Mutual Interaction of Clinical Factors and Specific microRNAs to Predict Mild Cognitive Impairment in Patients Receiving Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
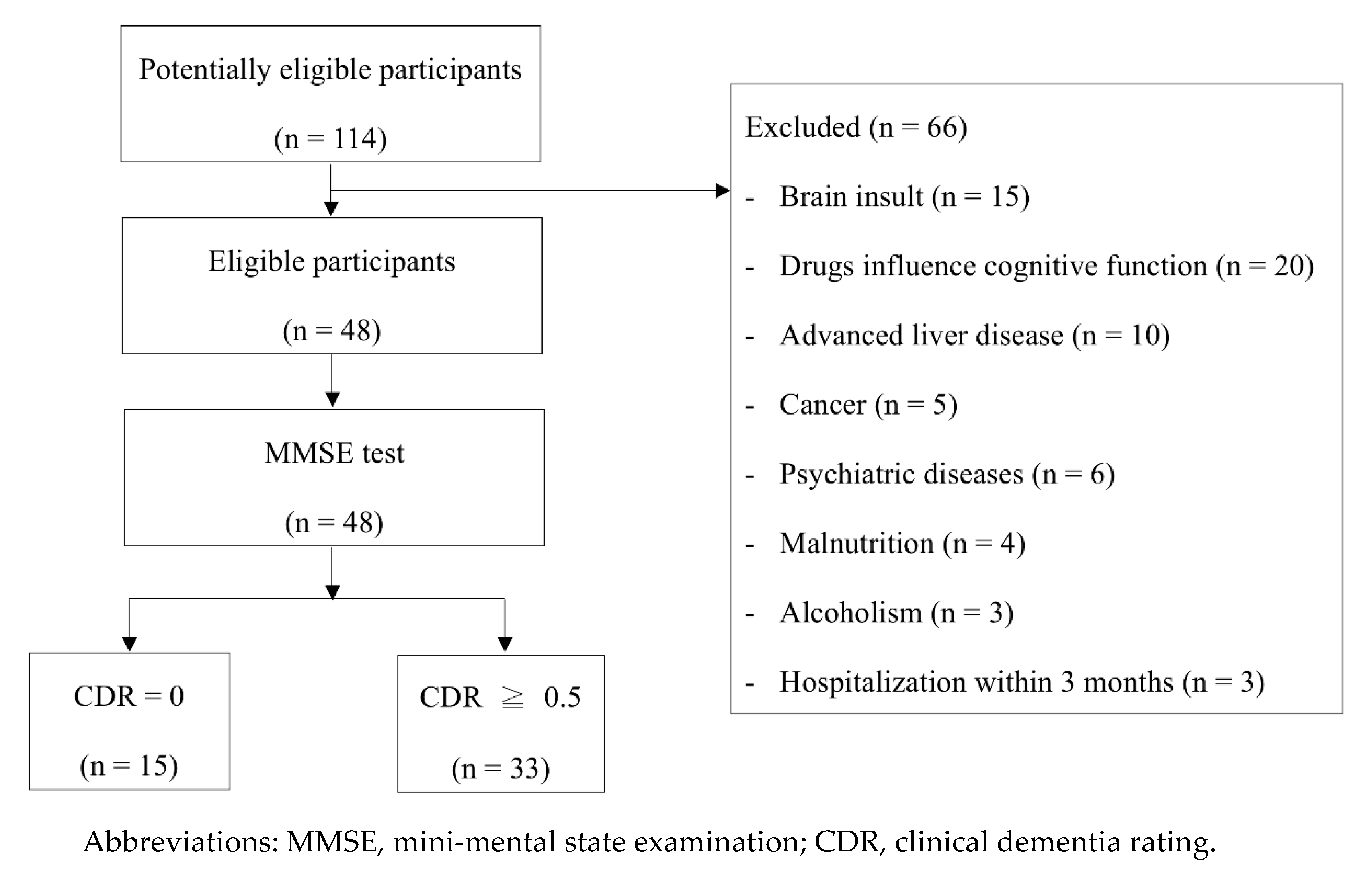
2.1. Participants
2.2. Laboratory Measurement
2.3. Nerve-Injury Proteins
2.4. Measurement of Serum miRNAs Levels
2.5. Method of NGS
2.6. Quantitative PCR for miRNAs
2.7. Mini-Mental State Examination
2.8. Statistical Analyses
3. Results
Baseline Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kurella Tamura, M.; Yaffe, K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011, 79, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Tighiouart, H.; Scott, T.M.; Lou, K.V.; Sorensen, E.P.; Giang, L.M.; Drew, D.A.; Shaffi, K.; Strom, J.A.; Singh, A.K.; et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology 2013, 80, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Ackerson, L.; Tamura, M.K.; Ma, P.L.B.; Kusek, J.W.; Sehgal, A.R.; Cohen, D.; Anderson, C.; Appel, L.; DeSalvo, K.; et al. Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J. Am. Geriatr. Soc. 2010, 58, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, S.E.; Koudstaal, P.J.; Oudkerk, M.; Hofman, A.; Breteler, M.M. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam scan study. Stroke 2002, 33, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.M. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv. Chronic Kidney Dis. 2008, 15, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nagasawa, H.; Iseki, C.; Takahashi, Y.; Sato, H.; Arawaka, S.; Kawanami, T.; Kurita, K.; Daimon, M.; Kato, T. Cerebral small vessel disease and chronic kidney disease (CKD): Results of a cross-sectional study in community-based Japanese elderly. J. Neurol. Sci. 2008, 272, 36–42. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, H.; Hou, B.; You, H.; Yuan, J.; Luo, K.; Chen, L.; Li, M.; Xu, Q.; Zhu, Y.; et al. Malnutrition-inflammation is a risk factor for cerebral small vessel diseases and cognitive decline in peritoneal dialysis patients: A cross-sectional observational study. BMC Nephrol. 2017, 18, 366. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Tan, J.; Salter, M.L.; Gross, A.; Meoni, L.A.; Jaar, B.G.; Kao, W.-H.L.; Parekh, R.S.; Segev, D.L.; Sozio, S.M. Frailty and cognitive function in incident hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 2181–2189. [Google Scholar] [CrossRef]
- Lu, R.; Kiernan, M.C.; Murray, A.; Rosner, M.H.; Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 2015, 11, 707–719. [Google Scholar] [CrossRef]
- Seidel, U.K.; Gronewold, J.; Volsek, M.; Todica, O.; Kribben, A.; Bruck, H.; Hermann, D.M. Physical, cognitive and emotional factors contributing to quality of life, functional health and participation in community dwelling in chronic kidney disease. PLoS ONE 2014, 9, e91176. [Google Scholar] [CrossRef]
- Banerjee, G.; Karia, S.; Varley, J.; Brown, E.A. Cognitive impairment in elderly renal inpatients: An under-identified phenomenon. Nephron Clin. Pract. 2014, 126, 19–23. [Google Scholar] [CrossRef]
- Moorhouse, P.; Rockwood, K. Vascular cognitive impairment: Current concepts and clinical developments. Lancet Neurol. 2008, 7, 246–255. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, T.; Nakayama, M. Cerebro-renal interactions: Impact of uremic toxins on cognitive function. Neurotoxicology 2014, 44, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.F.; Chen, J.B.; Hsieh, K.C.; Liou, C.W. Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery 2008, 143, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Gul, A.; Sarnak, M.J. Cognitive function in chronic kidney disease. Semin. Dial. 2008, 21, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, M.H.; Kleinveld, H.A.; Dekker, F.W.; Van Munster, B.C.; Rabelink, T.J.; Van Buren, M.; Mooijaart, S.P. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD—A systematic review. Clin. J. Am. Soc. Nephrol. 2016, 11, 1624–1639. [Google Scholar] [CrossRef]
- Drew, D.A.; Weiner, D.E. Cognitive impairment in chronic kidney disease: Keep vascular disease in mind. Kidney Int. 2014, 85, 505–507. [Google Scholar] [CrossRef]
- Florkowski, C.M. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: Communicating the performance of diagnostic tests. Clin. Biochem. Rev. 2008, 29 (Suppl. 1), S83–S87. [Google Scholar]
- Hajian-Tilaki, K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Pan, C.-T.; Tsai, K.-W.; Hung, T.-M.; Lin, W.-C.; Pan, C.-Y.; Yu, H.-R.; Li, S.-C. miRSeq: A user-friendly standalone toolkit for sequencing quality evaluation and miRNA profiling. Biomed Res. Int. 2014, 2014, 462135. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Yu, R.-L.; Lee, W.-J.; Li, J.-Y.; Chang, Y.-Y.; Chen, C.-C.; Lin, J.-J.; Sung, Y.-F.; Lin, T.-K.; Fuh, J.-L. Evaluating mild cognitive dysfunction in patients with Parkinson’s disease in clinical practice in Taiwan. Sci. Rep. 2020, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.C.; Wilson, R.S.; Tang, Y.; Dong, X.; Murray, A.; Bennett, D.A. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology 2009, 32, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.C.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Hemoglobin level in older persons and incident Alzheimer disease: Prospective cohort analysis. Neurology 2011, 77, 219–226. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Sojkova, J.; Beason-Held, L.L.; An, Y.; Longo, D.L.; Ferrucci, L.; Resnick, S.M. Patterns of regional cerebral blood flow associated with low hemoglobin in the Baltimore longitudinal study of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 963–969. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Bahrainwala, Z.; Wityk, R.J.; Hillis, A.E. Neglect is more common and severe at extreme hemoglobin levels in right hemispheric stroke. Stroke 2010, 41, 1641–1645. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Ojo, J.O.; Rezaie, P.; Gabbott, P.L.; Stewart, M.G. Impact of age-related neuroglial cell responses on hippocampal deterioration. Front. Aging Neurosci. 2015, 7, 57. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Zhang, X.; Wu, B.; Liu, X.; Shen, Z. The spatial association of gene expression evolves from synchrony to asynchrony and stochasticity with age. PLoS ONE 2011, 6, e24076. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Guarente, L. Genetics and the specificity of the aging process. Science 2003, 299, 1351–1354. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lee, H.-J.; Yang, S.-C.; Chen, T.-F.; Lin, K.-N.; Lin, C.-C.; Wang, P.-N.; Tang, L.-Y.; Chiu, M.-J. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS ONE 2014, 9, e100303. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Kim, W.-K.; Choi, Y.-B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Kommer, T.N.V.D.; Dik, M.; Comijs, H.; Jonker, C.; Deeg, D. Homocysteine and inflammation: Predictors of cognitive decline in older persons? Neurobiol. Aging 2010, 31, 1700–1709. [Google Scholar] [CrossRef]
- Sheinerman, K.S.; Tsivinsky, V.G.; Abdullah, L.; Crawford, F.; Umansky, S.R. Plasma microRNA biomarkers for detection of mild cognitive impairment: Biomarker validation study. Aging 2013, 5, 925–938. [Google Scholar] [CrossRef]
- Moller, R.S.; Dahl, H.A.; Helbig, I. The contribution of next generation sequencing to epilepsy genetics. Expert Rev. Mol. Diagn. 2015, 15, 1531–1538. [Google Scholar] [CrossRef]
- Renkema, K.Y.; Stokman, M.F.; Giles, R.H.; Knoers, N.V. Next-generation sequencing for research and diagnostics in kidney disease. Nat. Rev. Nephrol. 2014, 10, 433–444. [Google Scholar] [CrossRef]
- Glatt, K.; Glatt, H.; Lalande, M. Structure and organization of GABRB3 and GABRA5. Genomics 1997, 41, 63–69. [Google Scholar] [CrossRef]
- Cook, E.H.; Courchesne, R.Y.; Cox, N.J.; Lord, C.; Gonen, D.; Guter, S.J.; Lincoln, A.; Nix, K.; Haas, R.; Leventhal, B.L.; et al. Linkage-disequilibrium mapping of autistic disorder, with 15q11-13 markers. Am. J. Hum. Genet. 1998, 62, 1077–1083. [Google Scholar] [CrossRef]
- Trzybulska, D.; Eckersten, D.; Giwercman, A.; Christensson, A.; Tsatsanis, C. Alterations in serum MicroRNA profile during hemodialysis—Potential biological implications. Cell. Physiol. Biochem. 2018, 46, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Seifter, J.L.; Samuels, M.A. Uremic encephalopathy and other brain disorders associated with renal failure. Semin. Neurol. 2011, 31, 139–143. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, P.P.; Vanholder, R.; Eloot, S.; Glorieux, G. Guanidino compounds as uremic (neuro)toxins. Semin. Dial. 2009, 22, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Ingrosso, D.; Satta, E.; Lombardi, C.; Galletti, P.; D’Aniello, A.; de Santo, N.G. Plasma protein aspartyl damage is increased in hemodialysis patients: Studies on causes and consequences. J. Am. Soc. Nephrol. 2004, 15, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Schmechel, D.E.; Brightman, M.W.; Marangos, P.J. Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res. 1980, 190, 195–214. [Google Scholar] [CrossRef]
- Marangos, P.J.; Schmechel, D.E.; Parma, A.M.; Goodwin, F.K. Developmental profile of neuron-specific (NSE) and non-neuronal (NNE) enolase. Brain Res. 1980, 190, 185–193. [Google Scholar] [CrossRef]
- Kirino, T.; Brightman, M.W.; Oertel, W.H.; Schmechel, D.E.; Marangos, P.J. Neuron-specific enolase as an index of neuronal regeneration and reinnervation. J. Neurosci. 1983, 3, 915–923. [Google Scholar] [CrossRef]
| Variables | CDR = 0 (n = 15) | CDR ≥ 0.5 (n = 33) | p | Cohen’s d | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 59.1 | ±8.1 | 64.0 | ±9.5 | 0.092 | −0.50 |
| Gender (men, %) | 5 | 33.33% | 18 | 54.55% | 0.173 | |
| Education level | 0.786 | |||||
| No | 0 | 0.00% | 2 | 6.06% | ||
| Primary school | 5 | 33.33% | 6 | 18.18% | ||
| Elementary school | 2 | 13.33% | 4 | 12.12% | ||
| High school | 5 | 33.33% | 11 | 33.33% | ||
| Bachelor | 2 | 13.33% | 4 | 12.12% | ||
| Unknown | 1 | 6.67% | 6 | 18.18% | ||
| Laboratory measurement | ||||||
| Kt/V | 1.83 | ±0.39 | 1.82 | ±0.41 | 0.968 | −0.001 |
| Hb (g/dL) | 9.92 | ±0.9 | 10.83 | ±1.15 | 0.010 | −0.97 |
| Albumin (g/dL) | 3.80 | ±0.24 | 3.84 | ±0.36 | 0.685 | −0.14 |
| GOT (U/L) (median, interquartile range) | 19 | 14–25 | 18.5 | 15–27.5 | 0.404 | −0.26 |
| Small water-soluble solutes | ||||||
| ADMA (µmol/L) (median, interquartile range) | 3.28 | 0.9–5.74 | 3.28 | 0.63–5.95 | 0.511 | −0.23 |
| 8OHDG (ng/mL) | 27.48 | ±0.67 | 27.07 | ±0.69 | 0.058 | 0.52 |
| BUN (mg/dL) | 75.00 | ±23.38 | 59.36 | ±15.9 | 0.009 | 0.88 |
| Cr (mg/dL) | 10.72 | ±4.29 | 9.31 | ±2.4 | 0.149 | 0.45 |
| Ca (mg/dL) | 9.51 | ±0.68 | 9.22 | ±1.77 | 0.492 | −0.08 |
| P (mg/dL) | 5.76 | ±2.21 | 5.00 | ±0.45 | 0.661 | 0.50 |
| K (mEq/L) | 4.47 | ±0.84 | 4.36 | ±0.89 | 0.551 | −0.21 |
| Protein-bound solutes | ||||||
| PCS (µg/mL) | 25.14 | ±16.81 | 27.00 | ±21.18 | 0.766 | −0.22 |
| IS (µg/mL) | 46.05 | ±20.94 | 38.85 | ±18.83 | 0.241 | 0.28 |
| Homocysteine (µmol/mL) | 26.82 | ±7.9 | 29.70 | ±10.15 | 0.351 | −0.29 |
| Middle molecules | ||||||
| IL-1β(pg/mL) (median, interquartile range) | 0.76 | 0.65–0.95 | 0.66 | 0.65–0.85 | 0.728 | -0.13 |
| IL-6(pg/mL) (median, interquartile range) | 6.47 | 2.42–10.92 | 4.18 | 2.8–5.34 | 0.275 | 0.31 |
| IL-18(ng/mL) (median, interquartile range) | 111.99 | 90.13–166.47 | 108.64 | 90.78–140.18 | 0.122 | 0.50 |
| TNF-α (pg/mL) | 35.39 | ±10.98 | 34.88 | ±12.34 | 0.893 | −0.03 |
| iPTH (pg/dL) | 260.7 | 108.7–715.8 | 190.85 | 121.55–507.4 | 0.392 | 0.18 |
| Beta-2-microglobulin (µg/L) (median, interquartile range) | 27,700 | 20,740–31,619.5 | 25,933.95 | 20,695.75–31,985.15 | 0.835 | 0.13 |
| Molecular markers of nerve injury | ||||||
| NSE (ng/mL) (median, interquartile range) | 1556.04 | 936.89–2952.33 | 2418.23 | 1084.04–3520.94 | 0.896 | −0.04 |
| HSP 70 (ng/mL) (median, interquartile range) | 0.14 | 0.08–0.16 | 0.13 | 0.11–0.14 | 0.294 | −0.38 |
| S100B (pg/mL) (median, interquartile range) | 83.58 | 57.05–142.02 | 83.58 | 25.26–157.98 | 0.892 | −0.13 |
| MicroRNA | ||||||
| miR-134 (median, interquartile range) | 0.53 | 0.33–1.77 | 0.51 | 0.15–2.48 | 0.563 | −0.17 |
| miR-182 (median, interquartile range) | 0.09 | 0.03–0.36 | 0.06 | 0.04–0.23 | 0.970 | −0.04 |
| miR-451 (median, interquartile range) | 4.92 | 0.36–10.69 | 1.9 | 0.49–10.04 | 0.284 | −0.32 |
| miR-486 (median, interquartile range) | 32.38 | 22.18–188.2 | 111.14 | 33.96–269.09 | 0.643 | −0.07 |
| Variable | AUC | Best Cutoff Value | Sensitivity | Specificity | Correctly Classified |
|---|---|---|---|---|---|
| Age | 0.708 | 63 | 69.70% | 66.67% | 68.75% |
| Gender | 0.606 | Male | 54.55% | 66.67% | 58.33% |
| Education level | 0.517 | Above Elementary school | 70.37% | 35.71% | 58.54% |
| Laboratory measurement | |||||
| Kt/V | 0.470 | 1.3 | 100.00% | 13.33% | 72.34% |
| Hb | 0.792 | 10.7 | 66.67% | 93.33% | 75.00% |
| Albumin | 0.503 | 4.08 | 28.13% | 86.67% | 46.81% |
| Small water-soluble solutes | |||||
| ADMA | 0.476 | 1.85 | 60.61% | 46.67% | 56.25% |
| 8OHDG | 0.339 | 25.6 | 100.00% | 0.00% | 68.75% |
| BUN | 0.297 | 31 | 96.97% | 0.00% | 66.67% |
| Cr | 0.409 | 11.2 | 33.33% | 73.33% | 45.83% |
| Ca | 0.487 | 10.3 | 24.24% | 93.33% | 45.83% |
| P | 0.406 | 3.8 | 84.85% | 20.00% | 64.58% |
| Protein-bound solutes | |||||
| PCS | 0.497 | 53.8 | 15.15% | 100.00% | 41.67% |
| IS | 0.398 | 21.1 | 93.94% | 13.33% | 68.75% |
| Homocysteine | 0.571 | 27.97 | 65.63% | 57.14% | 63.04% |
| Middle molecules | |||||
| IL-1β | 0.405 | 16.41 | 3.03% | 100.00% | 33.33% |
| IL-6 | 0.393 | 2.49 | 81.82% | 26.67% | 64.58% |
| IL-18 | 0.439 | 99.6 | 60.61% | 46.67% | 56.25% |
| TNF-α | 0.481 | 53.5 | 9.09% | 100.00% | 37.50% |
| iPTH | 0.464 | 54.4 | 87.50% | 20.00% | 65.96% |
| Beta-2-microglobulin | 0.504 | 29040 | 40.63% | 73.33% | 51.06% |
| Molecular markers of nerve injury | |||||
| NSE | 0.565 | 2418.23 | 51.52% | 73.33% | 58.33% |
| HSP 70 | 0.477 | 0.06 | 96.97% | 13.33% | 70.83% |
| S100B | 0.477 | 227.27 | 18.18% | 93.33% | 41.67% |
| MicroRNA | |||||
| miR-134 | 0.501 | 1.22 | 40.63% | 73.33% | 51.06% |
| miR-182 | 0.483 | 0.02 | 93.75% | 14.29% | 69.57% |
| miR-451 | 0.503 | 0.93 | 69.70% | 46.67% | 62.50% |
| miR-486 | 0.614 | 32.68 | 78.79% | 53.33% | 70.83% |
| Cumulated Top-Ranked Variables *,1 | Variable | Cumulative AUC | Standard Error | 95% Confidence Interval |
|---|---|---|---|---|
| 2 | Hb and Age | 0.837 | 0.065 | 0.71–0.965 |
| 3 | Above plus miR-486 | 0.897 | 0.047 | 0.806–0.988 |
| 4 | Above plus Gender | 0.874 | 0.054 | 0.768–0.981 |
| 5 | Above plus Homocysteine | 0.835 | 0.070 | 0.698–0.971 |
| 6 | Above plus NSE | 0.848 | 0.063 | 0.725–0.971 |
| 7 | Above plus Education level | 0.828 | 0.064 | 0.702–0.954 |
| 8 | Above plus Beta-2-microglobulin | 0.824 | 0.065 | 0.697–0.951 |
| 9 | Above plus Albumin | 0.827 | 0.068 | 0.694–0.959 |
| 10 | Above plus miR-451 | 0.798 | 0.072 | 0.657–0.939 |
| 11 | Above plus miR-134 | 0.794 | 0.076 | 0.644–0.943 |
| 12 | Above plus PCS | 0.799 | 0.075 | 0.652–0.946 |
| 13 | Above plus Ca | 0.819 | 0.070 | 0.682–0.955 |
| 14 | Above plus miR-182 | 0.800 | 0.073 | 0.658–0.943 |
| 15 | Above plus TNF-α | 0.806 | 0.071 | 0.666–0.945 |
| 16 | Above plus HSP 70 | 0.799 | 0.074 | 0.655–0.943 |
| 17 | Above plus S100B | 0.800 | 0.075 | 0.653–0.947 |
| 18 | Above plus ADMA | 0.815 | 0.073 | 0.671–0.958 |
| 19 | Above plus Kt/V | 0.823 | 0.073 | 0.681–0.965 |
| 20 | Above plus iPTH | 0.833 | 0.073 | 0.69–0.976 |
| 21 | Above plus IL-18 | 0.835 | 0.068 | 0.701–0.968 |
| 22 | Above plus Cr | 0.810 | 0.074 | 0.664–0.955 |
| 23 | Above plus P | 0.812 | 0.075 | 0.666–0.958 |
| 24 | Above plus IL-1β | 0.812 | 0.075 | 0.666–0.958 |
| 25 | Above plus IS | 0.804 | 0.075 | 0.657–0.951 |
| 26 | Above plus IL-6 | 0.808 | 0.074 | 0.662–0.954 |
| 27 | Above plus 8OHDG | 0.808 | 0.074 | 0.662–0.954 |
| 28 | Above plus BUN | 0.804 | 0.075 | 0.658–0.951 |
| Number of Dichotomized Variables * | Sensitivity (95% CI) | Specificity (95% CI) | Youden’s Index | Correctly Classified | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|
| S1 | 100% (89.4%-100%) | 26.67% (7.79%-55.1%) | 26.67% | 77.08% | 1.36 (1.01–1.85) | - |
| S2 | 81.82% (64.5%-93%) | 86.67% (59.5%-98.3%) | 68.49% | 83.33% | 6.14 (1.67–22.5) | 0.21 (0.1–0.44) |
| S3 | 27.27% (13.3%-45.5%) | 100% (78.2%-100%) | 27.27% | 50.00% | - | 0.73 (0.59–0.9) |
| Cumulated Risk Score | Total | Hb ≥ 10.7 | Age ≥ 63 | miR-486 ≥ 32.68 | |||
|---|---|---|---|---|---|---|---|
| (n = 21) | (n = 28) | (n = 33) | |||||
| n | n | % | n | % | n | % | |
| S0 | 4 | - | - | - | - | - | - |
| S1 | 15 | 2 | 13.3 | 3 | 20.0 | 10 | 66.7 |
| S2 | 20 | 10 | 50.0 | 16 | 80.0 | 14 | 70.0 |
| S3 | 9 | 9 | 100.0 | 9 | 100.0 | 9 | 100.0 |
| Variable | OR (95%CI) | p |
|---|---|---|
| Univariate | ||
| Hb | 2.29 (1.13–4.64) | 0.022 |
| Age | 1.06 (0.99–1.13) | 0.106 |
| miR-486 | 4.24 (1.14–15.79) | 0.031 |
| Multivariate | ||
| Hb | 2.74 (1.13–6.67) | 0.026 |
| Age | 1.04 (0.96–1.13) | 0.351 |
| miR-486 | 7.54 (1.47–38.6) | 0.015 |
| 2-Order Interaction | ||
| Hb*Age | 1.01 (1.001–1.01) | 0.019 |
| Hb*miR-486 | 1.17 (1.03–1.32) | 0.016 |
| Age*miR-486 | 1.03 (1.004–1.05) | 0.019 |
| 3-Order Interaction | ||
| Hb*Age*miR-486 | Omitted | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-B.; Chang, C.-C.; Li, L.-C.; Lee, W.-C.; Lin, C.-N.; Li, S.-C.; Moi, S.-H.; Yang, C.-H. Mutual Interaction of Clinical Factors and Specific microRNAs to Predict Mild Cognitive Impairment in Patients Receiving Hemodialysis. Cells 2020, 9, 2303. https://doi.org/10.3390/cells9102303
Chen J-B, Chang C-C, Li L-C, Lee W-C, Lin C-N, Li S-C, Moi S-H, Yang C-H. Mutual Interaction of Clinical Factors and Specific microRNAs to Predict Mild Cognitive Impairment in Patients Receiving Hemodialysis. Cells. 2020; 9(10):2303. https://doi.org/10.3390/cells9102303
Chicago/Turabian StyleChen, Jin-Bor, Chiung-Chih Chang, Lung-Chih Li, Wen-Chin Lee, Chia-Ni Lin, Sung-Chou Li, Sin-Hua Moi, and Cheng-Hong Yang. 2020. "Mutual Interaction of Clinical Factors and Specific microRNAs to Predict Mild Cognitive Impairment in Patients Receiving Hemodialysis" Cells 9, no. 10: 2303. https://doi.org/10.3390/cells9102303
APA StyleChen, J.-B., Chang, C.-C., Li, L.-C., Lee, W.-C., Lin, C.-N., Li, S.-C., Moi, S.-H., & Yang, C.-H. (2020). Mutual Interaction of Clinical Factors and Specific microRNAs to Predict Mild Cognitive Impairment in Patients Receiving Hemodialysis. Cells, 9(10), 2303. https://doi.org/10.3390/cells9102303








