Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche
Abstract
1. Introduction
2. Methods
2.1. Mice
2.2. Immunohistochemistry
2.3. Cell Lysis and OPN/trOPN Quantification via ELISA
2.4. Fetal Liver HSC In vitro Proliferation Analysis
2.5. Fetal Liver HSC Homing Analysis
2.6. Cell Cycle Analysis on Fetal HSC and Progenitors
2.7. Expression of α4β1 and α9β1 on Fetal HSC and Progenitors
2.8. Flow Cytometry
2.9. Quantification of Calcium, Magnesium and Manganese using Inductively Coupled Plasma Mass Spectrometry (ICPMS)
2.10. Statistical Analysis and Data Presentation
3. Results
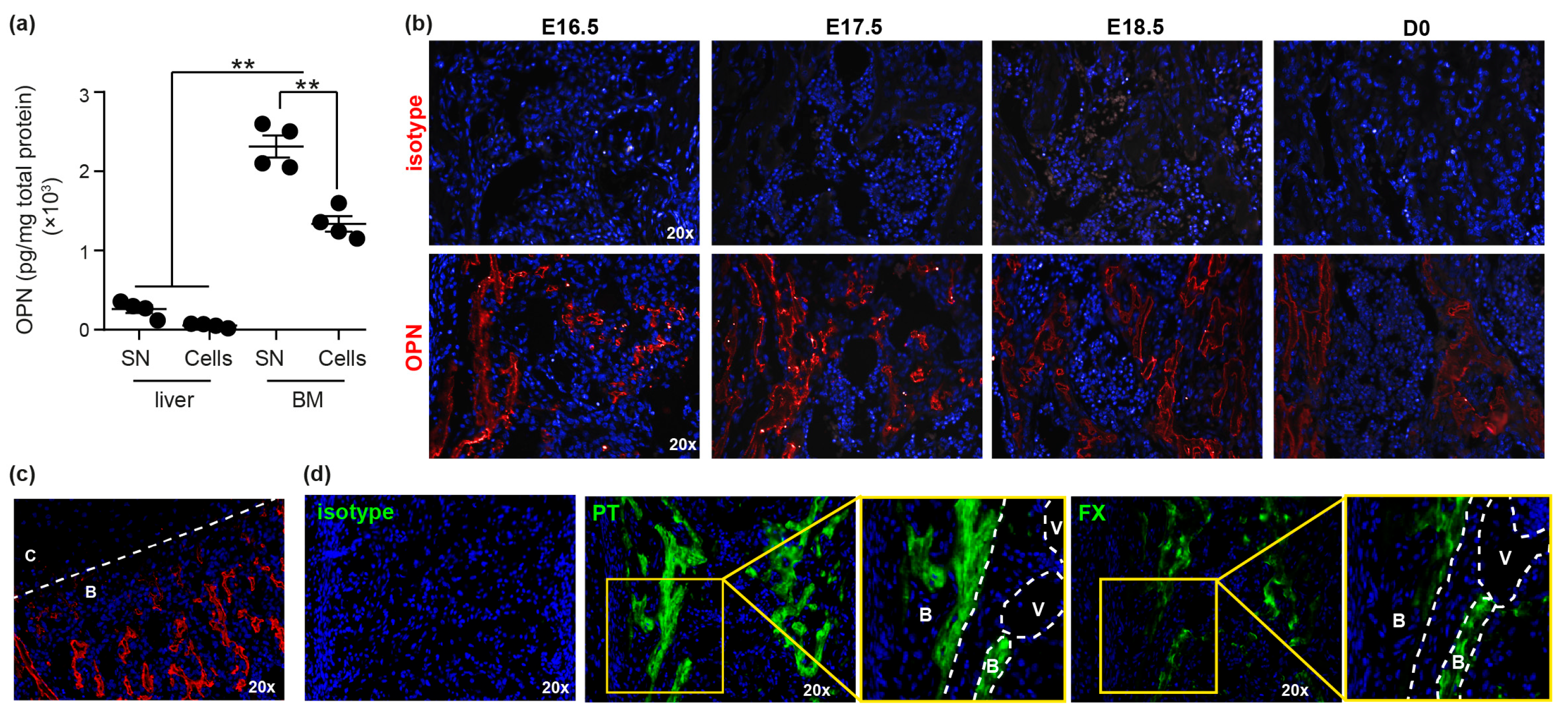
3.1. OPN and Specifically trOPN is Highly Expressed in Fetal BM
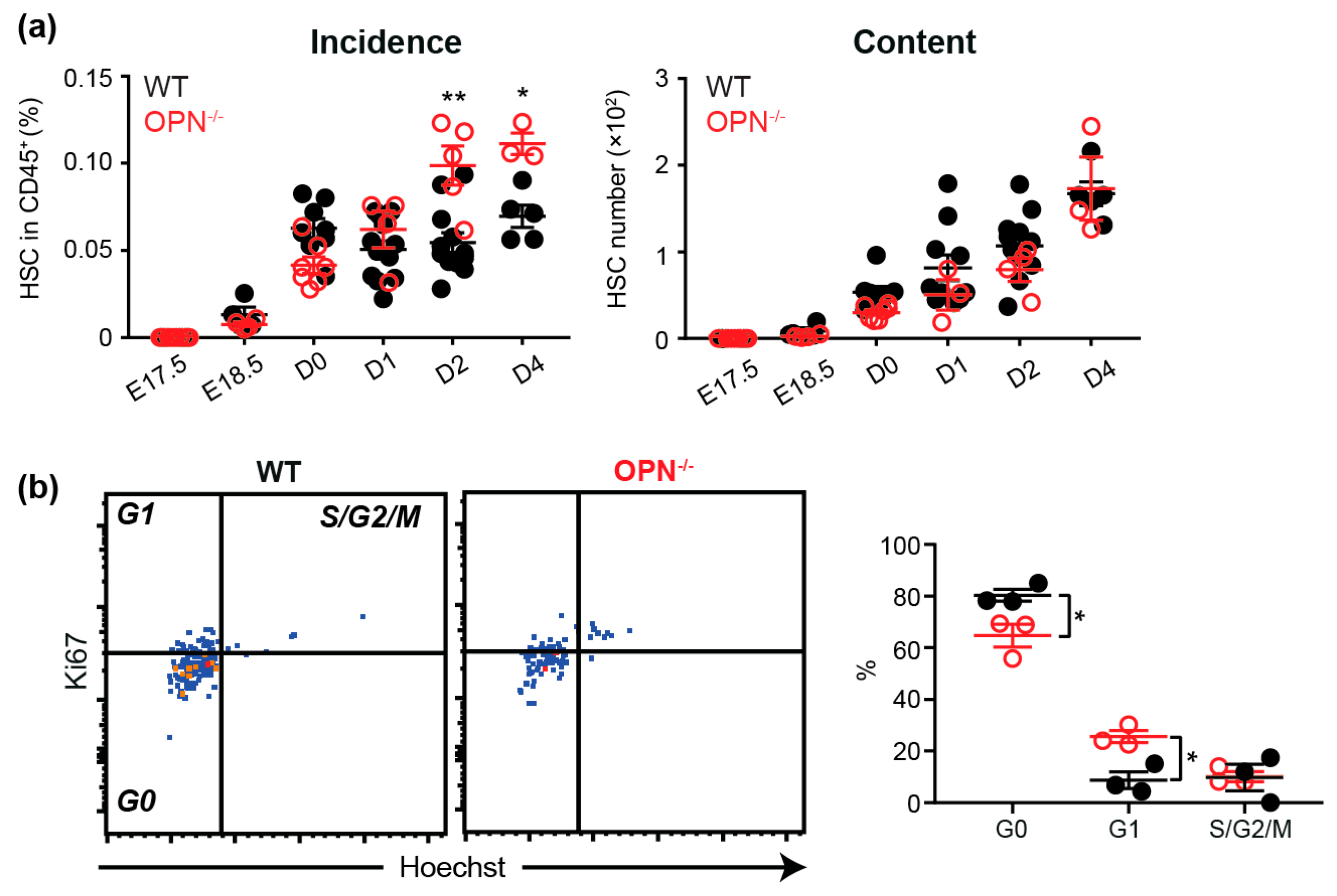
3.2. OPN is Important in Maintaining the Fetal BM Progenitor Pool
3.3. OPN Regulates the HSC Pool in Neonatal BM.
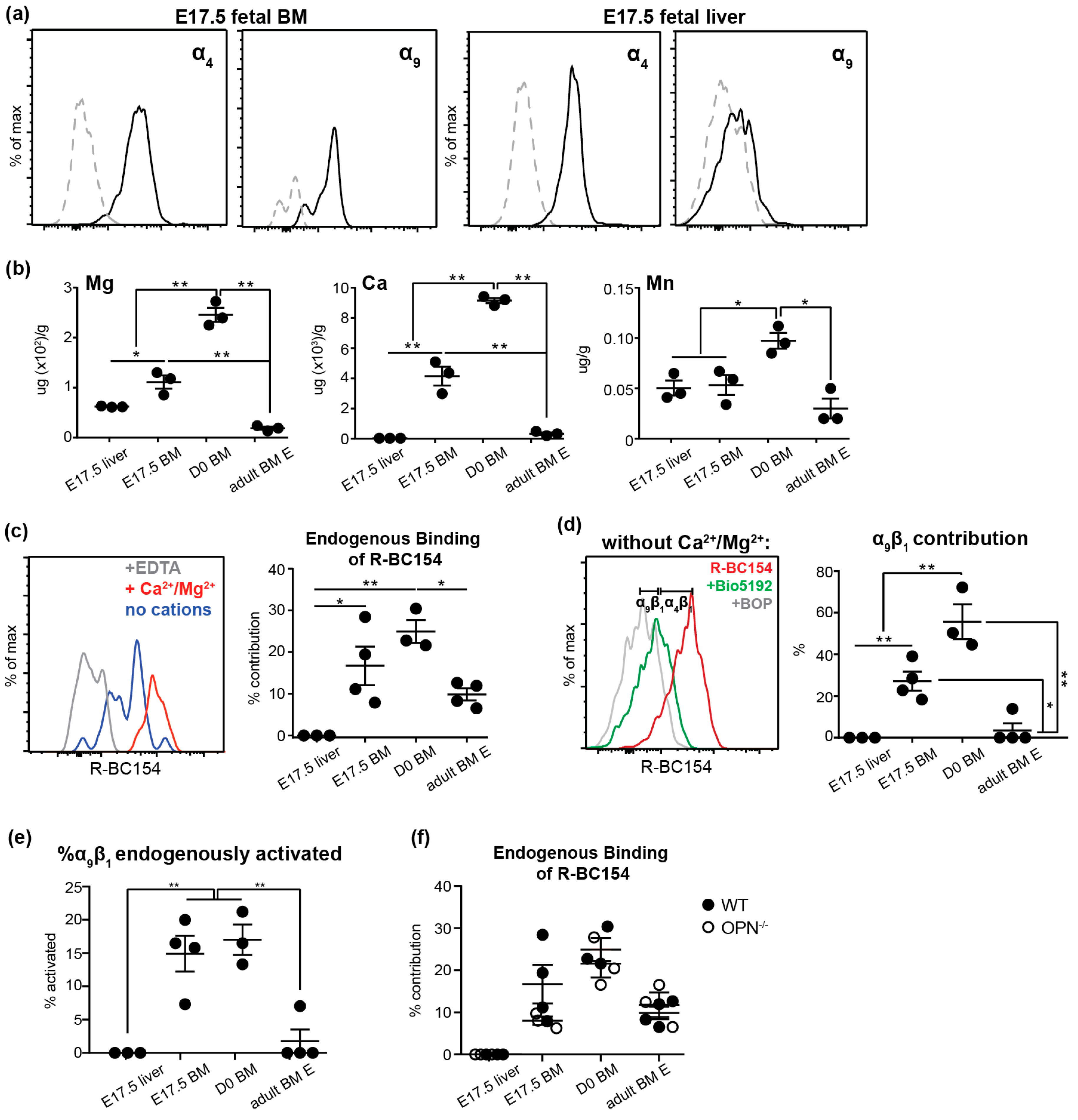
3.4. Endogenous Activation of α9β1 is Up-Regulated on Fetal BM Stem and Progenitors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef] [PubMed]
- Kazanecki, C.C.; Kowalski, A.J.; Ding, T.; Rittling, S.R.; Denhardt, D.T. Characterization of anti-osteopontin monoclonal antibodies: Binding sensitivity to post-translational modifications. J. Cell. Biochem. 2007, 102, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Reinholt, F.P.; Hultenby, K.; Oldberg, A.; Heinegard, D. Osteopontin—A possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA 1990, 87, 4473–4475. [Google Scholar] [CrossRef] [PubMed]
- Asou, Y.; Rittling, S.R.; Yoshitake, H.; Tsuji, K.; Shinomiya, K.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology 2001, 142, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Stier, S.; Ko, Y.; Forkert, R.; Lutz, C.; Neuhaus, T.; Grunewald, E.; Cheng, T.; Dombkowski, D.; Calvi, L.M.; Rittling, S.R.; et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 2005, 201, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Suzuki, K.; Goldberg, H.A.; Rittling, S.R.; Denhardt, D.T.; McCulloch, C.A.; Sodek, J. Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: Evidence of a role for an intracellular form of osteopontin. J. Cell. Physiol. 2004, 198, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Kipari, T.; Ophascharoensuk, V.; Wesson, J.A.; Johnson, R.; Hughes, J. Osteopontin—A molecule for all seasons. QJM 2002, 95, 3–13. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef]
- Mark, M.P.; Prince, C.W.; Oosawa, T.; Gay, S.; Bronckers, A.L.; Butler, W.T. Immunohistochemical demonstration of a 44-KD phosphoprotein in developing rat bones. J. Histochem. Cytochem. 1987, 35, 707–715. [Google Scholar] [CrossRef]
- Mark, M.P.; Butler, W.T.; Prince, C.W.; Finkelman, R.D.; Ruch, J.V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone gamma-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation 1988, 37, 123–136. [Google Scholar] [CrossRef]
- Dodds, R.A.; Connor, J.R.; James, I.E.; Rykaczewski, E.L.; Appelbaum, E.; Dul, E.; Gowen, M. Human osteoclasts, not osteoblasts, deposit osteopontin onto resorption surfaces: An in vitro and ex vivo study of remodeling bone. J. Bone Miner. Res. 1995, 10, 1666–1680. [Google Scholar] [CrossRef]
- Iwata, M.; Awaya, N.; Graf, L.; Kahl, C.; Torok-Storb, B. Human marrow stromal cells activate monocytes to secrete osteopontin, which down-regulates Notch1 gene expression in CD34+ cells. Blood 2004, 103, 4496–4502. [Google Scholar] [CrossRef][Green Version]
- Nilsson, S.K.; Johnston, H.M.; Whitty, G.A.; Williams, B.; Webb, R.J.; Denhardt, D.T.; Bertoncello, I.; Bendall, L.J.; Simmons, P.J.; Haylock, D.N. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005, 106, 1232–1239. [Google Scholar] [CrossRef]
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R., Jr.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef]
- McKee, M.D.; Farach-Carson, M.C.; Butler, W.T.; Hauschka, P.V.; Nanci, A. Ultrastructural immunolocalization of noncollagenous (osteopontin and osteocalcin) and plasma (albumin and alpha 2HS-glycoprotein) proteins in rat bone. J. Bone Miner. Res. 1993, 8, 485–496. [Google Scholar] [CrossRef]
- Domingues, M.J.; Cao, H.; Heazlewood, S.Y.; Cao, B.; Nilsson, S.K. Niche Extracellular Matrix Components and Their Influence on HSC. J. Cell. Biochem. 2017, 118, 1984–1993. [Google Scholar] [CrossRef]
- Grassinger, J.; Haylock, D.N.; Storan, M.J.; Haines, G.O.; Williams, B.; Whitty, G.A.; Vinson, A.R.; Be, C.L.; Li, S.; Sorensen, E.S.; et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood 2009, 114, 49–59. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, X.; Huang, Z.; Weber, G.F.; Zhang, G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS ONE 2011, 6, e23831. [Google Scholar] [CrossRef]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef]
- Lin, Y.H.; Huang, C.J.; Chao, J.R.; Chen, S.T.; Lee, S.F.; Yen, J.J.; Yang-Yen, H.F. Coupling of osteopontin and its cell surface receptor CD44 to the cell survival response elicited by interleukin-3 or granulocyte-macrophage colony-stimulating factor. Mol. Cell. Biol. 2000, 20, 2734–2742. [Google Scholar] [CrossRef]
- Storan, M.J.; Heazlewood, S.Y.; Heazlewood, C.K.; Haylock, D.N.; Alexander, W.S.; Neaves, R.J.; Oteiza, A.; Nilsson, S.K. Brief Report: Factors Released by Megakaryocytes Thrombin Cleave Osteopontin to Negatively Regulate Hematopoietic Stem Cells. Stem Cells 2015, 33, 2351–2357. [Google Scholar] [CrossRef]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Spencer, T.E. Osteopontin: Roles in implantation and placentation. Biol. Reprod. 2003, 69, 1458–1471. [Google Scholar] [CrossRef]
- Martin, M.A.; Bhatia, M. Analysis of the human fetal liver hematopoietic microenvironment. Stem Cells Dev. 2005, 14, 493–504. [Google Scholar] [CrossRef]
- Pietras, E.M.; Warr, M.R.; Passegue, E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011, 195, 709–720. [Google Scholar] [CrossRef]
- Cao, H.; Oteiza, A.; Nilsson, S.K. Understanding the role of the microenvironment during definitive hemopoietic development. Exp. Hematol. 2013, 41, 761–768. [Google Scholar] [CrossRef]
- Coskun, S.; Chao, H.; Vasavada, H.; Heydari, K.; Gonzales, N.; Zhou, X.; de Crombrugghe, B.; Hirschi, K.K. Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Rep. 2014, 9, 581–590. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef]
- Rittling, S.R.; Matsumoto, H.N.; McKee, M.D.; Nanci, A.; An, X.R.; Novick, K.E.; Kowalski, A.J.; Noda, M.; Denhardt, D.T. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J. Bone Miner. Res. 1998, 13, 1101–1111. [Google Scholar] [CrossRef]
- Cao, H.; Heazlewood, S.Y.; Williams, B.; Cardozo, D.; Nigro, J.; Oteiza, A.; Nilsson, S.K. The role of CD44 in fetal and adult hematopoietic stem cell regulation. Haematologica 2016, 101, 26–37. [Google Scholar] [CrossRef]
- Grassinger, J.; Haylock, D.N.; Williams, B.; Olsen, G.H.; Nilsson, S.K. Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood 2010, 116, 3185–3196. [Google Scholar] [CrossRef]
- Cao, H.; Williams, B.; Nilsson, S.K. Investigating the Interaction between Hemopoietic Stem Cells and Their Niche during Embryonic Development: Optimizing the Isolation of Fetal and Newborn Stem Cells From Liver, Spleen, and Bone Marrow. In Hematopoietic Stem Cell Protocols; Bunting, K., Ed.; Humana Press: Totowa, NJ, USA, 2014. [Google Scholar]
- Cao, B.; Zhang, Z.; Grassinger, J.; Williams, B.; Heazlewood, C.K.; Churches, Q.I.; James, S.A.; Li, S.; Papayannopoulou, T.; Nilsson, S.K. Therapeutic targeting and rapid mobilization of endosteal HSC using a small molecule integrin antagonist. Nat. Commun. 2016, 7, 11007. [Google Scholar] [CrossRef]
- Cao, H.; Williams, B.; Nilsson, S.K. Investigating the interaction between hematopoietic stem cells and their niche during embryonic development: Optimizing the isolation of fetal and newborn stem cells from liver, spleen, and bone marrow. Methods Mol. Biol. 2014, 1185, 9–20. [Google Scholar]
- Sommer, B.; Bickel, M.; Hofstetter, W.; Wetterwald, A. Expression of matrix proteins during the development of mineralized tissues. Bone 1996, 19, 371–380. [Google Scholar] [CrossRef]
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef]
- Askari, J.A.; Buckley, P.A.; Mould, A.P.; Humphries, M.J. Linking integrin conformation to function. J. Cell Sci. 2009, 122, 165–170. [Google Scholar] [CrossRef]
- Cao, B.; Hutt, O.E.; Zhang, Z.; Li, S.; Heazlewood, S.Y.; Williams, B.; Smith, J.A.; Haylock, D.N.; Savage, G.P.; Nilsson, S.K. Design, synthesis and binding properties of a fluorescent alpha(9)beta(1)/alpha(4)beta(1) integrin antagonist and its application as an in vivo probe for bone marrow haemopoietic stem cells. Org. Biomol. Chem. 2014, 12, 965–978. [Google Scholar] [CrossRef]
- Ramirez, P.; Rettig, M.P.; Uy, G.L.; Deych, E.; Holt, M.S.; Ritchey, J.K.; DiPersio, J.F. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood 2009, 114, 1340–1343. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Matsuura, N.; Sasaki, T.; Murakami, I.; Schneider, H.; Higashiyama, S.; Saitoh, Y.; Yamakido, M.; Taooka, Y.; Sheppard, D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J. Biol. Chem. 1999, 274, 36328–36334. [Google Scholar] [CrossRef]
- Papayannopoulou, T.; Priestley, G.V.; Nakamoto, B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood 1998, 91, 2231–2239. [Google Scholar]
- Morrison, S.J.; Weissman, I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1994, 1, 661–673. [Google Scholar] [CrossRef]
- Morrison, S.J.; Hemmati, H.D.; Wandycz, A.M.; Weissman, I.L. The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1995, 92, 10302–10306. [Google Scholar] [CrossRef]
- Manesia, J.K.; Franch, M.; Tabas-Madrid, D.; Nogales-Cadenas, R.; Vanwelden, T.; Van Den Bosch, E.; Xu, Z.; Pascual-Montano, A.; Khurana, S.; Verfaillie, C.M. Distinct Molecular Signature of Murine Fetal Liver and Adult Hematopoietic Stem Cells Identify Novel Regulators of Hematopoietic Stem Cell Function. Stem Cells Dev. 2017, 26, 573–584. [Google Scholar] [CrossRef]
- Zhang, C.C.; Kaba, M.; Ge, G.; Xie, K.; Tong, W.; Hug, C.; Lodish, H.F. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 2006, 12, 240–245. [Google Scholar] [CrossRef]
- Zhang, C.C.; Lodish, H.F. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood 2004, 103, 2513–2521. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Charbord, P.; Tavian, M.; Humeau, L.; Peault, B. Early ontogeny of the human marrow from long bones: An immunohistochemical study of hematopoiesis and its microenvironment. Blood 1996, 87, 4109–4119. [Google Scholar]
- Tavian, M.; Peault, B. Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. 2005, 49, 243–250. [Google Scholar] [CrossRef]
- Weintraub, A.S.; Lin, X.; Itskovich, V.V.; Aguinaldo, J.G.; Chaplin, W.F.; Denhardt, D.T.; Fayad, Z.A. Prenatal detection of embryo resorption in osteopontin-deficient mice using serial noninvasive magnetic resonance microscopy. Pediatr. Res. 2004, 55, 419–424. [Google Scholar] [CrossRef]
- Chen, C.; Huang, X.; Atakilit, A.; Zhu, Q.S.; Corey, S.J.; Sheppard, D. The Integrin alpha9beta1 contributes to granulopoiesis by enhancing granulocyte colony-stimulating factor receptor signaling. Immunity 2006, 25, 895–906. [Google Scholar] [CrossRef][Green Version]
- Choi, S.I.; Kim, S.Y.; Lee, J.H.; Kim, J.Y.; Cho, E.W.; Kim, I.G. Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget 2017, 8, 101284–101297. [Google Scholar] [CrossRef]
- Behera, R.; Kumar, V.; Lohite, K.; Karnik, S.; Kundu, G.C. Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 2010, 31, 192–200. [Google Scholar] [CrossRef]
- Guidi, N.; Sacma, M.; Standker, L.; Soller, K.; Marka, G.; Eiwen, K.; Weiss, J.M.; Kirchhoff, F.; Weil, T.; Cancelas, J.A.; et al. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J. 2017, 36, 840–853. [Google Scholar] [CrossRef]
- Li, J.; Carrillo Garcia, C.; Riedt, T.; Brandes, M.; Szczepanski, S.; Brossart, P.; Wagner, W.; Janzen, V. Murine hematopoietic stem cell reconstitution potential is maintained by osteopontin during aging. Sci. Rep. 2018, 8, 2833. [Google Scholar] [CrossRef]
- Hui, T.; Sorensen, E.S.; Rittling, S.R. Osteopontin binding to the alpha 4 integrin requires highest affinity integrin conformation, but is independent of post-translational modifications of osteopontin. Matrix Biol. 2015, 41, 19–25. [Google Scholar] [CrossRef]
- Bouet, G.; Bouleftour, W.; Juignet, L.; Linossier, M.T.; Thomas, M.; Vanden-Bossche, A.; Aubin, J.E.; Vico, L.; Marchat, D.; Malaval, L. The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS ONE 2015, 10, e0117402. [Google Scholar] [CrossRef]
- Sugars, R.V.; Olsson, M.L.; Marchner, S.; Hultenby, K.; Wendel, M. The glycosylation profile of osteoadherin alters during endochondral bone formation. Bone 2013, 53, 459–467. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Cao, B.; Heazlewood, C.K.; Domingues, M.; Sun, X.; Debele, E.; McGregor, N.E.; Sims, N.A.; Heazlewood, S.Y.; Nilsson, S.K. Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche. Cells 2019, 8, 985. https://doi.org/10.3390/cells8090985
Cao H, Cao B, Heazlewood CK, Domingues M, Sun X, Debele E, McGregor NE, Sims NA, Heazlewood SY, Nilsson SK. Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche. Cells. 2019; 8(9):985. https://doi.org/10.3390/cells8090985
Chicago/Turabian StyleCao, Huimin, Benjamin Cao, Chad K. Heazlewood, Melanie Domingues, Xuan Sun, Emmanuel Debele, Narelle E. McGregor, Natalie A. Sims, Shen Y. Heazlewood, and Susan K. Nilsson. 2019. "Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche" Cells 8, no. 9: 985. https://doi.org/10.3390/cells8090985
APA StyleCao, H., Cao, B., Heazlewood, C. K., Domingues, M., Sun, X., Debele, E., McGregor, N. E., Sims, N. A., Heazlewood, S. Y., & Nilsson, S. K. (2019). Osteopontin is An Important Regulative Component of the Fetal Bone Marrow Hematopoietic Stem Cell Niche. Cells, 8(9), 985. https://doi.org/10.3390/cells8090985




