Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mouse Embryo Collection
2.3. In Vitro Implantation Model Using Mouse Blastocysts
2.4. Immunofluorescence Staining
2.5. Fluorescence Microscopy
2.6. RNA Extraction and Quantitative PCR
2.7. Statistical Analysis
3. Results
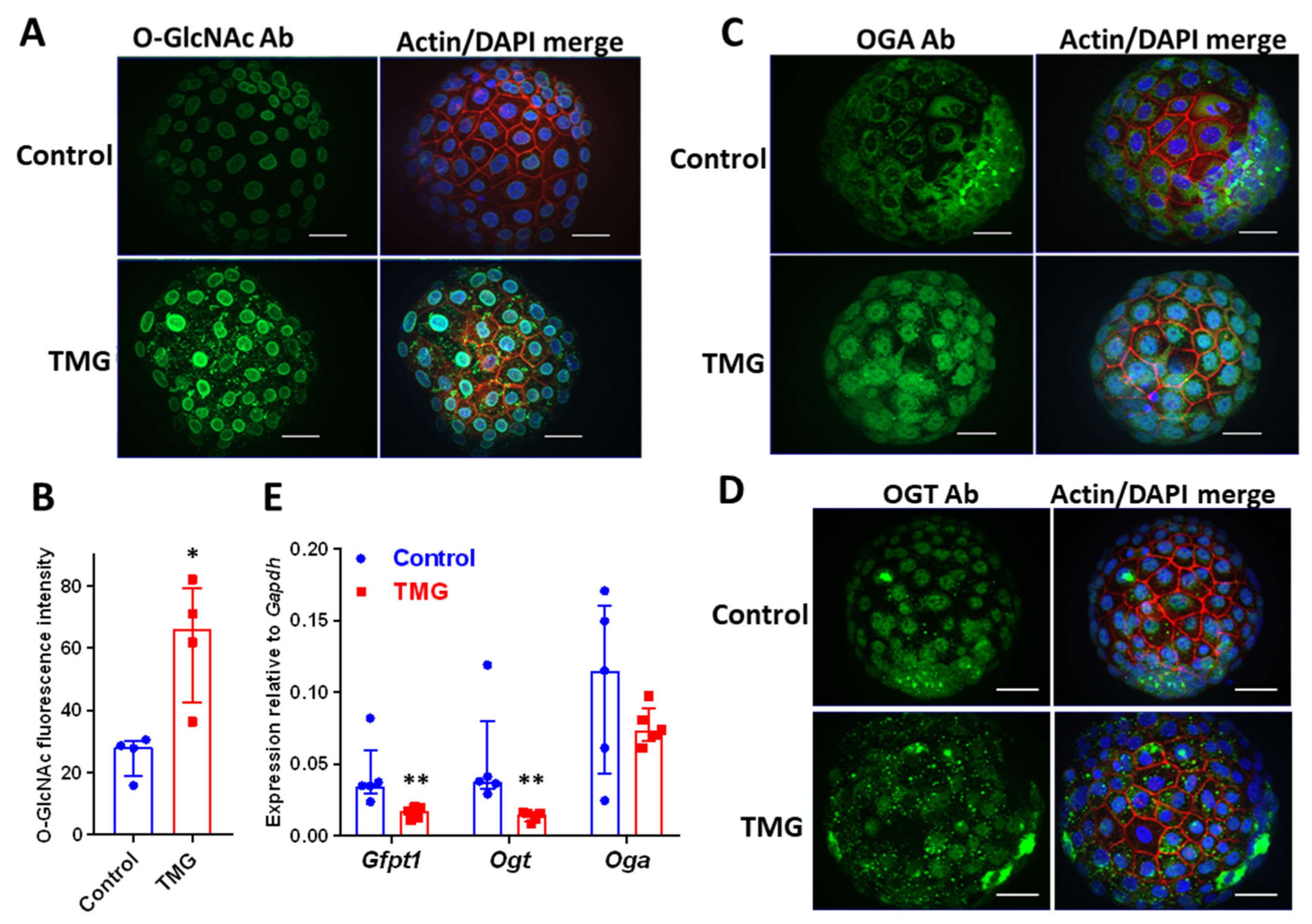
3.1. TMG Increases O-GlcNAcylation and Alters OGT and OGA Localization in Mouse Blastocysts
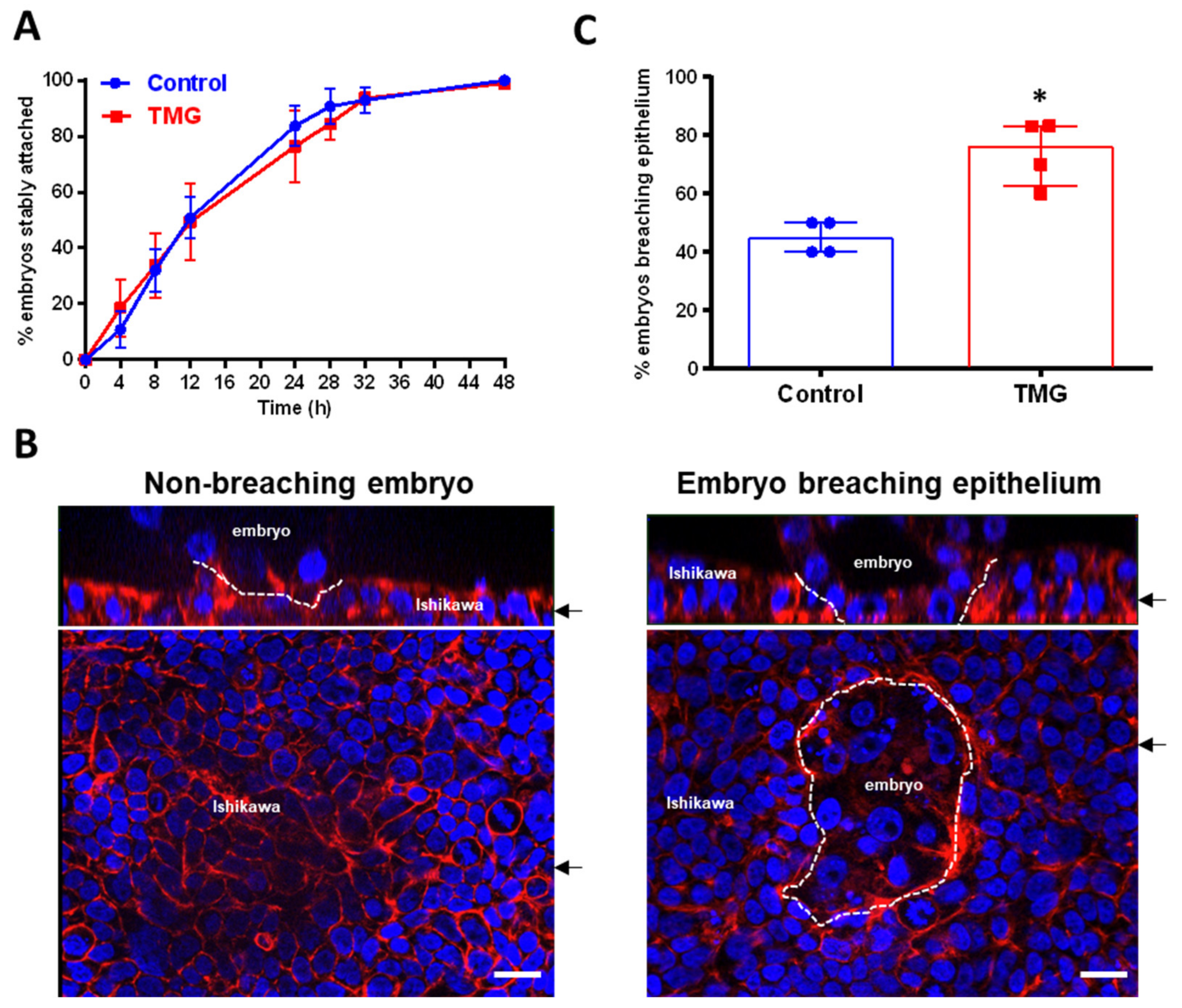
3.2. TMG Treatment of Blastocysts Promotes Invasion in a Model of Implantation
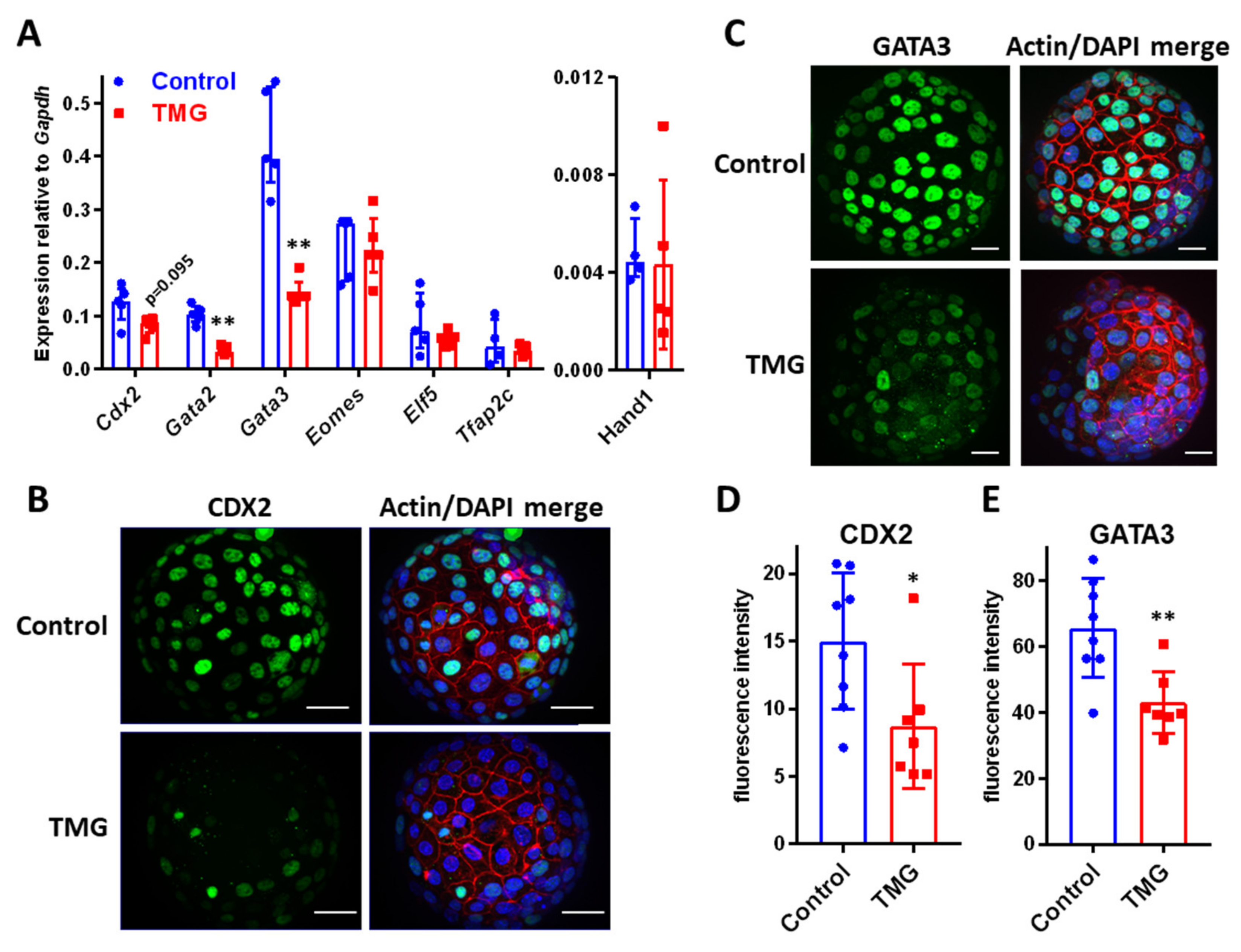
3.3. TMG Alters TE Transcription Factor Expression in Mouse Blastocysts
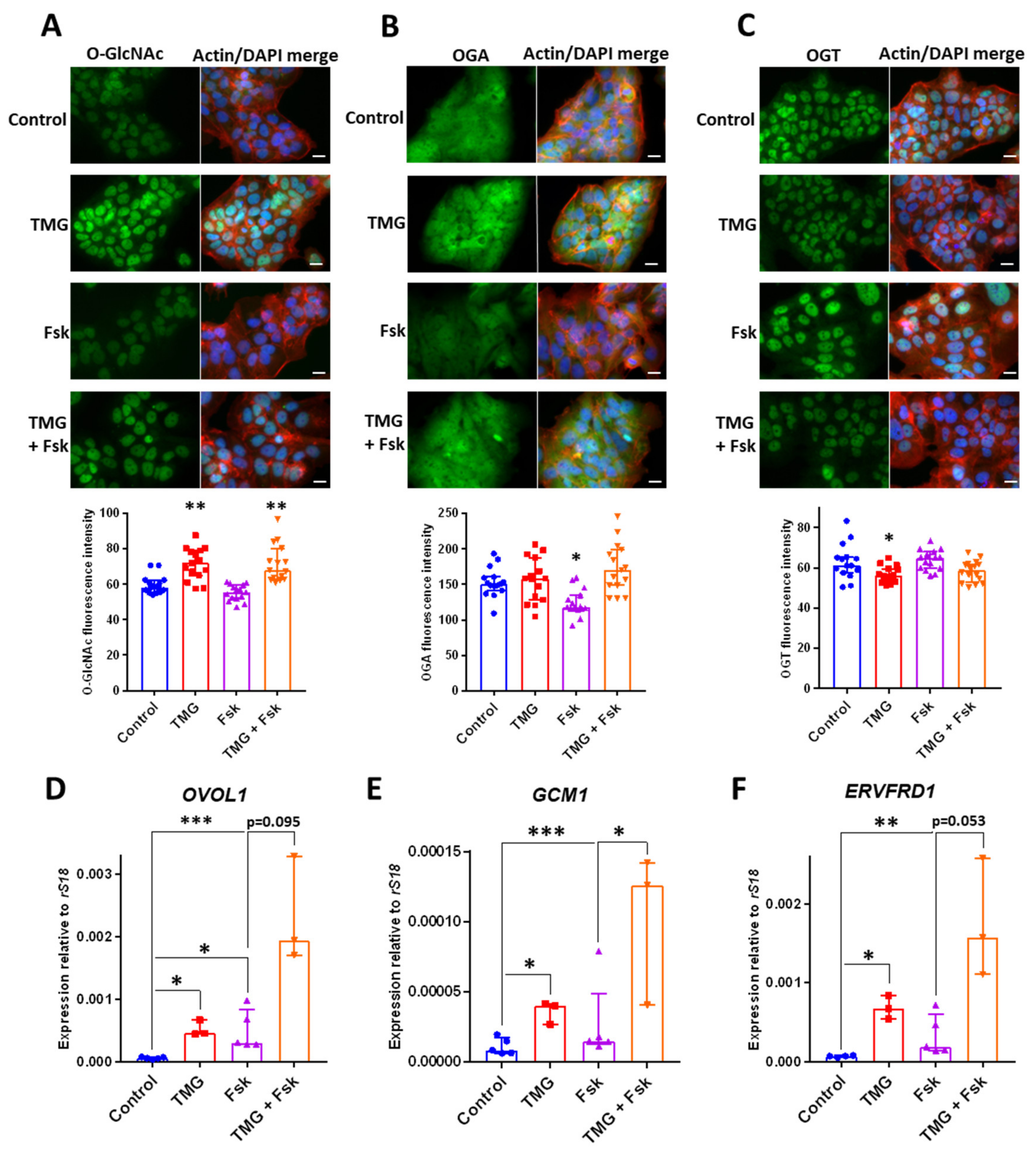
3.4. TMG Stimulates Syncytiotrophoblast Transcription Factor Expression in a Human Trophoblast Cell Line
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, M.D.; Zenker, J.; Bissière, S.; Plachta, N. Instructions for Assembling the Early Mammalian Embryo. Dev. Cell 2018, 45, 667–679. [Google Scholar] [CrossRef]
- Aplin, J.D.; Ruane, P.T. Embryo–epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef]
- Rappolee, D.A.; Zhou, S.; Puscheck, E.E.; Xie, Y. Stress responses at the endometrial-placental interface regulate labyrinthine placental differentiation from trophoblast stem cells. Reproduction 2013, 145, R139–R155. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.; Katari, S.; Gerson, L.F.; Chalian, R.; Foster, M.W.; Gaughan, J.P.; Coutifaris, C.; Sapienza, C. Inter- and Intra-Individual Variation in Allele-Specific DNA Methylation and Gene Expression in Children Conceived using Assisted Reproductive Technology. PLoS Genet. 2010, 6, e1001033. [Google Scholar] [CrossRef] [PubMed]
- Haavaldsen, C.; Tanbo, T.; Eskild, A. Placental weight in singleton pregnancies with and without assisted reproductive technology: A population study of 536 567 pregnancies. Hum. Reprod. 2012, 27, 576–582. [Google Scholar] [CrossRef]
- Nelissen, E.C.; Dumoulin, J.C.; Busato, F.; Ponger, L.; Eijssen, L.M.; Evers, J.L.; Tost, J.; Van Montfoort, A.P. Altered gene expression in human placentas after IVF/ICSI. Hum. Reprod. 2014, 29, 2821–2831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, S.; Ghosh, J.; Mainigi, M.A.; Turan, N.; Weinerman, R.; Truongcao, M.; Coutifaris, C.; Sapienza, C. DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clin. Epigenetics 2015, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, N.; Schneider, E.; Lehnen, H.; Haaf, T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction 2014, 148, R111–R120. [Google Scholar] [CrossRef]
- Puscheck, E.E.; Awonuga, A.O.; Yang, Y.; Jiang, Z.; Rappolee, D. Molecular Biology of the Stress Response in the Early Embryo and its Stem Cells. Adv. Exp. Med. Biol. 2015, 843, 77–128. [Google Scholar] [CrossRef]
- Ruane, P.T.; Koeck, R.; Berneau, S.C.; Kimber, S.J.; Westwood, M.; Brison, D.R.; Aplin, J.D. Osmotic stress induces JNK-dependent embryo invasion in a model of implantation. Reproduction 2018, 156, 421–428. [Google Scholar] [CrossRef]
- Burton, G.J.E.; Jauniaux, D.S. Charnock-Jones, The influence of the intrauterine environment on human placental development. Int. J. Dev. Biol. 2010, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.J.; Porter, R.; Watkins, A.J.; Burt, E.; Brooks, S.; Leese, H.J.; Humpherson, P.G.; Cameron, I.T.; Fleming, T.P. Metabolic Induction and Early Responses of Mouse Blastocyst Developmental Programming following Maternal Low Protein Diet Affecting Life-Long Health. PLoS ONE 2013, 7, e52791. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Awonuga, A.; Liu, J.; Rings, E.; Puscheck, E.E.; Rappolee, D.A. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]. Stem Cells Dev. 2013, 22, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Velazquez, M.A.; Marfy-Smith, S.; Sheth, B.; Cox, A.; Johnston, D.A.; Smyth, N.; Fleming, T. Mouse early extra-embryonic lineages activate compensatory endocytosis in response to poor maternal nutrition. Development 2014, 141, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Gürke, J.; Schindler, M.; Pendzialek, M.; Thieme, R.; Grybel, K.J.; Heller, R.; Spengler, K.; Fleming, T.P.; Fischer, B.; Santos, A.N.; et al. Maternal diabetes promotes mTORC1 downstream signalling in rabbit preimplantation embryos. Reproduction 2016, 151, 465–476. [Google Scholar] [CrossRef]
- Watkins, A.J.; Lucas, E.S.; Marfy-Smith, S.; Bates, N.; Kimber, S.J.; Fleming, T.P. Maternal nutrition modifies trophoblast giant cell phenotype and fetal growth in mice. Reproduction 2015, 149, 563–575. [Google Scholar] [CrossRef]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar]
- Watkins, A.J.; Platt, D.; Papenbrock, T.; Wilkins, A.; Eckert, J.J.; Kwong, W.Y.; Osmond, C.; Hanson, M.; Fleming, T.P. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl. Acad. Sci. USA 2007, 104, 5449–5454. [Google Scholar] [CrossRef]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Hart, G.W.; Marth, J.D. DynamicO-GlcNAc Modification of Nucleocytoplasmic Proteins in Response to Stress. J. Boil. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef]
- Butkinaree, C.; Park, K.; Hart, G.W. O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim. Biophys. Acta 2010, 1800, 96–106. [Google Scholar] [CrossRef]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Boil. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Medford, H.M.; Chatham, J.C.; Marsh, S.A. Chronic ingestion of a Western diet increases O-linked-beta-N-acetylglucosamine (O-GlcNAc) protein modification in the rat heart. Life Sci. 2012, 90, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.R.; Hanover, J.A. O-GlcNAc Cycling: A Link Between Metabolism and Chronic Disease. Annu. Rev. Nutr. 2013, 33, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.; Giachini, F.R.; Matsumoto, T.; Li, W.; Bressan, A.F.M.; Chawla, D.; Webb, R.C.; Ergul, A.; Tostes, R.C. High-fat diet increases O-GlcNAc levels in cerebral arteries: A link to vascular dysfunction associated with hyperlipidaemia/obesity? Clin. Sci. 2016, 130, 871–880. [Google Scholar] [CrossRef]
- Pantaleon, M.; Scott, J.; Kaye, P.L. Nutrient Sensing by the Early Mouse Embryo: Hexosamine Biosynthesis and Glucose Signaling During Preimplantation Development1. Boil. Reprod. 2008, 78, 595–600. [Google Scholar] [CrossRef]
- Pantaleon, M.; Tan, H.Y.; Kafer, G.R.; Kaye, P.L. Toxic Effects of Hyperglycemia Are Mediated by the Hexosamine Signaling Pathway and O-Linked Glycosylation in Early Mouse Embryos1. Boil. Reprod. 2010, 82, 751–758. [Google Scholar] [CrossRef]
- Chi, F.; Sharpley, M.S.; Nagaraj, R.; Roy, S.S.; Banerjee, U. Glycolysis-Independent Glucose Metabolism Distinguishes TE from ICM Fate during Mammalian Embryogenesis. Dev. Cell 2020, 53, 9.e4–26.e4. [Google Scholar] [CrossRef]
- Nagaraj, R.; Sharpley, M.S.; Chi, F.; Braas, D.; Zhou, Y.; Kim, R.; Clark, A.T.; Banerjee, U. Nuclear Localization of Mitochondrial TCA Cycle Enzymes as a Critical Step in Mammalian Zygotic Genome Activation. Cell 2017, 168, 210–223. [Google Scholar] [CrossRef]
- Brown, H.M.; Green, E.S.; Tan, T.C.Y.; Gonzalez, M.B.; Rumbold, A.R.; Hull, M.L.; Norman, R.J.; Packer, N.H.; Robertson, S.A.; Thompson, J.G. Periconception onset diabetes is associated with embryopathy and fetal growth retardation, reproductive tract hyperglycosylation and impaired immune adaptation to pregnancy. Sci. Rep. 2018, 8, 2114. [Google Scholar] [CrossRef]
- Howerton, C.L.; Morgan, C.P.; Fischer, D.B.; Bale, T.L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl. Acad. Sci. USA 2013, 110, 5169–5174. [Google Scholar] [CrossRef]
- Yang, Y.R.; Jang, H.-J.; Lee, Y.H.; Kim, I.S.; Lee, H.; Ryu, S.H.; Suh, P.-G. O-GlcNAc cycling enzymes control vascular development of the placenta by modulating the levels of HIF-1α. Placenta 2015, 36, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.V.; Justina, V.D.; Dos Passos, R.R.J.; Volpato, G.T.; Souto, P.C.D.S.; Martin, S.S.; Giachini, F.R. O-GlcNAc Modification During Pregnancy: Focus on Placental Environment. Front. Physiol. 2018, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Bergós, J.; Tardio, L.; Larrañaga-Vera, A.; Gómez, R.; Herrero-Beaumont, G.; Largo, R. The Increase in O-Linked N-Acetylglucosamine Protein Modification Stimulates Chondrogenic Differentiation Both in Vitro and in Vivo*. J. Boil. Chem. 2012, 287, 33615–33628. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.M.; Sun, Y.; Yuan, K.; Bradley, W.E.; Litovsky, S.; Dell’Italia, L.J.; Chatham, J.C.; Wu, H.; Chen, Y. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ. Res. 2014, 114, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Ouyang, X.; Benavides, G.A.; Redmann, M.; Cofield, S.S.; Shacka, J.J.; Chatham, J.C.; Darley-Usmar, V.; Zhang, J. O-GlcNAc regulation of autophagy and alpha-synuclein homeostasis; implications for Parkinson’s disease. Mol. Brain 2017, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Ruane, P.T.; Berneau, S.C.; Koeck, R.; Watts, J.; Kimber, S.J.; Brison, D.R.; Westwood, M.; Aplin, J.D. Apposition to endometrial epithelial cells activates mouse blastocysts for implantation. Mol. Hum. Reprod. 2017, 23, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Berneau, S.C.; Ruane, P.T.; Brison, D.R.; Kimber, S.J.; Westwood, M.; Aplin, J.D. Characterisation of Osteopontin in an In Vitro Model of Embryo Implantation. Cells 2019, 8, 432. [Google Scholar] [CrossRef]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef]
- Orendi, K.; Gauster, M.; Moser, G.; Meiri, H.; Huppertz, B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010, 140, 759–766. [Google Scholar] [CrossRef]
- Renaud, S.J.; Chakraborty, D.; Mason, C.W.; Rumi, M.A.K.; Vivian, J.L.; Soares, M.J. OVO-like 1 regulates progenitor cell fate in human trophoblast development. Proc. Natl. Acad. Sci. USA 2015, 112, E6175–E6184. [Google Scholar] [CrossRef]
- Martinez, M.R.; Dias, T.B.; Natov, P.S.; Zachara, N.E. Stress-induced O-GlcNAcylation: An adaptive process of injured cells. Biochem. Soc. Trans. 2017, 45, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Yuzwa, S.A.; Macauley, M.S.; Heinonen, J.E.; Shan, X.; Dennis, R.J.; He, Y.; Whitworth, G.E.; Stubbs, K.A.; McEachern, E.J.; Davies, G.J.; et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Methods 2008, 4, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Yuzwa, S.A.; Shan, X.; Macauley, M.S.; Clark, T.; Skorobogatko, Y.; Vosseller, K.; Vocadlo, D.J. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Methods 2012, 8, 393–399. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, X.; Li, D.; Hao, J. Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-kappaB p65 signaling. J. Cereb. Blood Flow Metab. 2016, 37, 2938–2951. [Google Scholar] [CrossRef]
- Jiang, M.; Yu, S.; Yu, Z.; Sheng, H.; Li, Y.; Liu, S.; Warner, D.S.; Paschen, W.; Yang, W. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation Is Neuroprotective in Ischemic Stroke in Young Mice and Its Impairment in Aged Mice Is Rescued by Thiamet-G. Stroke 2017, 48, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.T.; Romar, R.; Pavone, M.E.; Soriano-Úbeda, C.; Zhang, J.; Slawson, C.; Duncan, F.E. Disruption of O -GlcNAc homeostasis during mammalian oocyte meiotic maturation impacts fertilization. Mol. Reprod. Dev. 2019, 86, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.F.; El-Karim, E.G.; Wells, L. Dissecting PUGNAc-mediated inhibition of the pro-survival action of insulin. Glycobiology 2016, 26, 1198–1208. [Google Scholar] [CrossRef]
- Eustice, M.; Bond, M.R.; Hanover, J.A. O-GlcNAc cycling and the regulation of nucleocytoplasmic dynamics. Biochem. Soc. Trans. 2017, 45, 427–436. [Google Scholar] [CrossRef]
- Leturcq, M.; Lefebvre, T.; Vercoutter-Edouart, A.-S. O-GlcNAcylation and chromatin remodeling in mammals: An up-to-date overview. Biochem. Soc. Trans. 2017, 45, 323–338. [Google Scholar] [CrossRef]
- Love, D.C.; Kochan, J.; Cathey, R.L.; Shin, S.-H.; Hanover, J.A.; Kochran, J. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J. Cell Sci. 2003, 116, 647–654. [Google Scholar] [CrossRef]
- Keembiyehetty, C.N.; Krześlak, A.; Love, N.C.; Hanover, J.A. A lipid-droplet-targeted O-GlcNAcase isoform is a key regulator of the proteasome. J. Cell Sci. 2011, 124, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.A.; Lane, M.D.; Hart, G.W. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J. Biol. Chem. 2008, 283, 21411–21417. [Google Scholar] [CrossRef] [PubMed]
- Bullen, J.W.; Balsbaugh, J.L.; Chanda, D.; Shabanowitz, J.; Hunt, N.F.; Neumann, D.; Hart, G.W. Cross-talk between Two Essential Nutrient-sensitive Enzymes. J. Boil. Chem. 2014, 289, 10592–10606. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.G.; Kim, H.B.; Kang, M.J.; Ryum, J.H.; Yi, E.C.; Cho, J.W. Identification of the nuclear localisation signal of O-GlcNAc transferase and its nuclear import regulation. Sci. Rep. 2016, 6, 34614. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.; Gao, Y.; Mahoney, J.A.; Vosseller, K.; Chen, C.; Rosen, A.; Hart, G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: Further characterization of the nucleocytoplasmic beta-N-acetylglucosaminidase, O-GlcNAcase. J. Boil. Chem. 2002, 277, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Parker, M.P.; Graw, S.; Novikova, L.V.; Fedosyuk, H.; Fontes, J.D.; Koestler, D.C.; Peterson, K.R.; Slawson, C. O-GlcNAc homeostasis contributes to cell fate decisions during hematopoiesis. J. Boil. Chem. 2019, 294, 1363–1379. [Google Scholar] [CrossRef]
- McColgan, N.M.; Feeley, M.N.; Woodward, A.M.; Guindolet, D.; Argüeso, P. The O-GlcNAc modification promotes terminal differentiation of human corneal epithelial cells. Glycobiology 2020. [Google Scholar] [CrossRef]
- Yu, C.; Shen, K.; Lin, M.; Chen, P.; Lin, C.; Chang, G.-D.; Chen, H. GCMa Regulates the Syncytin-mediated Trophoblastic Fusion. J. Boil. Chem. 2002, 277, 50062–50068. [Google Scholar] [CrossRef]
- Liang, C.-Y.; Wang, L.-J.; Chen, C.-P.; Chen, L.-F.; Chen, Y.-H.; Chen, H. GCM1 Regulation of the Expression of Syncytin 2 and Its Cognate Receptor MFSD2A in Human Placenta1. Boil. Reprod. 2010, 83, 387–395. [Google Scholar] [CrossRef]
- Xie, S.; Jin, N.; Gu, J.; Shi, J.; Sun, J.; Chu, D.; Zhang, L.; Dai, C.-L.; Gu, J.-H.; Gong, C.-X.; et al. O-GlcNAcylation of protein kinase A catalytic subunits enhances its activity: A mechanism linked to learning and memory deficits in Alzheimer’s disease. Aging Cell 2016, 15, 455–464. [Google Scholar] [CrossRef]
- Hirosawa, M.; Hayakawa, K.; Yoneda, C.; Arai, D.; Shiota, H.; Suzuki, T.; Tanaka, S.; Dohmae, N.; Shiota, K. Novel O-GlcNAcylation on Ser40 of canonical H2A isoforms specific to viviparity. Sci. Rep. 2016, 6, 31785. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.M.; Harper, J.; Roberts, S.A.; O’Neill, H.C.; Johnstone, E.D.; Brison, D.R. The impact of selected embryo culture conditions on ART treatment cycle outcomes: A UK national study. Hum. Reprod. Open 2020, 2020, hoz031. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef] [PubMed]
| Antibody (Clone/Catalogue Number) | Source |
|---|---|
| O-GlcNAc (RL2) | Biolegend (London, UK) |
| OGA (14711-1-AP) | Proteintech (Manchester, UK) |
| OGT (D1D8Q) | Cell Signaling Technologies (London, UK) |
| CDX2 (D11D10) | Cell Signaling Technologies |
| GATA3 (MAB6330) | R & D Systems (Abingdon, UK) |
| Gene | Primers (5′–3′) |
|---|---|
| Gfpt1 | CACCAATCGTGTCATCTTTCTGG GCAGTTCGTTTAATTCGGTGGAT |
| Ogt | TTGGCAATTAAACAGAATCCCCT GGCATGTCGATAATGCTCGAT |
| Oga | CATAGGATGTTTTGGCGAGAGAT CCTGGCGAAATAGCATAGATGAA |
| Cdx2 | CAAGGACGTGAGCATGTATCC GTAACCACCGTAGTCCGGGTA |
| Gata2 | CACCCCGCCGTATTGAATG CCTGCGAGTCGAGATGGTTG |
| Gata3 | CTCGGCCATTCGTACATGGAA GGATACCTCTGCACCGTAGC |
| Eomes | GCGCATGTTTCCTTTCTTGAG GGTCGGCCAGAACCACTTC |
| Elf5 | ACCGATCTGTTCAGCAATGAAG CGCTTGGTCCAGTATTCAGG |
| Tfap2c | ATCCCTCACCTCTCCTCTCC CCAGATGCGAGTAATGGTCGG |
| Hand1 | CTACCAGTTACATCGCCTACTTG ACCACCATCCGTCTTTTTGAG |
| Gapdh | AGGTCGGTGTGAACGGATTTG GGGGTCGTTGATGGCAACA |
| OVOL1 | TGAACCGCCACATGAAGTGTC GACGTGTCTCTTGAGGTCGAA |
| GCM1 | CCAATTCCAGCGGGTAATCTT GGTGAATGGTATGCAGGAGAC |
| ERVFRD1 | ACCGCCATCCTGATTTCCC GAGGCTGGATAAGCTGCTCC |
| S18 | CGGCTACCACATCCAAGGAA GCTGGAATTACCGCGGCT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruane, P.T.; Tan, C.M.J.; Adlam, D.J.; Kimber, S.J.; Brison, D.R.; Aplin, J.D.; Westwood, M. Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation. Cells 2020, 9, 2246. https://doi.org/10.3390/cells9102246
Ruane PT, Tan CMJ, Adlam DJ, Kimber SJ, Brison DR, Aplin JD, Westwood M. Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation. Cells. 2020; 9(10):2246. https://doi.org/10.3390/cells9102246
Chicago/Turabian StyleRuane, Peter T., Cheryl M. J. Tan, Daman J. Adlam, Susan J. Kimber, Daniel R. Brison, John D. Aplin, and Melissa Westwood. 2020. "Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation" Cells 9, no. 10: 2246. https://doi.org/10.3390/cells9102246
APA StyleRuane, P. T., Tan, C. M. J., Adlam, D. J., Kimber, S. J., Brison, D. R., Aplin, J. D., & Westwood, M. (2020). Protein O-GlcNAcylation Promotes Trophoblast Differentiation at Implantation. Cells, 9(10), 2246. https://doi.org/10.3390/cells9102246






