Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of (Non-)Degradable Nanogels and Non-Crosslinked, Soluble Polymers
2.4. Nanobody Conjugation to (Non-)Degradable Nanogels and Non-Crosslinked, Soluble Polymers
2.5. Uptake of Anti-MMR Nanobody-Functionalized (Non-)Degradable Nanogels and Non-Crosslinked, Soluble Polymers by CHOMMR+ and CHOMMR− Cells
2.5.1. Cell Culture
2.5.2. Flow Cytometry
2.5.3. Confocal Microscopy
2.5.4. Statistical Analysis
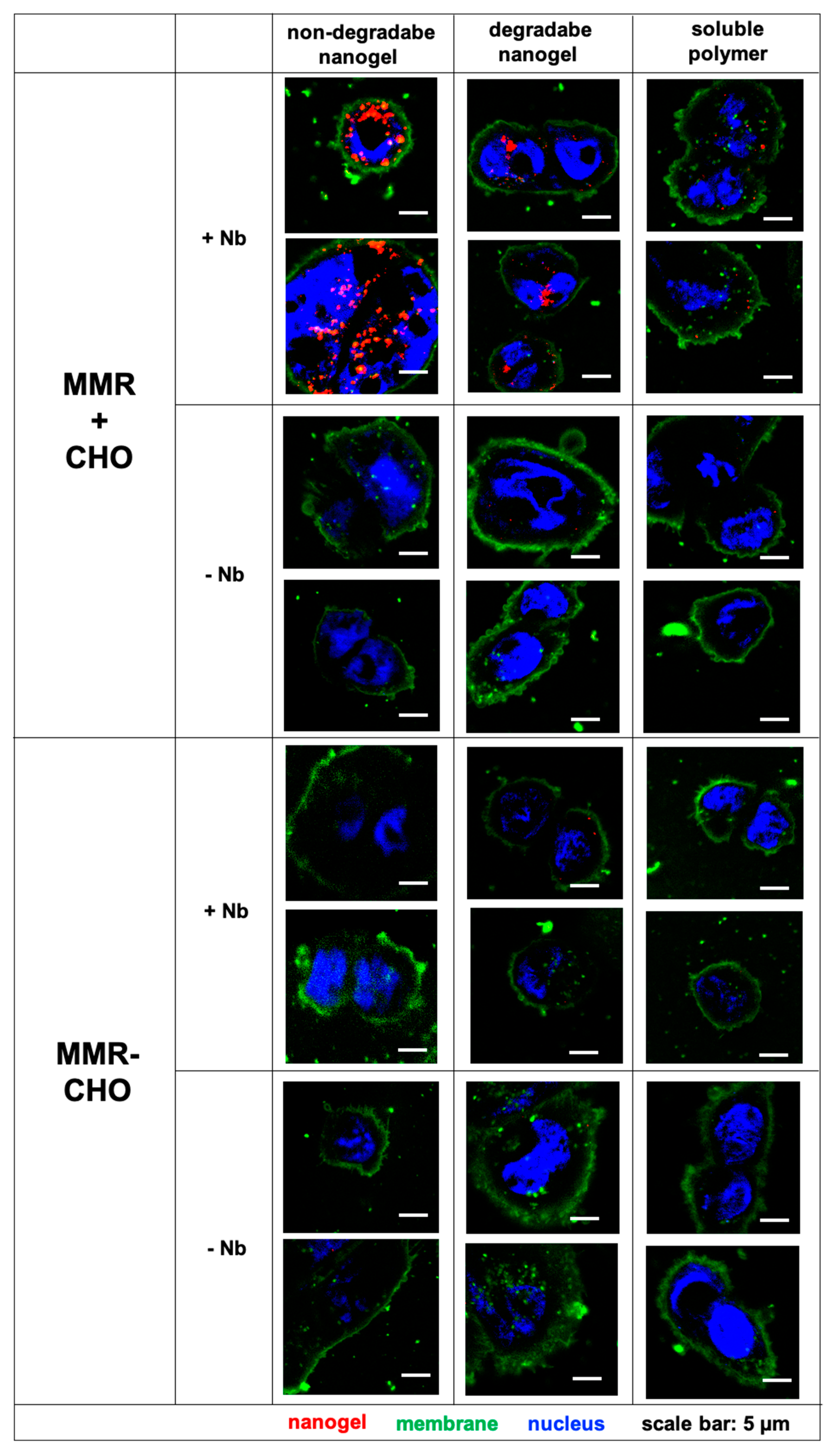
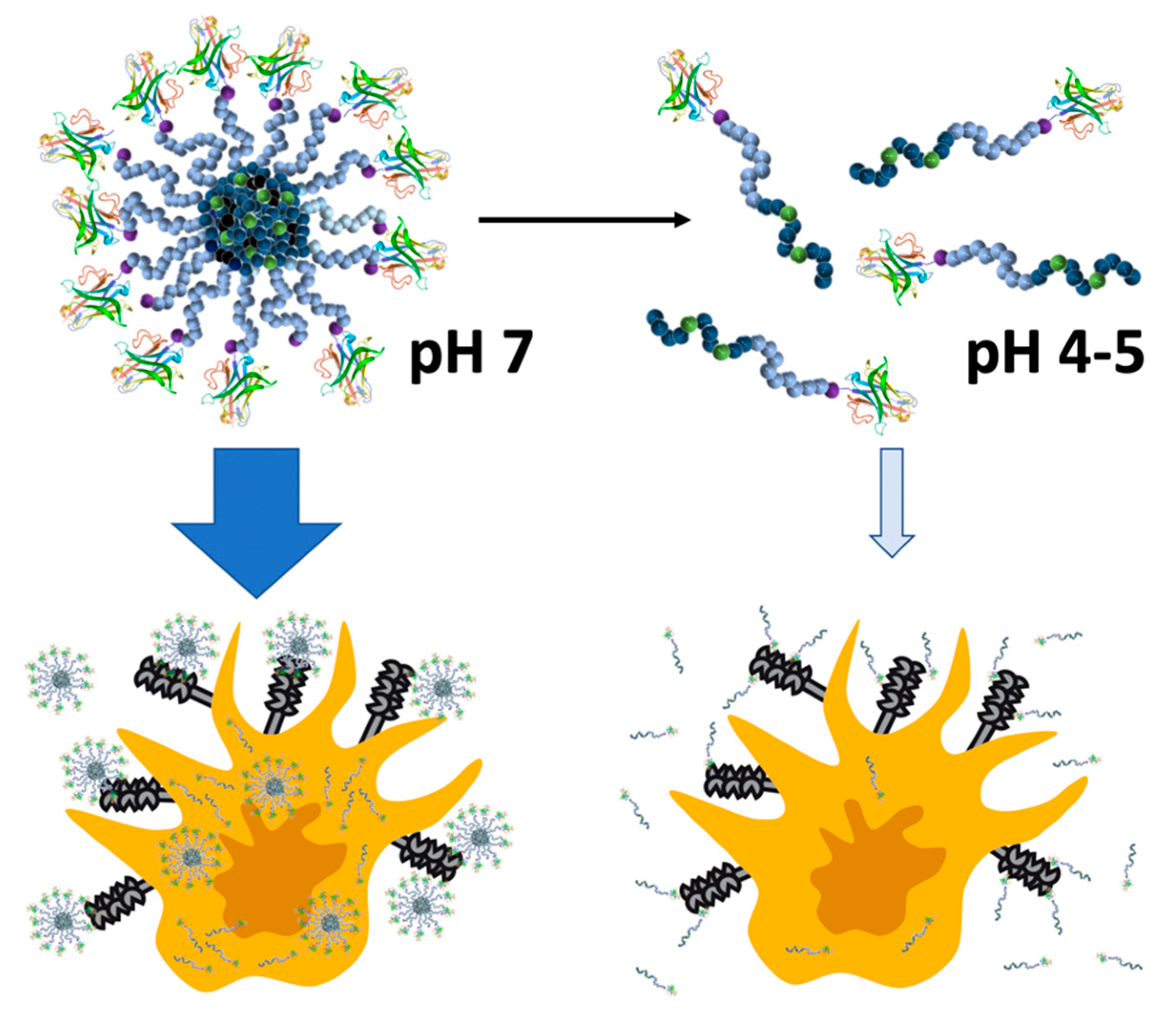
3. Results and Discussion
4. Summary and Conclusions
Author Contributions
Funding
Acknowledgment
Conflicts of Interest
References
- Farokhzad, O.C.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Matsumura, Y. EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv. Drug Deliv. Rev. 2010, 3, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Gros, L.; Ringsdorf, H.; Schupp, H. Polymeric Antitumor Agents on a Molecular and on a Cellular Level? Angew. Chem. Int. Ed. 1981, 20, 305–325. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farahavar, G.; Abolmaali, S.S.; Gholijani, N.; Nejatollahi, F. Antibody-guided nanomedicines as novel breakthrough therapeutic, diagnostic and theranostic tools. Biomater. Sci. 2019, 7, 4000–4016. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.W.K.; Allen, T.M. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: A comparison of whole monoclonal antibody, Fab′ fragments and single chain Fv. J. Control. Release 2008, 126, 50–58. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Bajyana Songa, E.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Arbabi Ghahroudi, M.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Richards, D.A.; Maruani, A.; Chudasama, V. Antibody fragments as nanoparticle targeting ligands: A step in the right direction. Chem. Sci. 2017, 8, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Talelli, M.; Rijcken, C.J.F.; Oliveira, S.; Van Der Meel, R.; Van Bergen En Henegouwen, P.M.P.; Lammers, T.; Van Nostrum, C.F.; Storm, G.; Hennink, W.E. Reprint of “nanobody–Shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting”. J. Control. Release 2011, 153, 93–102. [Google Scholar] [CrossRef]
- Talelli, M.; Oliveira, S.; Rijcken, C.J.F.; Pieters, E.H.E.; Etrych, T.; Ulbrich, K.; van Nostrum, R.C.F.; Storm, G.; Hennink, W.E.; Lammers, T. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials 2013, 34, 1255–1260. [Google Scholar] [CrossRef]
- Van Audenhove, I.; Gettemans, J. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Liu, C.; Muyldermans, S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front. Immunol. 2017, 8, 1442. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Abarghooi Kahaki, F.; Ahangarzadeh, S.; Yaghoobi, H.; Yarian, F.; Arezumand, R.; Ranjbari, J.; Mokhtarzadeh, A.; de la Guardia, M. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J. Control. Release 2017, 268, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Yuen, D.; Chen, M.Z.; Porter, C.J.H.; Johnston, A.P.R. Pointing in the Right Direction: Controlling the Orientation of Proteins on Nanoparticles Improves Targeting Efficiency. Nano Lett. 2019, 19, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.W.; Yuen, D.; Chen, M.Z.; Johnston, A.P.R. Engineering the Orientation, Density, and Flexibility of Single-Domain Antibodies on Nanoparticles to Improve Cell Targeting. ACS Appl. Mater. Interfaces 2020, 12, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Laoui, D.; Van Overmeire, E.; Conza, G.D.; Aldeni, C.; Keirsse, J.; Morias, Y.; Movahedi, K.; Houbracken, I.; Schouppe, E.; Elkrim, Y.; et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014, 74, 24–30. [Google Scholar] [CrossRef] [Green Version]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Chittezhath, M.; Dhillon, M.K.; Lim, J.Y.; Laoui, D.; Shalova, I.N.; Teo, Y.L.; Chen, J.; Kamaraj, R.; Raman, L.; Lum, J.; et al. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Cancer Cell 2014, 41, 815–829. [Google Scholar] [CrossRef] [Green Version]
- Movahedi, K.; Schoonooghe, S.; Laoui, D.; Houbracken, I.; Waelput, W.; Breckpot, K.; Bouwens, L.; Lahoutte, T.; De Baetselier, P.; Raes, G.; et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012, 72, 4165–4177. [Google Scholar] [CrossRef] [Green Version]
- Put, S.; Schoonooghe, S.; Devoogdt, N.; Schurgers, E.; Avau, A.; Mitera, T.; D’Huyvetter, M.; De Baetselier, P.; Raes, G.; Lahoutte, T.; et al. SPECT Imaging of Joint Inflammation with Nanobodies Targeting the Macrophage Mannose Receptor in a Mouse Model for Rheumatoid Arthritis. J. Nucl. Med. 2013, 54, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Bolli, E.; D’Huyvetter, M.; Murgaski, A.; Berus, D.; Stangé, G.; Clappaert, E.J.; Arnouk, S.; Pombo Antunes, A.R.; Krasniqi, A.; Lahoutte, T.; et al. Stromal-targeting radioimmunotherapy mitigates the progression of therapy-resistant tumors. J. Control. Release 2019, 314, 1–11. [Google Scholar] [CrossRef]
- De Vlaeminck, Y.; Lecocq, Q.; Giron, P.; Heirman, C.; Geeraerts, X.; Bolli, E.; Movahedi, K.; Massa, S.; Schoonooghe, S.; Thielemans, K.; et al. Single-domain antibody fusion proteins can target and shuttle functional proteins into macrophage mannose receptor expressing macrophages. J. Control. Release 2019, 299, 107–120. [Google Scholar] [CrossRef]
- Sun, X.; Gao, D.; Gao, L.; Zhang, C.; Yu, X.; Jia, B.; Wang, F.; Liu, Z. Molecular imaging of tumor-infiltrating macrophages in a preclinical mouse model of breast cancer. Theranostics 2015, 5, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, L.; Cai, Y.; Liu, H.; Gao, D.; Lai, J.; Jia, B.; Wang, F.; Liu, Z. Inhibition of tumor growth and metastasis by photoimmunotherapy targeting tumor-associated macrophage in a sorafenib-resistant tumor model. Biomaterials 2016, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al Faraj, A.; Shaik, A.S.; Afzal, S.; Al Sayed, B.; Halwani, R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int. J. Nanomed. 2014, 9, 1491–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuhn, L.; Bolli, E.; Massa, S.; Vandenberghe, I.; Movahedi, K.; Devreese, B.; Van Ginderachter, J.A.; De Geest, B.G. Targeting Protumoral Tumor-Associated Macrophages with Nanobody-Functionalized Nanogels through Strain Promoted Azide Alkyne Cycloaddition Ligation. Bioconjug. Chem. 2018, 29, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Nuhn, L.; Hirsch, M.; Krieg, B.; Koynov, K.; Fischer, K.; Schmidt, M.; Helm, M.; Zentel, R. Cationic Nanohydrogel Particles as Potential siRNA Carriers for Cellular Delivery. ACS Nano 2012, 6, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Leber, N.; Nuhn, L.; Zentel, R. Cationic Nanohydrogel Particles for Therapeutic Oligonucleotide Delivery. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stickdorn, J.; Nuhn, L. Reactive-ester derived polymer nanogels for cancer immunotherapy. Eur. Polym. J. 2020, 124, 109481. [Google Scholar] [CrossRef]
- Hartmann, S.; Nuhn, L.; Palitzsch, B.; Glaffig, M.; Stergiou, N.; Gerlitzki, B.; Schmitt, E.; Kunz, H.; Zentel, R. CpG-Loaded Multifunctional Cationic Nanohydrogel Particles as Self-Adjuvanting Glycopeptide Antitumor Vaccines. Adv. Healthc. Mater. 2015, 4, 522–527. [Google Scholar] [CrossRef]
- Leber, N.; Kaps, L.; Aslam, M.; Schupp, J.; Brose, A.; Schäffel, D.; Fischer, K.; Diken, M.; Strand, D.; Koynov, K.; et al. SiRNA-mediated in vivo gene knockdown by acid-degradable cationic nanohydrogel particles. J. Control. Release 2017, 248, 10–23. [Google Scholar] [CrossRef]
- Kaps, L.; Leber, N.; Klefenz, A.; Choteschovsky, N.; Zentel, R.; Nuhn, L.; Schuppan, D. In Vivo siRNA Delivery to Immunosuppressive Liver Macrophages by α-Mannosyl-Functionalized Cationic Nanohydrogel Particles. Cells 2020, 9, 1905. [Google Scholar] [CrossRef]
- Vanparijs, N.; Nuhn, L.; Paluck, S.J.; Kokkinopoulou, M.; Lieberwirth, I.; Maynard, H.D.; De Geest, B.G. Core/shell protein-reactive nanogels via a combination of RAFT polymerization and vinyl sulfone postmodification. Nanomedicine 2016, 11, 2631–2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuhn, L.; Kaps, L.; Diken, M.; Schuppan, D.; Zentel, R. Reductive Decationizable Block Copolymers for Stimuli-Responsive mRNA Delivery. Macromol. Rapid Commun. 2016, 37, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [Green Version]
- Nuhn, L.; Van Herck, S.; Best, A.; Deswarte, K.; Kokkinopoulou, M.; Lieberwirth, I.; Koynov, K.; Lambrecht, B.N.; De Geest, B.G. FRET Monitoring of Intracellular Ketal Hydrolysis in Synthetic Nanoparticles. Angew. Chem. Int. Ed. 2018, 57, 10760–10764. [Google Scholar] [CrossRef]
- Kockelmann, J.; Stickdorn, J.; Kasmi, S.; De Vrieze, J.; Pieszka, M.; Ng, D.Y.W.; David, S.A.; De Geest, B.G.; Nuhn, L. Control over Imidazoquinoline Immune Stimulation by pH-Degradable Poly(norbornene) Nanogels. Biomacromolecules 2020, 21, 2246–2257. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pomares, L.; Reid, D.M.; Brown, G.D.; Taylor, P.R.; Stillion, R.J.; Linehan, S.A.; Zamze, S.; Gordon, S.; Wong, S.Y.C. Analysis of mannose receptor regulation by IL-4, IL-10, and proteolytic processing using novel monoclonal antibodies. J. Leukoc. Biol. 2003, 73, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Bakker, T.; Harris, J.; Tsang, C.; Brown, G.D.; Wormald, M.R.; Gordon, S.; Dwek, R.A.; Rudd, P.M.; Martinez-Pomares, L. Glycosylation influences the lectin activities of the macrophage mannose receptor. J. Biol. Chem. 2005, 280, 32811–32820. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.B.; Azad, A.K.; Torrelles, J.B.; Kaufman, T.M.; Beharka, A.; Tibesar, E.; DesJardin, L.E.; Schlesinger, L.S. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 2005, 202, 987–999. [Google Scholar] [CrossRef]
- Sweet, L.; Singh, P.P.; Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S.; Schorey, J.S. Mannose receptor-dependent delay in phagosome maturation by Mycobacterium avium glycopeptidolipids. Infect. Immun. 2010, 78, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Vigerust, D.J.; Vick, S. Stable Expression and Characterization of an Optimized Mannose Receptor. J. Clin. Cell. Immunol. 2015, 6, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
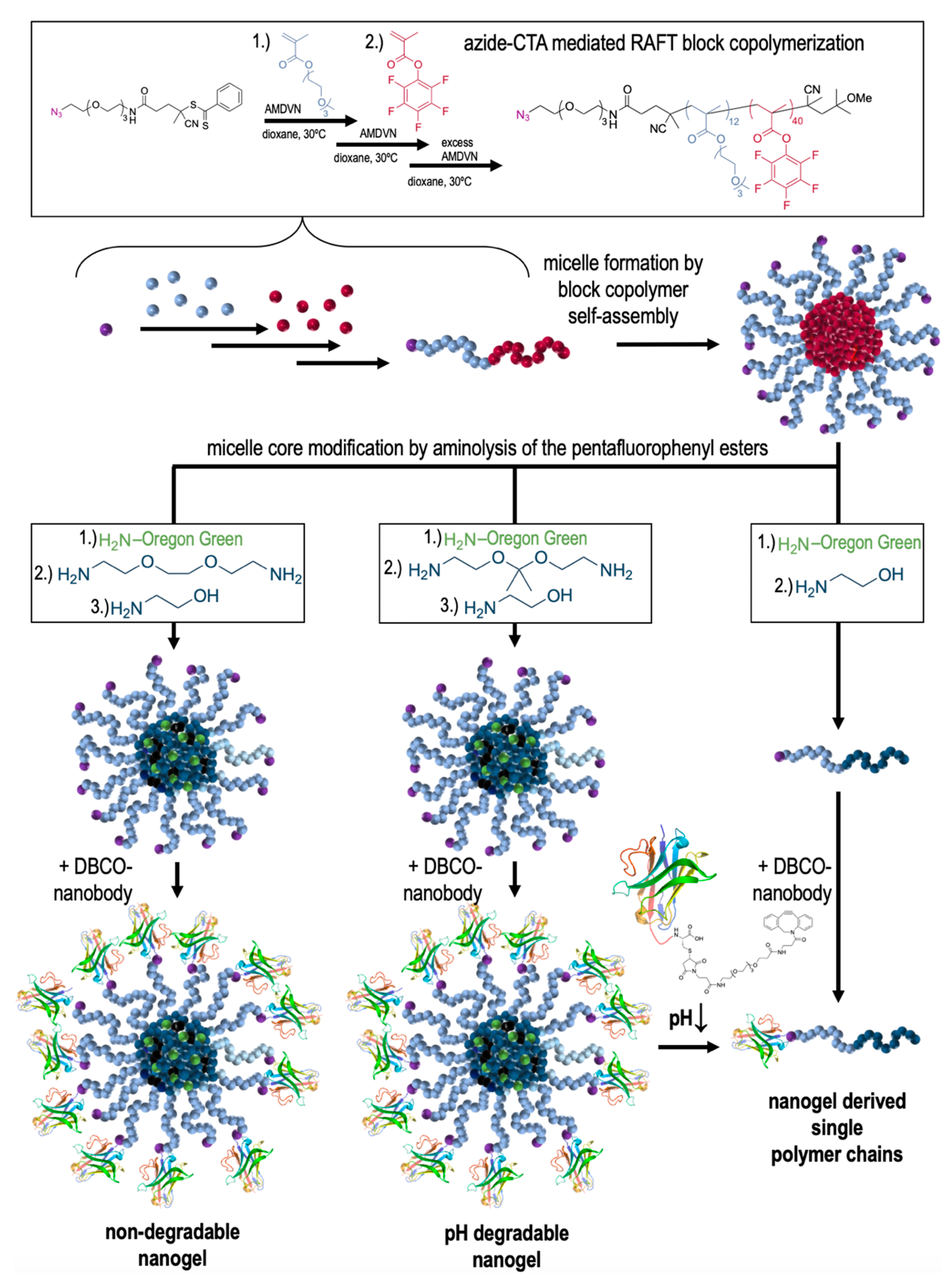
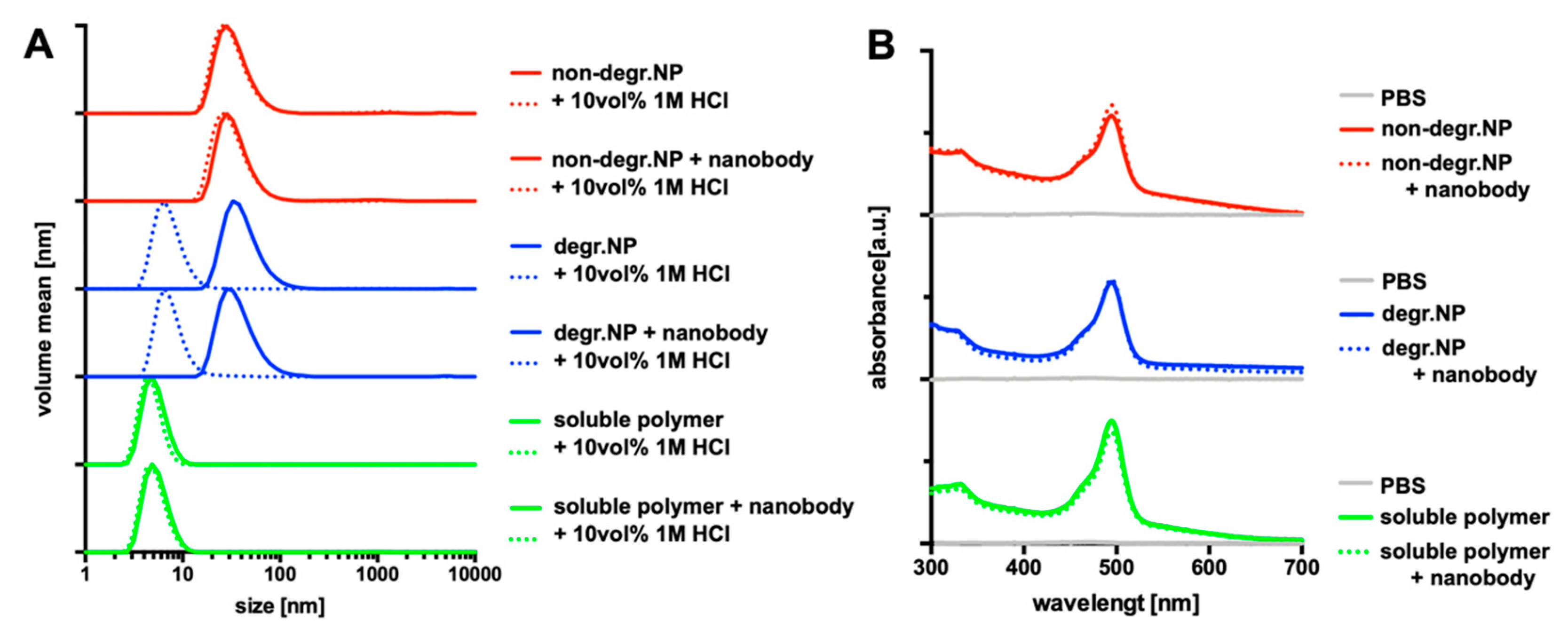
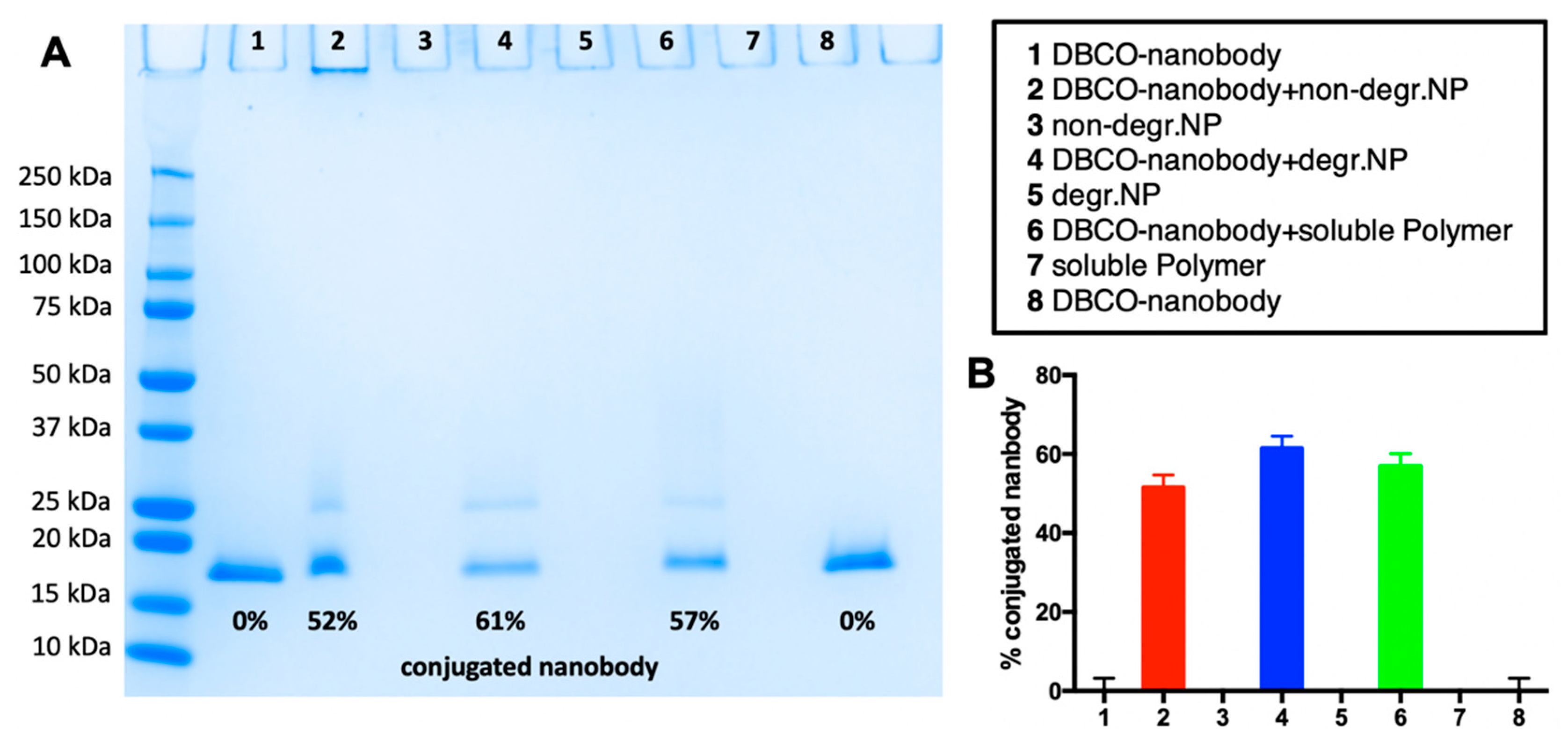
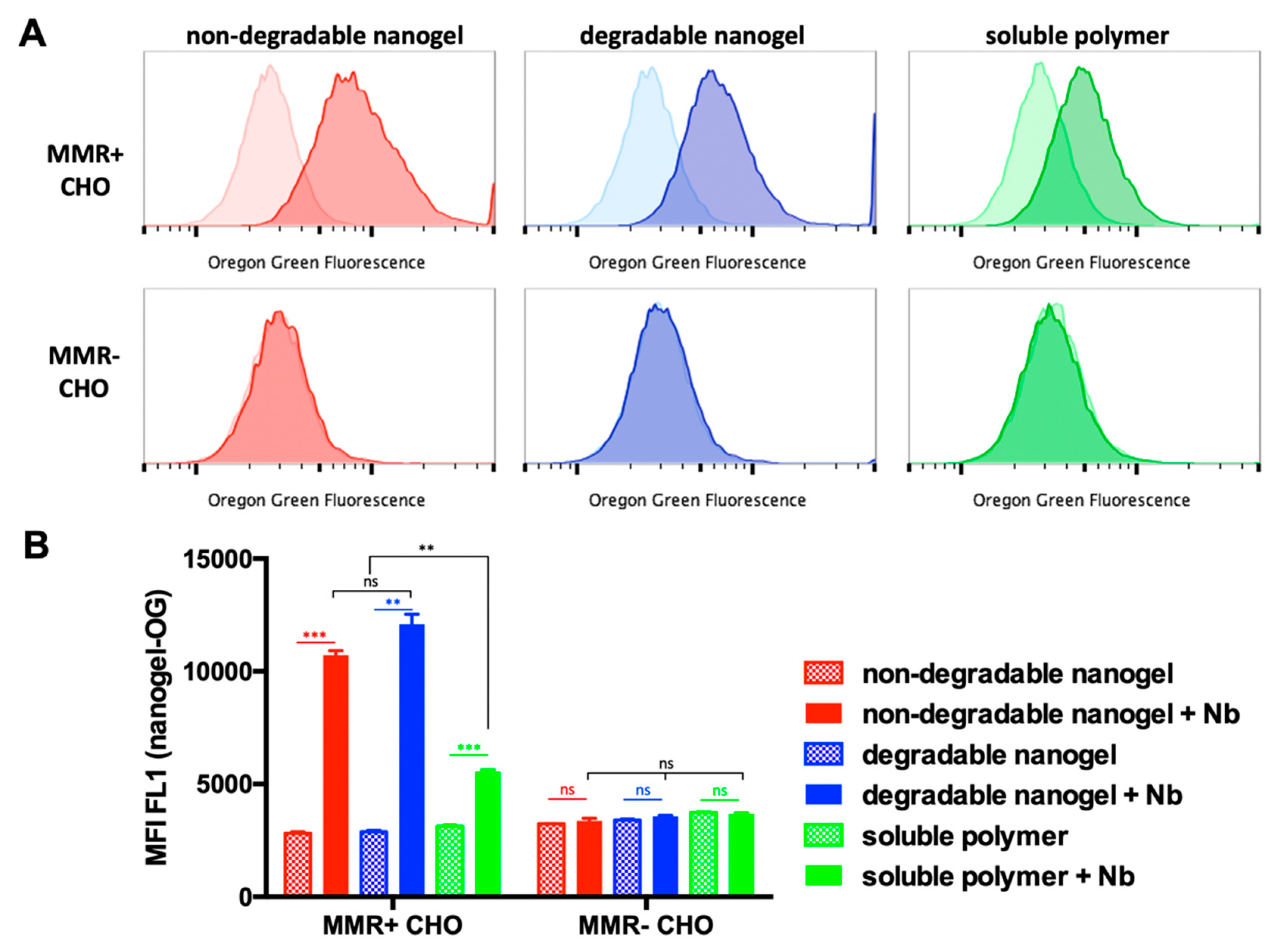
| Sample | Precursor Polymer | Crosslinker | Hydrodynamic Diameter [Nm] * | Poly-Dispersity * |
|---|---|---|---|---|
| non-degradable nanogel | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2,2′ -(ethylenedioxy) bis(ethylamine) | 51.2 ± 0.5 | 0.23 ± 0.01 |
| non-degradable nanogel + nanobody | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2,2′ -(ethylenedioxy) bis(ethylamine) | 57.6 ± 0.6 | 0.33 ± 0.01 |
| degradable nanogel | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2,2-bis(amino-ethoxy)propane | 61.9 ± 0.5 | 0.20 ± 0.01 |
| degradable nanogel + nanobody | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2,2-bis(amino-ethoxy)propane | 58.3 ± 0.5 | 0.22 ± 0.01 |
| soluble polymer | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2-ethanolamine (non-crosslinking) | 9.4 ± 0.6 # | 0.64 ± 0.26 |
| soluble polymer + nanobody | N3-(mTEGMA)12 -b-P(PFPMA)40 | 2-ethanolamine (non-crosslinking) | 8.9 ± 3.2 # | 0.57 ± 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherger, M.; Bolli, E.; Antunes, A.R.P.; Arnouk, S.; Stickdorn, J.; Van Driessche, A.; Schild, H.; Grabbe, S.; De Geest, B.G.; Van Ginderachter, J.A.; et al. Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels. Cells 2020, 9, 2222. https://doi.org/10.3390/cells9102222
Scherger M, Bolli E, Antunes ARP, Arnouk S, Stickdorn J, Van Driessche A, Schild H, Grabbe S, De Geest BG, Van Ginderachter JA, et al. Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels. Cells. 2020; 9(10):2222. https://doi.org/10.3390/cells9102222
Chicago/Turabian StyleScherger, Maximilian, Evangelia Bolli, Ana Rita Pombo Antunes, Sana Arnouk, Judith Stickdorn, Alexandra Van Driessche, Hansjörg Schild, Stephan Grabbe, Bruno G. De Geest, Jo A. Van Ginderachter, and et al. 2020. "Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels" Cells 9, no. 10: 2222. https://doi.org/10.3390/cells9102222
APA StyleScherger, M., Bolli, E., Antunes, A. R. P., Arnouk, S., Stickdorn, J., Van Driessche, A., Schild, H., Grabbe, S., De Geest, B. G., Van Ginderachter, J. A., & Nuhn, L. (2020). Transient Multivalent Nanobody Targeting to CD206-Expressing Cells via PH-Degradable Nanogels. Cells, 9(10), 2222. https://doi.org/10.3390/cells9102222






