Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis
Abstract
1. Introduction
- the dependencies of mean dwell-times of (conducting/non-conducting) states on the duration of the previous state (here called “conditional mean dwell-times”),
- Hurst exponent by the R/S method [46],
- generalized Hurst exponent (scaling exponent) by the detrended fluctuation analysis (DFA) [47],
- spectral parameters obtained by MFDFA analysis [48].
2. Materials and Methods
2.1. Cell Culture
2.2. Mitoplast Preparation
2.3. Electrophysiological Recordings
2.4. Analysis of Experimental Data
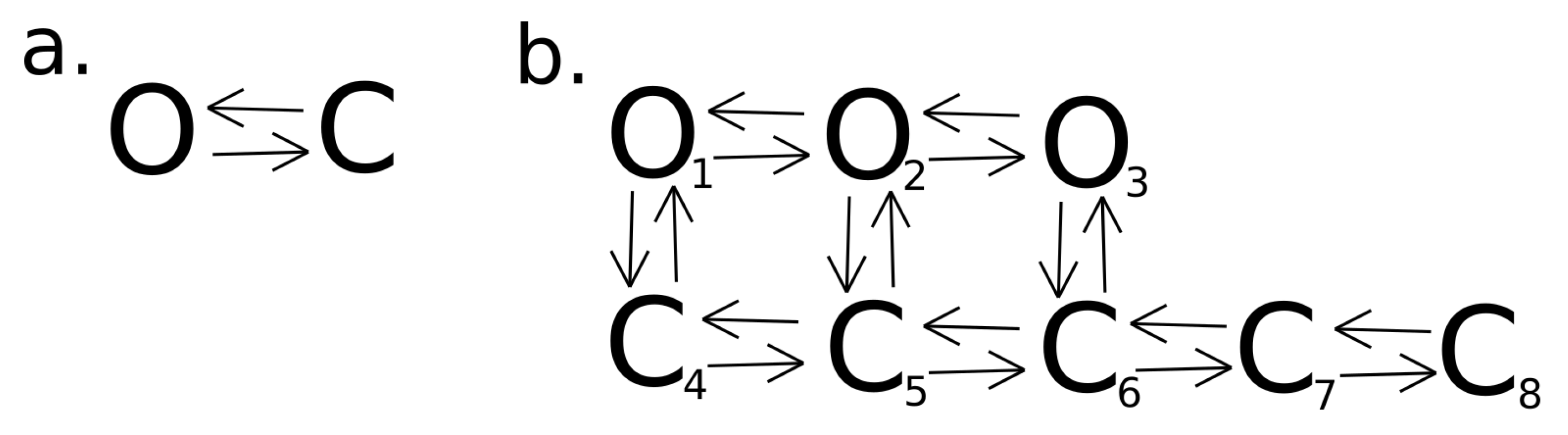
2.4.1. Construction of Dwell-Time Series
- plotting the probability density function (PDF) of ionic current approximated by the nonparametric kernel density estimate with Epanechnikov kernel with logarithmic scales on both the horizontal and vertical axes,
- finding the intervals, where the power-law scaling is satisfied,
- finding the point intersection of the power-law plots (between the unimodal densities representing functionally open and closed states of a channel). The current value corresponding to this point of intersection indicates the .
2.4.2. Conditional Mean Dwell-Times of Conducting/Non-Conducting States
2.4.3. Hurst R/S Analysis
2.4.4. Detrended Fluctuation Analysis (DFA)
2.4.5. Multifractal Detrended Fluctuation Analysis (MDFA)
2.5. Statistical Analysis
3. Results and Discussion
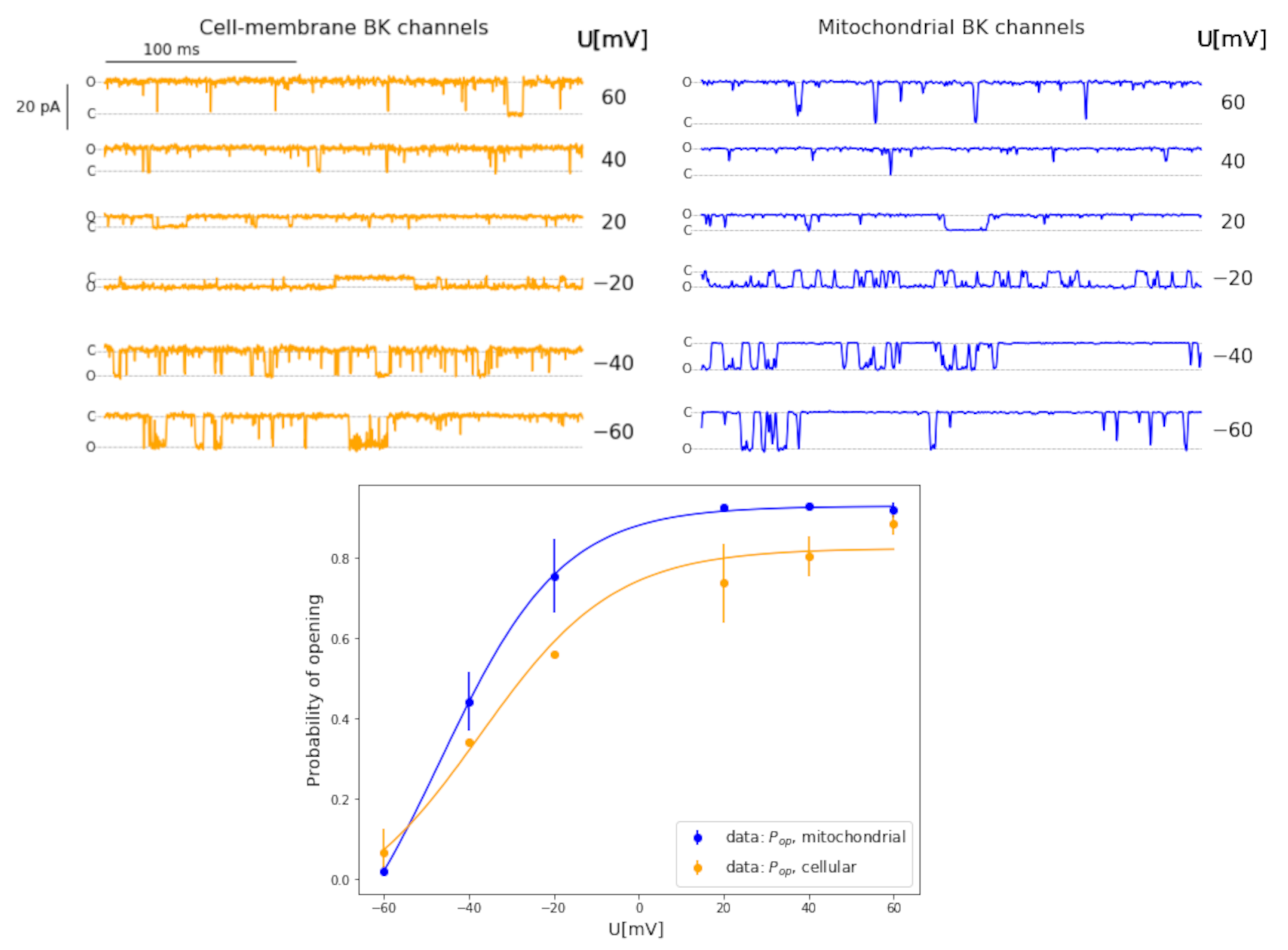
3.1. Kinetic Features
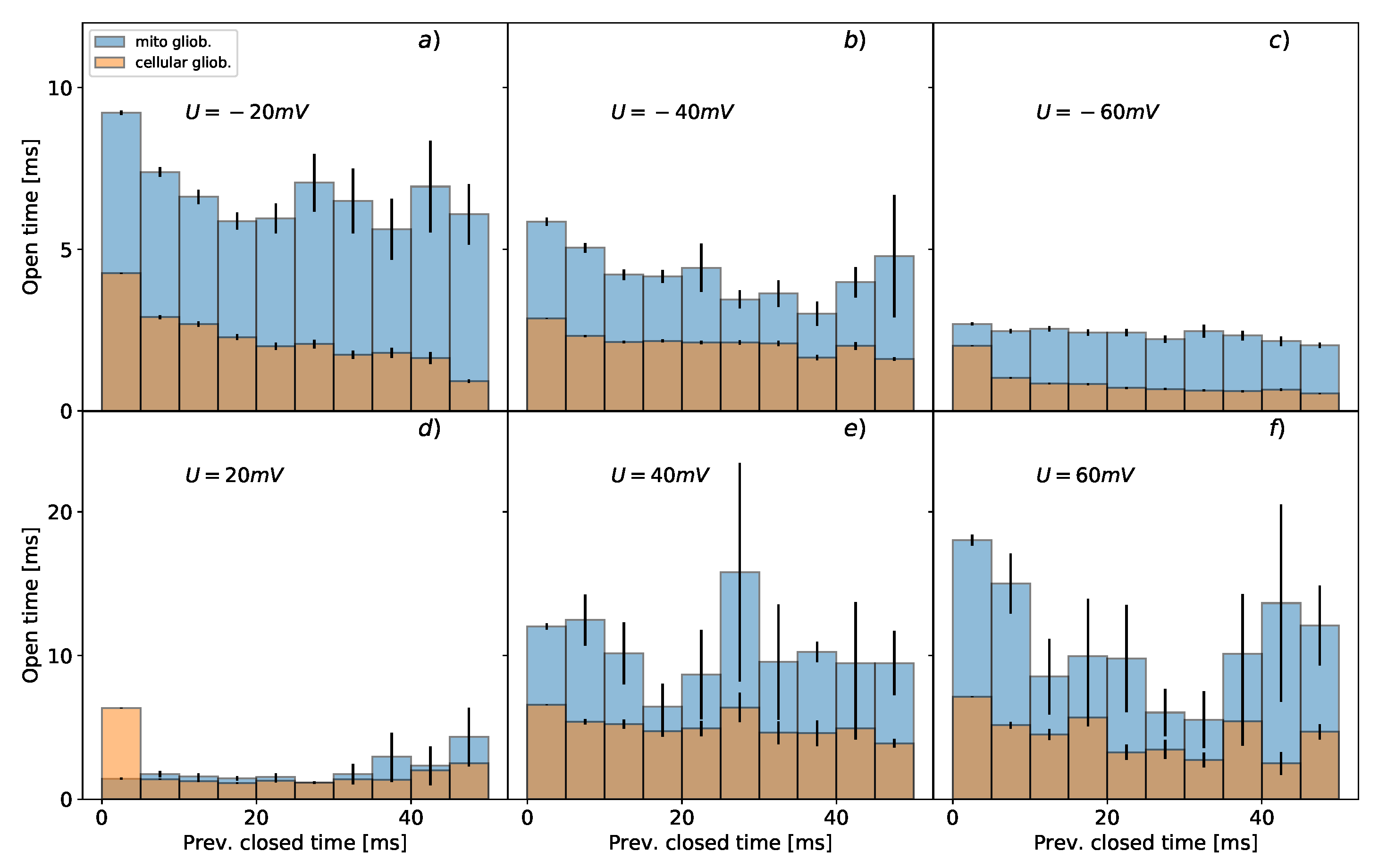
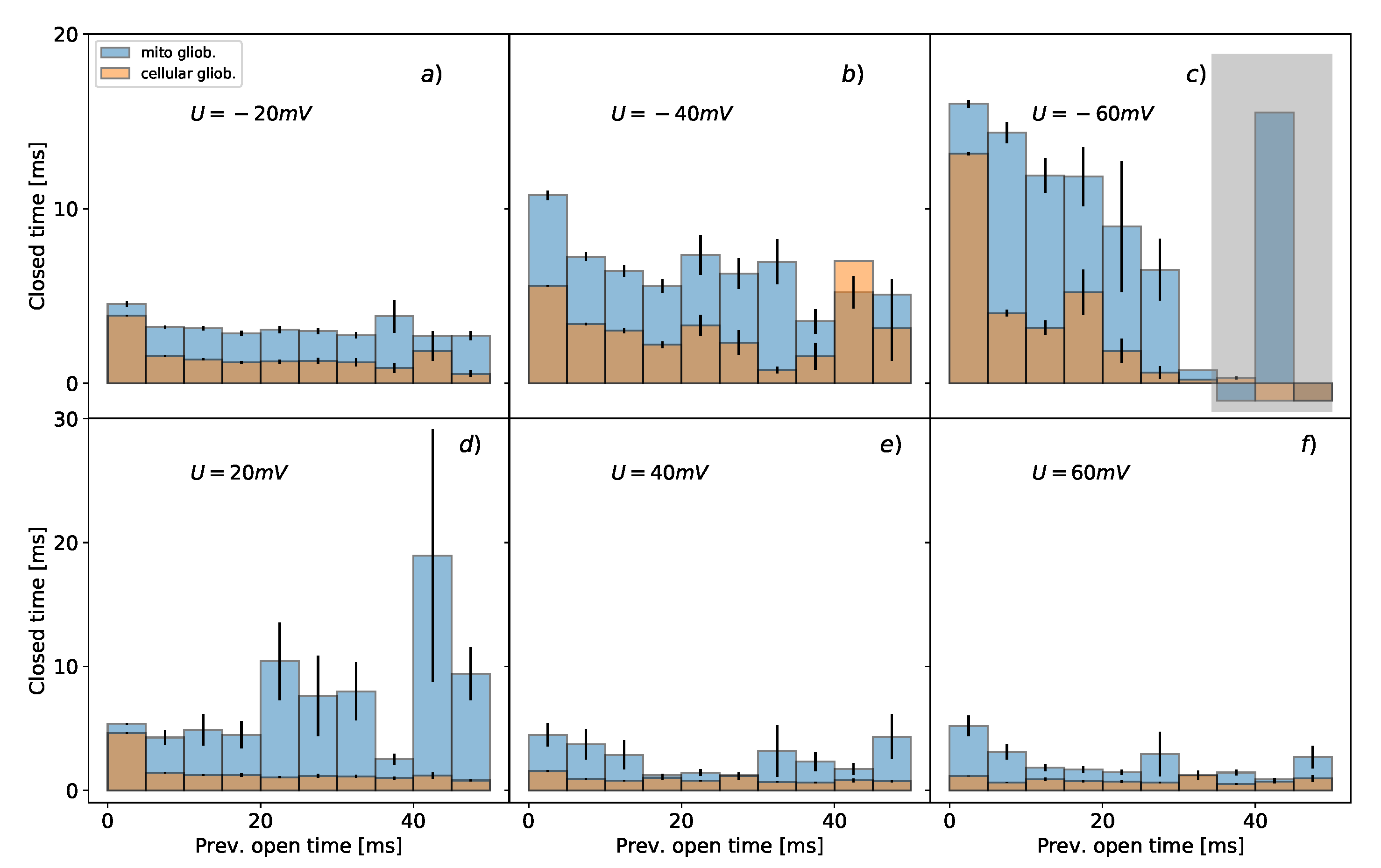
3.2. Conditional Mean Dwell-Times of Conducting/Non-Conducting States
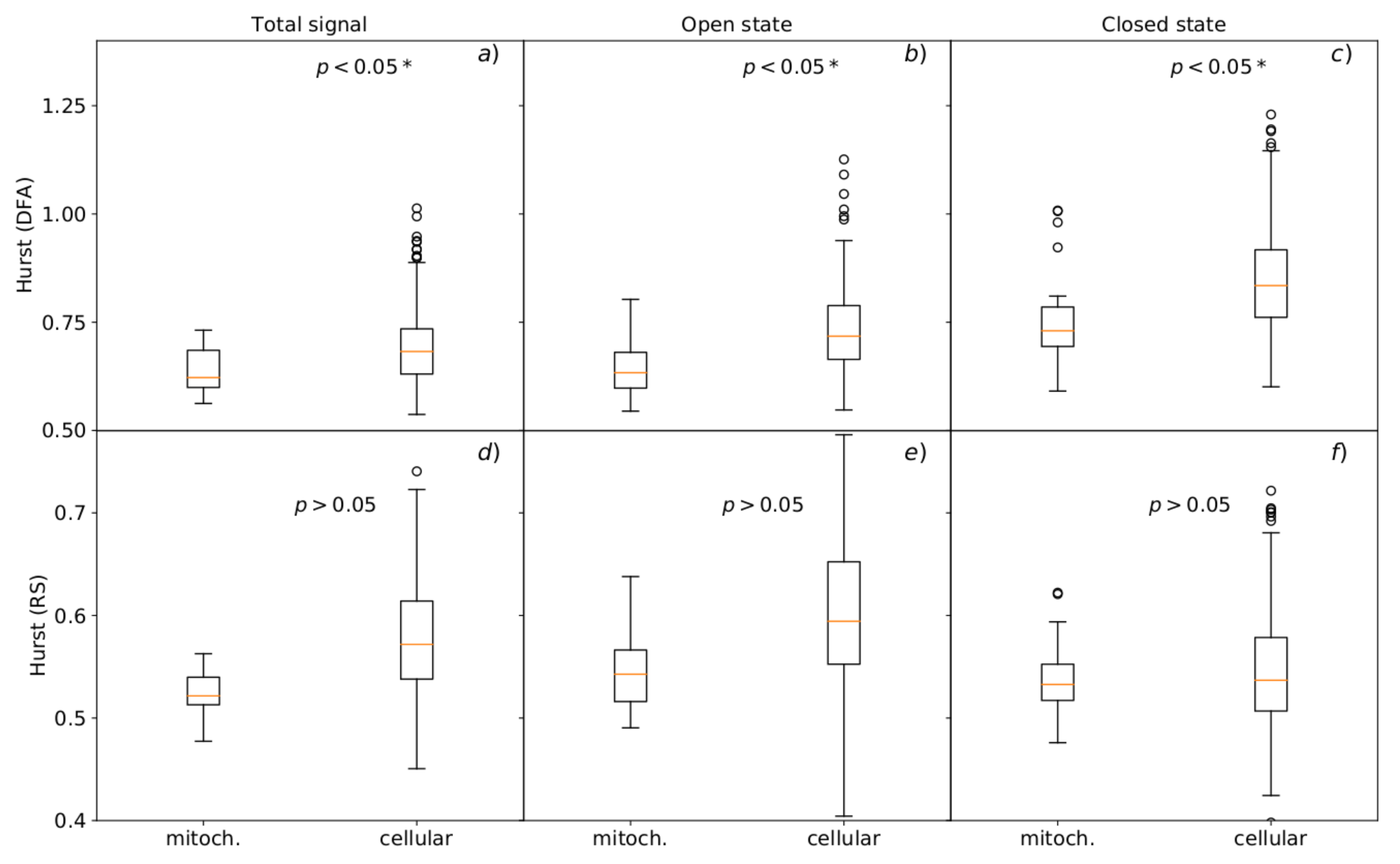
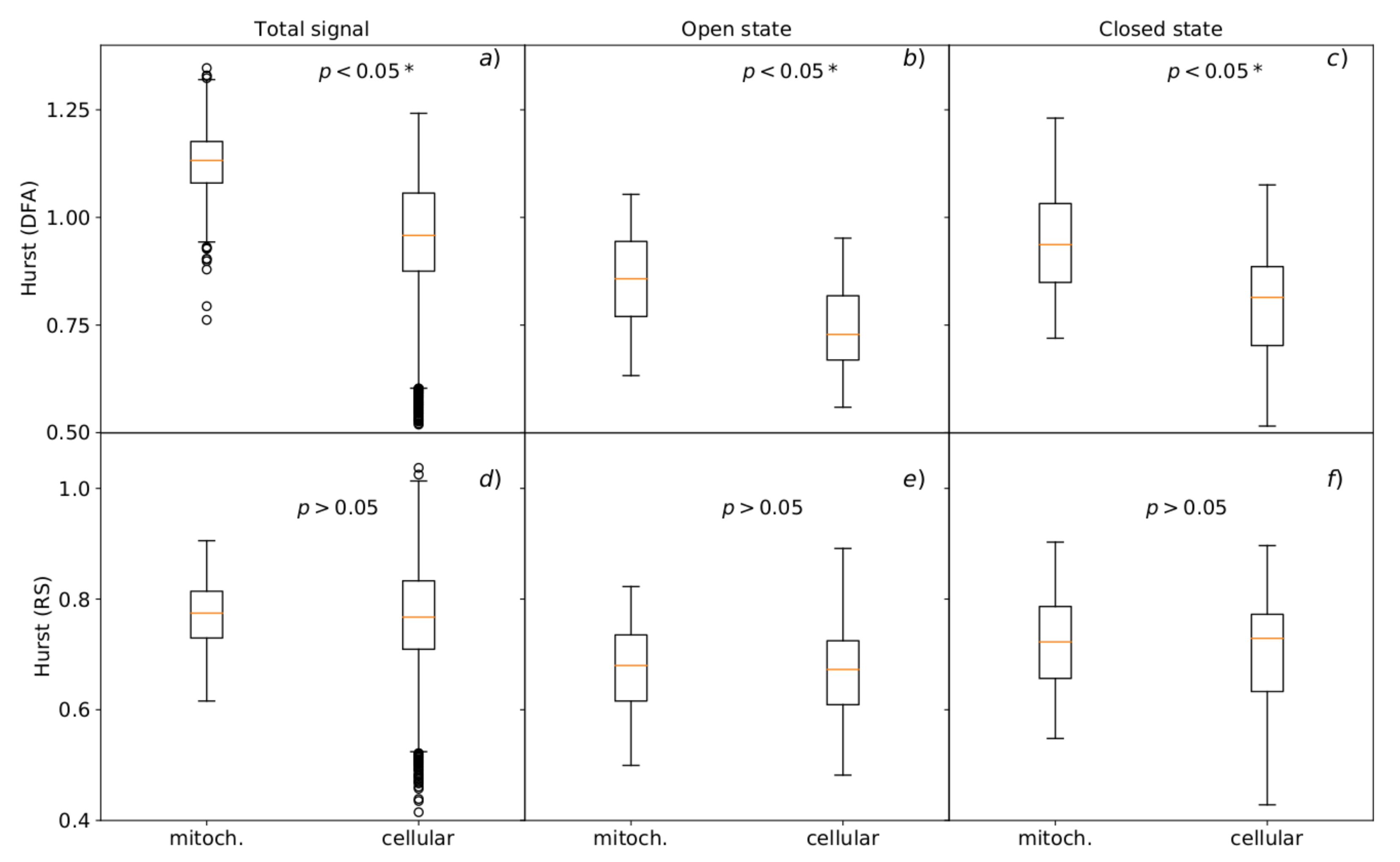
3.3. Hurst R/S and DFA Analyses
3.3.1. Analysis of Dwell-Time Series of Channel States
3.3.2. Analysis of Single Channel Currents
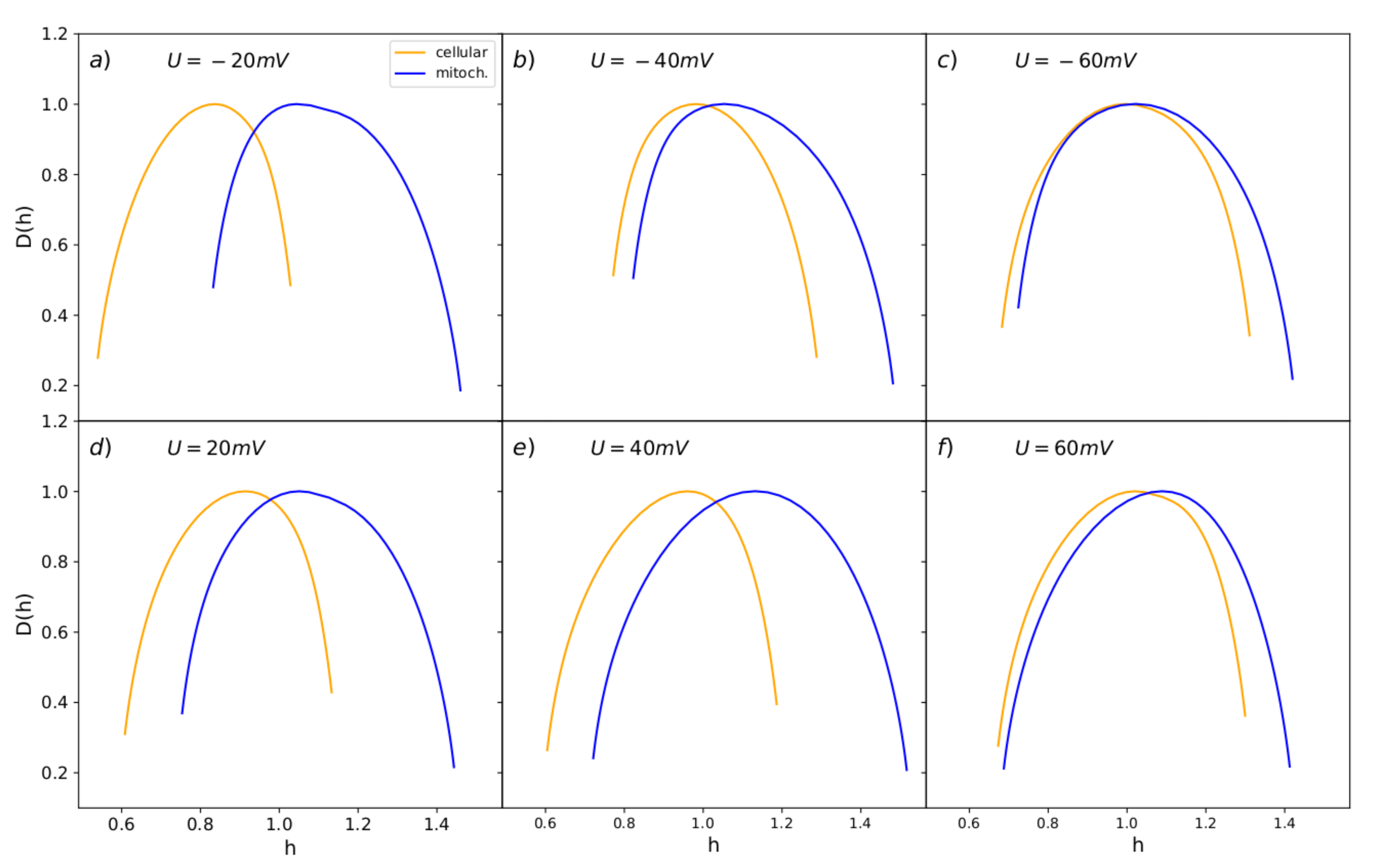
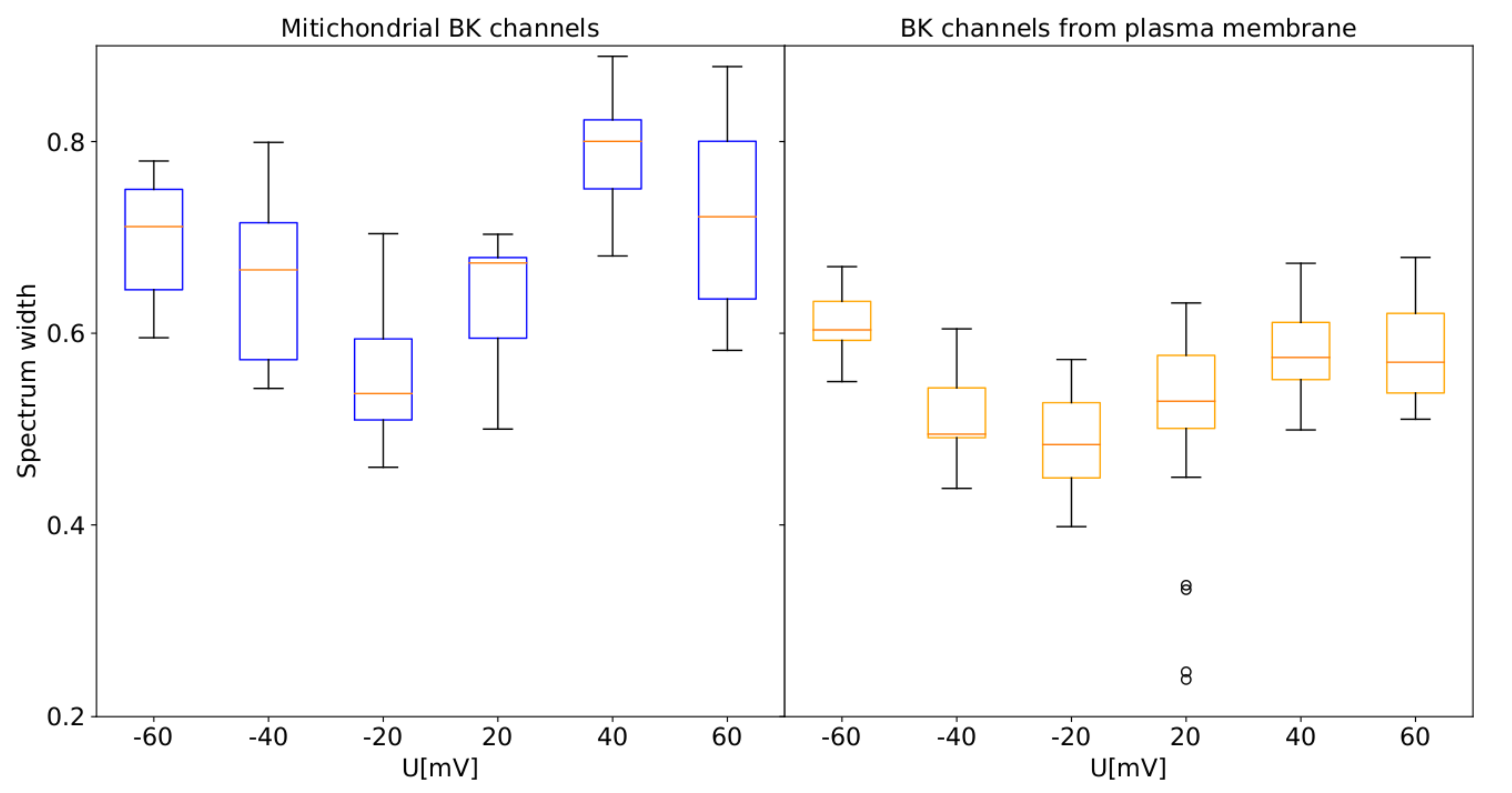
3.4. MFDFA Analysis of Experimental Single-Channel Recordings
3.5. Analysis of Randomized Series
4. Conclusions
- short-range correlations between the lifespan of subsequent channel states confirmed by the analysis of the conditional dwell-time duration as shown in Figure 2 and Figure 3, which suggest the connectivity between the channel’s substates of different longevity (i.e., the number of routes between the substates within the manifold of functionally open or closed states) [63,64],
- multifractal complex dynamics indicated by the MFDFA analysis (Figure 6), where the spectral width exhibits an analogous dependence on the membrane potential for both types of BK channels variants (broader spectrum at highly hyper- or depolarized membranes than at the close to zero) (Figure 8), suggesting similar changes of the conformational diffusive space with .
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| the scaling exponent | |
| DFA | Detrended Fluctuation Analysis |
| the spectral width | |
| gBK | large-conductance voltage- and Ca-activated channel from the glioblastoma cells |
| H | the Hurst exponent |
| MFDFA | Multifractal Detrended Fluctuation Analysis |
| R/S | rescaled-range method of the Hurst exponent estimation |
| membrane potential |
References
- Marty, A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 1981, 291, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, B.S.; Magleby, K.L.; Barrett, J.N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature 1981, 293, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.G.; Petersen, O.H. Modulation of Ca2+-and voltage-activated K+ channels by internal Mg2+ in salivary acinar cells. Biochim. Biophys. Acta (BBA) Biomembr. 1987, 899, 171–175. [Google Scholar] [CrossRef]
- Tang, X.D.; Xu, R.; Reynolds, M.F.; Garcia, M.L.; Heinemann, S.H.; Hoshi, T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 2003, 425, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Krien, U.; Gagov, H. Protons inhibit the BKCa channel of rat small artery smooth muscle cells. J. Vasc. Res. 2001, 38, 30–38. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Cui, J. BK channels: Multiple sensors, one activation gate. Front. Physiol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Dworakowska, B.; Grzywna, Z.J. The temperature dependence of the BK channel activity–kinetics, thermodynamics, and long-range correlations. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1805–1814. [Google Scholar] [CrossRef]
- Allard, B.; Couble, M.L.; Magloire, H.; Bleicher, F. Characterization and gene expression of high conductance calcium-activated potassium channels displaying mechanosensitivity in human odontoblasts. J. Biol. Chem. 2000, 275, 25556–25561. [Google Scholar] [CrossRef]
- Tseng-Crank, J.; Foster, C.D.; Krause, J.D.; Mertz, R.; Godinot, N.; DiChiara, T.J.; Reinhart, P.H. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron 1994, 13, 1315–1330. [Google Scholar] [CrossRef]
- Fury, M.; Marx, S.O.; Marks, A.R. Molecular BKology: The study of splicing and dicing. Sci. Stke 2002, 2002, pe12. [Google Scholar] [CrossRef]
- Schubert, R.; Nelson, M.T. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol. Sci. 2001, 22, 505–512. [Google Scholar] [CrossRef]
- Li, M.; Tanaka, Y.; Alioua, A.; Wu, Y.; Lu, R.; Kundu, P.; Sanchez-Pastor, E.; Marijic, J.; Stefani, E.; Toro, L. Thromboxane A2 receptor and MaxiK-channel intimate interaction supports channel trans-inhibition independent of G-protein activation. Proc. Natl. Acad. Sci. USA 2010, 107, 19096–19101. [Google Scholar] [CrossRef] [PubMed]
- Shipston, M.J. Alternative splicing of potassium channels: A dynamic switch of cellular excitability. Trends Cell Biol. 2001, 11, 353–358. [Google Scholar] [CrossRef]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular determinants of BK channel functional diversity and functioning. Physiol. Rev. 2017, 97, 39–87. [Google Scholar] [CrossRef]
- Kyle, B.D.; Braun, A.P. The regulation of BK channel activity by pre-and post-translational modifications. Front. Physiol. 2014, 5, 316. [Google Scholar] [CrossRef]
- Poulsen, A.N.; Wulf, H.; Hay-Schmidt, A.; Jansen-Olesen, I.; Olesen, J.; Klaerke, D.A. Differential expression of BK channel isoforms and β-subunits in rat neuro-vascular tissues. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 380–389. [Google Scholar] [CrossRef]
- Contreras, G.F.; Neely, A.; Alvarez, O.; Gonzalez, C.; Latorre, R. Modulation of BK channel voltage gating by different auxiliary β subunits. Proc. Natl. Acad. Sci. USA 2012, 109, 18991–18996. [Google Scholar] [CrossRef]
- Balderas, E.; Zhang, J.; Stefani, E.; Toro, L. Mitochondrial BKCa channel. Front. Physiol. 2015, 6, 104. [Google Scholar] [CrossRef]
- Singh, H.; Lu, R.; Bopassa, J.C.; Meredith, A.L.; Stefani, E.; Toro, L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc. Natl. Acad. Sci. USA 2013, 110, 10836–10841. [Google Scholar] [CrossRef]
- Colbeau, A.; Nachbaur, J.; Vignais, P. Enzymac characterization and lipid composition of rat liver subcellular membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1971, 249, 462–492. [Google Scholar] [CrossRef]
- Tillman, T.S.; Cascio, M. Effects of membrane lipids on ion channel structure and function. Cell Biochem. Biophys. 2003, 38, 161–190. [Google Scholar] [CrossRef]
- Duncan, A.L.; Reddy, T.; Koldsø, H.; Hélie, J.; Fowler, P.W.; Chavent, M.; Sansom, M.S. Protein crowding and lipid complexity influence the nanoscale dynamic organization of ion channels in cell membranes. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Morales, J.F.; Vásquez, V. How lipids contribute to ion channel function, a fat perspective on direct and indirect interactions. Curr. Opin. Struct. Biol. 2018, 51, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta (BBA) Biomembr. 2004, 1666, 62–87. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.F.; Chawla, U.; Perera, S.M. Membrane Lipid-Protein Interactions. In The Biophysics of Cell Membranes; Springer Nature: Singapore, 2017; pp. 61–84. [Google Scholar]
- Laskowski, M.; Augustynek, B.; Bednarczyk, P.; Żochowska, M.; Kalisz, J.; O’rourke, B.; Szewczyk, A.; Kulawiak, B. Single-channel properties of the ROMK-pore-forming subunit of the mitochondrial ATP-sensitive potassium channel. Int. J. Mol. Sci. 2019, 20, 5323. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative structural and functional coupling of the mitochondrial BK Ca channel to the respiratory chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef]
- Rosa, P.; Sforna, L.; Carlomagno, S.; Mangino, G.; Miscusi, M.; Pessia, M.; Franciolini, F.; Calogero, A.; Catacuzzeno, L. Overexpression of large-conductance calcium-activated potassium channels in human glioblastoma stem-like cells and their role in cell migration. J. Cell. Physiol. 2017, 232, 2478–2488. [Google Scholar] [CrossRef]
- Ransom, C.B.; Liu, X.; Sontheimer, H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia 2002, 38, 281–291. [Google Scholar] [CrossRef]
- Molenaar, R.J. Ion channels in glioblastoma. ISRN Neurol. 2011, 2011, 590249. [Google Scholar] [CrossRef]
- Weaver, A.K.; Bomben, V.C.; Sontheimer, H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006, 54, 223–233. [Google Scholar] [CrossRef]
- Edalat, L.; Stegen, B.; Klumpp, L.; Haehl, E.; Schilbach, K.; Lukowski, R.; Kühnle, M.; Bernhardt, G.; Buschauer, A.; Zips, D.; et al. BK K+ channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget 2016, 7, 14259. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; Catacuzzeno, L.; Sforna, L.; Mangino, G.; Carlomagno, S.; Mincione, G.; Petrozza, V.; Ragona, G.; Franciolini, F.; Calogero, A. BK channels blockage inhibits hypoxia-induced migration and chemoresistance to cisplatin in human glioblastoma cells. J. Cell. Physiol. 2018, 233, 6866–6877. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Kampa, R.P.; Daniluk, J.; Sek, A.; Jarmuszkiewicz, W.; Szewczyk, A.; Bednarczyk, P. Regulation of the mitochondrial BKCa channel by the citrus flavonoid naringenin as a potential means of preventing cell damage. Molecules 2020, 25, 3010. [Google Scholar] [CrossRef]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Augustynek, B.; Kulawiak, B.; Koprowski, P.; Bednarczyk, P.; Jarmuszkiewicz, W.; Szewczyk, A. What do we not know about mitochondrial potassium channels? Biochim. Biophys. Acta (BBA) Bioenerg. 2016, 1857, 1247–1257. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Huang, Q.; Jin, W.; Chen, J. Immunotherapy for malignant glioma: Current status and future directions. Trends Pharmacol. Sci. 2020, 41, 123–138. [Google Scholar] [CrossRef]
- Szewczyk, A.; Skalska, J.; Głąb, M.; Kulawiak, B.; Malińska, D.; Koszela-Piotrowska, I.; Kunz, W.S. Mitochondrial potassium channels: From pharmacology to function. Biochim. Biophys. Acta (BBA) Bioenerg. 2006, 1757, 715–720. [Google Scholar] [CrossRef]
- Szewczyk, A.; Kajma, A.; Malinska, D.; Wrzosek, A.; Bednarczyk, P.; Zabłocka, B.; Dołowy, K. Pharmacology of mitochondrial potassium channels: Dark side of the field. FEBS Lett. 2010, 584, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Stefani, E.; Toro, L. Intracellular BKCa (iBKCa) channels. J. Physiol. 2012, 590, 5937–5947. [Google Scholar] [CrossRef] [PubMed]
- Walewska, A.; Kulawiak, B.; Szewczyk, A.; Koprowski, P. Mechanosensitivity of mitochondrial large-conductance calcium-activated potassium channels. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1859, 797–805. [Google Scholar] [CrossRef]
- Ransom, C.B.; Sontheimer, H. BK channels in human glioma cells. J. Neurophysiol. 2001, 85, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.E. The problem of long-term storage in reservoirs. Hydrol. Sci. J. 1956, 1, 13–27. [Google Scholar] [CrossRef]
- Peng, C.K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Goldberger, A.L. Mosaic organization of DNA nucleotides. Phys. Rev. 1994, 49, 1685. [Google Scholar] [CrossRef]
- Kantelhardt, J.W.; Zschiegner, S.A.; Koscielny-Bunde, E.; Havlin, S.; Bunde, A.; Stanley, H.E. Multifractal detrended fluctuation analysis of nonstationary time series. Phys. A Stat. Mech. Appl. 2002, 316, 87–114. [Google Scholar] [CrossRef]
- Nogueira, R.; Varanda, W.; Liebovitch, L. Hurst analysis in the study of ion channel kinetics. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 1995, 28, 491–496. [Google Scholar]
- De Oliveira, R.C.; Barbosa, C.; Consoni, L.; Rodrigues, A.; Varanda, W.; Nogueira, R. Long-term correlation in single calcium-activated potassium channel kinetics. Phys. A Stat. Mech. Appl. 2006, 364, 13–22. [Google Scholar] [CrossRef]
- Varanda, W.A.; Liebovitch, L.S.; Figueiroa, J.N.; Nogueira, R.A. Hurst analysis applied to the study of single calcium-activated potassium channel kinetics. J. Theor. Biol. 2000, 206, 343–353. [Google Scholar] [CrossRef]
- Borys, P. Long term Hurst memory that does not die at long observation times—Deterministic map to describe ion channel activity. Chaos Solitons Fractals 2020, 132, 109560. [Google Scholar] [CrossRef]
- Bahramian, A.; Nouri, A.; Baghdadi, G.; Gharibzadeh, S.; Towhidkhah, F.; Jafari, S. Introducing a chaotic map with a wide range of long-term memory as a model of patch-clamped ion channels current time series. Chaos Solitons Fractals 2019, 126, 361–368. [Google Scholar] [CrossRef]
- Wawrzkiewicz, A.; Pawelek, K.; Borys, P.; Dworakowska, B.; Grzywna, Z.J. On the simple random-walk models of ion-channel gate dynamics reflecting long-term memory. Eur. Biophys. J. 2012, 41, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Siwy, Z.; Mercik, S.; Weron, K.; Ausloos, M. Application of dwell-time series in studies of long-range correlation in single channel ion transport: Analysis of ion current through a big conductance locust potassium channel. Phys. A Stat. Mech. Appl. 2001, 297, 79–96. [Google Scholar] [CrossRef]
- Siwy, Z.; Ausloos, M.; Ivanova, K. Correlation studies of open and closed state fluctuations in an ion channel: Analysis of ion current through a large-conductance locust potassium channel. Phys. Rev. E 2002, 65, 031907. [Google Scholar] [CrossRef]
- Lan, T.H.; Gao, Z.Y.; Abdalla, A.N.; Cheng, B.; Wang, S. Detrended fluctuation analysis as a statistical method to study ion single channel signal. Cell Biol. Int. 2008, 32, 247–252. [Google Scholar] [CrossRef]
- Kazachenko, V.; Astashev, M.; Grinevich, A. Multifractal analysis of K+ channel activity. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2007, 1, 169–175. [Google Scholar] [CrossRef]
- Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Dworakowska, B.; Machura, Ł. Multifractal Properties of BK Channel Currents in Human Glioblastoma Cells. J. Phys. Chem. B 2020, 124, 2382–2391. [Google Scholar] [CrossRef]
- Mercik, S.; Weron, K.; Siwy, Z. Statistical analysis of ionic current fluctuations in membrane channels. Phys. Rev. E 1999, 60, 7343. [Google Scholar] [CrossRef]
- Mukli, P.; Nagy, Z.; Eke, A. Multifractal formalism by enforcing the universal behavior of scaling functions. Phys. A Stat. Mech. Its Appl. 2015, 417, 150–167. [Google Scholar] [CrossRef]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28–30 June 2010; Volume 445, pp. 51–56. [Google Scholar]
- Colquhoun, D.; Hawkes, A. A note on correlations in single ion channel records. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1987, 230, 15–52. [Google Scholar]
- Colquhoun, D.; Hawkes, A.G. The principles of the stochastic interpretation of ion-channel mechanisms. In Single-Channel Recording; Plenum Press: New York, NY, USA, 1995; pp. 397–482. [Google Scholar]
- Geng, Y.; Magleby, K.L. Single-channel kinetics of BK (Slo1) channels. Front. Physiol. 2015, 5, 532. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, B.S.; Magleby, K.L. Gating Kinetics of Single Large-Conductance Ca2+-Activated K+ Channels in High Ca2+ Suggest a Two-Tiered Allosteric Gating Mechanism. J. Gen. Physiol. 1999, 114, 93–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Aldrich, R.W. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry 2000, 39, 15612–15619. [Google Scholar] [CrossRef] [PubMed]
- Bunde, A.; Havlin, S. Fractals and Disordered Systems; Springer: Berlin, Germany, 2012. [Google Scholar]
- Chen, Y.; Huang, L. Spatial measures of urban systems: From entropy to fractal dimension. Entropy 2018, 20, 991. [Google Scholar] [CrossRef]
- Peyronnet, R.; Nerbonne, J.M.; Kohl, P. Cardiac mechano-gated ion channels and arrhythmias. Circ. Res. 2016, 118, 311–329. [Google Scholar] [CrossRef]
- N’gouemo, P. Targeting BK (big potassium) channels in epilepsy. Expert Opin. Ther. Targets 2011, 15, 1283–1295. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzkiewicz-Jałowiecka, A.; Trybek, P.; Borys, P.; Dworakowska, B.; Machura, Ł.; Bednarczyk, P. Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis. Cells 2020, 9, 2305. https://doi.org/10.3390/cells9102305
Wawrzkiewicz-Jałowiecka A, Trybek P, Borys P, Dworakowska B, Machura Ł, Bednarczyk P. Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis. Cells. 2020; 9(10):2305. https://doi.org/10.3390/cells9102305
Chicago/Turabian StyleWawrzkiewicz-Jałowiecka, Agata, Paulina Trybek, Przemysław Borys, Beata Dworakowska, Łukasz Machura, and Piotr Bednarczyk. 2020. "Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis" Cells 9, no. 10: 2305. https://doi.org/10.3390/cells9102305
APA StyleWawrzkiewicz-Jałowiecka, A., Trybek, P., Borys, P., Dworakowska, B., Machura, Ł., & Bednarczyk, P. (2020). Differences in Gating Dynamics of BK Channels in Cellular and Mitochondrial Membranes from Human Glioblastoma Cells Unraveled by Short- and Long-Range Correlations Analysis. Cells, 9(10), 2305. https://doi.org/10.3390/cells9102305







