The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review
Abstract
1. Introduction
1.1. Basics
1.2. Diabetes Mellitus and Musculoskeletal Disturbances
1.3. Diabetes Mellitus and Intervertebral Disc Degeneration
1.4. Rodents as Experimental Diabetic Models in IVDD
1.5. Study’s Basic Idea
2. Materials and Methods
2.1. Data Search: Literature Sources
2.2. Characteristics of the Initially Obtained Literature
2.3. Literature Filtration and Selection
2.4. Study’s Admission Requirements
2.5. Data Extraction
2.6. Evaluation of the Studies
2.7. Study Scoring System (SSS)
2.8. Evidence Assessment System (EAS)
3. Results
3.1. Identified Studies
3.2. Origin of Included Studies
3.3. Data Assortment, Analysis, and Evaluation
Quality of the Studies Included
3.4. Evidence Assortment and Assessment
4. Discussion
4.1. Study Strength
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM-TS | A disintegrin and metalloproteinase with thrombospondin motifs |
| AF | Annulus fibrosus |
| AFCs | Annulus fibrosis cells |
| AGE | Advanced glycation end-products |
| AI | Apoptotic index |
| ALA | 5-Aminolevulinic acid |
| ALSL | Anterior longitudinal spinal ligament |
| Atg3 | Autophagy related 3 |
| Bax | Bcl-2 associated protein X |
| BB | Biobreeding |
| Bcl-2 | B-cell lymphoma 2 |
| BKS | black Kaliss‘s mice |
| BMD | Bone mass density |
| BSA | Bovine serum albumin |
| CEPCs | Cartilage endplate cells |
| CML | Ne-carboxymethyl lysine |
| CT | Computer tomography |
| Db/db rats | diabetic rats |
| DHI | Disc height index |
| DM | Diabetes mellitus |
| DMTII | Diabetes mellitus type II |
| DRG | Dorsal root ganglion |
| EAS | Evidence assessment system |
| ECM | Extracellular matrix |
| EMBASE | Excerpta Medica dataBASE |
| EP | Endplate |
| ERK | Extracellular signal-regulated kinase |
| FCD | Fixed charge density |
| GAG | Glycosaminoglycan |
| GAP43 | Growth-associated protein 43 |
| GK | Goto-Kakizaki |
| H2DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| Iba1 | Ionized calcium-binding adapter molecule-1 |
| IGF-1 | Insulin-like growth factor-1 |
| IGF-1R | Insulin-like growth factor-1 receptor |
| IDDM | Insulin-dependent diabetes |
| IVD | Intervertebral disc |
| IVDD | Intervertebral disc degeneration |
| IVDR | Intervertebral disc regeneration mellitus |
| LBP | Low back pain |
| LCMV | Lymphocytic choriomeningitis virus |
| LC3 | Microtubule-associated protein light chain 3 |
| LDH | Lumbar disc herniation |
| LETO | Long–Evans Tokushima Otsuka |
| LETL | Long–Evans Tokushima lean leprdb leptin receptor-deficient diabetic |
| LSD | Lysergic acid diethylamide |
| LSL | Longitudinal spinal ligament |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| MAP | Mitogen activated protein kinase |
| MG | Methylglyoxal |
| MMP | Matrix-metalloproteinase |
| MnSOD | Manganese superoxide dismutase |
| MVD | Microvascular density |
| µCT | Microcomputed tomography |
| NOD | Non-obese diabetic mouse |
| NOS | Newcastle-Ottawa scale |
| NP | Nucleus pulposus |
| NPCs | Nucleus pulposus cells |
| NZO | New Zealand obese |
| OLETF | Otsuka Long-Evans Tokushima fatty |
| OSD | Obese Sprague Dawley |
| PCR | Polymerase chain reaction |
| PLSL | Posterior longitudinal spinal ligament |
| PI | Propidium iodide |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| p38MAPK | p38 mitogen-activated protein kinases |
| pRB | Retinoblastoma protein |
| QA | Quality assessment |
| RAGE | Receptor for advanced glycation end-products |
| RF | Risk factor |
| ROP-Os | Radiation-induced oligosyndactyly mice |
| ROS | Reactive oxygen species |
| RQ | Research quality |
| SSS | Study scoring system |
| SA-β-Gal | Senescence-associated beta-galactosidase |
| SD | Sprague Dawley rats |
| SDT | Spontaneously diabetic Torii |
| SHG | Second-harmonic generation |
| SHROB | Spontaneous hypertensive obese rat |
| SIRT | Sirtuin |
| SOX9 | Sex-determining region SRY of the Y chromosome |
| SSS | Study scoring system |
| STZ | Streptozotocin |
| T2DM | Diabetes mellitus type II |
| TEM | Transmission electron microscope |
| TIMP1 | Tissue inhibitor of metalloproteinases 1 |
| TNF-α | Tumor necrosis factor α |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| UCD-T2DM | University California Davis type 2 diabetes mellitus |
| WT mice | Wild type mice |
| ZDF | Zucker diabetic fatty (German) |
References
- Andersson, G.B. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef]
- Battie, M.C.; Videman, T. Lumbar Disc Degeneration: Epidemiology and Genetics. J. Bone Jt. Surg. Am. Vol. 2006, 88, 3. [Google Scholar] [CrossRef]
- Krishnamoorthy, D.; Hoy, R.; Natelson, D.M.; Torre, O.M.; Laudier, D.M.; Iatridis, J.C.; Illien-Jünger, S. Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs. Dis. Model. Mech. 2018, 11, dmm036012. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- Liu, X.; Pan, F.; Ba, Z.; Wang, S.-J.; Wu, D. The potential effect of type 2 diabetes mellitus on lumbar disc degeneration: A retrospective single-center study. J. Orthop. Surg. Res. 2018, 13, 52. [Google Scholar] [CrossRef]
- Oxland, T.R. Fundamental biomechanics of the spine—What we have learned in the past 25 years and future directions. J. Biomech. 2016, 49, 817–832. [Google Scholar] [CrossRef]
- Rawls, A.; Fisher, R.E. Development and Functional Anatomy of the Spine. Genet. Dev. Scoliosis 2009, Chapter 2, 21–46. [Google Scholar] [CrossRef]
- Arthroscopic, P.K. Endoscopic Spinal Surgery: Text and Atlas, 2nd ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 29–47. [Google Scholar]
- Urban, J.P.G.; Roberts, S.; Ralphs, J.R. The Nucleus of the Intervertebral Disc from Development to Degeneration. Am. Zool. 2000, 40, 53–61. [Google Scholar] [CrossRef]
- Fennell, A.J.; Jones, A.P.; Hukins, D.W.L. Migration of the Nucleus Pulposus Within the Intervertebral Disc During Flexion and Extension of the Spine. Spine 1996, 21, 2753–2757. [Google Scholar] [CrossRef]
- Alexander, L.; Hancock, E.; Agouris, I.; Smith, F.W.; Macsween, A. The Response of the Nucleus Pulposus of the Lumbar Intervertebral Discs to Functionally Loaded Positions. Spine 2007, 32, 1508–1512. [Google Scholar] [CrossRef]
- Prescher, A. Anatomy and pathology of the aging spine. Eur. J. Radiol. 1998, 27, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Nerurkar, N.L.; Choi, K.-S.; Harfe, B.D.; Elliott, D.M. Degeneration and regeneration of the intervertebral disc: Lessons from development. Dis. Model. Mech. 2010, 4, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Harnsberger, H.O.; Ross, J.; Moore, K.R. Diagnostic and Surgical Imaging and Anatomy: Brain, Head & Neck, Spine; Amirsys: Salt Lake City, UT, USA, 2006. [Google Scholar]
- Fields, A.J.; Berg-Johansen, B.; Metz, L.N.; Miller, S.; La, B.; Liebenberg, E.C.; Coughlin, D.G.; Graham, J.L.; Stanhope, K.L.; Havel, P.J.; et al. Alterations in intervertebral disc composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes. J. Orthop. Res. 2015, 33, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, C.; Sylvia, M.; Schaible, E.; Graham, J.L.; Stanhope, K.L.; Metz, L.N.; Gludovatz, B.; Schwartz, A.V.; Ritchie, R.O.; Alliston, T.; et al. Contributions of Material Properties and Structure to Increased Bone Fragility for a Given Bone Mass in the UCD-T2DM Rat Model of Type 2 Diabetes. J. Bone Miner. Res. 2018, 33, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.Y.; Bishop, P.B. Preliminary Evaluation of a Scheme for Grading the Gross Morphology of the Human Intervertebral Disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Malucelli, D.A.; Malucelli, F.J.; Fonseca, V.R.; Zeigeboim, B.; Ribas, A.; De Trotta, F.; Da Silva, T.P. Hearing loss prevalence in patients with diabetes mellitus type 1. Braz. J. Otorhinolaryngol. 2012, 78, 105–115. [Google Scholar]
- Gomes, M.F.; Amorim, J.B.; Giannasi, L.C.; Salgado, M.A.C. Biomaterials for Tissue Engineering Applications in Diabetes Mellitus. Biomater. Regen. Med. 2018, 409–435. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar]
- Silva, M.B.; Skare, T.L. Musculoskeletal disorders in diabetes mellitus. Rev. Bras. Reumatol. 2012, 52, 601–609. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetes Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Haligur, M.; Topsakal, S.; Ozmen, O. Early Degenerative Effects of Diabetes Mellitus on Pancreas, Liver, and Kidney in Rats: An Immunohistochemical Study. Exp. Diabetes Res. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Douloumpakas, I.; Pyrpasopoulou, A.; Triantafyllou, A.; Sampanis, C.; Aslanidis, S. Prevalence of musculoskeletal disorders in patients with type 2 diabetes mellitus: A pilot study. Hippokratia 2007, 11, 216–218. [Google Scholar] [PubMed]
- Center, P.A.E.E.; Sozen, T.; Calik Basaran, N.; Tinazli, M.; Ozisik, L. Musculoskeletal problems in diabetes mellitus. Eur. J. Rheumatol. 2018, 5, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Schiavone, C.; Pelotti, P.; Salini, V. Limited Joint Mobility in Diabetes and Ageing: Recent Advances in Pathogenesis and Therapy. Int. J. Immunopathol. Pharmacol. 2010, 23, 997–1003. [Google Scholar] [CrossRef]
- Jakoi, A.M.; Pannu, G.; D’Oro, A.; Buser, Z.; Pham, M.H.; Patel, N.N.; Hsieh, P.C.; Liu, J.C.; Acosta, F.L.; Hah, R.; et al. The Clinical Correlations between Diabetes, Cigarette Smoking and Obesity on Intervertebral Degenerative Disc Disease of the Lumbar Spine. Asian Spine J. 2017, 11, 337–347. [Google Scholar] [CrossRef]
- Aprill, C.; Bogduk, N. Mbbs High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br. J. Radiol. 1992, 65, 361–369. [Google Scholar] [CrossRef]
- Schellhas, K.P.; Pollei, S.R.; Gundry, C.R.; Heithoff, K.B. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine 1996, 21, 79–86. [Google Scholar] [CrossRef]
- Schellhas, K.P.; Smith, M.D.; Gundry, C.R.; Pollei, S.R. Cervical discogenic pain. Prospective correlation of magnetic resonance imaging and discography in asymptomatic subjects and pain sufferers. Spine 1996, 21, 300–311. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, Z.; Figini, M.; Shangguan, J.; Zhang, Z.; Chen, W. Multi-parameter evaluation of lumbar intervertebral disc degeneration using quantitative magnetic resonance imaging techniques. Am. J. Transl. Res. 2018, 10, 444–454. [Google Scholar] [PubMed]
- Griffith, J.F.; Wang, Y.-X.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.; Leung, P.C. Modified Pfirrmann Grading System for Lumbar Intervertebral Disc Degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Balian, G.; Li, X.J. Animal models for disc degeneration-an update. Histol. Histopathol. 2017, 33, 543–554. [Google Scholar] [PubMed]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. BioMed Res. Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.B.; Kettler, A.; Masuda, K.; Melrose, J.; Ralphs, J.R.; Stokes, I.A.; Wilke, H.-J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2007, 17, 2–19. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Lin, C.-H.; Huang, T.-H.; Chuang, S.-Y. In Vivo Rodent Models of Type 2 Diabetes and Their Usefulness for Evaluating Flavonoid Bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef]
- Cefalu, W.T. Animal Models of Type 2 Diabetes: Clinical Presentation and Pathophysiological Relevance to the Human Condition. ILAR J. 2006, 47, 186–198. [Google Scholar] [CrossRef]
- Fajardo, R.; Karim, L.; Calley, V.I.; Bouxsein, M.L. A review of rodent models of type 2 diabetic skeletal fragility. J. Bone Miner. Res. 2014, 29, 1025–1040. [Google Scholar] [CrossRef]
- Chatzigeorgiou, A.; Halapas, A.; Kalafatakis, K.; Kamper, E. The use of animal models in the study of diabetes mellitus. Vivo 2009, 23, 245–258. [Google Scholar]
- King, A. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef]
- Ziv, I.; Moskowitz, R.W.; Kraise, I.; Adler, J.H.; Maroudas, A. Physicochemical properties of the aging and diabetic sand rat intervertebral disc. J. Orthop. Res. 1992, 10, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Dar, M.A.; Gani, I.; Mir, S.A. Animal Models in Diabetes Mellitus: An Overview. J. Drug Deliv. Ther. 2019, 9, 472–475. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Devlin, M.J.; Robbins, A.; Cosman, M.; Moursi, C.; Cloutier, A.; Louis, L.; Van Vliet, M.; Conlon, C.; Bouxsein, M. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female C57BL/6J vs. FVB/NJ Mice. Bone Rep. 2018, 8, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Wang, Y.; Cao, F.; Chen, Z.; Hu, Z.; Yu, B.; Feng, H.; Ba, Z.; Liu, T.; et al. Intervertebral disc degeneration in mice with type II diabetes induced by leptin receptor deficiency. BMC Musculoskelet. Disord. 2020, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Laboratory, J. 2020. Available online: https://www.jax.org/strain/000632 (accessed on 25 May 2020).
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; A Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.S.B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2017. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 May 2020).
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Lu, Y.; Qureshi, S.A.; Hecht, A.C.; Cai, W.; Vlassara, H.; Striker, G.E.; Iatridis, J.C. Chronic Ingestion of Advanced Glycation End Products Induces Degenerative Spinal Changes and Hypertrophy in Aging Pre-Diabetic Mice. PLoS ONE 2015, 10, e0116625. [Google Scholar] [CrossRef]
- Chen, S.; Liao, M.; Li, J.; Peng, H.; Xiong, M. The correlation between microvessel pathological changes of the endplate and degeneration of the intervertebral disc in diabetic rats. Exp. Ther. Med. 2012, 5, 711–717. [Google Scholar] [CrossRef][Green Version]
- Park, E.-Y.; Park, J.-B. High glucose-induced oxidative stress promotes autophagy through mitochondrial damage in rat notochordal cells. Int. Orthop. 2013, 37, 2507–2514. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, J.-B.; Park, I.-J.; Park, E.-Y. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int. Orthop. 2014, 38, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-G.; Park, J.-B.; Kim, M.S.; Park, E.-Y. High Glucose Accelerates Autophagy in Adult Rat Intervertebral Disc Cells. Asian Spine J. 2014, 8, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-G.; Park, J.-B.; Lee, D.; Park, E.-Y. Effect of High Glucose on Stress-Induced Senescence of Nucleus Pulposus Cells of Adult Rats. Asian Spine J. 2015, 9, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, J.; Nisar, M.; Chen, T.; Xu, T.; Zheng, G.; Wang, C.; Jin, H.; Chen, J.; Gao, W.; et al. The Sirt1/P53 Axis in Diabetic Intervertebral Disc Degeneration Pathogenesis and Therapeutics. Oxid. Med. Cell. Longev. 2019, 2019, 7959573. [Google Scholar] [CrossRef]
- Park, J.-B.; Byun, C.-H.; Park, E.-Y. Rat Notochordal Cells Undergo Premature Stress-Induced Senescence by High Glucose. Asian Spine J. 2015, 9, 495–502. [Google Scholar] [CrossRef]
- Park, E.-Y.; Park, J.-B. Dose- and time-dependent effect of high glucose concentration on viability of notochordal cells and expression of matrix degrading and fibrotic enzymes. Int. Orthop. 2013, 37, 1179–1186. [Google Scholar] [CrossRef]
- Park, J.-B.; Park, E.-Y. Increased Apoptosis, Expression of Matrix Degrading Enzymes and Inflammatory Cytokines of Annulus Fibrosus Cells in Genetically Engineered Diabetic Rats: Implication for Intervertebral Disc Degeneration. Glob. Spine J. 2016, 6, 415–423. [Google Scholar] [CrossRef]
- Illien-Jünger, S.; Grosjean, F.; Laudier, D.M.; Vlassara, H.; Striker, G.E.; Iatridis, J.C. Combined Anti-Inflammatory and Anti-AGE Drug Treatments Have a Protective Effect on Intervertebral Discs in Mice with Diabetes. PLoS ONE 2013, 8, e64302. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Ho, N.Y.-J.; Lin, Y.-T.; Lai, P.-L.; Fu, T.-S.; Niu, C.-C.; Chen, L.-H.; Chen, W.-J.; Pang, J.-H.S. Advanced glycation end products in degenerative nucleus pulposus with diabetes. J. Orthop. Res. 2013, 32, 238–244. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Zheng, X.; Ru, A.; Ni, X.; Wu, Y.; Tian, N.; Huang, Y.; Xue, E.; Wang, X.; et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J. Orthop. Res. 2012, 31, 692–702. [Google Scholar] [CrossRef]
- Cheng, X.; Ni, B.; Zhang, F.; Hu, Y.; Zhao, J. High Glucose-Induced Oxidative Stress Mediates Apoptosis and Extracellular Matrix Metabolic Imbalances Possibly via p38 MAPK Activation in Rat Nucleus Pulposus Cells. J. Diabetes Res. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Jiang, Z.; Zeng, Q.; Li, D.; Ding, L.; Lu, W.; Bian, M.; Wu, J. Long noncoding RNA MALAT1 promotes high glucoseinduced rat cartilage endplate cell apoptosis via the p38/MAPK signalling pathway. Mol. Med. Rep. 2020, 21, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lu, W.; Zeng, Q.; Li, D.; Ding, L.; Wu, J. High glucose-induced excessive reactive oxygen species promote apoptosis through mitochondrial damage in rat cartilage endplate cells. J. Orthop. Res. 2018, 36, 2476–2483. [Google Scholar] [CrossRef]
- An, J.L.; Zhang, W.; Zhang, J.; Lian, L.C.; Shen, Y.; Ding, W.Y. Vitamin D improves the content of TGF-beta and IGF-1 in intervertebral disc of diabetic rats. Exp. Biol. Med. 2017, 242, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Sekiguchi, M.; Kaneuchi, Y.; Konno, S.-I. Investigation of the Effect of Diabetes on Radiculopathy Induced by Nucleus Pulposus Application to the DRG in a Spontaneously Diabetic Rat Model. Spine 2017, 42, 1749–1756. [Google Scholar] [CrossRef]
- Natelson, D.M.; Lai, A.; Krishnamoorthy, D.; Hoy, R.C.; Iatridis, J.C.; Illien-Jünger, S. Leptin signaling and the intervertebral disc: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model. PLoS ONE 2020, 15, e0227527. [Google Scholar] [CrossRef]
- Terashima, Y.; Kakutani, K.; Yurube, T.; Takada, T.; Maeno, K.; Hirata, H.; Miyazaki, S.; Ito, M.; Kakiuchi, Y.; Takeoka, Y.; et al. Expression of adiponectin receptors in human and rat intervertebral disc cells and changes in receptor expression during disc degeneration using a rat tail temporary static compression model. J. Orthop. Surg. Res. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Zhou, K.-L.; Zhou, Y.-F.; Wu, K.; Tian, N.-F.; Wu, Y.-S.; Wang, Y.-L.; Chen, D.-H.; Zhou, B.; Wang, X.-Y.; Xu, H.-Z.; et al. Stimulation of autophagy promotes functional recovery in diabetic rats with spinal cord injury. Sci. Rep. 2015, 5, 17130. [Google Scholar] [CrossRef]
- Gu, C.; Yang, Y.; Xiang, H.; Li, S.; Liang, L.; Sui, H.; Zhan, L.; Lu, X. Deciphering bacterial community changes in zucker diabetic fatty rats based on 16S rRNA gene sequences analysis. Oncotarget 2016, 7, 48941–48952. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Mirjolet, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Kawano, K.; Hirashima, T.; Mori, S.; Natori, T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: A new NIDDM rat strain. Diabetes Res. Clin. Pract. 1994, 24, S317–S320. [Google Scholar] [CrossRef]
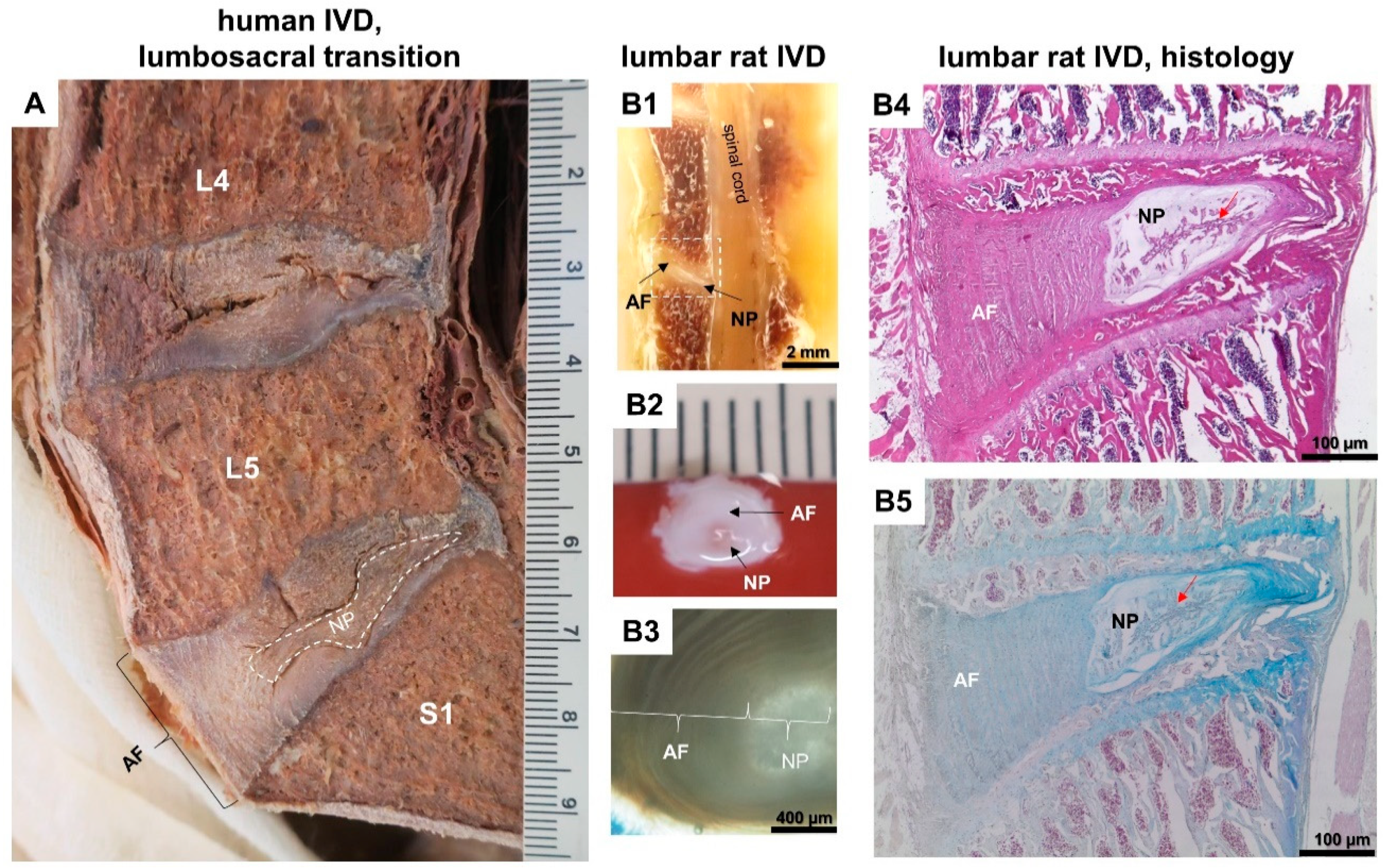
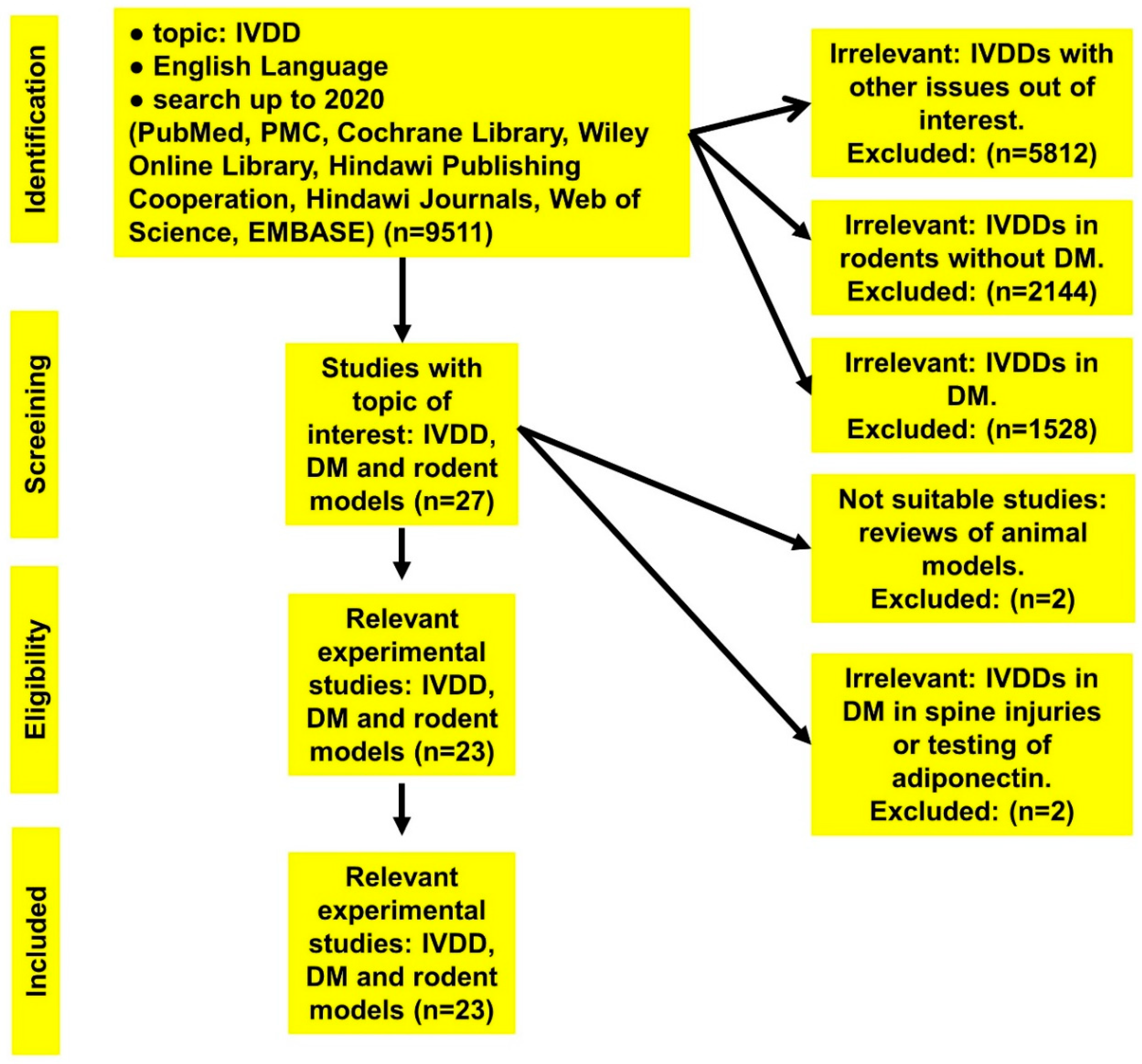
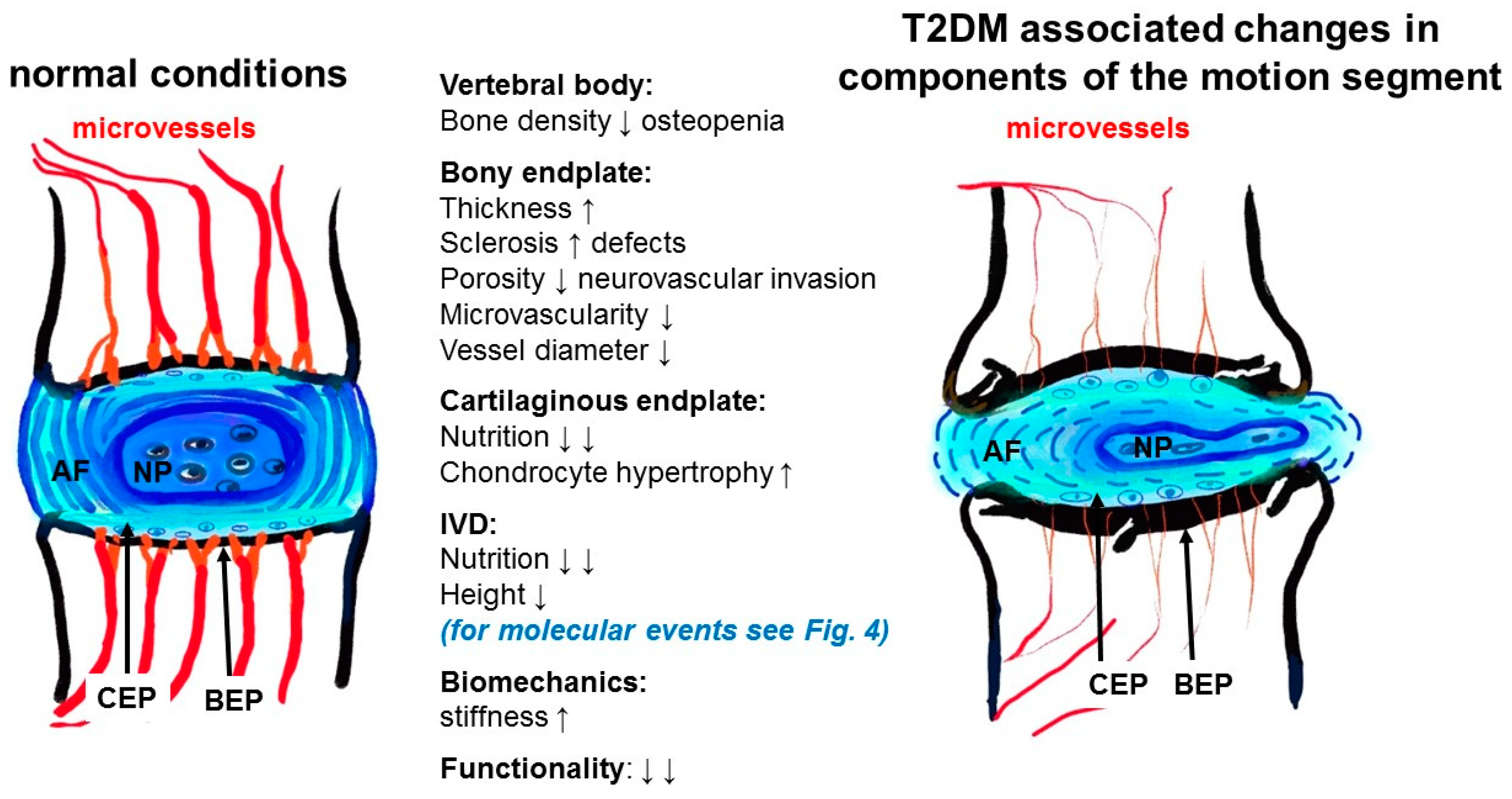
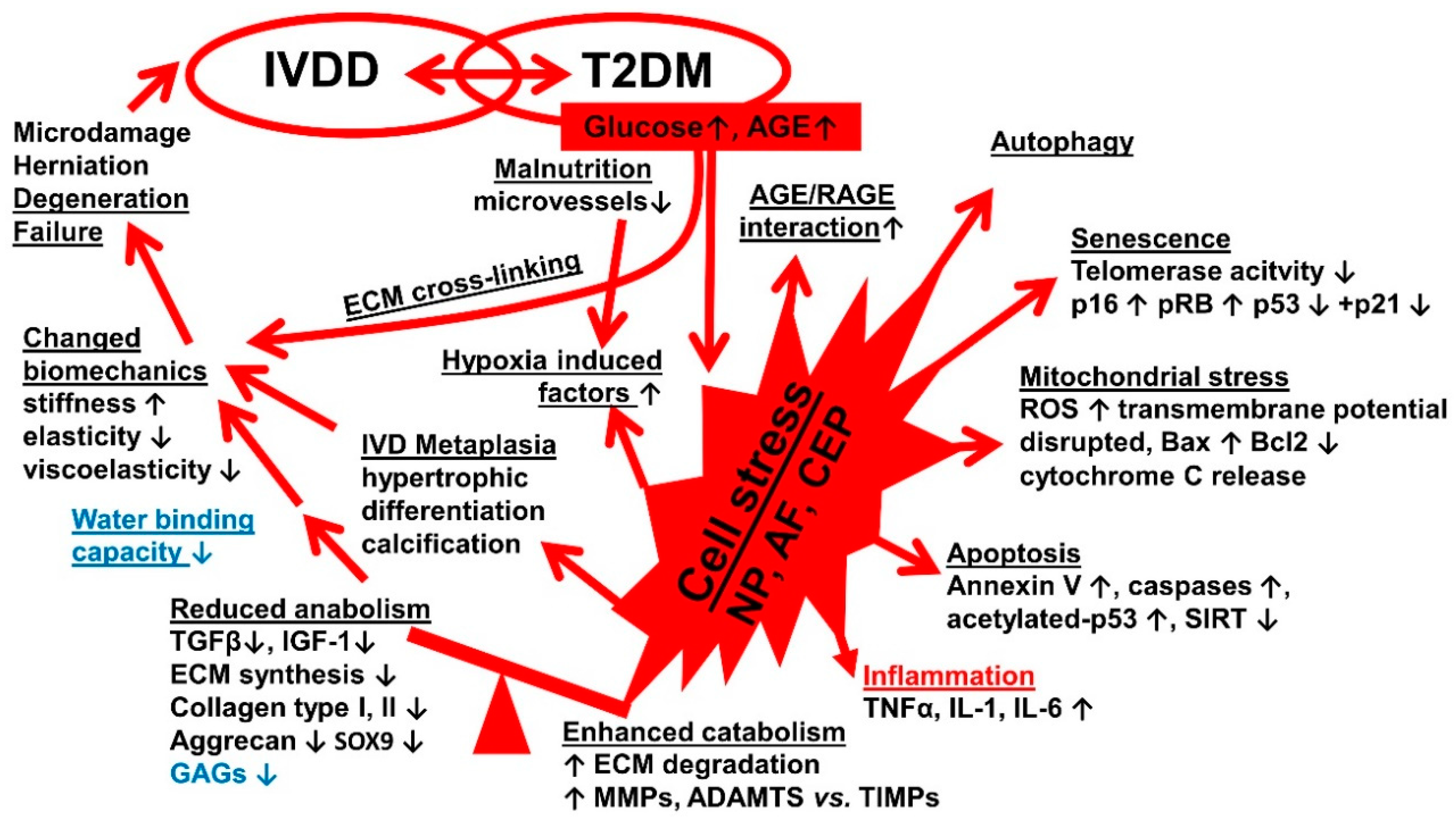
| Topic | Number of Collected Publications |
|---|---|
| IVDD | 9511 |
| IVDD in rats | 2144 |
| IVDD in diabetic cases | 1528 |
| IVDD in diabetic rodent models | 27 |
| Studies (n) | Status | Cause | Type |
|---|---|---|---|
| 9484 | excluded | irrelevant | Reviews, retrospective, and prospective clinical, epidemiological, cross-sectional studies, etc. |
| 2 | excluded | not applicable | Reviews of animal models (no experiments) |
| 2 | excluded | irrelevant | Experimental: |
| 1st study: IVDD, rat model, and adiponectin | |||
| 2nd study: DM, rat model, and spine injury | |||
| 23 | included | relevant | Experimental (DM, IVDD, and rodents) |
| Inclusion Criteria (V/V) | Exclusion Criteria (I–V/V) |
|---|---|
| Experimental with clearly described methods (I) | Non-experimental (review, clinical, case report, etc.) (I) |
| T2DM (II) | Deficient of DM (II) or IVDD (III) or rodent model (IV) |
| IVDD (III) | Unclear methods (V) |
| Rodent models (IV) | |
| Study of pathogenesis, RF, treatment, prophylactic agent Available in English (V) | |
| Remark: to be included, the selected papers should fulfill five criteria (V/V) | Remark: one criterion up to four criteria is/are enough to exclude the papers (I-V/V) |
| Authors | Title | Aim | Focus | Rodent Model Type | Methods | Key Results |
|---|---|---|---|---|---|---|
| (1) Ziv et al., 1992 [43] USA & Israel | Physicochemi-cal Properties of the Aging and Diabetic Sand Rat Intervertebral Disc | Understanding of the changes in the physiochemical properties of intervertebral discs (IVDs) in the aged and diabetic rats | Physiochemical properties (in vivo) | 180 IVDs were extracted from forty-five desert sand rats (gender information not provided) divided into three equal groups: -Young healthy -Old healthy -Young diabetics | -Rat DM model -Blood glucose and insulin levels -Tissue harvest: lumbar IVD -Metabolic studies | -Discs of young diabetic rats demonstrated decreased hydration, fixed charged density and ability to resist compression under osmotic pressures as compared with the young and healthy discs and were more similar to the discs from old rats and from human -IVD: most affected musculoskeletal tissue in sand rats with aging and DM |
| (2) Chen et al., 2013 [53] China | The correlation between microvessel pathological changes of the endplate (EP) and degeneration of the intervertebral disc in diabetic rats | Identifying the possible mechanism, by which DM induces degeneration of the INDs with focus on microvessel density (MVD) in the EP | Pathogenesis: microvessel density in the EP (in vivo) | 30 three-month-old male adult Sprague Dawley (SD) rats. Rats were divided randomly in two groups (n = 15 rats/group): -Streptozotocin (STZ) -induced DM group -Control group | -Rat DM model -Fasting blood glucose -Tissue harvest (lumbar spine) -Histopathology -Immunohistochemistry: collagen types Ⅰ and Ⅱ, factor Ⅷ-related antigen (von Willebrand Factor, vWF) -Transmission electron microscopy (TEM) | -Expression of collagen type I in the DM group was higher than in controls in contrast to collagen type Ⅱ - vWF was expressed in both, but was low in the DM group - MVD of the DM group was smaller compared to that of the controls -The apoptotic index (AI) in the DM group was significantly higher compared to that of the controls -Negative correlation between the MVD of EP and the AI of notochordal cells -Compared to controls, the EP MVD and the vessel width decreased or disappeared in DM rats |
| (3) Illien-Junger et al., 2013 [62] USA | Combined Anti-Inflammatory and Anti- Advanced Glycation End-products (AGE) Drug Treatments Have a Protective Effect on IVDs in Mice with Diabetes | Investigation of the effectivity of oral treatments with a combination of anti-inflammatory and anti-AGE drugs in preventing diabetes-induced degenerative changes to the spine (IVD and vertebral bone density) | Prophylaxis (in vivo) | Three age-matched groups of 21 female ROP-Os mice (group size 6-8 animals): -Non-diabetic group -Diabetic group (STZ- induced) -Diabetic mice treated with pentosan-polysulfate & pyridoxamine | -Mice DM model -Blood glucose -Tissue harvest: lumbar spine -Micro-computed tomography (µCT)-Histopathology -Immunohistochemistry: Ne-carboxymethyl lysine [CML], methylglyoxal [MG], tumor necrosis factor (TNF)α, Matrix-metalloproteinase (MMP)-13 and a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS)-5 -GAG measurement | -Diabetic mice exhibited pathological changes: IVD height↓, vertebral bone mass↓, glycosaminoglycans (GAGs)↓ and morphological alterations of IVDs with focal highly expressed TNFα, MMP-13 and ADAMTS-5 -Accumulation of larger MG amounts suggested that AGE accumulation was associated with these diabetic degenerative changes -Treatment prevented / reduced DM induced degeneration of vertebrae and IVD |
| (4) Jiang et al., 2013 [64] China | Apoptosis, Senescence, and Autophagy in Rat Nucleus Pulposus Cells: Implications for Diabetic IVD Degeneration | Studying of the mechanisms by which DM aggravates IVDD and discussing of the relationship between autophagy and IDD in NP cells | Pathogenesis (in vivo) | Two groups of thirty-four 2-month-old male SD rats (STZ) -Control (citrate buffer) -diabetic (STZ) | -Rat DM model -Fasting blood glucose (plasma) -Tissue harvest: lumbar discs -Histopathology -TEM -Immunohistochemistry: collagen type II, cleaved caspase-3, p16lnk4A, Microtubule-associated protein 1A/1B-light chain 3 (LC-3) -polymerase chain reaction (PCR): collagen types I, II, aggrecan -Westernblot: caspase-8, -9, -3, p16lnk4A, p62, Beclin-1 -terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay | -Higher levels of autophagy in NP cells of diabetic rats than control rats (statistically significant) -Proteoglycan and collagen type II in the ECM and the aggrecan and collagen type II mRNA expression in NP cells of diabetic rats were decreased compared with the control group -DM increased apoptosis of NP cells and led to activations of initiators of intrinsic (caspases-9) and extrinsic (caspase-8) pathways as well as their common executioner (caspase-3) -Cellular senescence was increased about twofold in NP of diabetic rats |
| (5) Fields et al., 2015 [15] USA | Alterations in IVD composition, matrix homeostasis and biomechanical behavior in the UCD-T2DM rat model of type 2 diabetes | Approving of the role of DM in causation of IVDD and in turn low back pain (LBP) | Pathogenesis Patho-biomechanics (in vivo) | One diabetic and two non-diabetic groups (gender not mentioned): -Six-month-old lean SD rats (“control”), -obese SD rats (“obese”), -UCD-T2DM rats (“diabetic”); n = 6 | -Rat DM model -Blood glucose (not fasted) -Tissue harvest: coccygeal -Cell harvest & cell culture -Blood glucose measurement -µCT: EP microarchitecture -Histopathology: EP vascular supply -Biomechanical assessment of creep characteristics -Biochemical analysis: GAGs-Immunoassay: AGE -PCR: genes of ECM homeostasis | -DM: GAG and water contents↓ vertebral EP thickness↑, EP porosity↓, AGE level↑ -Discs from diabetic rats were stiffer and less compressible -Expression of hypoxia-inducible genes↑, catabolic markers↑ in AF and NP from DM rats |
| (6) Illien-Junger et al., 2015 [52] USA | Chronic Ingestion of Advanced Glycation End Products (AGEs) Induces Degenerative Spinal Changes and Hypertrophy in Aging Pre-Diabetic Mice | Investigation of the role of specific AGE precursors on IVDD and vertebral pathologies in aging mice that were fed isocaloric diets with standard or reduced amounts of MG-derivatives | Risk factor (Modern diets contain high levels of AGEs) (in vivo) | Two groups of aging C57BL/6 mice (gender not mentioned) received: -either a low AGE chow produced without the use of heat (n = 12), or -a low AGE chow supplemented with the synthetic AGE-precursor methylglyoxal-bovine serum albumin (MG-BSA), (n = 9) Mice were insulin resistant but not hyperglycemic | -Mice prediabetic model -Fasting blood glucose -Tissue: lumbar spine -µCT -Histopathology -Immunohistochemistry: MG, CML, TNFα, collagen type X, ADAMTS-5 | Chronic exposure to dietary MG/AGEs leads to: -Cortical-thickness and cortical surface area↑ -AGE accumulation & ectopic calcification in vertebral EPs -IVD calcification & hypertrophic differentiation -GAG loss -Accelerated IVDD and vertebral alterations with insulin resistance - IVD height↓ NP cells from dMG+ mice exhibited increased collagen type X staining |
| (7) Park et al., 2016 [61] Korea | Increased Apoptosis, Expression of Matrix Degrading Enzymes and Inflammatory Cytokines of AF Cells in Genetically Engineered Diabetic Rats: Implication for IVDD | Investigation of the effect of DM on apoptosis, expression of matrix degrading enzymes and inflammatory cytokines in cells of IVDs derived from genetically engineered OLETF (diabetic) and LETO (control) rats | Pathogenesis (in vivo) | Two rat groups: 6-month old male OLETF (diabetic) and LETO (control) rats (10 per each group) | -Rat DM model -Glucose tolerance tested -Tissue harvest: lumbar spine -Histopathology -TUNEL assay (apoptosis: AF cells) -Western blot: MMP-1, -2, -3, -13, tissue inhibitor of metalloproteinase (TIMP)-1, -2 -PCR: IL-1, -6 and TNF-α | -OLETF rats showed increased body weight and abnormal 2-h glucose tolerance tests compared to LETO rats -The AI and the degree of Fas expression by AF and the -Expression of MMP-1, -2, -3, -13, TIMPs-1 and -2 was statistically higher in OLETF rats -Expression of IL-1, -6 and TNF-α was statistically higher in OLETF rats -Histological analysis showed more severe fibrosis and loss of lamellar pattern in AF tissues of OLETF rats |
| (8) An et al., 2017 [68] China | Vitamin D (calcitriol) improves the content of transforming growth factor (TGF)-β and insulin-like growth factor (IGF)-1 in IVD of diabetic rats | Testing of protective effect of Vit. D against IVDD in DM | Prophylaxis (in vivo) | 55 SD rats were divided into three groups (gender not mentioned): -Experimental group (STZ+calcitriol) (n = 20) -Control group (STZ+citrate buffer) (n = 20) -Normal group (citrate buffer) (n = 15) | -Rat DM model -Fasting blood glucose -Tissue harvest: lumbar spine -Histopathology -Immunohistochemistry: TGFβ, IGF-1 -Western blot: TGFβ, IGF-1 | -Histology revealed degenerative changes in discs of experimental and control group at three different time points, while there were no changes in discs in normal group -Content of TGFβ & IGF-1 in experimental and control group was significantly lower than in normal group at three different time points, but there were also significant lower values in control compared with the experimental group |
| (9) Kameda et al., 2017 [69] Japan | Investigation of the effect of diabetes on radiculopathy induced by NP application to the dorsal root ganglion (DRG) in a spontaneously diabetic rat model | Evaluation of the effect of DM on radiculopathy due to lumbar disc herniation (LDH), by investigating pain-related behavior and the expression of TNF-α and growth-associated protein (GAP)43 in type 2 diabetic rats following application of NP to the dorsal root ganglion (DRG) | Pathogenesis (in vivo) | Two groups: -A total of 129 13-weeks old male Wistar rats -A total of 126 13-weeks old, male GK rats. The GK rat is a spontaneous model of T2DM Large-sized test and control group -Surgical NP group -Sham group -Naïve rats | -Rat DM models -Blood glucose: refers to previous study -Tissue harvest: lumbar spine -Measurement of mechanical withdrawal thresholds -Immunohistochemistry: Ionized calcium-binding adapter molecule-1 [Iba-1], TNF-α, RAGE -PCR: GAP43 -Western blot: GAP43 | -Mechanical withdrawal threshold significantly declined in the non-DM NP group compared to the non-DM sham group for 28 days, whereas the decline in threshold extended to 35 days in the DM NP group compared to the DM sham group -RAGE and TNF-α expression in DRGs was co-localized in Iba-1 positive cells -Non-DM NP rats had higher TNF-α protein expression levels vs. the non-DM sham rats on day 7, the DM NP group had higher levels vs. the DM sham group on days 7 and 14 -Non-DM NP group had higher GAP43 mRNA expression compared to the non-DM sham group for 28 days, while the DM NP group had a higher level than the DM sham group for 35 days |
| (10) Krishna-moorthy et al., 2018 [3] USA | Dietary AGE consumption leads to mechanical stiffening of murine IVDs | Testing the hypothesis that chronic consumption of high AGE diets results in sex-specific IVD structural disruption and functional changes | Risk factor (chronic consumption of high AGE diets) (in vivo) | mice model: 21 females and 23 males C57BL/6J mice, each assigned to two isocaloric diet groups, receiving either a low AGE or high AGE chow, generated via high-temperature heating | -Mice DM model (both sexes) -Fasting blood glucose and serum -Tissue harvest: coccygeal and lumbar spine -Histopathology -Biomechanical testing - AGE quantification (western blot: serum, IVD) -Molecular assessment of collagen (collagen peptide hybridization) -Second harmonic generation (SHG) imaging with multi photon laser-scanning microscope: collagen fiber orientation | -High AGE diet resulted in AGE accumulation in IVDs and increased IVD compressive stiffness, particularly in females -IVD biomechanical changes result from increased AGE crosslinking in AF -Increased collagen damage did not appear to influence biomechanical properties -High AGE diet has greater influence on females |
| (11) Li et al., 2020 [47] China | IVDD in mice with type II diabetes induced by leptin receptor deficiency | Studying of the effects of T2DM on IVDD in leptin receptor-deficient knockout mice model. Observation of the effects of T2DM and glucose-lowering treatment on IVDD by IGF-1 injection | Therapy Specific Pathogenesis (in vivo) | Three groups: -wild-type male C57BL/6J mice, -leptin receptor gene knockout, db/db mice -IGF-1 group Only male mice were included because of the DM protective effect of the female sex steroids | -Mice DM model -Fasting blood glucose -IVD harvest (coccygeal + lumbar) -µCT -Histopathology -Immunohistochemistry: MMP-3 -Western blot: leptin receptor -Tunnel assay: apoptosis -PCR: sex-determining region SRY of the Y chromosome (SOX9), aggrecan, MMP-3 | -Blood glucose levels were significantly higher in the db/db mice -T2DM in db/db group showed an association with significantly decreased vertebral bone mass and increased IVDD when compared to WT mice - db/db mice showed a higher percentage of MMP-3 expression and cell apoptosis than wild type mice -IGF-1 treatment partly reversed the findings |
| (12) Natelson et al., 2020 [70] USA | Leptin signaling and the IVD: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a T2DM mouse model | Investigating, if obesity and DM type II cause spinal pathology in a sex-specific manner using in vivo diabetic and dietary mouse models | Risk factor (hypercaloric Western diets in cases of T2DM, obesity and leptin receptor deficiency) (in vivo) | Four groups of mice models were used: -Two groups of leptin receptor-deficient mice on a C57BL/6J background (B6.BKS (D)-Leprdb/J (Db/Db) (15 females, 19 males) -Two non-diabetic control groups (21 females, 27 males) | -Mice DM model -Fasting blood glucose -Tissue harvest: lumbar IVD -Histopathology -Metabolic studies, (HbA1c level) -µCT: detection of IVD disc height index (DHI) -Biomechanical examination | -Dietary effects on bone structure in Db/Db mice were sex-dependent and evident in females but not males -IVDs of female (but not male) Db/Db mice exhibited morphological changes, but no IVDD -Leptin receptor deficiency did not cause IVDD in 3 months old mice - DHI was not changed in any group -No biomechanical changes, except diminished torsional properties in leptin deficient mice. |
| (13) Tsai et al., 2014 [63] Taiwan | AGEs in Degenerative NP with Diabetes | Investigation of the effect of AGEs on the degeneration process in diabetic NP and NP cells in rats and humans | Pathogenesis (in vivo and in vitro) Risk factor Peculiarity: Parallel experiments using human and rat-derived NP cells | Nine 8-week-old male SD-rats were divided into two groups:-Non-diabetic (n = 4) -Diabetic group, STZ (n = 5). Human NP tissue: Diabetic (n = 3) Non-diabetic (n = 3) | -Rat DM model -Fasting blood glucose -Tissue harvest: coccygeal discs -Cell isolation & cell culture -Histopathology -Immunohistochemistry: AGE -PCR: MMP-2, RAGE -Zymography: MMP-2 -Western blot: extracellular signal regulated kinase [ERK] | -Immunohistochemical expression of AGEs was significantly↑ in diabetic human and rat-derived discs - MMP-2↑ and RAGE↑, at both mRNA and protein expression levels and phosphorylated ERK↑ in diabetic NP cells as a response to AGEs (human+rat) -AGEs and DM are obviously associated with IVDD in both humans & rats -Hyperglycemia in diabetes enhances the accumulation of AGEs in the NP and triggers IVDD |
| (14) Zhang et al., 2019 [58] China | The sirtuin (Sirt)1/p53 Axis in Diabetic IVDD Pathogenesis and Therapeutics | Understanding of the relation between DM and IVDD, in particular the Sirt1/p53 axis in NP cells which may be involved in the pathogenesis of diabetic IDD and may also serve as a therapeutic target for diabetic IDD | Specific pathogenesis Therapy (in vivo and in vitro) | Forty-eight adult male SD rats divided into four groups (12 males for group): -DM (STZ) -DM (STZ)+IDD (AF puncture) -Butein treated DM+IDD-Control Isolated NP cells from young rats exposed to high glucose (5.5, 25, 50, 100, and 150 μM) | -Rat DM model -Blood glucose -IVD harvest (probably coccygeal) -NP cell isolation & cell culture -Cell viability assay (cell counting kit-8) -MRI -Histopathology -Immunohistochemistry: Sirt1, acetyl-p53, cleaved caspase-3 -Western blot: Bax, Bcl-2, acetyl p53/p53, p16INK4a, p21WAF1, Sirt1 -TUNEL Assay -Sirt1 Expression & Activity Assay -Reactive Oxygen Species Assay | -High glucose may promote the incidence of apoptosis and senescence in NP cells in vitro -Acetylation of p53 was found increased in diabetic NP cells in vitro. -Hyperglycemia could suppress the expression and activity of Sirt1 in NP cells (in vitro and in vivo) -Butein may inhibit acetylation of p53 and protect NP cells against hyperglycemia-induced apoptosis and senescence through Sirt1 activation |
| (15) Park et al., 2013 [54] Korea | High glucose-induced oxidative stress promotes autophagy through mitochondrial damage in rat notochordal cells | Evaluation of the effects of high glucose concentrations (0.1, 0.2 M glucose) on the induction of oxidative stress and autophagy through mitochondrial damage in rat notochordal cells | Pathogenesis (in vitro) | Only one non-diabetic group (four-week-old male SD rats), from which the NPs have been harvested before exposed to hyperglycemic or normoglycemic conditions. | -Rat DM model -Tissue harvest: lumbar spine -NP cell isolation and culture under hyper- or normoglycemic conditions -Western blot: autophagy markers -Immunofluorescence: detection of mitochondrial damage and of manganese superoxide dismutase (MnSOD) -Flow cytometry: Intracellular reactive oxygen species (ROS) -Measurement of catalase level | -An enhanced disruption of mitochondrial transmembrane potential, which indicates mitochondrial damage, was identified in rat notochordal cells treated with both high glucose concentrations. -Both high glucose concentrations increased production of ROS by rat notochordal cells in a dose- and time-dependent manner -Two high glucose solutions also enhanced in rat notochordal cells in a dose- and time-dependent manner: -Compensatory expressions of anti-oxidative MnSOD and catalase -Ratio of autophagy markers (LC3-II/LC3-I) |
| (16) Park et al., 2013 [60] Korea | Dose- and time-dependent effect of high glucose concentration on viability of notochordal cells and expression of matrix degrading and fibrotic enzymes | Understanding of the effect of the duration and severity of DM (using high glucose concentrations: 0.1, 0.2, 0.4 M glucose) on viability of notochordal cells and IVDD | Risk factor (Duration and severity of DM) (in vitro) | Only one non-diabetic group (four-week-old male SD rats), from which the NPs have been harvested before exposed to hyper- or normoglycemic conditions. | -Rat DM model -Lumbar IVD harvest -Cell isolation and culture under hyper- or normoglycemic conditions -Immunohistochemistry -Evaluation of cell proliferation -Evaluation of cell apoptosis (DNA stain) -Western blot: MMP-1,2,-3,-7,-9,-13, TIMP-1,-2, caspase-3, -9, cytochrome-c, Akt, Phospho-p38 MAPK and poly (ADP) ribose polymerase (PARP) | -High glucose significantly decreased proliferation and increased apoptosis of notochordal cells from culture days one to seven in a dose-dependent manner -Compared with normoglycemic group, caspase-9 & -3 activities and cleavage of Bid and cytochrome-c were significantly increased in each three high glucose concentrations, accompanied by increased expression of MMP-1, -2, -3, -7, -9 and -13 and TIMP-1 & -2 |
| (17) Kong et al., 2014 [56] Korea | High Glucose Accelerates Autophagy in Adult Rat IVD Cells | Investigation of the effect of high glucose (0.1, 0.2 M) on autophagy in adult rat AF and NP cells | Pathogenesis (in vitro) | One group of 24-week-old male SD rats, from which the NP and AF cells have been harvested before exposed to hyper- or normoglycemic conditions. | -Rat DM model -Tissue harvest: lumbar spine -Cell isolation and culture under hyper- or normoglycemic conditions -Western blot: autophagy markers (beclin-1, LC3-I and LC3-II, and Atg 3, 5, 7, and 12) | -High glucose significantly increased the expressions of autophagy markers beclin-1, LC3-II, Atg3, 5, 7, and 12 in adult rat NP and AF cells in a dose- and time-dependent manner -Ratio of LC3-II/LC3-I expression↑ (dose- respectively time-dependently) |
| (18) Park et al., 2014 [55] Korea | Accelerated premature stress-induced senescence of young AF cells of rats by high glucose-induced oxidative stress | Investigation of the effect of high glucose (0.1, 0.2 M glucose) on mitochondrial damage, oxidative stress and senescence of young AF cells | Pathogenesis (in vitro) | Only one non-diabetic group (four-week-old male SD rats), from which the AFs have been harvested before exposed to hyper- or normoglycemic conditions. | -Rat DM model -Lumbar IVD harvest -Cell isolation and culture under hyper- or normoglycemic onditions -Immunohistochemistry: p16, retinoblastom (pRB), p53, p21 -Mitochondrial damage (with Mitotracker in Mitochondrion-selective Probes) -Intracellular ROS measurement with H2DCF-DA -SA-β-Gal activity assay -Telomerase activity using a TeloTAGG (PCR/ELISA) | -High glucose enhanced in a dose- and time-dependent manner: -Mitochondrial damage in young rat AF cells, resulting in enhanced ROS release for one and three days compared to normal control -Senescence of young AF cells -Telomerase activity declined in a dose- and time dependent manner Compared to controls hyperglycemia -Increased the expressions of p16 and pRB proteins and decreased that of p53+p21 in young rat AF cells for one and three days |
| (19) Kong et al., 2014 [57] Korea | Effect of High Glucose on Stress-Induced Senescence of NP Cells of Adult Rats | Investigation of the effect of diabetes mellitus (DM) on senescence of adult NP cells | Pathogenesis (in vitro) | One group of 24-week-old male SD rats, from which the NP cells have been harvested before exposed to hyper- or normoglycemic conditions. | -Rat DM model -Tissue harvest: lumbar spine -Cell isolation and culture under hyper- or normoglycemic conditions -Immunohistochemistry: p53, p21, pRB, and p16 -SA-β-Gal activity assay | High glucose: -increased the mean SA-β-Gal-positive cell percentage in adult rat NP cells dose- and time-dependently -Increased p16 and pRB and -impaired p53 and p21 proteins in adult rat NP cells |
| (20) Park et al., 2015 [59] Korea | Rat Notochordal Cells Undergo Premature Stress-Induced Senescence by High Glucose | Investigation of the effect of high glucose (0.1, 0.2 M) on premature stress-induced senescence of rat notochordal cells | Pathogenesis (in vitro) | One group of 4-week-old male SD rats, from which IVD notochordal cells have been harvested | -Rat DM model -Tissue harvest: lumbar spine -Notochordal cell isolation and culture under hyper- or normoglycemic conditions -Mitochondrial damage of notochordal cells (mitochondrial transmembrane potential and apoptosis detection kit) -Intracellular ROS measurement with H2DCF-DA -PCR/ELISA: Telomerase activity -Immunohistochemistry: MnSOD, p53, p21, pRB, and p16 -Expression of catalase -SA-β-Gal activity (SA-β-Gal staining kit) | High glucose enhanced in notochordal cells at 1 and 3 days: -Disruption of mitochondrial transmembrane potential and excessive generation of ROS. -Expressions of MnSOD and catalase - Occurrence of stress-induced senescence by p16-pRB pathways -Telomerase activity declined under high glucose conditions at 1 and 3 days |
| (21) Cheng et al., 2016 [65] China | High Glucose-Induced Oxidative Stress Mediates Apoptosis and ECM Metabolic Imbalances Possibly via p38 MAPK Activation in Rat NP Cells | To investigate whether high glucose-induced oxidative stress is implicated in apoptosis of rat NP cells and abnormal expression of critical genes involved in the metabolic balance of ECM | Pathogenesis (in vitro) | One 12-week-old male Wistar rats model group, from which NPs were harvested High glucose (5, 15, 25 mM) | -Rat DM model -Tissue harvest: lumbar discs -Cell isolation & culture -Flow cytometry: Annexin V+propidium iodide (PI) (apoptosis) -Measurement of intracellular ROS -Determination of NP cells viability -Western blot analysis (p38 kinase activation) -PCR: collagen type II, aggrecan, SOX9, MMP-3, TIMP-1 | High glucose -Reduced viability of NP cells, induced apoptosis -Resulted in increased ROS generation and p38MAPK activation. -Negatively regulated the expression of type II collagen, aggrecan, SOX9, and TIMP-1 and positively regulated MMP-3 gene expression |
| (22) Jiang et al., 2018 [67] China | High Glucose-Induced Excessive ROS Promote Apoptosis Through Mitochondrial Damage in Rat CEP cells | Evaluation of the effects of high glucose (0.1, 0.2 M) on CEP cells and to identify the mechanisms of those effects | Pathogenesis (in vitro) | A group of three 6-month-old male SD rats, from which CEPs were harvested -Negative control -0.1 M glucose -0.2 M glucose -0.2 M glucose + alpha-lipoic acid (ALA) 0.15M | -Rat DM model -Tissue harvest: lumbar discs -Cell isolation & culture -Flow cytometry: Intracellular ROS, apoptosis -Mitochondrial Membrane Potential (fluorescence microscopy) -Western blot: cleaved caspase-3, cleaved caspase-9, Bcl-2, Bax, and cytochrome c | -High glucose significantly increased apoptosis and ROS accumulation in CEP cells in a dose- and time-dependent manner. High glucose: -Induced a disrupted mitochondrial membrane potential. -Cleaved caspase-3, cleaved caspase-9, Bax, and cytochrome c↑ but anti-apoptotic protein Bcl-2↓ -ALA inhibited the expression of cleaved caspase-3, cleaved caspase-9, Bax, and cytochrome c but enhanced the expression of Bcl-2 and prevented the membrane potential disruption and apoptosis |
| (23) Jiang et al., 2020 [66] China | Long non-coding RNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) promotes high glucose-induced rat cartilage EP cell apoptosis via the p38/MAPK signaling pathway | Evaluation of the roles of MALAT1 in the apoptosis of CEP cells induced by high glucose (25 mM) and to explore the mechanisms underlying this effect | Specified Pathogenesis (in vitro) | A group of three 12-week-old male SD rats, from which CEPs were harvested -High glucose, -High glucose + MALAT1 -Negative control -High glucose + MALAT1 RNAi, -Normal control | -Rat DM model -Tissue harvest: lumbar discs -Cell isolation & culture -RNA interference/cell transfection -Flow cytometry: apoptosis, AnnexinV, PI -Western blot: p38 activation-PCR: MALAT1 | -Results revealed that high glucose concentration promoted apoptosis and enhanced expression of MALAT1 in CEP cells. -MALAT1 knockout decreased the expression levels of total and phosphorylated p38 and reduced the apoptosis of rat CEP cells. -Results obtained in the present study indicated that MALAT1 may serve as an important therapeutic target for curing or delaying IVDD in patients with DM |
| Authors | Abstract | Research | Aim | Methods | Results | Scoring | Quality | Evidence Strength of | |
|---|---|---|---|---|---|---|---|---|---|
| Question | IVDD and DM Relation | ||||||||
| (1) Ziv et al., 1992 [43] | 3 | 5 | 5 | 3 (in vivo) | 3 | 19 | Moderate | 3 (BC,B,S) | Moderate |
| (2) Chen et al., 2013 [53] | 4 | 5 | 5 | 5 (in vivo) | 5 | 24 | High | 3 (H,I,S) | Moderate |
| (3) Illien-Junger et al., 2013 [62] | 4 | 5 | 5 | 4 (in vivo) | 4 | 22 | High | 5 (H,I,BC,IM,S) | Moderate |
| (4) Jiang et al., 2013 [64] | 3 | 5 | 5 | 5 (in vivo) | 5 | 23 | High | 5 (H,I,BC,M,S) | Moderate |
| (5) Fields et al., 2015 [15] | 3 | 5 | 4 | 5 (in vivo) | 5 | 22 | High | 7(H,I,BC,M,B,IM,S) | Strong |
| (6) Illien-Junger et al., 2015 [52] | 3 | 5 | 5 | 5 (in vivo) | 5 | 23 | High | 5 (H,I,BC,IM,S) | Moderate |
| Analyses partly only qualitative | |||||||||
| (7) Park et al., 2016 [61] | 5 | 4 | 4 | 3 (in vivo) | 4 | 20 | Moderate | 5 (H,I,BC,M,S) | Strong |
| (8) An et al., 2017 [68] | 4 | 5 | 5 | 4 (in vivo) | 4 | 22 | High | 3 (H,I,S) | Moderate |
| (9) Kameda et al., 2017 [69] | 5 | 5 | 5 | 4 (in vivo) | 4 | 23 | High | 3 (I,C,S) | Moderate |
| (10) Krishnamoorthy et al., 2018 [3] | 3 | 4 | 4 | 3 (in vivo) | 4 | 18 | Moderate | 4 (H,I,B,S) | Moderate |
| (11) Li et al., 2020 [47] | 5 | 5 | 5 | 5 (in vivo) | 5 | 25 | High | 6(H,I,BC,M,IM,S) | Strong |
| (12) Natelson et al., 2020 [70] | 5 | 3 | 3 | 3 (in vivo) | 4 | 18 | Moderate | 0 | Absent |
| (13) Tsai et al., 2014 [63] | 4 | 5 | 5 | 5 (in vivo and in vitro) | 4 | 23 | High | 4 (I,BC,M,S) | Moderate |
| (14) Zhang et al., 2019 [58] | 3 | 4 | 4 | 4 (in vivo and in vitro) | 4 | 19 | Moderate | 5 (H,I,BC,IM,S) | Strong |
| (15) Park et al., 2013 [54] | 4 | 4 | 4 | 5 (in vitro) | 4 | 21 | High | 3 (I,BC,S) | Moderate |
| (16) Park et al., 2013 [60] | 5 | 2 | 2 | 3 (in vitro) | 3 | 15 | Low | 3 (I,BC,S) | Moderate |
| (17) Kong et al., 2014 [56] | 5 | 2 | 2 | 2 (in vitro) | 3 | 14 | Low | 2 (I,S) | Low |
| (18) Park et al., 2014 [55] | 5 | 2 | 2 | 3 (in vitro) | 3 | 15 | Low | 3 (I,BC,S) | Moderate |
| (19) Kong et al., 2015 [57] | 5 | 2 | 2 | 2 (in vitro) | 3 | 14 | Low | 3 (I,BC,S) | Moderate |
| (20) Park et al., 2015 [59] | 5 | 2 | 2 | 4 (in vitro) | 3 | 16 | Moderate | 3 (I,BC,S) | Moderate |
| (21) Cheng et al., 2016 [65] | 4 | 4 | 4 | 5 (in vitro) | 4 | 21 | High | 4 (I,M,BC,S) | Moderate |
| (22) Jiang et al., 2020 [67] | 3 | 4 | 4 | 4 (in vitro) | 4 | 15 | Low | 3 (I,BC,S) | Moderate |
| (23) Jiang et al., 2018 [66] | 4 | 4 | 4 | 5 (in vitro) | 5 | 22 | High | 3 (I,M,S) | Moderate |
| Remarks: | |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
| |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, M.; Kokozidou, M.; Auffarth, A.; Schulze-Tanzil, G. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells 2020, 9, 2208. https://doi.org/10.3390/cells9102208
Mahmoud M, Kokozidou M, Auffarth A, Schulze-Tanzil G. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells. 2020; 9(10):2208. https://doi.org/10.3390/cells9102208
Chicago/Turabian StyleMahmoud, Mohamed, Maria Kokozidou, Alexander Auffarth, and Gundula Schulze-Tanzil. 2020. "The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review" Cells 9, no. 10: 2208. https://doi.org/10.3390/cells9102208
APA StyleMahmoud, M., Kokozidou, M., Auffarth, A., & Schulze-Tanzil, G. (2020). The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells, 9(10), 2208. https://doi.org/10.3390/cells9102208






