization into the Secretory Pathway. Mol. Biol. Cell 2005, 16, 279–291. [Google Scholar] [CrossRef]
- Dancourt, J.; Barlowe, C. Protein Sorting Receptors in the Early Secretory Pathway. Annu. Rev. Biochem. 2010, 79, 777–802. [Google Scholar] [CrossRef]
- Kanapin, A.; Batalov, S.; Davis, M.J.; Gough, J.; Grimmond, S.; Kawaji, H.; Magrane, M.; Matsuda, H.; Schönbach, C.; Teasdale, R.D.; et al. Mouse Proteome Analysis. Genome Res. 2003, 13, 1335–1344. [Google Scholar] [CrossRef]
- Martínez, J.; Marmisolle, I.; Tarallo, D.; Quijano, C. Mitochondrial Bioenergetics and Dynamics in Secretion Processes. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef]
- Molinari, M.; Sitia, R. The Secretory Capacity of a Cell Depends on the Efficiency of Endoplasmic Reticulum-Associated Degradation. In Dislocation and Degradation of Proteins from the Endoplasmic Reticulum; Wiertz, E., Kikkert, M., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin\Heidelberg, Germany; Volume 300, pp. 1–15.
- Wang, B.; Stanford, K.R.; Kundu, M. ER-to-Golgi Trafficking and Its Implication in Neurological Diseases. Cells 2020, 9, 408. [Google Scholar] [CrossRef]
- Hetz, C.; Glimcher, L.H. Protein homeostasis networks in physiology and disease. Curr. Opin. Cell Biol. 2011, 23, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.; Rahman, J.; Achermann, J.C.; Dattani, M.T.; Rahman, S. Mitochondrial disease and endocrine dysfunction. Nat. Rev. Endocrinol. 2017, 13, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Devine, M.J.; Kittler, J.T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Haythorne, E.; Rohm, M.; van de Bunt, M.; Brereton, M.F.; Tarasov, A.I.; Blacker, T.S.; Sachse, G.; Silva dos Santos, M.; Terron Exposito, R.; Davis, S.; et al. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. Tumor Cells and the Onset of Cancer. In Molecular Cell Biology, 4th ed.; W. H. Freeman: New York, NY, USA, 2000; section 24.1. [Google Scholar]
- Bonnin, D.A.A.; Havrda, M.C.; Israel, M.A. Glioma Cell Secretion: A Driver of Tumor Progression and a Potential Therapeutic Target. Cancer Res. 2018, 78, 6031–6039. [Google Scholar] [CrossRef]
- Short, B. Determining the dynamics of cancer cell secretion. J. Gen. Physiol. 2019, 151, 1333. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pavlou, M.P.; Diamandis, E.P. Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol. Oncol. 2010, 4, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.M.; Fusenig, N.E. Friends or foes — bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 2004, 4, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Kano, A. Tumor cell secretion of soluble factor(s) for specific immunosuppression. Sci. Rep. 2015, 5, 8913. [Google Scholar] [CrossRef]
- Jensen, D.; Schekman, R. COPII-mediated vesicle formation at a glance. J. Cell Sci. 2011, 124, 1–4. [Google Scholar] [CrossRef]
- Budnik, A.; Stephens, D.J. ER exit sites – Localization and control of COPII vesicle formation. FEBS Lett. 2009, 583, 3796–3803. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Rowe, T.; Balch, W.E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996, 135, 19–35. [Google Scholar] [CrossRef]
- Appenzeller-Herzog, C.; Hauri, H.-P. The ER-Golgi intermediate compartment (ERGIC): In search of its identity and function. J. Cell Sci. 2006, 119, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Saraste, J.; Svensson, K. Distribution of the intermediate elements operating in ER to Golgi transport. J. Cell. Sci. 1991, 100, 415–430. [Google Scholar] [PubMed]
- Scales, S.J.; Pepperkok, R.; Kreis, T.E. Visualization of ER-to-Golgi Transport in Living Cells Reveals a Sequential Mode of Action for COPII and COPI. Cell 1997, 90, 1137–1148. [Google Scholar] [CrossRef]
- Murshid, A.; Presley, J.F. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell. Mol. Life Sci. 2004, 61, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Stalder, D.; Gershlick, D.C. Direct trafficking pathways from the Golgi apparatus to the plasma membrane. Semin. Cell Dev. Biol. 2020. [Google Scholar] [CrossRef]
- Ben-Tekaya, H.; Miura, K.; Pepperkok, R.; Hauri, H.-P. Live imaging of bidirectional traffic from the ERGIC. J. Cell Sci. 2005, 118, 357–367. [Google Scholar] [CrossRef]
- Luini, A.; Mavelli, G.; Jung, J.; Cancino, J. Control systems and coordination protocols of the secretory pathway. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef]
- Cole, N.B.; Ellenberg, J.; Song, J.; DiEuliis, D.; Lippincott-Schwartz, J. Retrograde Transport of Golgi-localized Proteins to the ER. J. Cell Biol. 1998, 140, 1–15. [Google Scholar] [CrossRef]
- Barlowe, C.K.; Miller, E.A. Secretory Protein Biogenesis and Traffic in the Early Secretory Pathway. Genetics 2013, 193, 383–410. [Google Scholar] [CrossRef]
- Xu, D.; Hay, J.C. Reconstitution of COPII vesicle fusion to generate a pre-Golgi intermediate compartment. J. Cell Biol. 2004, 167, 997–1003. [Google Scholar] [CrossRef]
- Presley, J.F.; Cole, N.B.; Schroer, T.A.; Hirschberg, K.; Zaal, K.J.M.; Lippincott-Schwartz, J. ER-to-Golgi transport visualized in living cells. Nature 1997, 389, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Dorner, A.J.; Kaufman, R.J. The Levels of Endoplasmic Reticulum Proteins and ATP Affect Folding and Secretion of Selective Proteins. Biologicals 1994, 22, 103–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dorner, A.J.; Wasley, L.C.; Kaufman, R.J. Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc. Natl. Acad. Sci. USA 1990, 87, 7429–7432. [Google Scholar] [CrossRef] [PubMed]
- Antoine, J.C.; Jouanne, C. Multiple effects of the phenylhydrazone derivative FCCP on the secretory pathway in rat plasma cells. Eur. J. Cell Biol. 1986, 42, 68–73. [Google Scholar] [PubMed]
- Argon, Y.; Burkhardt, J.K.; Leeds, J.M.; Milstein, C. Two steps in the intracellular transport of IgD are sensitive to energy depletion. J. Immunol. 1989, 142, 554–561. [Google Scholar]
- Depaoli, M.R.; Bischof, H.; Eroglu, E.; Burgstaller, S.; Ramadani-Muja, J.; Rauter, T.; Schinagl, M.; Waldeck-Weiermair, M.; Hay, J.C.; Graier, W.F.; et al. Live cell imaging of signaling and metabolic activities. Pharmacol. Ther. 2019, 202, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Depaoli, M.R.; Hay, J.C.; Graier, W.F.; Malli, R. The enigmatic ATP supply of the endoplasmic reticulum. Biol. Rev. Camb. Philos. Soc. 2019, 94, 610–628. [Google Scholar] [CrossRef]
- Depaoli, M.R.; Karsten, F.; Madreiter-Sokolowski, C.T.; Klec, C.; Gottschalk, B.; Bischof, H.; Eroglu, E.; Waldeck-Weiermair, M.; Simmen, T.; Graier, W.F.; et al. Real-Time Imaging of Mitochondrial ATP Dynamics Reveals the Metabolic Setting of Single Cells. Cell Rep. 2018, 25, 501–512. [Google Scholar] [CrossRef]
- Sargeant, J.; Costain, T.; Madreiter-Sokolowski, C.; Gordon, D.E.; Peden, A.A.; Mali, R.; Graier, W.F.; Hay, J.C. Calcium Sensors ALG-2 and Peflin Bind ER Exit Sites in Alternate States to Modulate Secretion in Response to Calcium Signaling. bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.02.22.944264v2.abstract (accessed on 9 October 2020).
- Bentley, M.; Nycz, D.C.; Joglekar, A.; Fertschai, I.; Malli, R.; Graier, W.F.; Hay, J.C. Vesicular Calcium Regulates Coat Retention, Fusogenicity, and Size of Pre-Golgi Intermediates. MBoC 2010, 21, 1033–1046. [Google Scholar] [CrossRef]
- Hay, J.C. Calcium: A fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007, 8, 236–240. [Google Scholar] [CrossRef]
- Helm, J.R.; Bentley, M.; Thorsen, K.D.; Wang, T.; Foltz, L.; Oorschot, V.; Klumperman, J.; Hay, J.C. Apoptosis-linked gene-2 (ALG-2)/Sec31 interactions regulate endoplasmic reticulum (ER)-to-Golgi transport: A potential effector pathway for luminal calcium. J. Biol. Chem. 2014, 289, 23609–23628. [Google Scholar] [CrossRef]
- Petersen, O.H.; Verkhratsky, A. Calcium and ATP control multiple vital functions. Phil. Trans. R. Soc. B 2016, 371. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.E.; Selvaggio, A.M.; Alexander, E.A.; Schwartz, J.H. Adenosine triphosphate depletion induces a rise in cytosolic free calcium in canine renal epithelial cells. J. Clin. Invest. 1988, 82, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Nhat, K.P.H.; Togawa, H.; Saito, K.; Iino, R.; Kato-Yamada, Y.; Nagai, T.; Noji, H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. PNAS 2009, 106, 15651–15656. [Google Scholar] [CrossRef]
- Vishnu, N.; Jadoon Khan, M.; Karsten, F.; Groschner, L.N.; Waldeck-Weiermair, M.; Rost, R.; Hallström, S.; Imamura, H.; Graier, W.F.; Malli, R. ATP increases within the lumen of the endoplasmic reticulum upon intracellular Ca2+ release. Mol. Biol. Cell 2014, 25, 368–379. [Google Scholar] [CrossRef]
- Verissimo, F.; Pepperkok, R. Imaging ER-to-Golgi transport: Towards a systems view. J. Cell Sci. 2013, 126, 5091–5100. [Google Scholar] [CrossRef] [PubMed]
- Presley, J.F. Imaging the secretory pathway: The past and future impact of live cell optical techniques. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1744, 259–272. [Google Scholar] [CrossRef]
- Boncompain, G.; Perez, F. Chapter 11 — Fluorescence-Based Analysis of Trafficking in Mammalian Cells. In Methods in Cell Biology; Perez, F., Stephens, D.J., Eds.; Methods for Analysis of Golgi Complex Function; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, pp. 179–194. [Google Scholar]
- Rollins, C.T.; Rivera, V.M.; Woolfson, D.N.; Keenan, T.; Hatada, M.; Adams, S.E.; Andrade, L.J.; Yaeger, D.; van Schravendijk, M.R.; Holt, D.A.; et al. A ligand-reversible dimerization system for controlling protein–protein interactions. Proc. Natl. Acad. Sci. USA 2000, 97, 7096–7101. [Google Scholar] [CrossRef]
- Rivera, V.M. Regulation of Protein Secretion Through Controlled Aggregation in the Endoplasmic Reticulum. Science 2000, 287, 826–830. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. The metabolism of cancer cells. Biochem. Z. 1924, 152, 319–344. [Google Scholar]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, S.; Bischof, H.; Gensch, T.; Stryeck, S.; Gottschalk, B.; Ramadani-Muja, J.; Eroglu, E.; Rost, R.; Balfanz, S.; Baumann, A.; et al. pH-Lemon, a Fluorescent Protein-Based pH Reporter for Acidic Compartments. ACS Sens. 2019, 4, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gerndt, S.; Chen, C.-C.; Chao, Y.-K.; Yuan, Y.; Burgstaller, S.; Scotto Rosato, A.; Krogsaeter, E.; Urban, N.; Jacob, K.; Nguyen, O.N.P.; et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. eLife 2020, 9, e54712. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; van Weeren, L.; Hink, M.A.; Vischer, N.O.E.; Jalink, K.; Gadella, T.W.J. Bright cyan fluorescent protein variants identified by fluorescence lifetime screening. Nat. Methods 2010, 7, 137–139. [Google Scholar] [CrossRef]
- Barlowe, C.; Helenius, A. Cargo Capture and Bulk Flow in the Early Secretory Pathway. Annu. Rev. Cell Dev. Biol. 2016, 32, 197–222. [Google Scholar] [CrossRef]
- Trahey, M.; Oh, H.S.; Cameron, C.E.; Hay, J.C. Poliovirus Infection Transiently Increases COPII Vesicle Budding. J. Virol. 2012, 86, 9675–9682. [Google Scholar] [CrossRef]
- Rayl, M.; Truitt, M.; Held, A.; Sargeant, J.; Thorsen, K.; Hay, J.C. Penta-EF-Hand Protein Peflin Is a Negative Regulator of ER-To-Golgi Transport. PLoS ONE 2016, 11, e0157227. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Sevier, C.S.; Weisz, O.A.; Davis, M.; Machamer, C.E. Efficient Export of the Vesicular Stomatitis Virus G Protein from the Endoplasmic Reticulum Requires a Signal in the Cytoplasmic Tail That Includes Both Tyrosine-based and Di-acidic Motifs. Mol. Biol. Cell 2000, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Jin, C.; Reed, J.C.; Tsien, R.Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. In Membrane Protein Complexes: Structure and Function; Harris, J.R., Boekema, E.J., Eds.; Subcellular Biochemistry; Springer: Singapore, 2018; pp. 229–258. ISBN 978-981-10-7757-9. [Google Scholar]
- Fusi, F.; Saponara, S.; Gagov, H.; Sgaragli, G. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca2+ channel via superoxide anion generation. Br. J. Pharmacol. 2001, 133, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Joyce, J.A. Pericellular proteolysis in cancer. Genes Dev. 2014, 28, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.D.; Joyce, J.A. Proteolytic Networks in Cancer. Trends Cell Biol. 2011, 21. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Misumi, Y.; Misumi, Y.; Miki, K.; Takatsuki, A.; Tamura, G.; Ikehara, Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 1986, 261, 11398–11403. [Google Scholar]
- Sausville, E.A.; Duncan, K.L.; Senderowicz, A.; Plowman, J.; Randazzo, P.A.; Kahn, R.; Malspeis, L.; Grever, M.R. Antiproliferative effect in vitro and antitumor activity in vivo of brefeldin A. Cancer J. Sci. Am. 1996, 2, 52–58. [Google Scholar]
- Tseng, C.-N.; Hong, Y.-R.; Chang, H.-W.; Yu, T.-J.; Hung, T.-W.; Hou, M.-F.; Yuan, S.-S.F.; Cho, C.-L.; Liu, C.-T.; Chiu, C.-C.; et al. Brefeldin A Reduces Anchorage-Independent Survival, Cancer Stem Cell Potential and Migration of MDA-MB-231 Human Breast Cancer Cells. Molecules 2014, 19, 17464–17477. [Google Scholar] [CrossRef]
- Tseng, C.-N.; Huang, C.-F.; Cho, C.-L.; Chang, H.-W.; Huang, C.-W.; Chiu, C.-C.; Chang, Y.-F. Brefeldin A Effectively Inhibits Cancer Stem Cell-Like Properties and MMP-9 Activity in Human Colorectal Cancer Colo 205 Cells. Molecules 2013, 18, 10242–10253. [Google Scholar] [CrossRef]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef]
- Tseng, P.-L.; Chen, C.-W.; Hu, K.-H.; Cheng, H.-C.; Lin, Y.-H.; Tsai, W.-H.; Cheng, T.-J.; Wu, W.-H.; Yeh, C.-W.; Lin, C.-C.; et al. The decrease of glycolytic enzyme hexokinase 1 accelerates tumor malignancy via deregulating energy metabolism but sensitizes cancer cells to 2-deoxyglucose inhibition. Oncotarget 2018, 9, 18949–18969. [Google Scholar] [CrossRef][Green Version]
- Sato, K.; Nakano, A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat. Struct. Mol. Biol. 2005, 12, 167–174. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Wang, X.; Zhao, W.; Chen, J.J. Expression and transcriptional profiling of the LKB1 tumor suppressor in cervical cancer cells. Gynecol. Oncol. 2014, 134, 372–378. [Google Scholar] [CrossRef]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; DePinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Weaver, D.; Hajnóczky, G. Control of mitochondrial motility and distribution by the calcium signal. J. Cell Biol. 2004, 167, 661–672. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauter, T.; Burgstaller, S.; Gottschalk, B.; Ramadani-Muja, J.; Bischof, H.; Hay, J.C.; Graier, W.F.; Malli, R. ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca2+ and Energy Stresses. Cells 2020, 9, 2311. https://doi.org/10.3390/cells9102311
Rauter T, Burgstaller S, Gottschalk B, Ramadani-Muja J, Bischof H, Hay JC, Graier WF, Malli R. ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca2+ and Energy Stresses. Cells. 2020; 9(10):2311. https://doi.org/10.3390/cells9102311
Chicago/Turabian StyleRauter, Thomas, Sandra Burgstaller, Benjamin Gottschalk, Jeta Ramadani-Muja, Helmut Bischof, Jesse C. Hay, Wolfgang F. Graier, and Roland Malli. 2020. "ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca2+ and Energy Stresses" Cells 9, no. 10: 2311. https://doi.org/10.3390/cells9102311
APA StyleRauter, T., Burgstaller, S., Gottschalk, B., Ramadani-Muja, J., Bischof, H., Hay, J. C., Graier, W. F., & Malli, R. (2020). ER-to-Golgi Transport in HeLa Cells Displays High Resilience to Ca2+ and Energy Stresses. Cells, 9(10), 2311. https://doi.org/10.3390/cells9102311






