Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tail Suspension Test
2.3. Forced Swim Test
2.4. Learned Helplessness Paradigm
2.5. Open Field
2.6. Nociceptive Behavior
2.7. Corticosterone Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Dexamethasone Suppression Test
2.9. Bromodeoxyuridine Administration
2.10. Immunohistochemistry
2.11. Mass Spectrometry
2.12. Cell Culture, Transfection, and Luciferase Activity Assay
3. Results
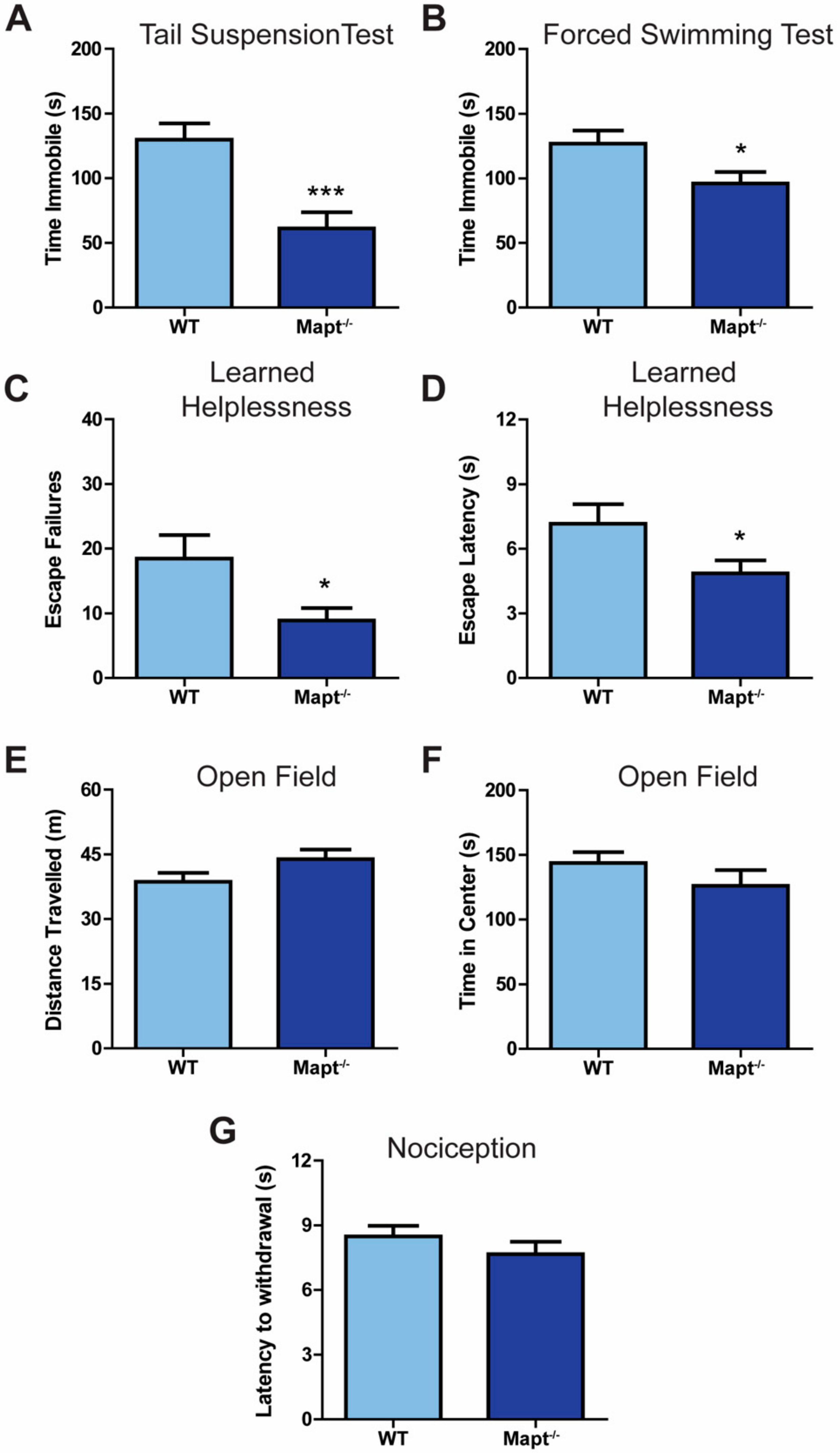
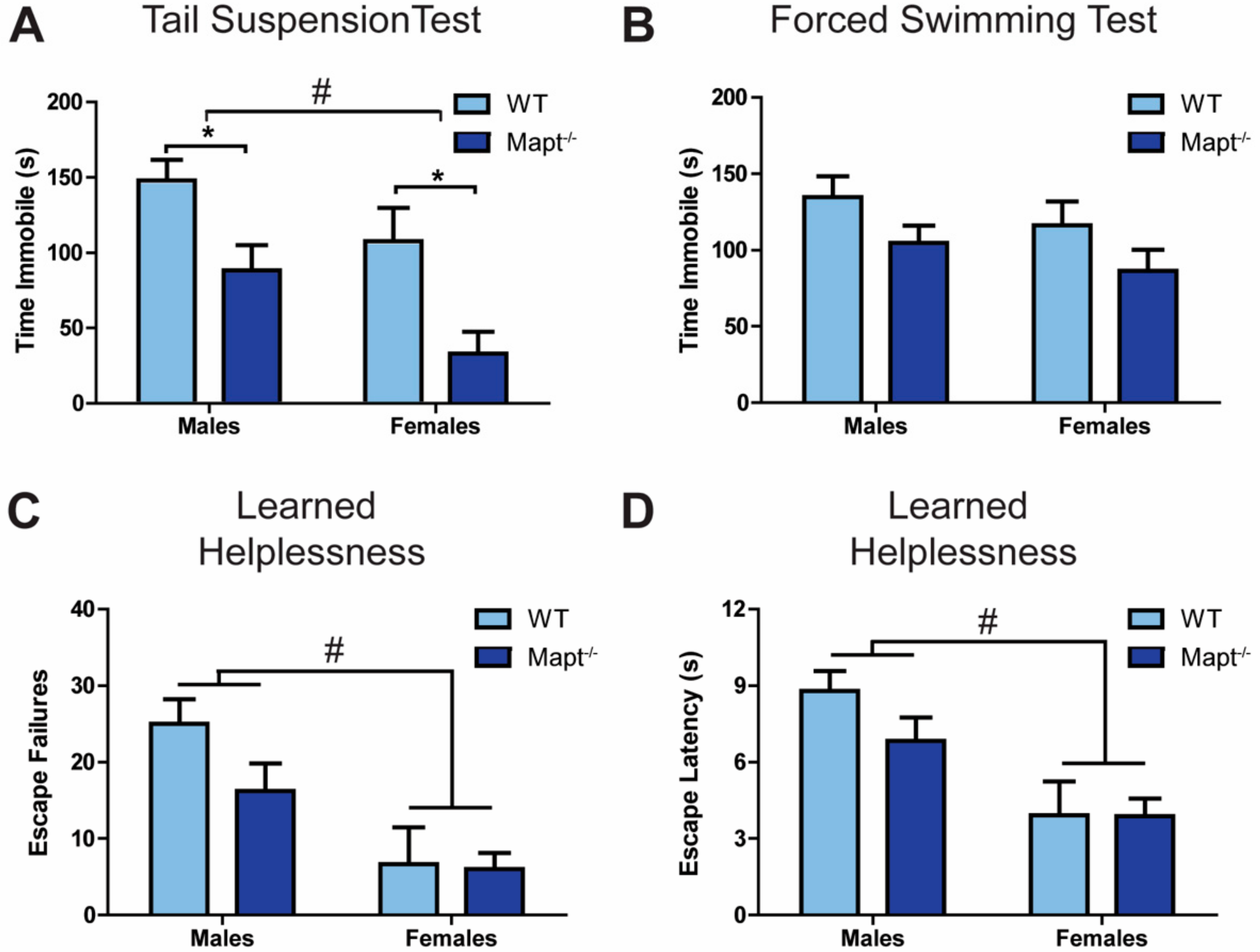
3.1. Mapt−/− Mice Display a Depression-Resistant Phenotype
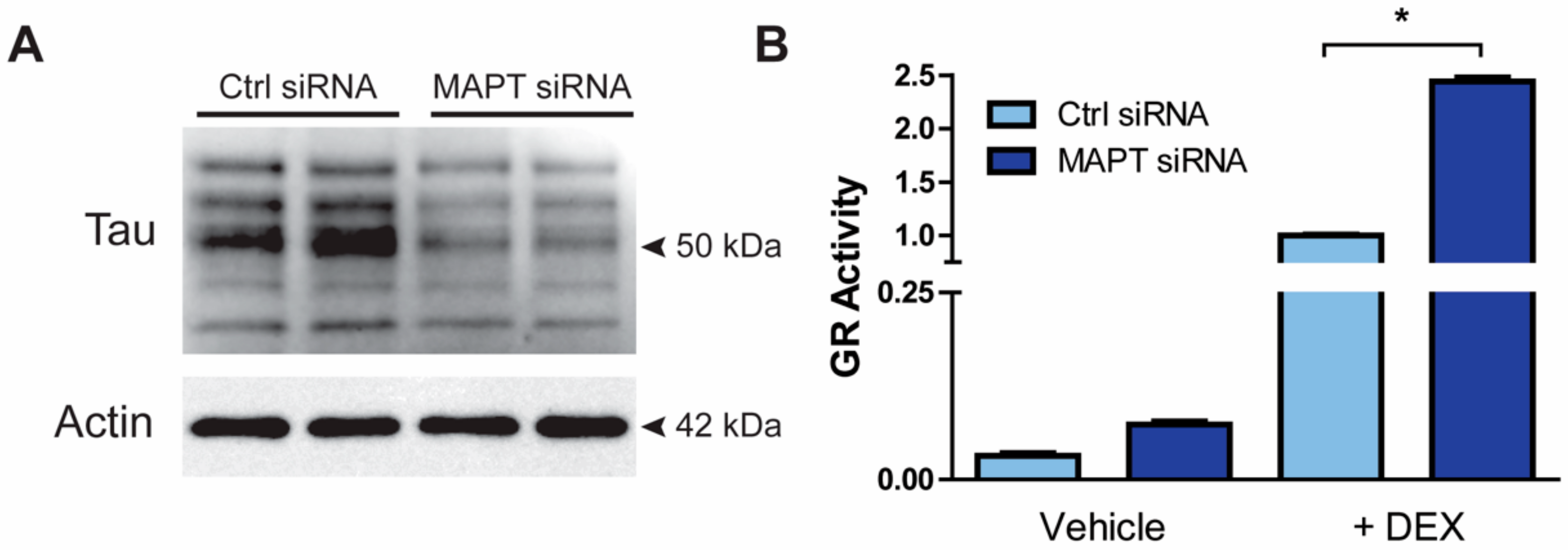
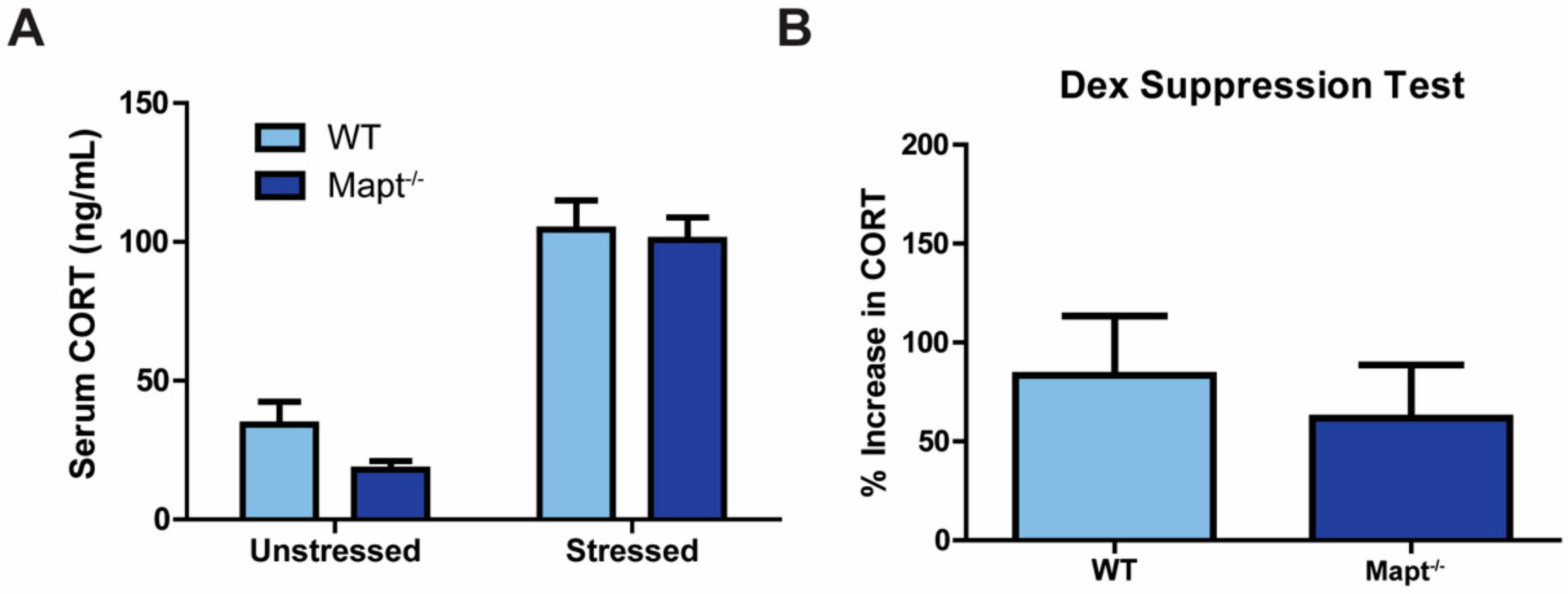
3.2. Resiliency to Depression Is Independent of the HPA Axis Function
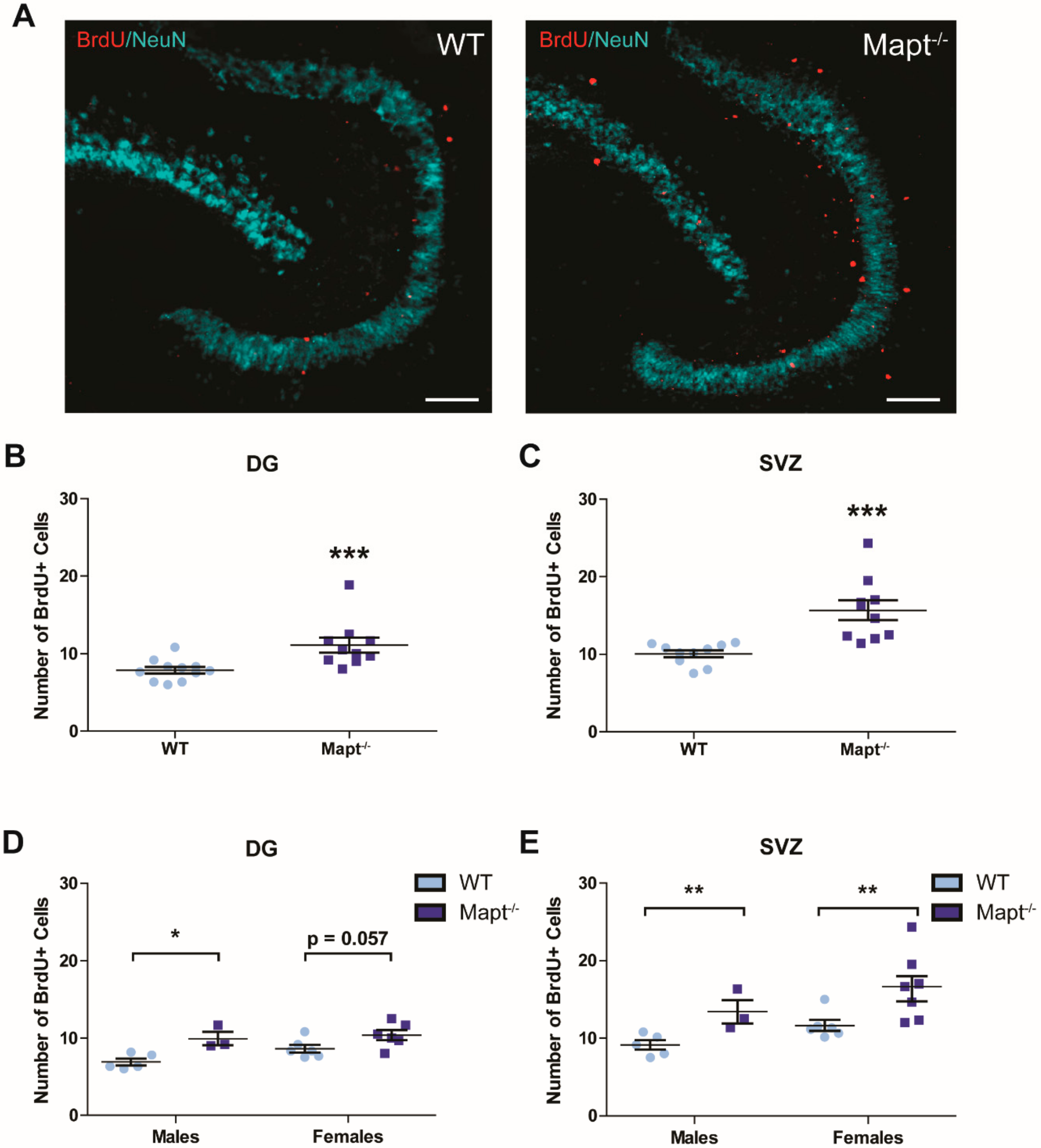
3.3. Tau Deletion Increases Neurogenesis in the Dentate Gyrus and Subventricular Zone
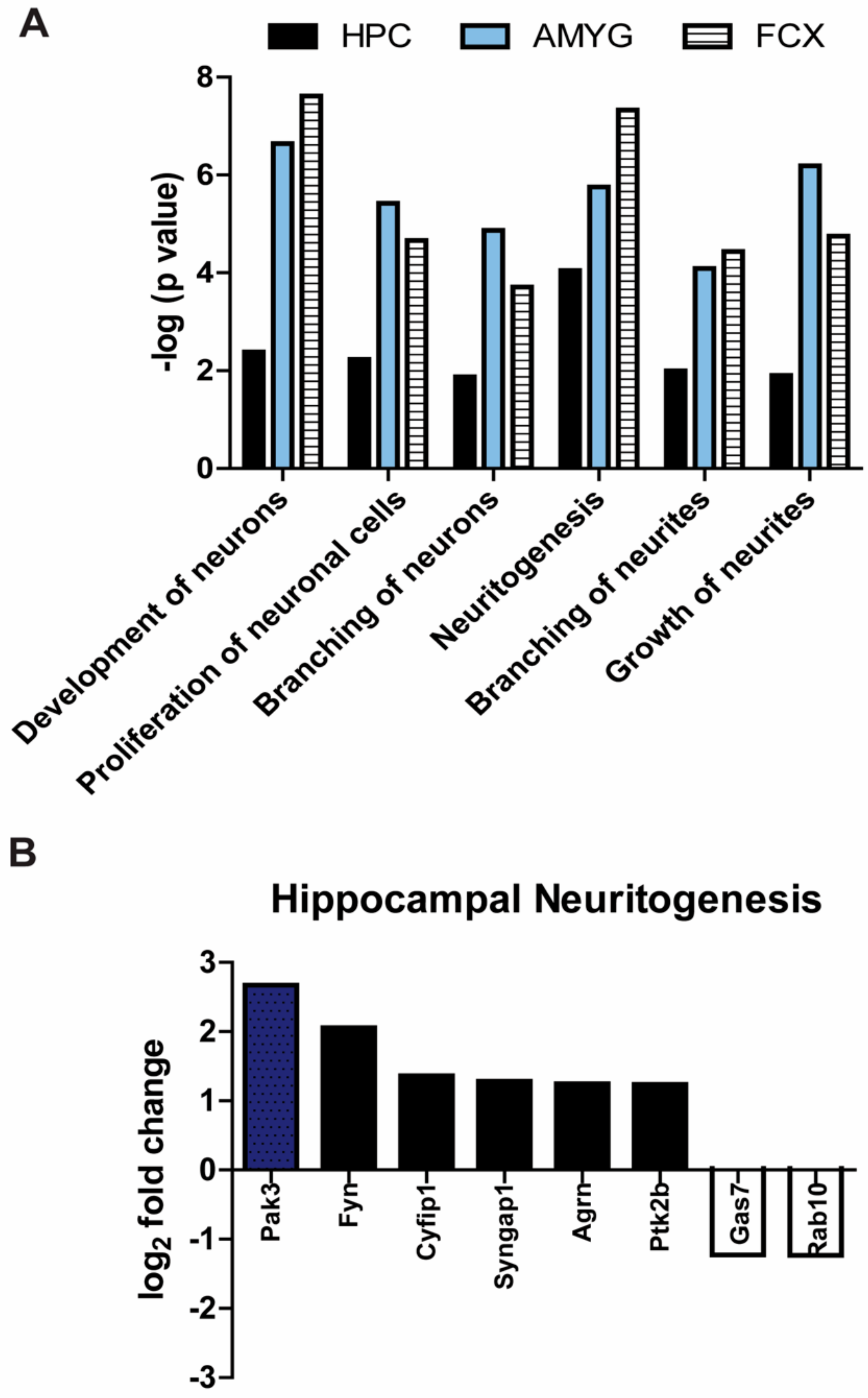
3.4. Processes Involved in Neuronal Development Are Significantly Upregulated in Mapt−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braak, H.; Braak, E. Staging of Alzheimer’s Disease-Related Neurofibrillary Changes. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Pozueta, J.; Lefort, R.; Shelanski, M.L. Synaptic Changes in Alzheimer’s Disease and Its Models. Neuroscience 2013, 251, 51–65. [Google Scholar] [CrossRef]
- Mufson, E.J.; Ward, S.; Binder, L. Prefibrillar Tau Oligomers in Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener. Dis. 2014, 13, 151–153. [Google Scholar] [CrossRef]
- Bierer, L.M.; Haroutunian, V.; Gabriel, S.; Knott, P.J.; Carlin, L.S.; Purohit, D.P.; Perl, D.P.; Schmeidler, J.; Kanof, P.; Davis, K.L. Neurochemical Correlates of Dementia Severity in Alzheimer’s Disease: Relative Importance of the Cholinergic Deficits. J. Neurochem. 2002, 64, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Petersen, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal Loss Correlates with but Exceeds Neurofibrillary Tangles in Alzheimer’s Disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Galts, C.P.C.; Bettio, L.E.B.; Jewett, D.C.; Yang, C.C.; Brocardo, P.S.; Rodrigues, A.L.S.; Thacker, J.S.; Gil-Mohapel, J. Depression in Neurodegenerative Diseases: Common Mechanisms and Current Treatment Options. Neurosci. Biobehav. Rev. 2019, 102, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Lara, E.; Haro, J.M.; Tang, M.-X.; Manly, J.; Stern, Y. Exploring the Excess Mortality Due to Depressive Symptoms in a Community-Based Sample: The Role of Alzheimer’s Disease. J. Affect. Disord. 2016, 202, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, J.D.; Waldorff, F.B.; Siersma, V.D.; Phung, T.K.T.; Bebe, A.C.K.M.; Waldemar, G. Major Depressive Symptoms Increase 3-Year Mortality Rate in Patients with Mild Dementia. Int. J. Alzheimers. Dis. 2017, 2017, 7482094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, H.; Nordström, A.; Nordström, P. Depression and Subsequent Risk of Parkinson Disease: A Nationwide Cohort Study. Neurology 2015, 84, 2422–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia-García, P.; de-la-Cámara, C.; Santabárbara, J.; Lopez-Anton, R.; Quintanilla, M.A.; Ventura, T.; Marcos, G.; Campayo, A.; Saz, P.; Lyketsos, C.; et al. Depression and Incident Alzheimer Disease: The Impact of Disease Severity. Am. J. Geriatr. Psychiatry 2015, 23, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Masters, M.C.; Morris, J.C.; Roe, C.M. Noncognitive Symptoms of Early Alzheimer Disease: A Longitudinal Analysis. Neurology 2015, 84, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringman, J.M.; Liang, L.-J.; Zhou, Y.; Vangala, S.; Teng, E.; Kremen, S.; Wharton, D.; Goate, A.; Marcus, D.S.; Farlow, M.; et al. Early Behavioural Changes in Familial Alzheimer’s Disease in the Dominantly Inherited Alzheimer Network. Brain 2015, 138, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panza, F.; Frisardi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Imbimbo, B.P.; Santamato, A.; Vendemiale, G.; Seripa, D.; Pilotto, A.; et al. Late-Life Depression, Mild Cognitive Impairment, and Dementia: Possible Continuum? Am. J. Geriatr. Psychiatry 2010, 18, 98–116. [Google Scholar] [CrossRef]
- Nie, L.; Wei, G.; Peng, S.; Qu, Z.; Yang, Y.; Yang, Q.; Huang, X.; Liu, J.; Zhuang, Z.; Yang, X. Melatonin Ameliorates Anxiety and Depression-like Behaviors and Modulates Proteomic Changes in Triple Transgenic Mice of Alzheimer’s Disease. BioFactors 2017, 43, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Pace, L.; Tempesta, B.; Lavecchia, A.M.; Macheda, T.; Bedse, G.; Petrella, A.; Cifani, C.; Serviddio, G.; Vendemiale, G.; et al. Depressive-like Behavior Is Paired to Monoaminergic Alteration in a Murine Model of Alzheimer’s Disease. Int. J. Neuropsychopharmacol. 2014, 18, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iascone, D.M.; Padidam, S.; Pyfer, M.S.; Zhang, X.; Zhao, L.; Chin, J. Impairments in Neurogenesis Are Not Tightly Linked to Depressive Behavior in a Transgenic Mouse Model of Alzheimer’s Disease. PLoS ONE 2013, 8, e79651. [Google Scholar] [CrossRef]
- Ledo, J.H.; Azevedo, E.P.; Clarke, J.R.; Ribeiro, F.C.; Figueiredo, C.P.; Foguel, D.; De Felice, F.G.; Ferreira, S.T. Amyloid-β Oligomers Link Depressive-like Behavior and Cognitive Deficits in Mice. Mol. Psychiatry 2013, 18, 1053–1054. [Google Scholar] [CrossRef] [Green Version]
- Filali, M.; Lalonde, R.; Rivest, S. Cognitive and Non-Cognitive Behaviors in an APPswe/PS1 Bigenic Model of Alzheimer’s Disease. Genes Brain Behav. 2009, 8, 143–148. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Jones, V.C.; Tabuchi, M.; Allan, S.M.; Knight, E.M.; LaFerla, F.M.; Oddo, S.; Verkhratsky, A. Impaired Adult Neurogenesis in the Dentate Gyrus of a Triple Transgenic Mouse Model of Alzheimer’s Disease. PLoS ONE 2008, 3, e2935. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, L.K.; Aumont, A.; Julien, C.; Vadnais, A.; Calon, F.; Fernandes, K.J.L. Widespread Deficits in Adult Neurogenesis Precede Plaque and Tangle Formation in the 3xTg Mouse Model of Alzheimer’s Disease. Eur. J. Neurosci. 2010, 32, 905–920. [Google Scholar] [CrossRef]
- Pan, H.; Wang, D.; Zhang, X.; Zhou, D.; Zhang, H.; Qian, Q.; He, X.; Liu, Z.; Liu, Y.; Zheng, T.; et al. Amyloid β Is Not the Major Factor Accounting for Impaired Adult Hippocampal Neurogenesis in Mice Overexpressing Amyloid Precursor Protein. Stem Cell Rep. 2016, 7, 707–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, H.N.; Ferreira, A.; Eyster, M.V.; Ghoshal, N.; Binder, L.I.; Vitek, M.P. Inhibition of Neuronal Maturation in Primary Hippocampal Neurons from Tau Deficient Mice. J. Cell Sci. 2001, 114, 179–1187. [Google Scholar]
- Lopes, S.; Vaz-Silva, J.; Pinto, V.; Dalla, C.; Kokras, N.; Bedenk, B.; Mack, N.; Czisch, M.; Almeida, O.F.X.; Sousa, N.; et al. Tau Protein Is Essential for Stress-Induced Brain Pathology. Proc. Natl. Acad. Sci. USA 2016, 113, E3755–E3763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiropoulos, I.; Catania, C.; Pinto, L.G.; Silva, R.; Pollerberg, G.E.; Takashima, A.; Sousa, N.; Almeida, O.F.X. Stress Acts Cumulatively to Precipitate Alzheimer’s Disease-like Tau Pathology and Cognitive Deficits. J. Neurosci. 2011, 31, 7840–7847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Quervain, D.J.-F.; Poirier, R.; Wollmer, M.A.; Grimaldi, L.M.E.; Tsolaki, M.; Streffer, J.R.; Hock, C.; Nitsch, R.M.; Mohajeri, M.H.; Papassotiropoulos, A. Glucocorticoid-Related Genetic Susceptibility for Alzheimer’s Disease. Hum. Mol. Genet. 2003, 13, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Iii, J.C.O.; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J.; et al. A New Anti-Depressive Strategy for the Elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6, e24840. [Google Scholar] [CrossRef]
- Chourbaji, S.; Zacher, C.; Sanchis-Segura, C.; Dormann, C.; Vollmayr, B.; Gass, P. Learned Helplessness: Validity and Reliability of Depressive-like States in Mice. Brain Res. Protoc. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Gebru, N.T.; Gould, L.A.; Smith, T.M.; Kim, S.; Blackburn, R.J.; Dickey, C.A.; Blair, L.J. Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. Int. J. Mol. Sci. 2019, 20, 2738. [Google Scholar] [CrossRef] [Green Version]
- De Felipe, C.; Herrero, J.F.; O’Brien, J.A.; Palmer, J.A.; Doyle, C.A.; Smith, A.J.H.; Laird, J.M.A.; Belmonte, C.; Cervero, F.; Hunt, S.P. Altered Nociception, Analgesia and Aggression in Mice Lacking the Receptor for Substance P. Nature 1998, 392, 394–397. [Google Scholar] [CrossRef]
- Sabbagh, J.J.; O’Leary, J.C.; Blair, L.J.; Klengel, T.; Nordhues, B.A.; Fontaine, S.N.; Binder, E.B.; Dickey, C.A. Age-Associated Epigenetic Upregulation of the FKBP5 Gene Selectively Impairs Stress Resiliency. PLoS ONE 2014, 9, e107241. [Google Scholar] [CrossRef]
- Abisambra, J.F.; Blair, L.J.; Hill, S.E.; Jones, J.R.; Kraft, C.; Rogers, J.; Iii, J.K.; Jinwal, U.K.; Lawson, L.; Johnson, A.G.; et al. Phosphorylation Dynamics Regulate Hsp27-Mediated Rescue of Neuronal Plasticity Deficits in Tau Transgenic Mice. J. Neurosci. 2010, 30, 15374–15382. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Sabbagh, J.J.; Blair, L.J.; Darling, A.L.; Wen, X.; Dickey, C.A. MicroRNA-511 Binds to FKBP5 MRNA, Which Encodes a Chaperone Protein, and Regulates Neuronal Differentiation. J. Biol. Chem. 2016, 291, 17897–17906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbagh, J.J.; Cordova, R.A.; Zheng, D.; Criado-Marrero, M.; Lemus, A.; Li, P.; Baker, J.D.; Nordhues, B.A.; Darling, A.L.; Martinez-Licha, C.; et al. Targeting the FKBP51/GR/Hsp90 Complex to Identify Functionally Relevant Treatments for Depression and PTSD. ACS Chem. Biol. 2018, 13, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N.; Wolf, J.M.; Wolf, O.T. Glucocorticoid Sensitivity of Cognitive and Inflammatory Processes in Depression and Posttraumatic Stress Disorder. Neurosci. Biobehav. Rev. 2010, 35, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Rhebergen, D.; Korten, N.C.M.; Penninx, B.W.J.H.; Stek, M.L.; van der Mast, R.C.; Oude Voshaar, R.; Comijs, H.C. Hypothalamic-Pituitary-Adrenal Axis Activity in Older Persons with and without a Depressive Disorder. Psychoneuroendocrinology 2015, 51, 341–350. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Burke, H.; Epel, E.S.; Reus, V.I. Glucocorticoids. Mood, Memory, and Mechanisms. Ann. N. Y. Acad. Sci. 2009, 1179, 19–40. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult Hippocampal Neurogenesis Buffers Stress Responses and Depressive Behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Houben, S.; Leroy, K.; Ando, K.; Yilmaz, Z.; Widomski, C.; Buée, L.; Brion, J.-P. Genetic Ablation of Tau in Postnatal Neurons Rescues Decreased Adult Hippocampal Neurogenesis in a Tauopathy Model. Neurobiol. Dis. 2019, 127, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Sacher, J.; Neumann, J.; Fünfstück, T.; Soliman, A.; Villringer, A.; Schroeter, M.L. Mapping the Depressed Brain: A Meta-Analysis of Structural and Functional Alterations in Major Depressive Disorder. J. Affect. Disord. 2012, 140, 142–148. [Google Scholar] [CrossRef]
- Ye, M.; Yang, T.; Qing, P.; Lei, X.; Qiu, J.; Liu, G. Changes of Functional Brain Networks in Major Depressive Disorder: A Graph Theoretical Analysis of Resting-State FMRI. PLoS ONE 2015, 10, e0133775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Y.; Chen, Y.; Su, D.-Y.; Marshall, C.; Xiao, M. Depressive- and Anxiety-like Phenotypes in Young Adult APPSwe/PS1dE9 Transgenic Mice with Insensitivity to Chronic Mild Stress. Behav. Brain Res. 2018, 353, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Fonseca, J.A.; Gosselink, K.L. Tauopathy and Neurodegeneration: A Role for Stress. Neurobiol. Stress 2018, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Geiszler, P.C.; Barron, M.R.; Pardon, M.-C. Impaired Burrowing Is the Most Prominent Behavioral Deficit of Aging Htau Mice. Neuroscience 2016, 329, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; Juster, R.P.; Raymond, C.; Marin, M.F. The Effects of Chronic Stress on the Human Brain: From Neurotoxicity, to Vulnerability, to Opportunity. Front. Neuroendocrinol. 2018, 49, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.; Silva, J.; Mota, C.; Vaz-Silva, J.; Veloso, A.; Pinto, V.; Sousa, N.; Cerqueira, J.; Sotiropoulos, I. Tau Mislocation in Glucocorticoid-Triggered Hippocampal Pathology. Mol. Neurobiol. 2016, 53, 4745–4753. [Google Scholar] [CrossRef] [Green Version]
- Zahodne, L.B.; Gongvatana, A.; Cohen, R.A.; Ott, B.R.; Tremont, G.; Alzheimer’s Disease Neuroimaging Initiative. Are Apathy and Depression Independently Associated with Longitudinal Trajectories of Cortical Atrophy in Mild Cognitive Impairment? Am. J. Geriatr. Psychiatry 2013, 21, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhang, J.; Liu, X.; Hou, H.; Cao, Y.; Wei, F.; Li, J.; Chen, X.; Shen, Y.; Chen, W. Neurometabolic Characteristics in the Anterior Cingulate Gyrus of Alzheimer’s Disease Patients with Depression: A 1H Magnetic Resonance Spectroscopy Study. BMC Psychiatry 2015, 15, 306. [Google Scholar] [CrossRef] [Green Version]
- Son, J.H.; Han, D.H.; Min, K.J.; Kee, B.S. Correlation between Gray Matter Volume in the Temporal Lobe and Depressive Symptoms in Patients with Alzheimer’s Disease. Neurosci. Lett. 2013, 548, 15–20. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Hoeijmakers, L.; Kakava-Georgiadou, N.; Meesters, A.; Lazic, S.E.; Lucassen, P.J.; Korosi, A. Chronic Early Life Stress Alters Developmental and Adult Neurogenesis and Impairs Cognitive Function in Mice. Hippocampus 2015, 25, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Rodrigues, A.J.; Silva, J.M.; Tronche, F.; Almeida, O.F.X.; Sousa, N.; Sotiropoulos, I. Chronic Stress and Glucocorticoids: From Neuronal Plasticity to Neurodegeneration. Neural Plast. 2016, 2016, 6391686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komuro, Y.; Xu, G.; Bhaskar, K.; Lamb, B.T. Human Tau Expression Reduces Adult Neurogenesis in a Mouse Model of Tauopathy. Neurobiol. Aging 2015, 36, 2034–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dioli, C.; Patrício, P.; Trindade, R.; Pinto, L.G.; Silva, J.M.; Morais, M.; Ferreiro, E.; Borges, S.; Mateus-Pinheiro, A.; Rodrigues, A.J.; et al. Tau-Dependent Suppression of Adult Neurogenesis in the Stressed Hippocampus. Mol. Psychiatry 2017, 22, 1110–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, A.; Oguchi, K.; Okabe, S.; Kuno, J.; Terada, S.; Ohshima, T.; Sato-Yoshitake, R.; Takei, Y.; Noda, T.; Hirokawa, N. Altered Microtubule Organization in Small-Calibre Axons of Mice Lacking Tau Protein. Nature 1994, 369, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.-L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Kiosea, N.C.; Nazari, S.; Chen, P.P.; Nothias, F.; et al. Loss of MAP Function Leads to Hippocampal Synapse Loss and Deficits in the Morris Water Maze with Aging. J. Neurosci. 2014, 34, 7124–7136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult Hippocampal Neurogenesis Is Abundant in Neurologically Healthy Subjects and Drops Sharply in Patients with Alzheimer’s Disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Stockmeier, C.A.; Mahajan, G.J.; Konick, L.C.; Overholser, J.C.; Jurjus, G.J.; Meltzer, H.Y.; Uylings, H.B.M.; Friedman, L.; Rajkowska, G. Cellular Changes in the Postmortem Hippocampus in Major Depression. Biol. Psychiatry 2004, 56, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Coupland, N.J.; Lebel, R.M.; Carter, R.; Seres, P.; Wilman, A.H.; Malykhin, N.V. Structural Changes in Hippocampal Subfields in Major Depressive Disorder: A High-Field Magnetic Resonance Imaging Study. Biol. Psychiatry 2013, 74, 62–68. [Google Scholar] [CrossRef]
- Frodl, T.M.; Carballedo, A.; Hughes, M.M.; Saleh, K.; Fagan, A.; Skokauskas, N.; McLoughlin, D.M.; Meaney, J.; O’Keane, V.; Connor, T.J. Reduced Expression of Glucocorticoid-Inducible Genes GILZ and SGK-1: High IL-6 Levels Are Associated with Reduced Hippocampal Volumes in Major Depressive Disorder. Transl. Psychiatry 2012, 2, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Marriott, M.; Nahmias, C.; Macqueen, G.M. Lower Hippocampal Volume in Patients Suffering From Depression: A Meta-Analysis. Am. J. Psychiatry 2004, 161, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, N.; Yücel, K.; Courtright, A.; Macmaster, F.P.; Sembo, M.; Macqueen, G. Subgenual Anterior Cingulate Cortex and Hippocampal Volumes in Depressed Youth: The Role of Comorbidity and Age. J. Affect. Disord. 2015, 190, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Dubos, A.; Combeau, G.; Bernardinelli, Y.; Barnier, J.-V.; Hartley, O.; Gaertner, H.; Boda, B.; Muller, D. Alteration of Synaptic Network Dynamics by the Intellectual Disability Protein PAK3. J. Neurosci. 2012, 32, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Hussain, N.K.; Thomas, G.M.; Luo, J.; Huganir, R.L. Regulation of AMPA Receptor Subunit GluA1 Surface Expression by PAK3 Phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E5883–E5890. [Google Scholar] [CrossRef] [Green Version]
- McPhie, D.L.; Coopersmith, R.; Hines-Peralta, A.; Chen, Y.; Ivins, K.J.; Manly, S.P.; Kozlowski, M.R.; Neve, K.A.; Neve, R.L. DNA Synthesis and Neuronal Apoptosis Caused by Familial Alzheimer Disease Mutants of the Amyloid Precursor Protein Are Mediated by the P21 Activated Kinase PAK3. J. Neurosci. 2003, 23, 6914–6927. [Google Scholar] [CrossRef] [Green Version]
- Boda, B.; Alberi, S.; Nikonenko, I.; Node-Langlois, R.; Jourdain, P.; Moosmayer, M.; Parisi-Jourdain, L.; Muller, D. The Mental Retardation Protein PAK3 Contributes to Synapse Formation and Plasticity in Hippocampus. J. Neurosci. 2004, 24, 10816–10825. [Google Scholar] [CrossRef] [Green Version]
- Fuchsova, B.; Alvarez Juliá, A.; Rizavi, H.S.; Frasch, A.C.; Pandey, G.N. Expression of P21-Activated Kinases 1 and 3 Is Altered in the Brain of Subjects with Depression. Neuroscience 2016, 333, 331–344. [Google Scholar] [CrossRef] [Green Version]
- Polydoro, M.; Acker, C.M.; Duff, K.; Castillo, P.E.; Davies, P. Age-Dependent Impairment of Cognitive and Synaptic Function in the Htau Mouse Model of Tau Pathology. J. Neurosci. 2009, 29, 10741–10749. [Google Scholar] [CrossRef] [Green Version]
- Kril, J.J.; Hodges, J.; Halliday, G. Relationship between Hippocampal Volume and CA1 Neuron Loss in Brains of Humans with and without Alzheimer’s Disease. Neurosci. Lett. 2004, 361, 9–12. [Google Scholar] [CrossRef]
- Oomen, C.A.; Mayer, J.L.; De Kloet, E.R.; Joëls, M.; Lucassen, P.J. Brief Treatment with the Glucocorticoid Receptor Antagonist Mifepristone Normalizes the Reduction in Neurogenesis after Chronic Stress. Eur. J. Neurosci. 2007, 26, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Oomen, C.; van Dam, A.-M.; Wester, J.; Zhou, J.-N.; Joëls, M.; Lucassen, P.J. A Single-Day Treatment with Mifepristone Is Sufficient to Normalize Chronic Glucocorticoid Induced Suppression of Hippocampal Cell Proliferation. PLoS ONE 2012, 7, e46224. [Google Scholar] [CrossRef] [PubMed]
- Baglietto-Vargas, D.; Medeiros, R.; Martinez-Coria, H.; LaFerla, F.M.; Green, K.N. Mifepristone Alters Amyloid Precursor Protein Processing to Preclude Amyloid Beta and Also Reduces Tau Pathology. Biol. Psychiatry 2013, 74, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulsin, A.C.; Herman, J.P.; Solomon, M.B. Mifepristone Decreases Depression-like Behavior and Modulates Neuroendocrine and Central Hypothalamic-Pituitary-Adrenocortical Axis Responsiveness to Stress. Psychoneuroendocrinology 2010, 35, 1100–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.-W.; David, D.J.; Monckton, J.E.; Battaglia, F.; Hen, R. Chronic Fluoxetine Stimulates Maturation and Synaptic Plasticity of Adult-Born Hippocampal Granule Cells. Neurobiol. Dis. 2008, 28, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.D.; Coplan, J.D.; Lisanby, S.H.; Lipira, C.M.; Arif, M.; Carpio, C.; Spitzer, G.; Santarelli, L.; Scharf, B.; Hen, R.; et al. Antidepressant-Induced Neurogenesis in the Hippocampus of Adult Nonhuman Primates. J. Neurosci. 2007, 27, 4894–4901. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-N.; Jia, Y.-F.; Zhang, L.; Zhang, Q.; Huang, Y.; Liu, X.-Z.; Hu, L.; Lan, W.; Chen, L.; Lesch, K.-P.; et al. Reducing Central Serotonin in Adulthood Promotes Hippocampal Neurogenesis. Sci. Rep. 2016, 6, 20338. [Google Scholar] [CrossRef] [Green Version]
- Wen, G.; Yao, H.; Li, Y.; Ding, R.; Ren, X.; Tan, Y.; Ren, W.; Yu, H.; Zhan, X.; Wang, X.; et al. Regulation of Tau Protein on the Antidepressant Effects of Ketamine in the Chronic Unpredictable Mild Stress Model. Front. Psychiatry 2019, 10, 287. [Google Scholar] [CrossRef]
- Caddy, C.; Amit, B.H.; McCloud, T.L.; Rendell, J.M.; Furukawa, T.A.; McShane, R.; Hawton, K.; Cipriani, A. Ketamine and Other Glutamate Receptor Modulators for Depression in Adults. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Costa, D.A.; Cracchiolo, J.R.; Bachstetter, A.D.; Hughes, T.F.; Bales, K.R.; Paul, S.M.; Mervis, R.F.; Arendash, G.W.; Potter, H. Enrichment Improves Cognition in AD Mice by Amyloid-Related and Unrelated Mechanisms. Neurobiol. Aging 2007, 28, 831–844. [Google Scholar] [CrossRef]
- Nichol, K.; Deeny, S.P.; Seif, J.; Camaclang, K.; Cotman, C.W. Exercise Improves Cognition and Hippocampal Plasticity in APOE Epsilon4 Mice. Alzheimers. Dement. 2009, 5, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.-S.; Xu, P.; Pigino, G.; Brady, S.T.; Larson, J.; Lazarov, O. Complex Environment Experience Rescues Impaired Neurogenesis, Enhances Synaptic Plasticity, and Attenuates Neuropathology in Familial Alzheimer’s Disease-Linked APPswe/PS1ΔE9 Mice. FASEB J. 2010, 24, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and TrkB MRNA in Rat Brain by Chronic Electroconvulsive Seizure and Antidepressant Drug Treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, W.; Kong, S.-Y. Antidepressants for Neuro-Regeneration: From Depression to Alzheimer’s Disease. Arch. Pharm. Res. 2013, 36, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Salas, M.; Pilar-Cuéllar, F.; Auberson, Y.P.; Adell, A. Signaling Pathways Responsible for the Rapid Antidepressant-like Effects of a GluN2A-Preferring NMDA Receptor Antagonist. Transl. Psychiatry 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, J.W.; Russo, S.J.; Ferguson, D.; Nestler, E.J.; Duman, R.S. Nuclear Factor-KappaB Is a Critical Mediator of Stress-Impaired Neurogenesis and Depressive Behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 2669–2674. [Google Scholar] [CrossRef] [Green Version]
- Bruel-Jungerman, E.; Davis, S.; Rampon, C.; Laroche, S. Long-Term Potentiation Enhances Neurogenesis in the Adult Dentate Gyrus. J. Neurosci. 2006, 26, 5888–5893. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.; Whitcomb, D.J.; Jo, J.; Regan, P.; Piers, T.; Heo, S.; Brown, C.; Hashikawa, T.; Murayama, M.; Seok, H.; et al. Microtubule-Associated Protein Tau Is Essential for Long-Term Depression in the Hippocampus. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130144. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.K.; Furgerson, M.; Crystal, J.D.; Fechheimer, M.; Furukawa, R.; Wagner, J.J. Alterations in Synaptic Plasticity Coincide with Deficits in Spatial Working Memory in Presymptomatic 3xTg-AD Mice. Neurobiol. Learn. Mem. 2015, 125, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Gassen, N.C.N.C.; Fries, G.R.; Zannas, A.S.; Hartmann, J.; Zschocke, J.; Hafner, K.; Carrillo-Roa, T.; Steinbacher, J.; Preißinger, S.N.; Hoeijmakers, L.; et al. Chaperoning Epigenetics: FKBP51 Decreases the Activity of DNMT1 and Mediates Epigenetic Effects of the Antidepressant Paroxetine. Sci. Signal. 2015, 8, 119. [Google Scholar] [CrossRef]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C.; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated Neurodegeneration through Chaperone-Mediated Oligomerization of Tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-T.; Wang, Z.-J.; Cai, H.-Y.; Yuan, L.; Hu, M.-M.; Wu, M.-N.; Qi, J.-S. Sex Differences in Neuropathology and Cognitive Behavior in APP/PS1/Tau Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2018, 34, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Quartey, M.O.; Nyarko, J.N.K.; Pennington, P.R.; Heistad, R.M.; Chaharyn, B.M.; Wei, Z.; Bainbridge, D.; Baker, G.B.; Mousseau, D.D. Age- and Sex-Dependent Profiles of APP Fragments and Key Secretases Align with Changes in Despair-like Behavior and Cognition in Young APPSwe/Ind Mice. Biochem. Biophys. Res. Commun. 2019, 511, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Di Lorenzo, F.; Bonnì, S.; Giacobbe, V.; Bozzali, M.; Caltagirone, C.; Martorana, A. Dopaminergic Modulation of Cortical Plasticity in Alzheimer’s Disease Patients. Neuropsychopharmacology 2014, 39, 2654–2661. [Google Scholar] [CrossRef] [Green Version]
- Colloby, S.J.; McParland, S.; O’Brien, J.T.; Attems, J. Neuropathological Correlates of Dopaminergic Imaging in Alzheimer’s Disease and Lewy Body Dementias. Brain 2012, 135, 2798–2808. [Google Scholar] [CrossRef] [Green Version]
- Marazziti, D. Understanding the Role of Serotonin in Psychiatric Diseases. F1000Research 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Oomen, C.A.; Girardi, C.E.N.; Cahyadi, R.; Verbeek, E.C.; Krugers, H.; Joëls, M.; Lucassen, P.J. Opposite Effects of Early Maternal Deprivation on Neurogenesis in Male versus Female Rats. PLoS ONE 2009, 4, e3675. [Google Scholar] [CrossRef] [Green Version]
- Bangasser, D.A.; Valentino, R.J. Sex Differences in Stress-Related Psychiatric Disorders: Neurobiological Perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Bourke, C.H.; Harrell, C.S.; Neigh, G.N. Stress-Induced Sex Differences: Adaptations Mediated by the Glucocorticoid Receptor. Horm. Behav. 2012, 62, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Sotiropoulos, I.; Lopes, A.T.; Pinto, V.; Lopes, S.; Carlos, S.; Duarte-Silva, S.; Neves-Carvalho, A.; Pinto-Ribeiro, F.; Pinheiro, S.; Fernandes, R.; et al. Selective Impact of Tau Loss on Nociceptive Primary Afferents and Pain Sensation. Exp. Neurol. 2014, 261, 486–493. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Criado-Marrero, M.; Sabbagh, J.J.; Jones, M.R.; Chaput, D.; Dickey, C.A.; Blair, L.J. Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice. Cells 2020, 9, 210. https://doi.org/10.3390/cells9010210
Criado-Marrero M, Sabbagh JJ, Jones MR, Chaput D, Dickey CA, Blair LJ. Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice. Cells. 2020; 9(1):210. https://doi.org/10.3390/cells9010210
Chicago/Turabian StyleCriado-Marrero, Marangelie, Jonathan J. Sabbagh, Margaret R. Jones, Dale Chaput, Chad A. Dickey, and Laura J. Blair. 2020. "Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice" Cells 9, no. 1: 210. https://doi.org/10.3390/cells9010210
APA StyleCriado-Marrero, M., Sabbagh, J. J., Jones, M. R., Chaput, D., Dickey, C. A., & Blair, L. J. (2020). Hippocampal Neurogenesis Is Enhanced in Adult Tau Deficient Mice. Cells, 9(1), 210. https://doi.org/10.3390/cells9010210




