Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
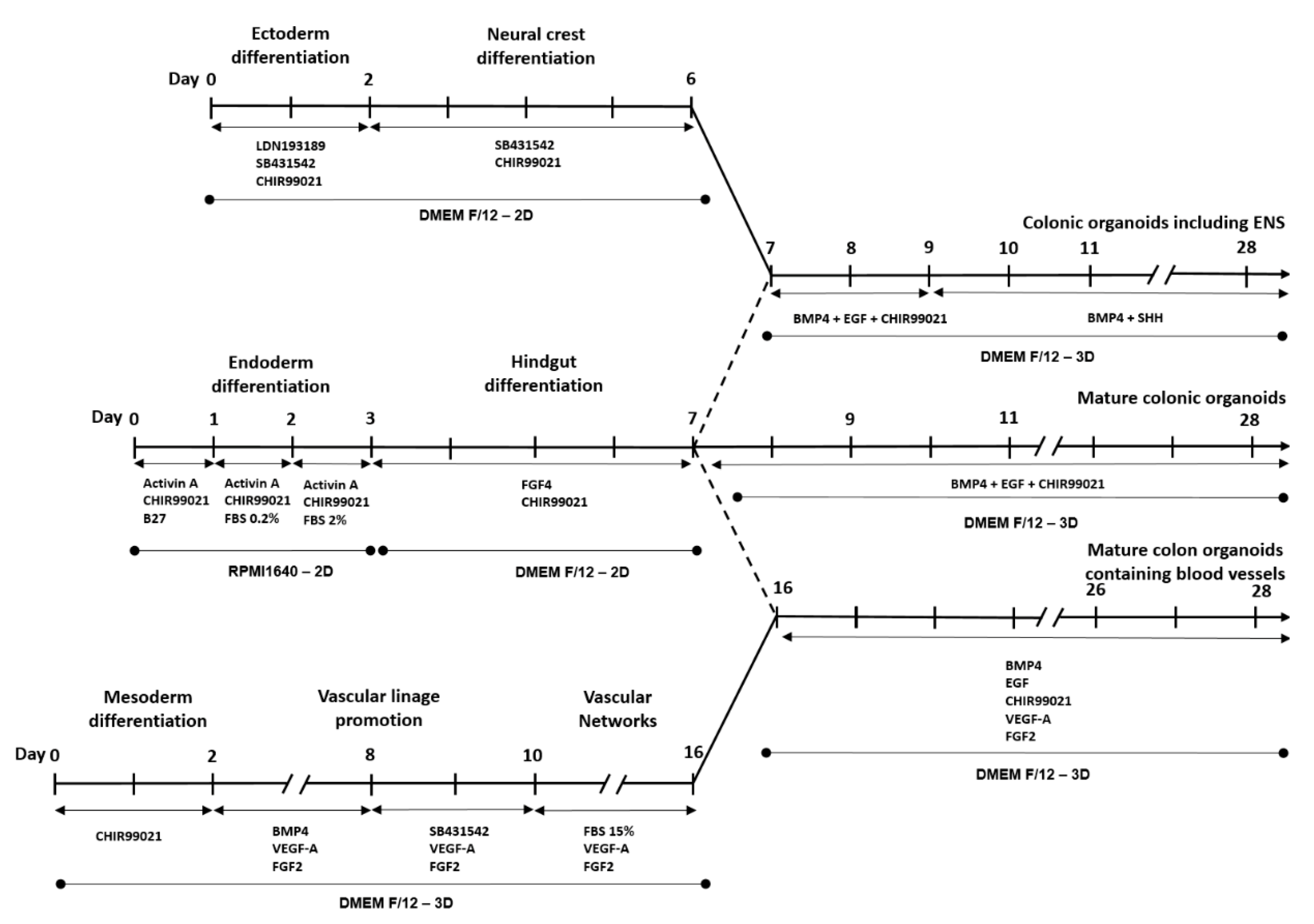
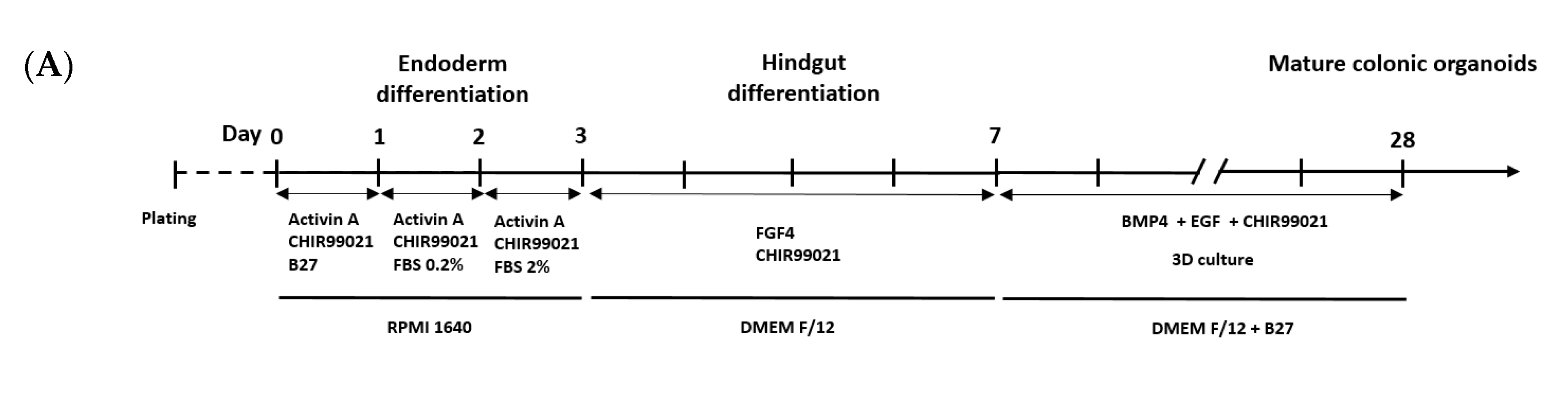
2.1. Colonic Organoid Development Using Human Embryonic Stem Cells
2.2. Development of Colonic Organoids Containing Enteric Nervous System
2.3. Development of Colonic Organoids Containing Blood Vessels
2.4. Colon Cancer Organoid Development
2.5. Total RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. Hematoxylin & Eosin Staining
2.8. Immunofluorescent Staining
2.9. Statistical Analysis
3. Results
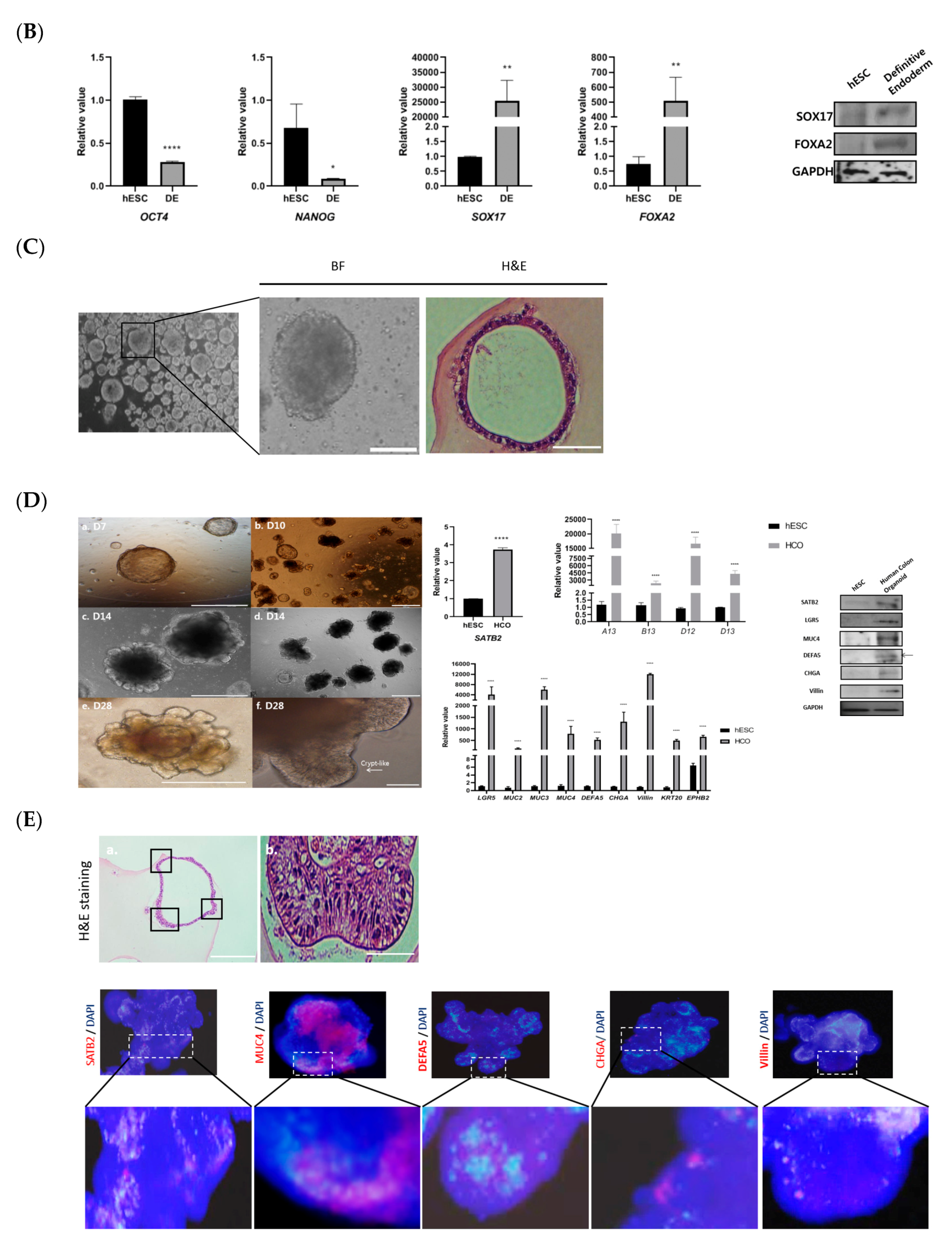
3.1. Development and Characterization of Colonic Organoids
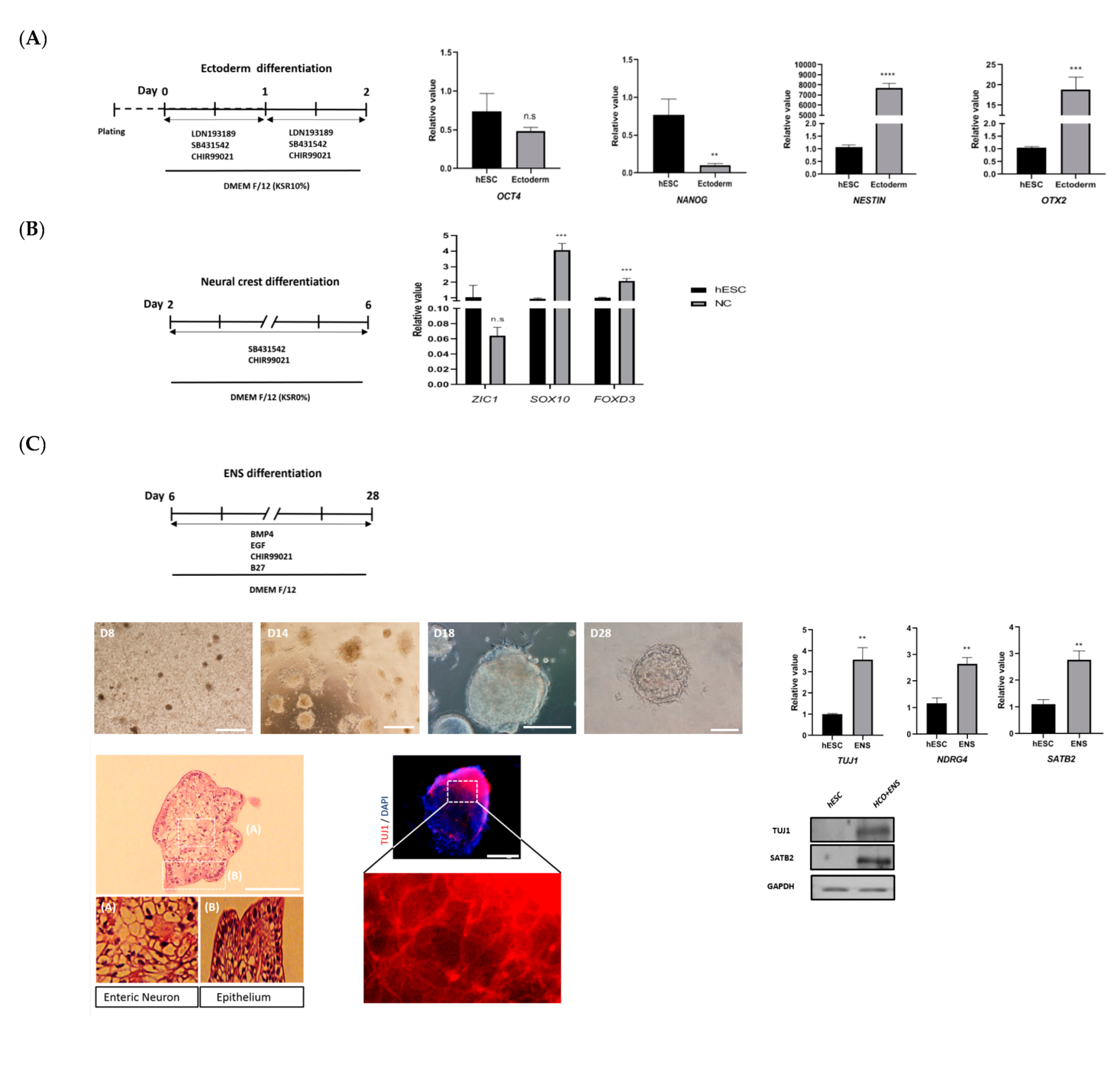
3.2. Development of Colonic Organoids Containing Enteric Nervous System
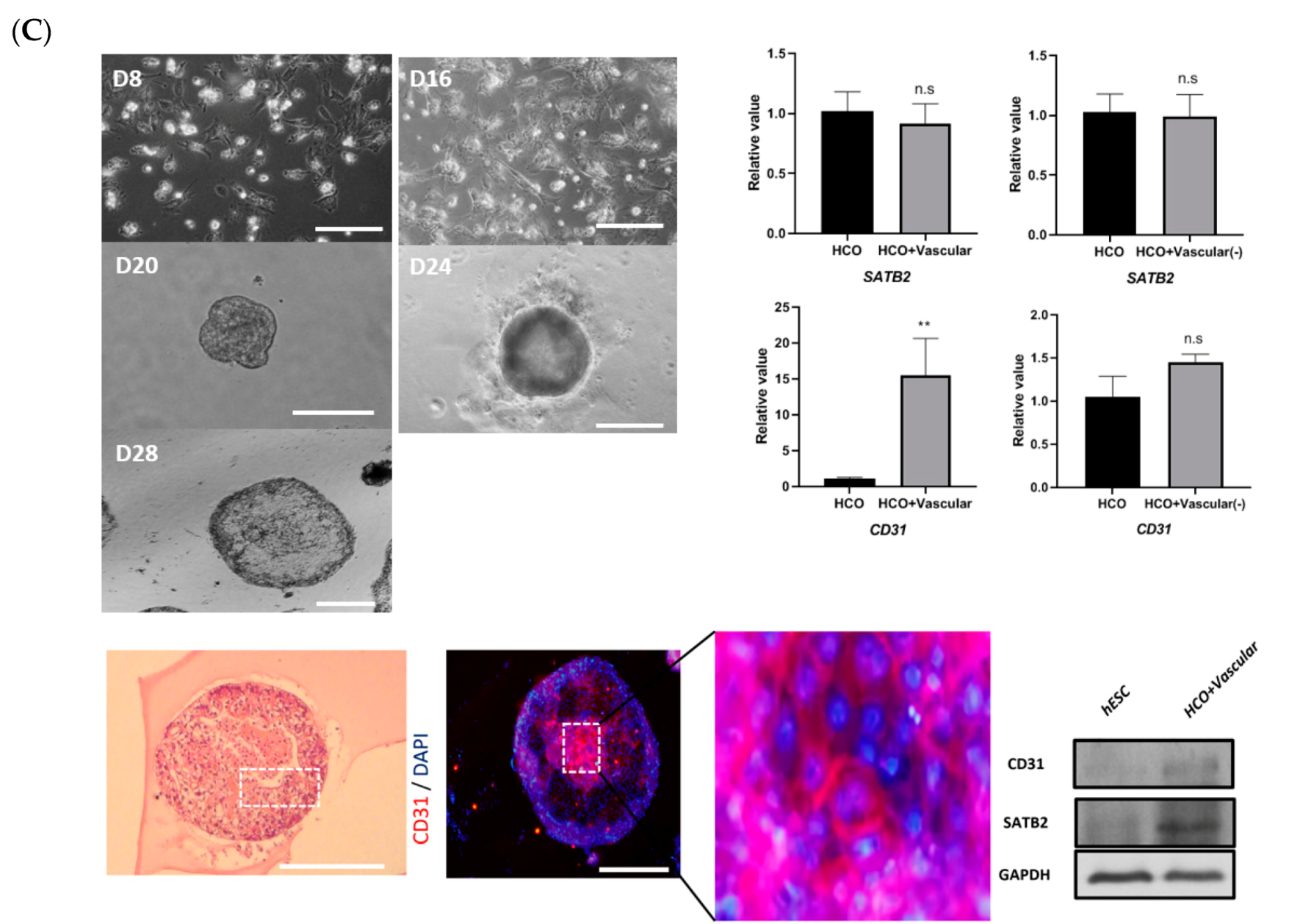
3.3. Development of Colonic Organoids Containing Blood Vessels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Of men, not mice. Nat. Med. 2013, 19, 379. [CrossRef] [PubMed] [Green Version]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Kondo, J.; Inoue, M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470. [Google Scholar] [CrossRef] [Green Version]
- Organoid Research. Nature Cell Biology. 2018. Available online: https://www.nature.com/collections/gglyjbyvdq (accessed on 14 June 2020).
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Mazzei, M.A.; Mazzei, F.G.; Marrelli, D.; Imbriaco, G.; Guerrini, S.; Vindigni, C.; Civitelli, S.; Roviello, F.; Grassi, R.; Volterrani, L. Computed tomographic evaluation of mesentery: Diagnostic value in acute mesenteric ischemia. J. Comput. Assist. Tomogr. 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Elder, K.; Lashner, B.A.; Al Solaiman, F. Clinical approach to colonic ischemia. Clevel. Clin. J. Med. 2009, 76, 401–409. [Google Scholar] [CrossRef]
- Pesce, M.; Borrelli, O.; Saliakellis, E.; Thapar, N. Gastrointestinal Neuropathies: New Insights and Emerging Therapies. Gastroenterol. Clin. N. Am. 2018, 47, 877–894. [Google Scholar] [CrossRef]
- Workman, M.J.; Mahe, M.M.; Trisno, S.; Poling, H.M.; Watson, C.L.; Sundaram, N.; Chang, C.-F.; Schiesser, J.; Aubert, P.; Stanley, E.G.; et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 2017, 23, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Kasendra, M.; Tovaglieri, A.; Sontheimer-Phelps, A.; Jalili-Firoozinezhad, S.; Bein, A.; Chalkiadaki, A.; Scholl, W.; Zhang, C.; Rickner, H.; Richmond, C.A.; et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep. 2018, 8, 2871. [Google Scholar] [CrossRef]
- Múnera, J.O.; Sundaram, N.; Rankin, S.A.; Hill, D.; Watson, C.; Mahe, M.; Vallance, J.E.; Shroyer, N.F.; Sinagoga, K.L.; Zarzoso-Lacoste, A.; et al. Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell 2017, 21, 51–64.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Shimizu, H.; Suzuki, K.; Kawamoto, A.; Takahashi, J.; Kawai, M.; Nagata, S.; Hiraguri, Y.; Takeoka, S.; Sugihara, H.Y.; et al. Organoid-based regenerative medicine for inflammatory bowel disease. Regen. Ther. 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-H.; Donowitz, M. Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World J. Gastroenterol. 2019, 25, 4125–4147. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burt, R.K.; Traynor, A.; Oyama, Y.; Craig, R. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood 2003, 101, 2064–2066. [Google Scholar] [CrossRef] [Green Version]
- Burt, R.K.; Craig, R.M.; Milanetti, F.; Quigley, K.; Gozdziak, P.; Bucha, J.; Testori, A.; Halverson, A.; Verda, L.; de Villiers, W.J.S.; et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: Long-term follow-up. Blood 2010, 116, 6123–6132. [Google Scholar] [CrossRef]
- Kessmann, J. Hirschsprung’s disease: Diagnosis and management. Am. Fam. Physician 2006, 74, 1319–1322. [Google Scholar]
- Badner, J.A.; Sieber, W.K.; Garver, K.L.; Chakravarti, A. A genetic study of Hirschsprung disease. Am. J. Hum. Genet. 1990, 46, 568–580. [Google Scholar]
- Stem, P.; Definitive, C.; Differentiation, E.; Wang, H.; Luo, X.; Yao, L.; Lehman, D.M.; Wang, P. Improvement of Cell Survival During Human. Stem Cells Dev. 2015, 24, 2536–2546. [Google Scholar]
- Tchieu, J.; Zimmer, B.; Fattahi, F.; Amin, S.; Zeltner, N.; Chen, S.; Studer, L. A Modular Platform for Differentiation of Human PSCs into All Major Ectodermal Lineages. Cell Stem Cell 2017, 21, 399–410.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Toni, L.S.; Garcia, A.M.; Jeffrey, D.A.; Jiang, X.; Stauffer, B.L.; Miyamoto, S.D.; Sucharov, C.C. Optimization of phenol-chloroform RNA extraction. MethodsX 2018, 5, 599–608. [Google Scholar] [CrossRef]
- Crespo, M.; Vilar, E.; Tsai, S.; Chang, K.; Amin, S.; Srinivasan, T.; Zhang, T.; Pipalia, N.H.; Chen, H.J.; Witherspoon, M.; et al. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017, 23, 878–884. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Teo, A.K.K.; Arnold, S.J.; Trotter, M.W.B.; Brown, S.; Ang, L.T.; Chng, Z.; Robertson, E.J.; Dunn, N.R.; Vallier, L. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011, 25, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Holtzinger, A.; Streeter, P.R.; Sarangi, F.; Hillborn, S.; Niapour, M.; Ogawa, S.; Keller, G. New markers for tracking endoderm induction and hepatocyte differentiation from human pluripotent stem cells. Development 2015, 142, 4253–4265. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-C.J.; Wani, M.A.; Whitsett, J.A.; Wells, J.M. Klf5 regulates lineage formation in the pre-implantation mouse embryo. Development 2010, 137, 3953–3963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Chen, J.W.; He, X.S.; Zhang, H.Z.; Ling, Y.H.; Wen, J.H.; Deng, W.H.; Li, P.; Yun, J.P.; Xie, D.; et al. SATB2 is a Promising Biomarker for Identifying a Colorectal Origin for Liver Metastatic Adenocarcinomas. EBioMedicine 2018, 28, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, B.; Gopurappilly, R.; Dadheech, N.; Gupta, S.; Bhonde, R.; Pal, R. Embryonic fibroblasts represent a connecting link between mesenchymal and embryonic stem cells. Dev. Growth Differ. 2013, 55, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mundell, N.A.; Labosky, P.A. Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development 2011, 138, 641–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Oron, E.; Nelson, B.; Razis, S.; Ivanova, N. Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell 2012, 10, 440–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plouhinec, J.; Roche, D.D.; Pegoraro, C.; Figueiredo, A.L.; Maczkowiak, F.; Brunet, L.J.; Milet, C.; Vert, J.-P.; Pollet, N.; Harland, R.M.; et al. Pax3 and Zic1 trigger the early neural crest gene regulatory network by the direct activation of multiple key neural crest specifiers. Dev. Biol. 2014, 386, 461–472. [Google Scholar] [CrossRef]
- Mora-Castilla, S.; Tejedo, J.R.; Tapia-Limonchi, R.; Díaz, I.; Hitos, A.B.; Cahuana, G.M.; Hmadcha, A.; Martín, F.; Soria, B.; Bedoya, F.J. Transient Downregulation of Nanog and Oct4 Induced by DETA/NO Exposure in Mouse Embryonic Stem Cells Leads to Mesodermal/Endodermal Lineage Differentiation. Stem Cells Int. 2014. [Google Scholar] [CrossRef]
- Fan, H.; Demirci, U.; Chen, P. Emerging organoid models: Leaping forward in cancer research. J. Hematol. Oncol. 2019, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs. 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [Green Version]
- van de Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; van Houdt, W.; van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Hunziker, W.; Choudhury, D. Engineering Microfluidic Organoid-on-a-Chip Platforms. Micromachines 2019, 10, 165. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.S.; Nguyen, L.P.; Yong, D. Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells. Cells 2020, 9, 2209. https://doi.org/10.3390/cells9102209
Park CS, Nguyen LP, Yong D. Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells. Cells. 2020; 9(10):2209. https://doi.org/10.3390/cells9102209
Chicago/Turabian StylePark, Chul Soon, Le Phuong Nguyen, and Dongeun Yong. 2020. "Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells" Cells 9, no. 10: 2209. https://doi.org/10.3390/cells9102209
APA StylePark, C. S., Nguyen, L. P., & Yong, D. (2020). Development of Colonic Organoids Containing Enteric Nerves or Blood Vessels from Human Embryonic Stem Cells. Cells, 9(10), 2209. https://doi.org/10.3390/cells9102209





