Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Monocytes Isolation and Culture
2.2. Cell Culture
2.3. Cell Characterization by Flow Cytometry
2.4. Reverse Transcription and Quantifying PCR (RT-qPCR)
2.5. 3D Co-Culture and Microencapsulation
2.6. Murine (BALB-c\SCID) Model of Neo-Angiogenesis
2.7. Immunofluorescence
2.8. Murine (BALB-c\SCID) Aorta Ex Vivo Model of Vascular Repair
2.9. Fluorescence In Situ Hybridization (FISH)
2.10. Immunohistochemistry (IHC)
2.11. Data Statistical Analysis
3. Results
3.1. In Vitro Monocytes Differentiate into Endothelial Cells (ECs)
3.2. Reactive Oxygen Species (ROS) Promote Monocytes Differentiation into Endothelial Cells (ECs)
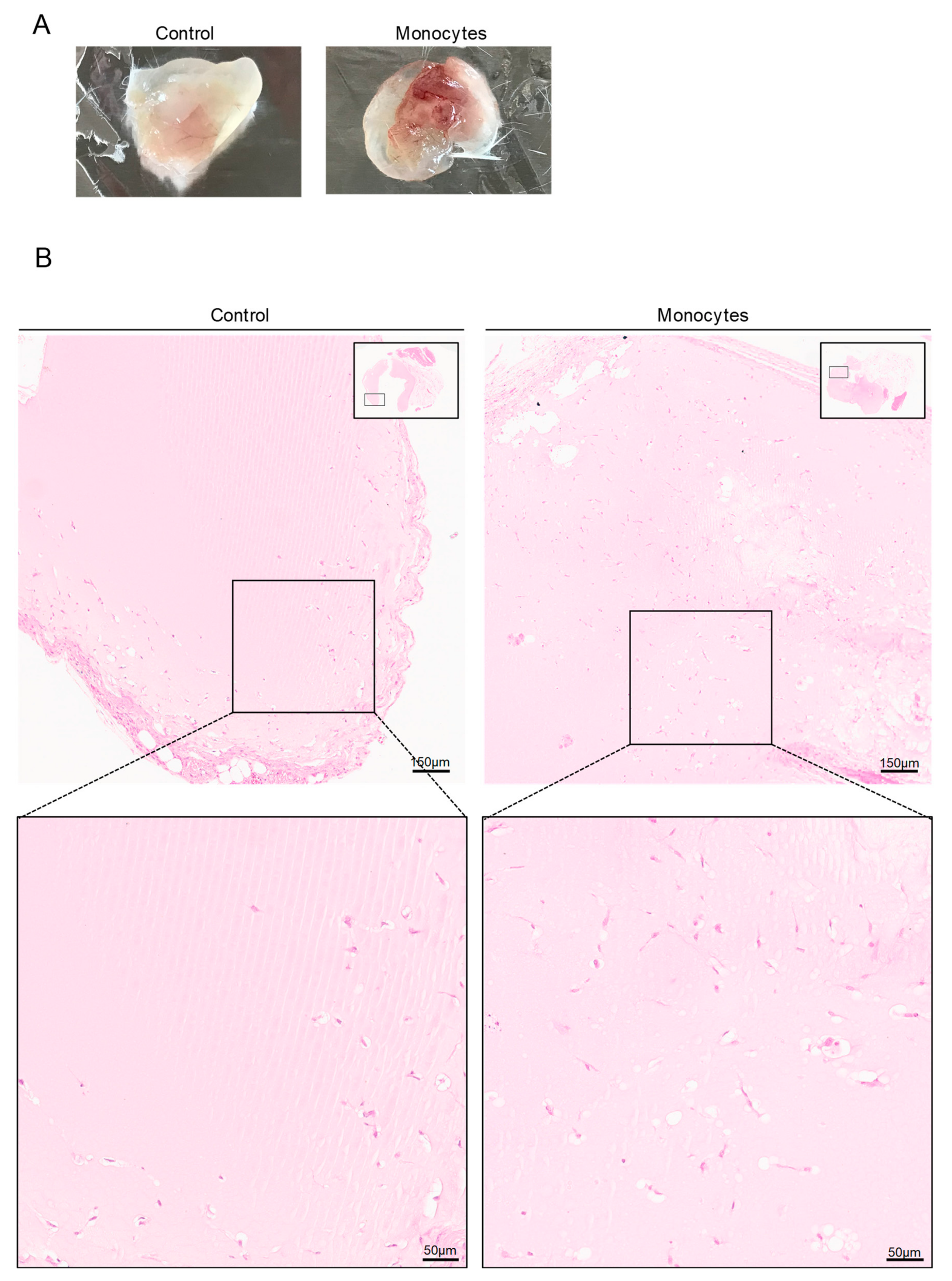
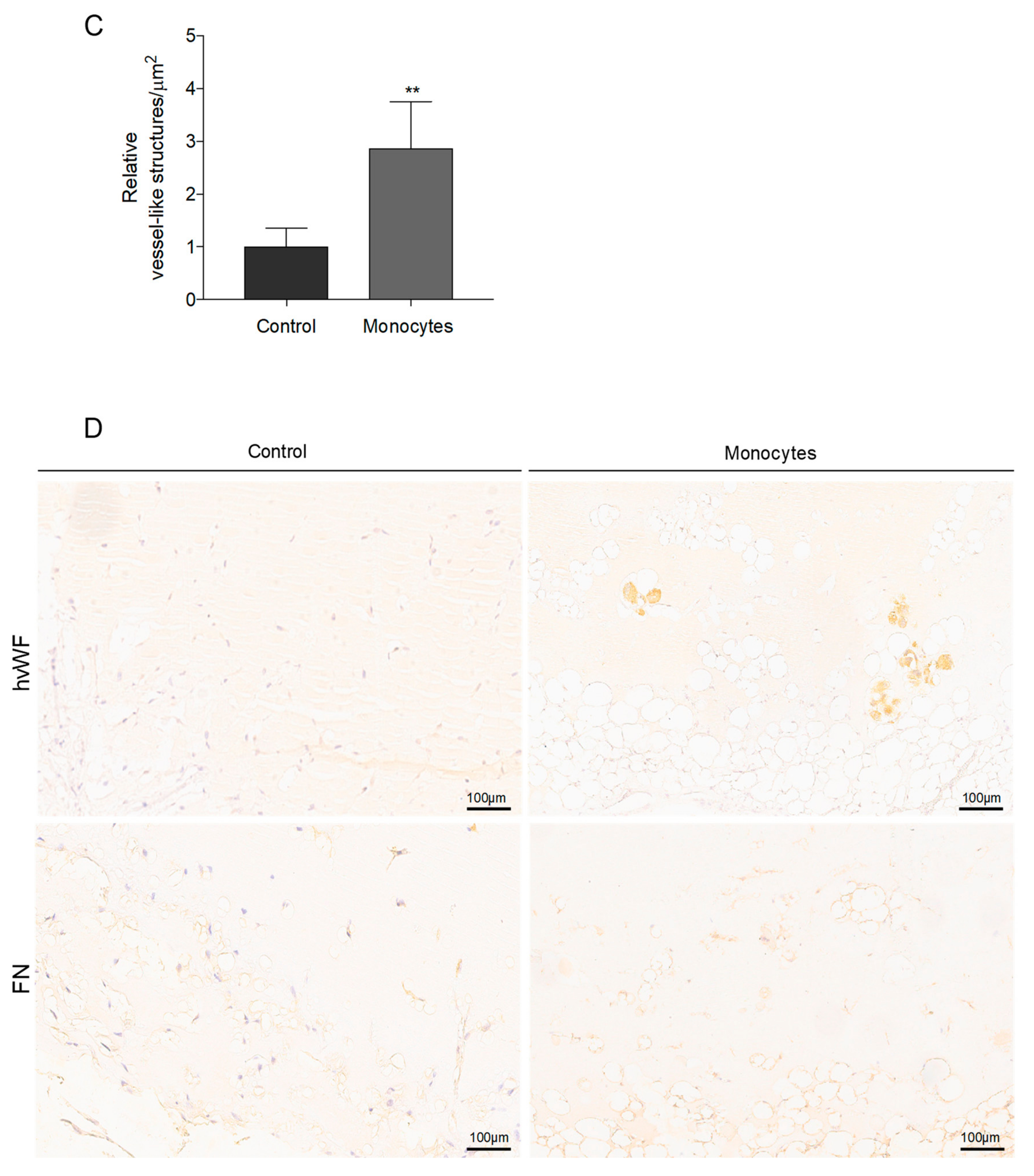
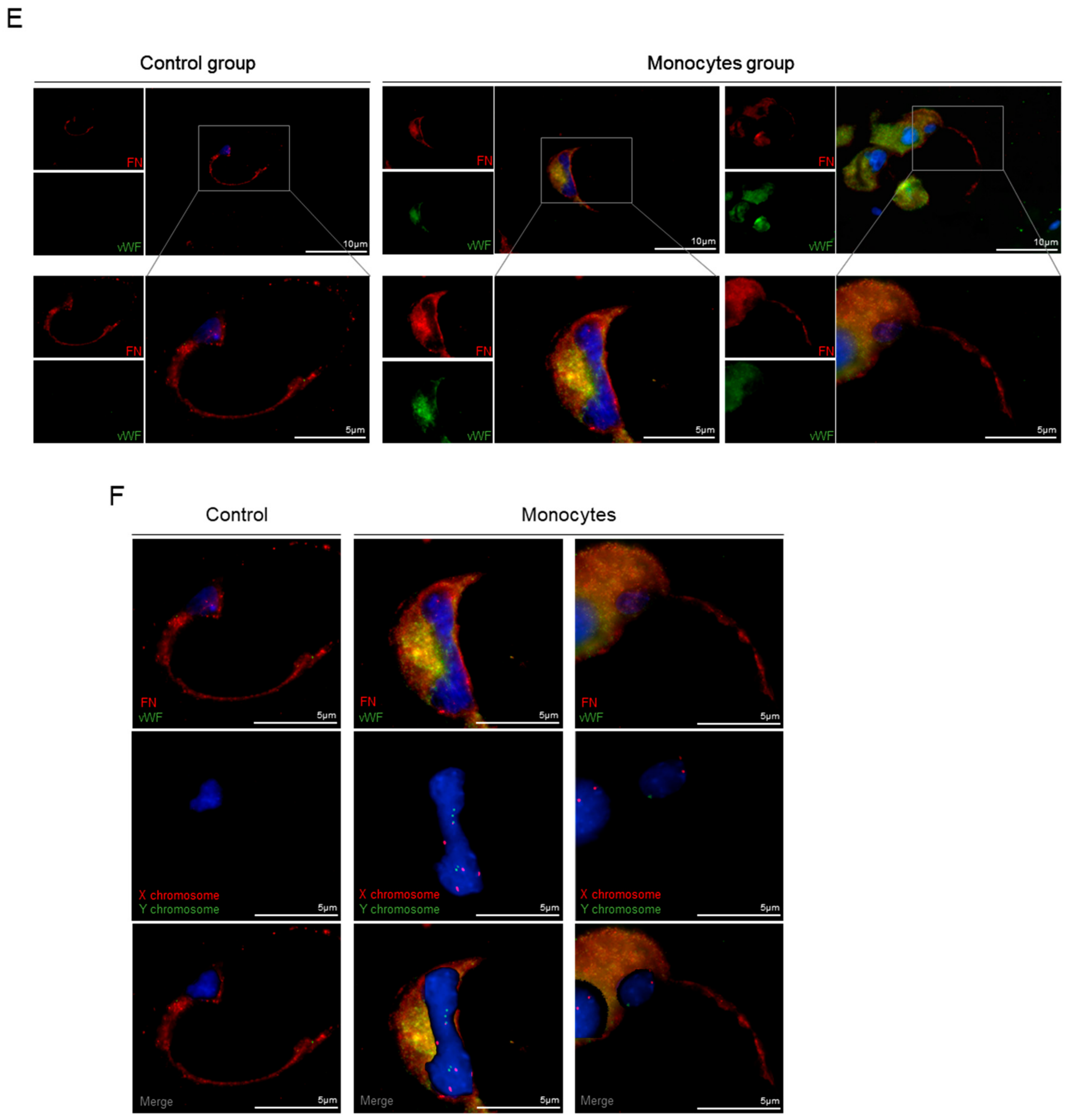
3.3. In Vivo, Monocytes Differentiate into ECs and Are Incorporated into Blood Vessels
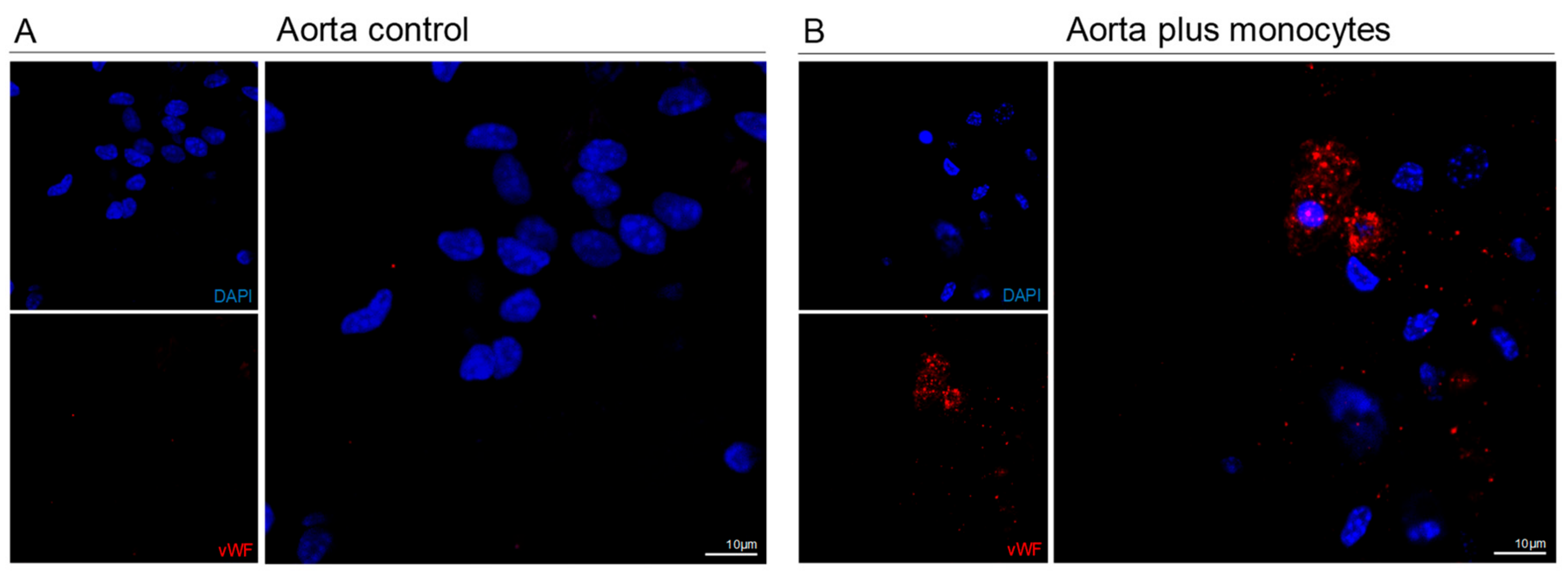
3.4. Monocytes Are Able to Repair Injured Aortas
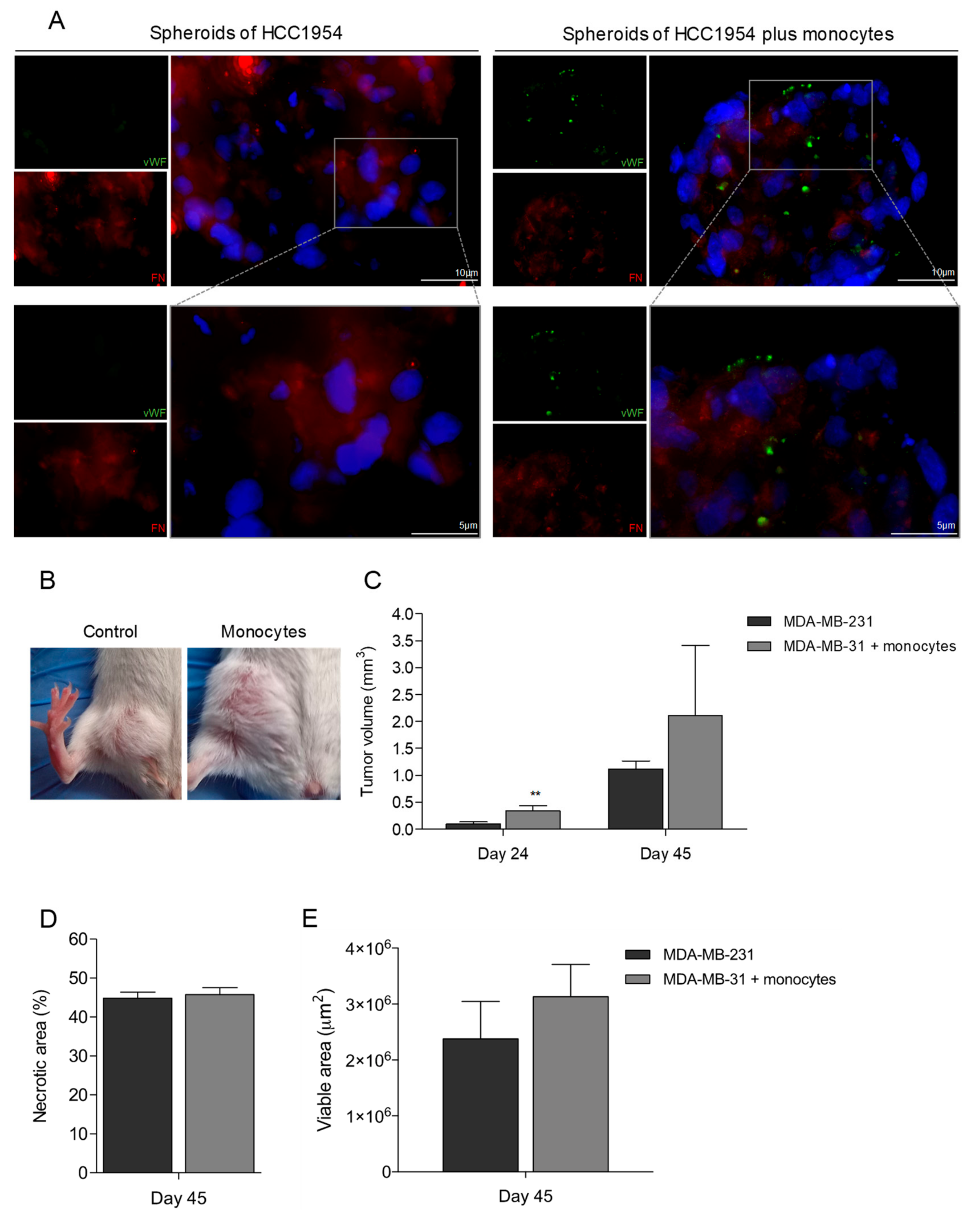
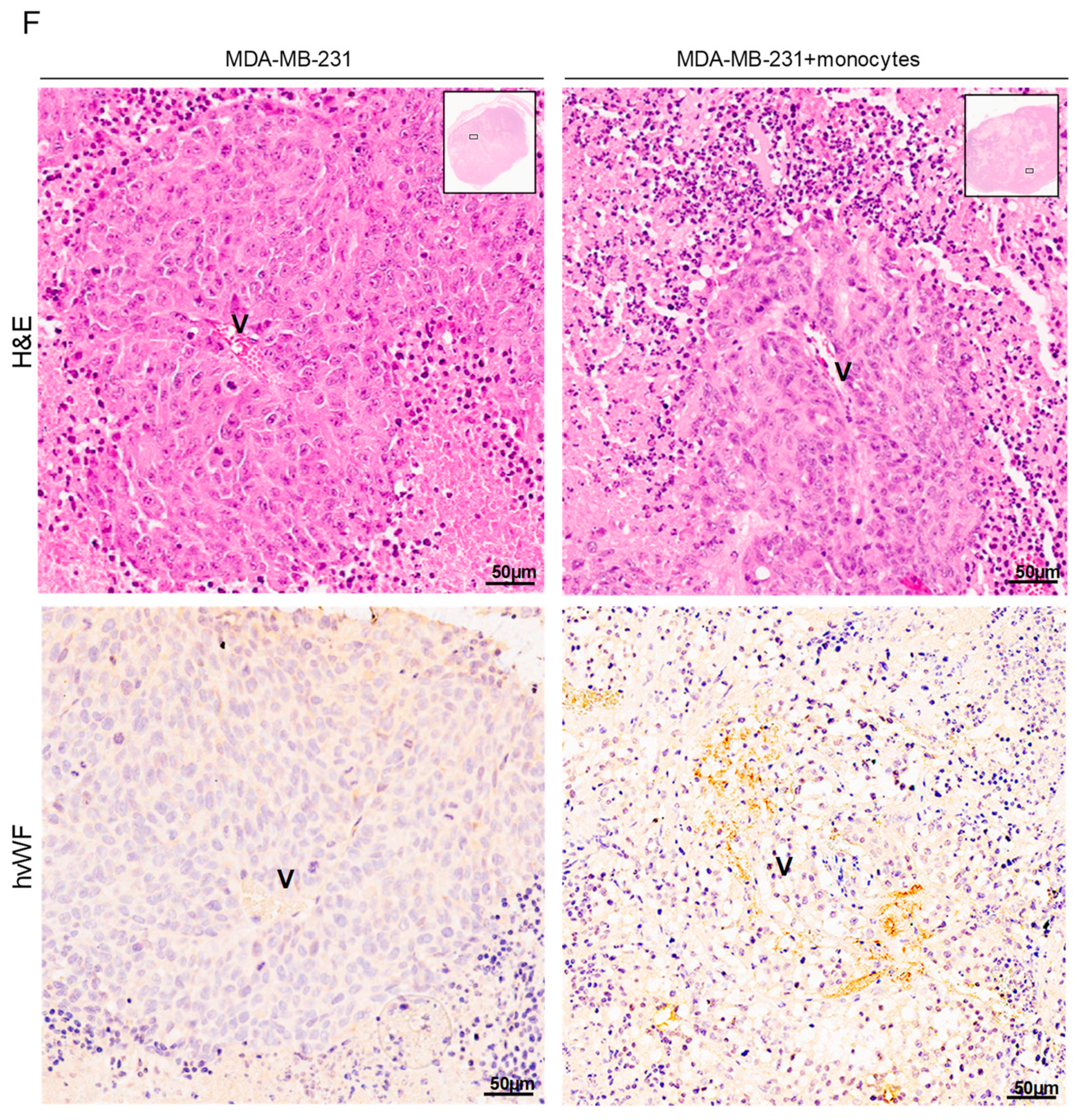
3.5. Monocytes Are Active Stakeholders in Neo-Vasculature Network Formation during Tumor Development
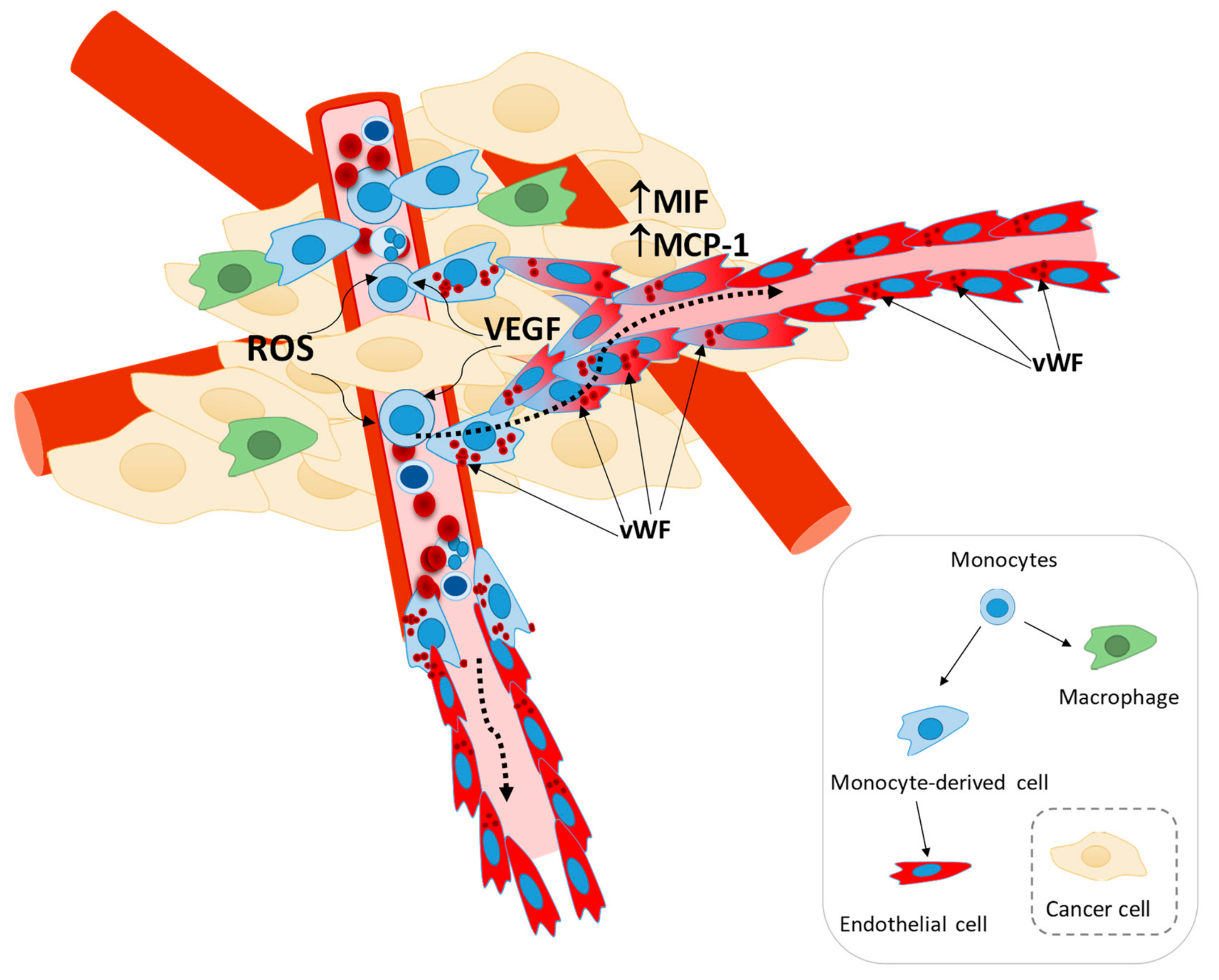
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour angiogenesis and angiogenic inhibitors: A review. J. Clin. Diagn. Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef] [PubMed]
- Marmé, D. Tumor Angiogenesis: A Key Target for Cancer Therapy. Oncol. Res. Treat. 2018, 41, 164. [Google Scholar] [CrossRef] [PubMed]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yang, J.; Sun, S.; Zhang, R.; Xu, Y. Endothelial Progenitor Cells’ Classification and Application in Neurological Diseases. Tissue Eng. Regen. Med. 2017, 14, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.R.; Yoder, M.C. Endothelial progenitor cells: Quo Vadis? J. Mol. Cell. Cardiol. 2011, 50, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Del Papa, N.; Quirici, N.; Soligo, D.; Scavullo, C.; Cortiana, M.; Borsotti, C.; Maglione, W.; Comina, D.P.; Vitali, C.; Fraticelli, P.; et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006, 54, 2605–2615. [Google Scholar] [CrossRef]
- Allanore, Y.; Batteux, F.; Avouac, J.; Assous, N.; Weill, B.; Kahan, A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin. Exp. Rheumatol. 2007, 25, 60–66. [Google Scholar]
- Avouac, J.; Juin, F.; Wipff, J.; Couraud, P.O.; Chiocchia, G.; Kahan, A.; Boileau, C.; Uzan, G.; Allanore, Y. Circulating endothelial progenitor cells in systemic sclerosis: Association with disease severity. Ann. Rheum. Dis. 2008, 67, 1455–1460. [Google Scholar] [CrossRef]
- Nevskaya, T.; Bykovskaia, S.; Lyssuk, E.; Shakhov, I.; Zaprjagaeva, M.; Mach, E.; Ananieva, L.; Guseva, N.; Nassonov, E. Circulating endothelial progenitor cells in systemic sclerosis: Relation to impaired angiogenesis and cardiovascular manifestations. Clin. Exp. Rheumatol. 2008, 26, 21–29. [Google Scholar]
- Plein, A.; Fantin, A.; Denti, L.; Pollard, J.W.; Ruhrberg, C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 2018, 562, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Heeschen, C.; Aicher, A.; Dernbach, E.; Zeiher, A.M.; Dimmeler, S. Relevance of Monocytic Features for Neovascularization Capacity of Circulating Endothelial Progenitor Cells. Circulation 2003, 108, 2511–2516. [Google Scholar] [CrossRef] [PubMed]
- Nucera, S.; Biziato, D.; de Palma, M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; De Palma, M.; Naldini, L. Tie2-expressing monocytes and tumor angiogenesis: Regulation by hypoxia and angiopoietin-2. Cancer Res. 2007, 67, 8429–8432. [Google Scholar] [CrossRef] [PubMed]
- Mazzieri, R.; Pucci, F.; Moi, D.; Zonari, E.; Ranghetti, A.; Berti, A.; Politi, L.S.; Gentner, B.; Brown, J.L.; Naldini, L.; et al. Targeting the ANG2/TIE2 Axis Inhibits Tumor Growth and Metastasis by Impairing Angiogenesis and Disabling Rebounds of Proangiogenic Myeloid Cells. Cancer Cell 2011, 19, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Lucas, T.; Van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Christofori, G. Corrupt policemen: Inflammatory cells promote tumor angiogenesis. Curr. Opin. Oncol. 2009, 21, 60–70. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation-and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44, 94–112. [Google Scholar] [CrossRef]
- Schmeisser, A.; Garlichs, C.D.; Zhang, H.; Eskafi, S.; Graffy, C.; Ludwig, J.; Strasser, R.H.; Daniel, W.G. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel® under angiogenic conditions. Cardiovasc. Res. 2001, 49, 671–680. [Google Scholar] [CrossRef]
- Yoder, M.C.; Mead, L.E.; Prater, D.; Krier, T.R.; Mroueh, K.N.; Li, F.; Krasich, R.; Temm, C.J.; Prchal, J.T.; Ingram, D.A. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 2007, 109, 1801–1809. [Google Scholar] [CrossRef]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Domingues, G.; Fernandes, S.; Salgado, D.; Nunes, S.; Pereira, S.; Coelho, F.; Silva, F.; Félix, A.; Serpa, J. Monocytes/Macrophages in Cancer, from Tumor Aggressors to Vascular Components—A new Insight for Anti-Angiogenic Therapy. In Proceedings of the EACR-AACR-SIC Special Conference on Anticancer Drug Action and Drug Resistance from Cancer Biology to the Clinic, Florence, Italy, 20–23 June 2015; pp. 98–99. [Google Scholar]
- Cai, H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms, and consequences. Cardiovasc. Res. 2005, 68, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Bretón-Romero, R.; Lamas, S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014, 2, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.H.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells exposed to physiologic shear stress. ASAIO J. 2007, 53, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Godo, S.; Saito, H.; Enkhjargal, B.; Shimokawa, H. Dual roles of vascular-derived reactive oxygen species-With a special reference to hydrogen peroxide and cyclophilin A-. J. Mol. Cell. Cardiol. 2014, 73, 50–56. [Google Scholar] [CrossRef]
- Park, W.H. The effects of exogenous H2O2 on cell death, reactive oxygen species and glutathione levels in calf pulmonary artery and human umbilical vein endothelial cells. Int. J. Mol. Med. 2013, 31, 471–476. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, X.; Cui, Y.; Zhang, J.; Liu, L.; Xie, X.; Hao, H.; He, G.; Kander, M.C.; Chen, M.; et al. Hydrogen peroxide inhibits proliferation and endothelial differentiation of bone marrow stem cells partially via reactive oxygen species generation. Life Sci. 2014, 112, 33–40. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ye, Z.; Jang, Y.Y. Roles of Reactive Oxygen Species in the Fate of Stem Cells. Antioxid. Redox Signal. 2014, 20, 1881–1890. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.Q.; Cao, Q.; Zhang, J.J.; Huang, L.Y.; Sang, T.T.; Liu, F.; Chen, S.Y. Hydrogen peroxide induced impairment of endothelial progenitor cell viability is mediated through a FoxO3a dependant mechanism. Microvasc. Res. 2013, 90, 48–54. [Google Scholar] [CrossRef]
- Ji, A.R.; Ku, S.Y.; Cho, M.S.; Kim, Y.Y.; Kim, Y.J.; Oh, S.K.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. Reactive oxygen species enhance differentiation of human embryonic stem cells into mesendodermal lineage. Exp. Mol. Med. 2010, 42, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Archidiacono, N.; Antonacci, R.; Marzella, R.; Finelli, P.; Lonoce, A.; Rocchi, M. Comparative mapping of human alphoid sequences in great apes using fluorescence in situ hybridization. Genomics 1995, 25, 477–484. [Google Scholar] [CrossRef]
- Cooke, H.J.; Schmidtke, J.; Gosden, J.R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma 1982, 87, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Fonseca, I.; Roque, L.; Pereira, T.; Ribeiro, C.; Bullerdiek, J.; Soares, J. PLAG1 gene alterations in salivary gland pleomorphic adenoma and carcinoma ex-pleomorphic adenoma: A combined study using chromosome banding, in situ hybridization and immunocytochemistry. Mod. Pathol. 2005, 18, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, G. Aldehyde Dehydrogenases: Not Just Markers, but Functional Regulators of Stem Cells. Stem Cells Int. 2019, 2019, 3904645. [Google Scholar] [CrossRef] [PubMed]
- Blix, E.S.; Kildal, A.B.; Bertelsen, E.; Waage, A.; Myklebust, J.H.; Kolstad, A.; Husebekk, A. Content of endothelial progenitor cells in autologous stem cellgrafts predict survival after transplantation for multiplemyeloma. Biol. Blood Marrow Transplant. 2015, 21, 840–847. [Google Scholar] [CrossRef][Green Version]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde Dehydrogenase Inhibitors: A Comprehensive Review of the Pharmacology, Mechanism of Action, Substrate Specificity, and Clinical Application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef]
- Kolachala, V.L.; Bajaj, R.; Wang, L.; Yan, Y.; Ritzenthaler, J.D.; Gewirtz, A.T.; Roman, J.; Merlin, D.; Sitaraman, S.V. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J. Biol. Chem. 2007, 282, 32965–32973. [Google Scholar] [CrossRef]
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS ONE 2016, 11, e0147600. [Google Scholar] [CrossRef]
- Cai, J.; Wei, J.; Li, S.; Suber, T.; Zhao, J. AM966, an Antagonist of Lysophosphatidic Acid Receptor 1, Increases Lung Microvascular Endothelial Permeability through Activation of Rho Signaling Pathway and Phosphorylation of VE-Cadherin. Mediat. Inflamm. 2017, 2017, 6893560. [Google Scholar] [CrossRef]
- Nogueras, S.; Merino, A.; Ojeda, R.; Carracedo, J.; Rodriguez, M.; Martin-Malo, A.; Ramírez, R.; Aljama, P. Coupling of endothelial injury and repair: An analysis using an in vivo experimental model. Am. J. Physiol 2008, 294, H708–H713. [Google Scholar] [CrossRef][Green Version]
- Teo, S.T.; Yung, Y.C.; Herr, D.R.; Chun, J. Lysophosphatidic acid in vascular development and disease. IUBMB Life 2009, 61, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.P.; Pinto, C.; Martins, T.R.; Harrer, N.; Estrada, M.F.; Loza-Alvarez, P.; Cabeçadas, J.; Alves, P.M.; Gualda, E.J.; Sommergruber, W.; et al. 3D-3-culture: A tool to unveil macrophage plasticity in the tumour microenvironment. Biomaterials 2018, 163, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wu, P.; Chen, J.; Guo, Y.; Wang, J.; Li, X.; Fang, Z. ANGPTL3 possibly promotes cardiac angiogenesis through improving proangiogenic ability of endothelial progenitor cells after myocardial infarction. Lipids Health Dis. 2018, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, F.S.; Correia, M.; Muccillo, F.; Souza Silva, C.G.E.; De Lorenzo, A. Associations between endothelial progenitor cells, clinical characteristics and coronary restenosis in patients undergoing percutaneous coronary artery intervention. BMC Res. Notes 2018, 11, 278. [Google Scholar] [CrossRef]
- Haider, K.H.; Aziz, S.; Al-Reshidi, M.A. Endothelial progenitor cells for cellular angiogenesis and repair: Lessons learned from experimental animal models. Regen. Med. 2017, 12, 969–982. [Google Scholar] [CrossRef]
- Pelosi, E.; Castelli, G.; Testa, U. Endothelial progenitors. Blood Cells Mol. Dis. 2014, 52, 186–194. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Circulating and Tissue Resident Endothelial Progenitor Cells. J. Cell. Physiol 2014, 229, 10–16. [Google Scholar] [CrossRef]
- Curry, C.V. Differential Blood Count: Reference Range, Interpretation, Collection and Panels; Medscape: Boston, MA, USA, 2016. [Google Scholar]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Tsutsumi, Y.; Ozono, R.; Masaki, H.; Mori, Y.; Iba, O.; Tateishi, E.; et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001, 104, 1046–1052. [Google Scholar] [CrossRef]
- Karcher, J.R.; Greene, A.S. Bone marrow mononuclear cell angiogenic competency is suppressed by a high-salt diet. Am. J. Physiol. Cell Physiol. 2013, 306, C123–C131. [Google Scholar] [CrossRef]
- Van Huyen, J.P.D.; Smadja, D.M.; Bruneval, P.; Gaussem, P.; Dal-Cortivo, L.; Julia, P.; Fiessinger, J.N.; Cavazzana-Calvo, M.; Aiach, M.; Emmerich, J. Bone marrow-derived mononuclear cell therapy induces distal angiogenesis after local injection in critical leg ischemia. Mod. Pathol. 2008, 21, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ciarrocchi, A.; Mellick, A.S.; Jaggi, J.S.; Bambino, K.; Gupta, S.; Heikamp, E.; McDevitt, M.R.; Scheinberg, D.A.; Benezra, R.; et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007, 21, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Barnett, F.H.; Rosenfeld, M.; Wood, M.; Kiosses, W.B.; Usui, Y.; Marchetti, V.; Aguilar, E.; Friedlander, M. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 2016, 11, 36659. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, M.J.; Buendía, P.; Velasco, F.; Aguirre, M.A.; Barbarroja, N.; Torres, L.A.; Khamashta, M.; López-Pedrera, C. Vascular endothelial growth factor expression in monocytes from patients with primary antiphospholipid syndrome. J. Thromb. Haemost. 2006, 4, 2461–2469. [Google Scholar] [CrossRef]
- Barleon, B.; Sozzani, S.; Zhou, D.; Weich, H.A.; Mantovani, A.; Marmé, D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996, 87, 3336–3343. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and antioxidants during ischemia-reperfusion injuries in cardiac and skeletal muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid. Med. Cell. Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef]
- Kumari, S.; Badana, A.K.; Murali Mohan, G.; Shailender, G.; Malla, R.R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights 2018, 13, 1177271918755391. [Google Scholar] [CrossRef]
- Pashkovskaia, N.; Gey, U.; Rödel, G. Mitochondrial ROS direct the differentiation of murine pluripotent P19 cells. Stem Cell Res. 2018, 30, 180–191. [Google Scholar] [CrossRef]
- McKenney, J.K.; Weiss, S.W.; Folpe, A.L. CD31 expression in intratumoral macrophages: A potential diagnostic pitfall. Am. J. Surg. Pathol. 2001, 25, 1167–1173. [Google Scholar] [CrossRef]
- Castaman, G.; Giacomelli, S.H.; Jacobi, P.M.; Obser, T.; Budde, U.; Rodeghiero, F.; Schneppenheim, R.; Haberichter, S.L. Reduced von Willebrand factor secretion is associated with loss of Weibel-Palade body formation. J. Thromb. Haemost. 2012, 10, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Eggermann, J.; Kliche, S.; Jarmy, G.; Hoffmann, K.; Mayr-Beyrle, U.; Debatin, K.M.; Waltenberger, J.; Beltinger, C. Endothelial progenitor cell culture and differentiation in vitro: A methodological comparison using human umbilical cord blood. Cardiovasc. Res. 2003, 58, 478–486. [Google Scholar] [CrossRef][Green Version]
- Chu, H.; Sun, Y.; Gao, Y.; Guan, X.; Yan, H.; Cui, X.; Zhang, X.; Li, X.; Li, H.; Cheng, M. Function of Krüppel like factor 2 in the shear stress induced cell differentiation of endothelial progenitor cells to endothelial cells. Mol. Med. Rep. 2019, 19, 1739–1746. [Google Scholar] [PubMed]
- Ge, Q.; Zhang, H.; Hou, J.; Wan, L.; Cheng, W.; Wang, X.; Dong, D.; Chen, C.; Xia, J.; Guo, J.; et al. VEGF secreted by Mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol. Med. Rep. 2018, 17, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Lopes-Coelho, F.; Gouveia-Fernandes, S.; Ramos, C.; Pereira, S.A.; Serpa, J. Cysteine boosters the evolutionary adaptation to CoCl2mimicked hypoxia conditions, favouring carboplatin resistance in ovarian cancer. BMC Evol. Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Ramos, C.; Lopes-Coelho, F.; Sequeira, C.O.; Silva, F.; Gouveia-Fernandes, S.; Rodrigues, A.; Guimarães, A.; Silveira, M.; Abreu, S.; et al. Cysteine allows ovarian cancer cells to adapt to hypoxia and to escape from carboplatin cytotoxicity. Sci. Rep. 2018, 8, 9513. [Google Scholar] [CrossRef]
- Nagano, M.; Yamashita, T.; Hamada, H.; Ohneda, K.; Kimura, K.I.; Nakagawa, T.; Shibuya, M.; Yoshikawa, H.; Ohneda, O. Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood 2007, 110, 151–160. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interface Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed]
- Kasama, T.; Ohtsuka, K.; Sato, M.; Takahashi, R.; Wakabayashi, K.; Kobayashi, K. Macrophage Migration Inhibitory Factor: A Multifunctional Cytokine in Rheumatic Diseases. Arthritis 2010, 20, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Fan, L.X.; Yao, Y.; Swenson-Fields, K.I.; Gadjeva, M.; Wallace, D.P.; Peters, D.J.M.; Yu, A.; Grantham, J.J.; et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease. J. Clin. Investig. 2015, 125, 2399–2412. [Google Scholar] [CrossRef]
- Nishihira, J. Macrophage migration inhibitory factor (MIF): Its essential role in the immune system and cell growth. J. Interface Cytokine Res. 2000, 20, 751–762. [Google Scholar] [CrossRef]
- Simpson, K.D.; Templeton, D.J.; Cross, J.V. Macrophage Migration Inhibitory Factor Promotes Tumor Growth and Metastasis by Inducing Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. J. Immunol. 2012, 189, 5533–5540. [Google Scholar] [CrossRef]
- Wang, S.S.; Cen, X.; Liang, X.H.; Tang, Y.L. Macrophage migration inhibitory factor: A potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget 2017, 8, 10650–10661. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene 2017, 36, 2095–2104. [Google Scholar] [CrossRef]
- He, L.J.; Xie, D.; Hu, P.J.; Liao, Y.J.; Deng, H.X.; Kung, H.F.; Zhu, S.L. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J. Gastroenterol. 2015, 21, 9916–9926. [Google Scholar] [CrossRef]
- Mangano, K.; Mazzon, E.; Basile, M.S.; Marco, R.D.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951–17970. [Google Scholar] [CrossRef]
- Girard, E.; Strathdee, C.; Trueblood, E.; Quéva, C. Macrophage migration inhibitory factor produced by the tumour stroma but not by tumour cells regulates angiogenesis in the B16-F10 melanoma model. Br. J. Cancer 2012, 107, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, F.; Wang, Y.; Liu, J.; Ming, X.; Hou, J.; Lv, B.; Fang, S.; Yu, B. Macrophage migration inhibitory factor promotes cardiac stem cell proliferation and endothelial differentiation through the activation of the PI3K/Akt/mTOR and AMPK pathways. Int. J. Mol. Med. 2016, 37, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Schinagl, A.; Thiele, M.; Douillard, P.; Völkel, D.; Kenner, L.; Kazemi, Z.; Freissmuth, M.; Scheiflinger, F.; Kerschbaumer, R.J. Oxidized macrophage migration inhibitory factor is a potential new tissue marker and drug target in cancer. Oncotarget 2016, 7, 73486. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Patel, M.; Sachdev, J.; Hart, L.; Halama, N.; Ramanathan, R.; Sarantopoulos, J.; Liu, X.; Yazji, S.; Jäger, D.; et al. Safety and efficacy analysis of imalumab, an anti-oxidized macrophage migration inhibitory factor (oxMIF) antibody, alone or in combination with 5-fluorouracil/leucovorin (5-FU/LV) or panitumumab, in patients with metastatic colorectal cancer (mCRC). Ann. Oncol. 2016, 27, ii105. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Eubank, T.D.; Galloway, M.; Montague, C.M.; Waldman, W.J.; Marsh, C.B. M-CSF Induces Vascular Endothelial Growth Factor Production and Angiogenic Activity From Human Monocytes. J. Immunol. 2003, 171, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.N.; Youkey, R.; Liu, X.; Jia, L.; Blatt, R.; Day, Y.J.; Sullivan, G.W.; Linden, J.; Tucker, A.L. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ. Res. 2007, 171, 2637–2643. [Google Scholar]
- Xu, J.; Shi, G.P. Vascular wall extracellular matrix proteins and vascular diseases. Biochim. Biophys. Acta 2014, 1842, 2106–2119. [Google Scholar] [CrossRef]
- Malinda, K.M. In vivo Matrigel Migration and Angiogenesis Assay. In Methods in Molecular Biology; Springer: Clifton, NJ, USA, 2009; pp. 287–294. [Google Scholar]
- Qin, M.; Guan, X.; Zhang, Y.; Shen, B.; Liu, F.; Zhang, Q.; Ma, Y.; Jiang, Y. Evaluation of ex vivo produced endothelial progenitor cells for autologous transplantation in primates. Stem Cell Res. Ther. 2018, 9, 14. [Google Scholar] [CrossRef]
- Kong, J.; Wang, F.; Zhang, J.; Cui, Y.; Pan, L.; Zhang, W.; Wen, J.; Liu, P. Exosomes of endothelial progenitor cells inhibit neointima formation after carotid artery injury. J. Surg. Res. 2018, 232, 398–407. [Google Scholar] [CrossRef]
- Zhao, W.N.; Xu, S.Q.; Liang, J.F.; Peng, L.; Liu, H.L.; Wang, Z.; Fang, Q.; Wang, M.; Yin, W.Q.; Zhang, W.J.; et al. Endothelial progenitor cells from human fetal aorta cure diabetic foot in a rat model. Metabolism 2016, 65, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Gao, X.P.; Zhao, Y.D.; Mirza, M.K.; Frey, R.S.; Kalinichenko, V.V.; Wang, I.C.; Costa, R.H.; Malik, A.B. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J. Clin. Investig. 2006, 116, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Karlmark, K.; Tacke, F.; Dunay, I. Monocytes in health and disease—Minireview. Eur. J. Microbiol. Immunol. 2012, 2, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Cortés, M.; Sanchez-Moral, L.; de Barrios, O.; Fernández-Aceñero, M.J.; Martínez-Campanario, M.; Esteve-Codina, A.; Darling, D.S.; Győrffy, B.; Lawrence, T.; Dean, D.C.; et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J. 2017, 36, 3336–33355. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef]
- Lopes-Coelho, F.; Gouveia-Fernandes, S.; Serpa, J. Metabolic cooperation between cancer and non-cancerous stromal cells is pivotal in cancer progression. Tumor Biol. 2018, 40, 1010428318756203. [Google Scholar] [CrossRef]
- Niu, Z.; Shi, Q.; Zhang, W.; Shu, Y.; Yang, N.; Chen, B.; Wang, Q.; Zhao, X.; Chen, J.; Cheng, N.; et al. Caspase-1 cleaves PPARγ for potentiating the pro-tumor action of TAMs. Nat. Commun. 2017, 8, 766. [Google Scholar] [CrossRef]
- Tal, R.; Dong, D.; Shaikh, S.; Mamillapalli, R.; Taylor, H.S. Bone-marrow-derived endothelial progenitor cells contribute to vasculogenesis of pregnant mouse uterus. Biol. Reprod. 2019, 100, 1228–1237. [Google Scholar] [CrossRef]
- Kaur, S.; Sehgal, R.; Shastry, S.M.; McCaughan, G.; McGuire, H.M.; St de Groth, B.F.; Sarin, S.; Trehanpati, N.; Seth, D. Circulating endothelial progenitor cells present an inflammatory phenotype and function in patients with alcoholic liver cirrhosis. Front. Physiol. 2018, 9, 556. [Google Scholar] [CrossRef]
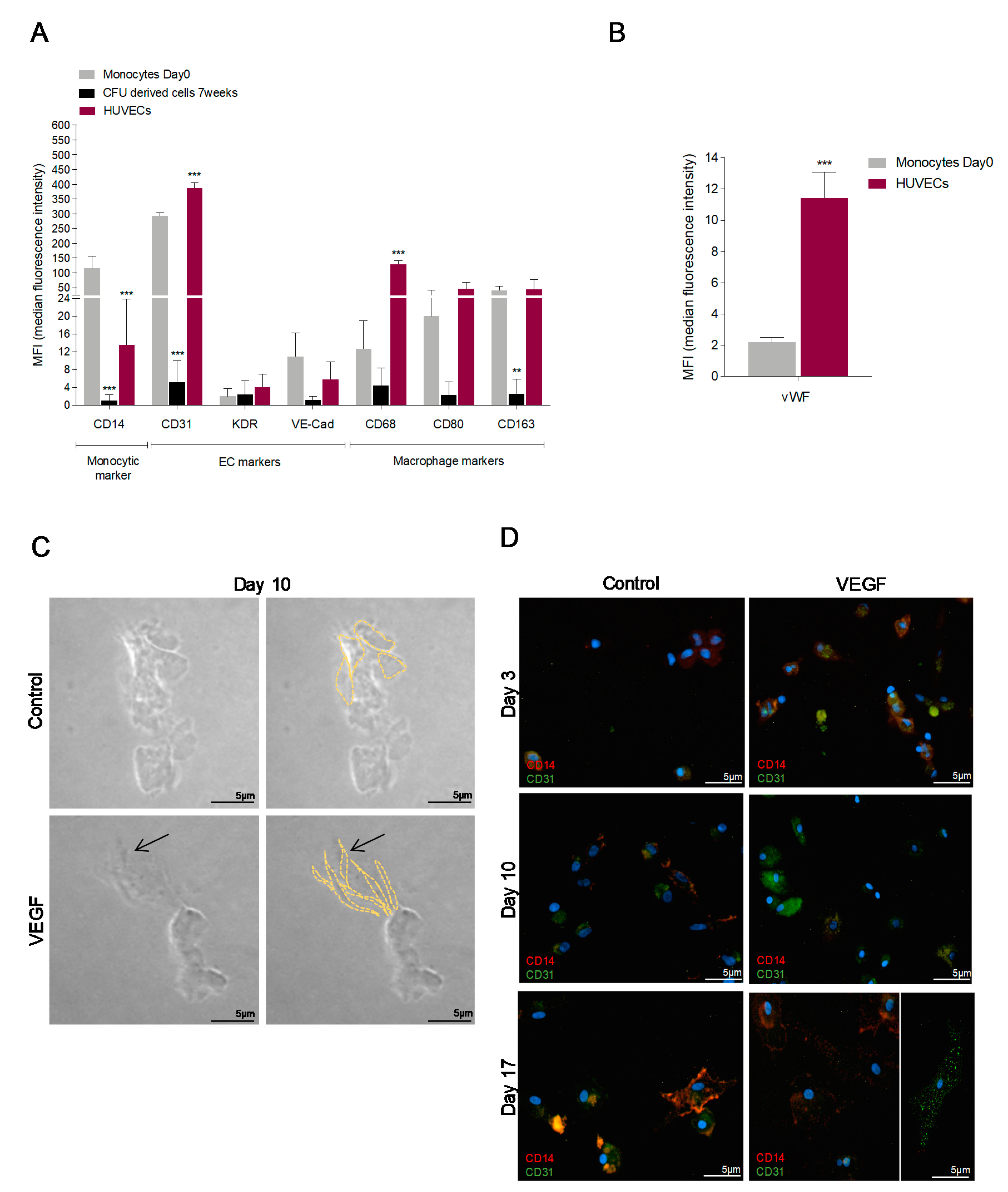
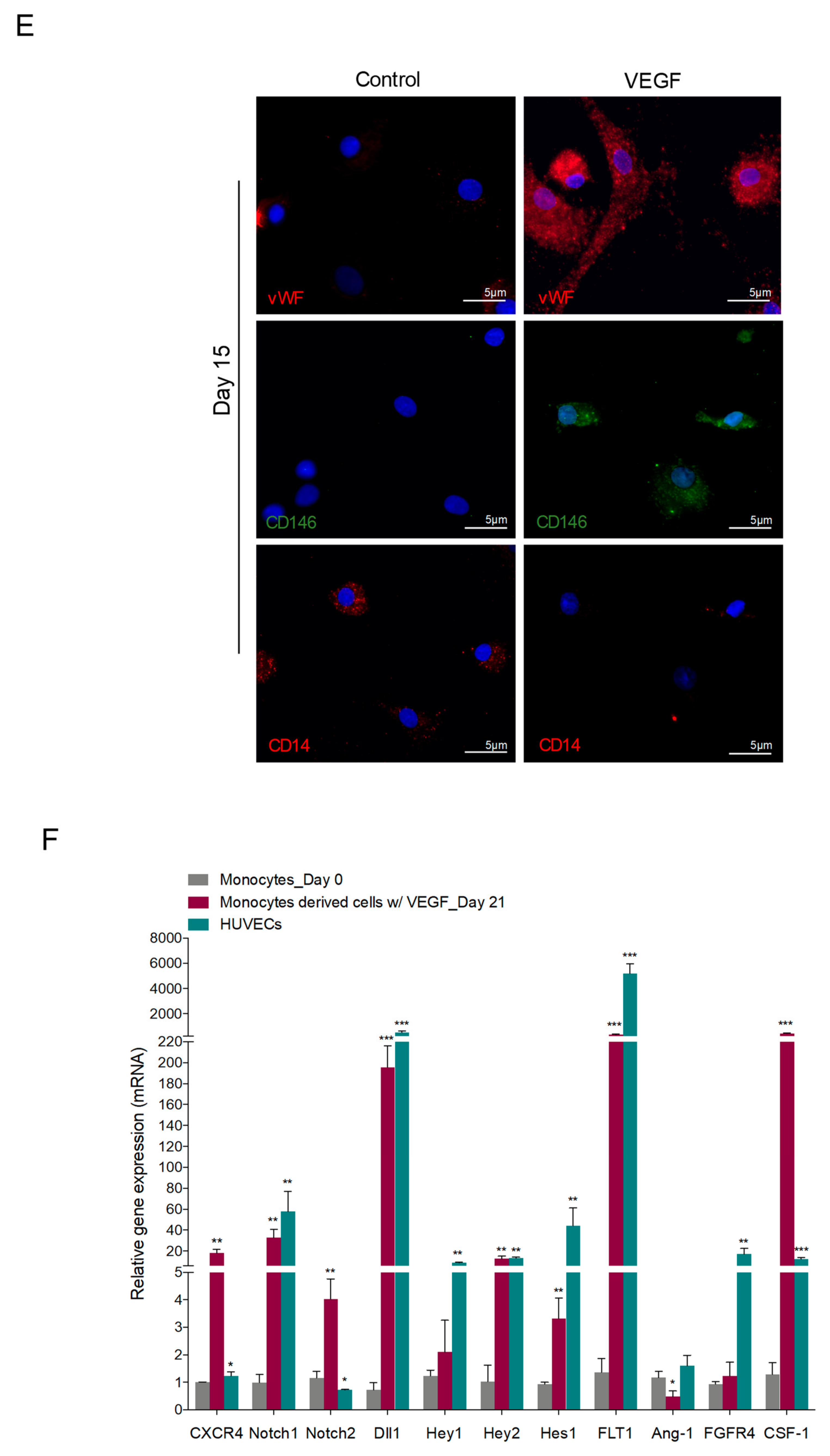
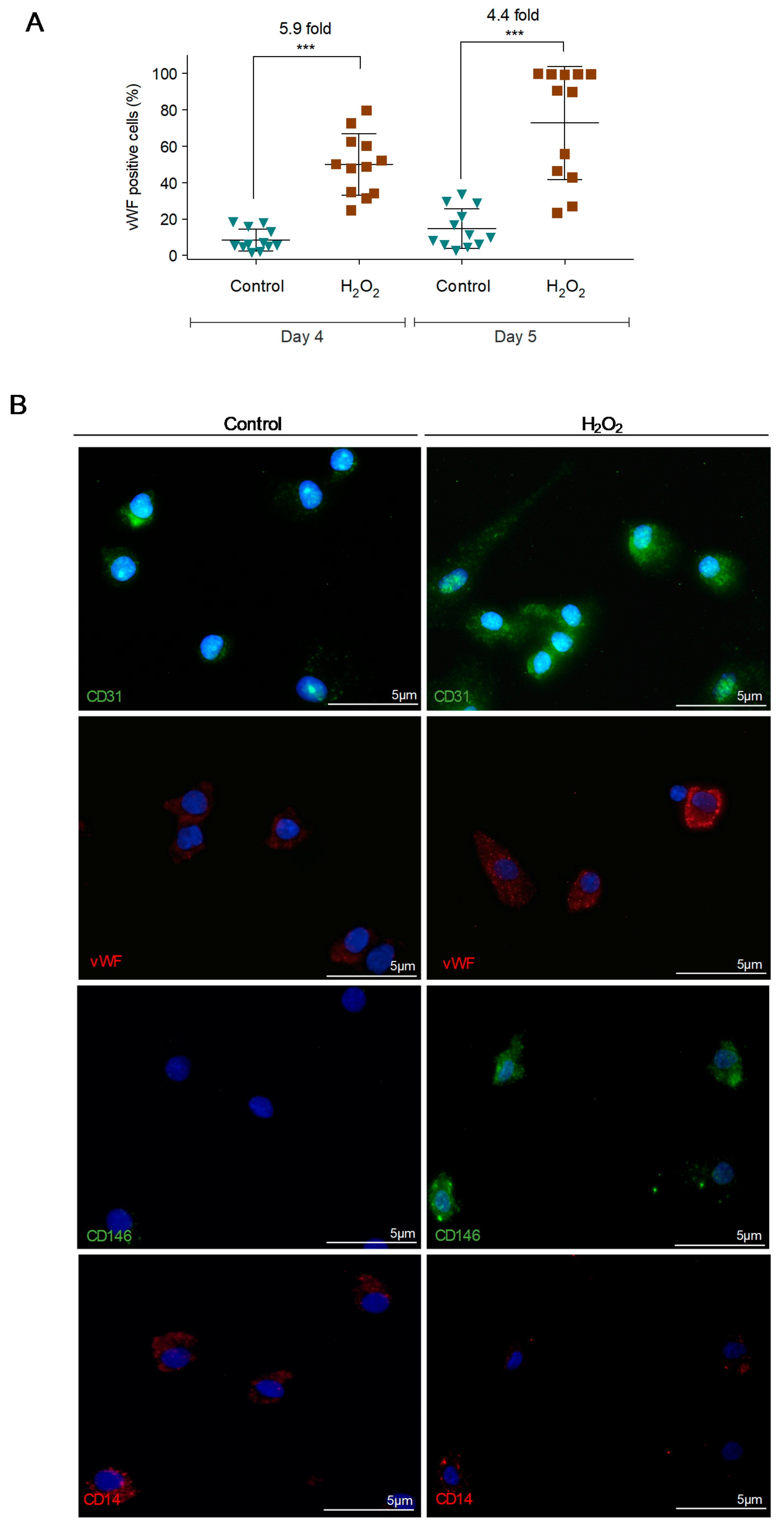
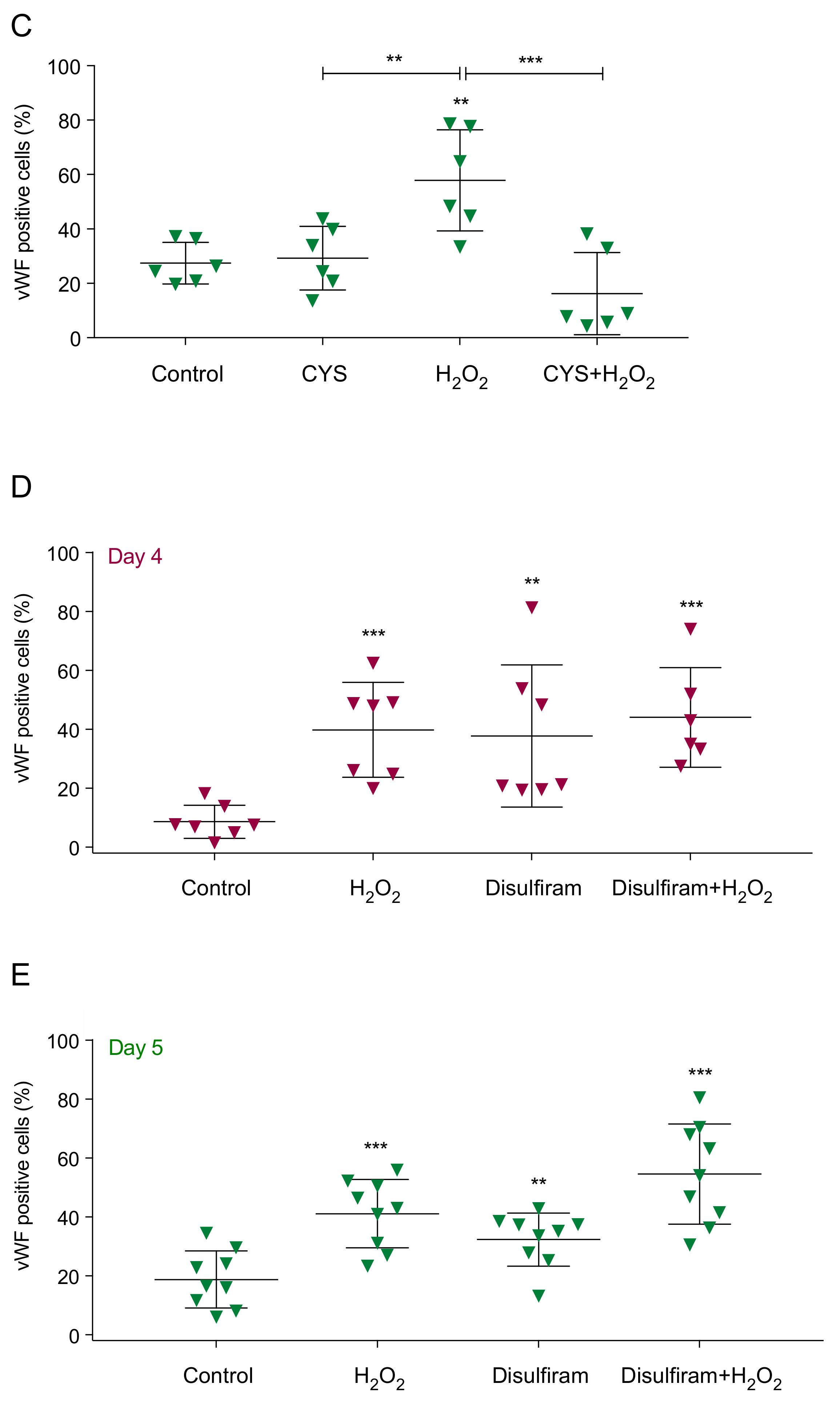
| Primer | Foward | Reverse |
|---|---|---|
| CXCR4 | CTCCAAGCTGTCACACTCCA | TCGATGCTGATCCCAATGTA |
| CSF-1 | GTCTTCCACCTGCTGGTGC | CCCTCTGGTTGCTCCAAGG |
| FLT1 | CACCAAGAGCGACGTGTG | TTTTGGGTCTCTGTGCCAG |
| Hes1 | ACGACACCGGATAAACCAAA | CGGAGGTGCTTCACTGTCAT |
| Ang1 | GGGGAGGTTGGACTGTAATAC | GCATGTACTGCCTCTGACTG |
| Dll1 | ATGCCTTCGGCCACTTCAC | CACATCCAGGCAGGCAGAT |
| Notch1 | TGGCGGGAAGTGTGAAGCGG | GTGCTGAGGCACGGGTTGGG |
| Notch2 | CCACAGGTGTCAGAATGGAG | GGCATTCATCCACATCCTCTG |
| Hey1 | GACGAGACCGGATCAATAACAG | GGTCATCTGCAGGATCTCG |
| Hey2 | GAGCGAGAACAATTACTCGGG | GTTATTTATCCGATCCCGACGC |
| FGFR4 | GATGCTCAAAGACAACGCCTC | GACACCAAGCAGGTTGATGATG |
| 18S | GCCCTATCAACTTTCGATGGT | CCGGAATCGAACCCTGATT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes-Coelho, F.; Silva, F.; Gouveia-Fernandes, S.; Martins, C.; Lopes, N.; Domingues, G.; Brito, C.; Almeida, A.M.; Pereira, S.A.; Serpa, J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells 2020, 9, 107. https://doi.org/10.3390/cells9010107
Lopes-Coelho F, Silva F, Gouveia-Fernandes S, Martins C, Lopes N, Domingues G, Brito C, Almeida AM, Pereira SA, Serpa J. Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells. 2020; 9(1):107. https://doi.org/10.3390/cells9010107
Chicago/Turabian StyleLopes-Coelho, Filipa, Fernanda Silva, Sofia Gouveia-Fernandes, Carmo Martins, Nuno Lopes, Germana Domingues, Catarina Brito, António M Almeida, Sofia A Pereira, and Jacinta Serpa. 2020. "Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis" Cells 9, no. 1: 107. https://doi.org/10.3390/cells9010107
APA StyleLopes-Coelho, F., Silva, F., Gouveia-Fernandes, S., Martins, C., Lopes, N., Domingues, G., Brito, C., Almeida, A. M., Pereira, S. A., & Serpa, J. (2020). Monocytes as Endothelial Progenitor Cells (EPCs), Another Brick in the Wall to Disentangle Tumor Angiogenesis. Cells, 9(1), 107. https://doi.org/10.3390/cells9010107









