PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Clinical Variables
2.3. Cutaneous Telangiectasia Biopsy
2.4. Histopathological Evaluation
2.5. Immunohistochemistry Studies
2.6. Statistical Analysis
3. Results
3.1. Histopathological Vascular Pattern in Human Cutaneous Telangiectasia Biopsies
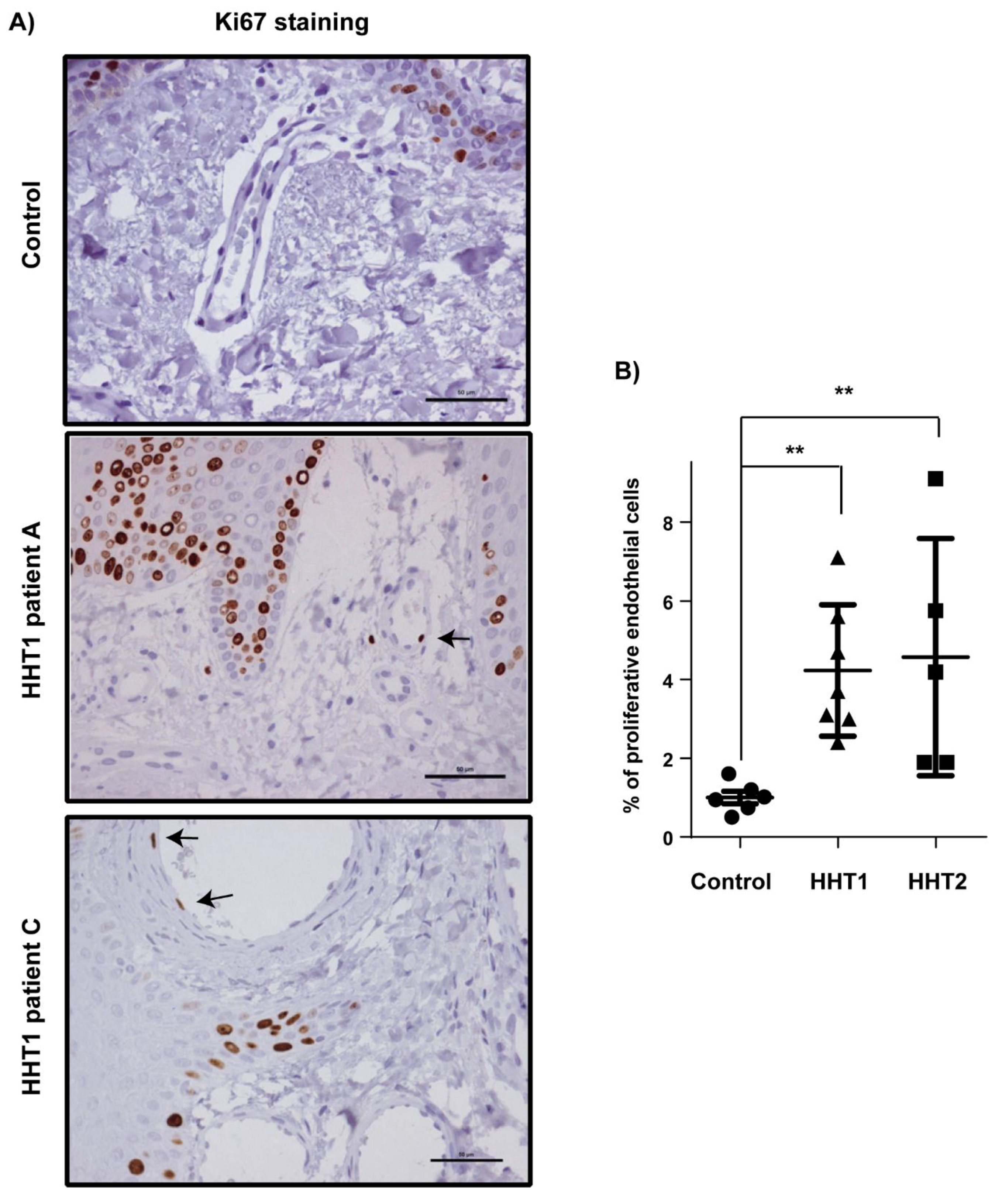
3.2. Vascular Size and Endothelial Cell Proliferation Are Increased in Cutaneous Telangiectasia Biopsies of Patients with HHT1 and HHT2 Compared to Controls
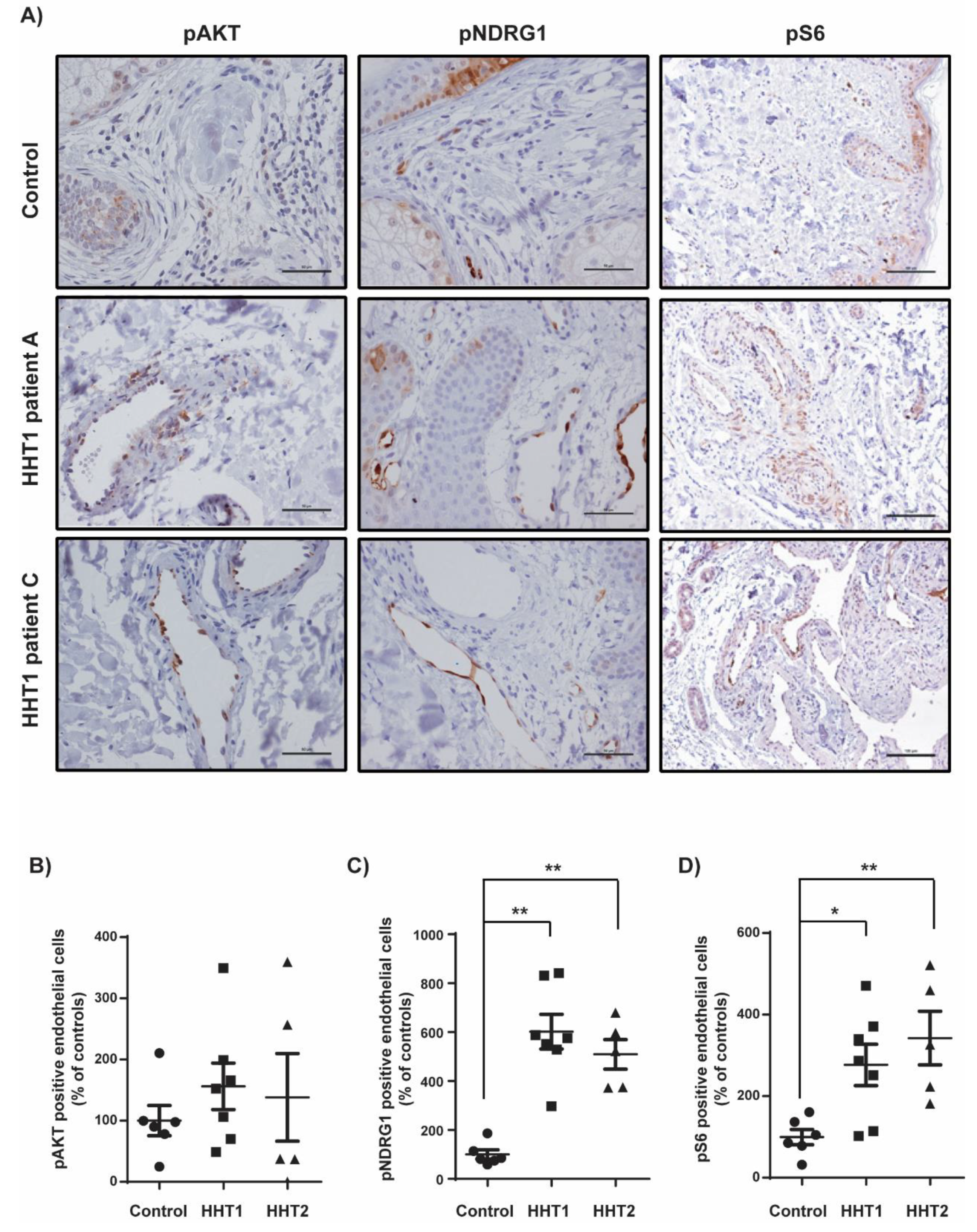
3.3. Overstimulation of the PI3K Pathway in Cutaneous Telangiectasia Biopsies of Patients with HHT1 Compared to Control Vessels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Portal for Rare Diseases and Orphan Drugs. Available online: https://www.orpha.net/consor/cgi-bin/index.php (accessed on 22 July 2019).
- Abdalla, S.A.; Letarte, M. Hereditary haemorrhagic telangiectasia: Current views on genetics and mechanisms of disease. J. Med. Genet. 2006, 43, 97–110. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Wooderchak-Donahue, W.; VanSant Webb, C.; Whitehead, K.; Stevenson, D.A.; Bayrak-Toydemir, P. Hereditary hemorrhagic telangiectasia: Genetics and molecular diagnostics in a new era. Front. Genet. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Tillet, E.; Bailly, S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front. Genet. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Allinson, K.R.; Zhai, Z.; Oakenfull, R.; Ghandi, P.; Adams, R.H.; Fruttiger, M.; Arthur, H.M. Pathogenesis of arteriovenous malformations in the absence of endoglin. Circ. Res. 2010, 106, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Bayrak-Toydemir, P.; McDonald, J.; Markewitz, B.; Lewin, S.; Miller, F.; Chou, L.S.; Gedge, F.; Tang, W.; Coon, H.; Mao, R. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: Mutations and manifestations. Am. J. Med. Genet. A 2006, 140, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Lesca, G.; Olivieri, C.; Burnichon, N.; Pagella, F.; Carette, M.F.; Gilbert-Dussardier, B.; Goizet, C.; Roume, J.; Rabilloud, M.; Saurin, J.C.; et al. French-Italian-Rendu-Osler Network. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: Data from the French-Italian HHT network. Genet. Med. 2007, 9, 14–22. [Google Scholar] [CrossRef]
- Riera-Mestre, A.; Mora-Luján, J.M.; Sanchez-Martínez, R.; Torralba, M.A.; Patier de la Peña, J.L.; Juyol-Rodrigo, M.C.; Lopez-Wolf, D.; Ojeda-Sosa, A.; Monserrat, L.; López-Rodríguez, M. Computerized registry of patients with hemorrhagic hereditary telangiectasia (RiHHTa Registry) in Spain: Objectives, methods, and preliminary results. Rev. Clin. Esp. 2018, 218, 468–476. [Google Scholar] [CrossRef]
- Braverman, I.M.; Keh, A.; Jacobson, B.S. Ultrastructure and three-dimensional organization of the telangiectases of hereditary hemorrhagic telangiectasia. J. Investig. Dermatol. 1990, 95, 422–427. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Buscarini, E.; Kjeldsen, A.D.; Mager, H.J.; Sabba, C.; Droege, F.; Geisthoff, U.; Ugolini, S.; Dupuis-Girod, S. European Reference Network for Rare Vascular Diseases (VASCERN) Outcome measures for Hereditary Haemorrhagic Telangiectasia (HHT). Orphanet J. Rare Dis. 2018, 13. [Google Scholar] [CrossRef]
- Riera-Mestre, A.; Ribas, J.; Castellote, J. Medical management of haemorrhagic hereditary telangiectasia in adult patients. Med. Clin. 2019, 152, 274–280. [Google Scholar] [CrossRef]
- Alsina-Sanchís, E.; García-Ibáñez, Y.; Figueiredo, A.M.; Riera-Domingo, C.; Figueras, A.; Matias-Guiu, X.; Casanovas, O.; Botella, L.M.; Pujana, M.A.; Riera-Mestre, A.; et al. ALK1 Loss Results in Vascular Hyperplasia in Mice and Humans Through PI3K Activation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1216–1229. [Google Scholar]
- Graupera, M.; Potente, M. Regulation of angiogenesis by PI3K signaling networks. Exp. Cell Res. 2013, 319, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Ola, R.; Dubrac, A.; Han, J.; Zhang, F.; Fang, J.S.; Larrivée, B.; Lee, M.; Urarte, A.A.; Kraehling, J.R.; Genet, G. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat. Commun. 2016, 29. [Google Scholar] [CrossRef] [PubMed]
- Faughnan, M.E.; Palda, V.A.; Garcia-Tsao, G.; Geisthoff, U.W.; McDonald, J.; Proctor, D.D.; Spears, J.; Brown, D.H.; Buscarini, E.; Chesnutt, M.S.; et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J. Med. Genet. 2011, 48, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med. Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Hoag, J.B.; Terry, P.; Mitchell, S.; Reh, D.; Merlo, C.A. An epistaxis severity score for hereditary hemorrhagia telangiectasia. Laryngoscope 2010, 120, 838–843. [Google Scholar] [CrossRef]
- Barzilai, B.; Waggoner, A.D.; Spessert, C.; Picus, D.; Goodenberger, D. Two-dimensional contrast echocardiography in the detection and follow-up of congenital pulmonary arteriovenous malformations. Am. J. Cardiol. 1991, 68, 1507–1510. [Google Scholar] [CrossRef]
- Khalid, S.K.; Garcia-Tsao, G. Hepatic vascular malformations in hereditary hemorrhagic telangiectasia. Semin. Liver Dis. 2008, 28, 247–258. [Google Scholar] [CrossRef]
- Torrado, M.; Maneiro, E.; Trujillo-Quintero, J.P.; Evangelista, A.; Mikhailov, A.T.; Monserrat, L. A Novel Heterozygous Intronic Mutation in the FBN1 Gene Contributes to FBN1 RNA Missplicing Events in the Marfan Syndrome. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Letteboer, T.G.; Mager, H.J.; Snijder, R.J.; Lindhout, D.; Ploos van Amstel, H.K.; Zanen, P.; Westermann, K.J. Genotype-phenotype relationship for localization and age distribution of telangiectases in hereditary hemorrhagic telangiectasia. Am. J. Med. Genet. A 2008, 146, 2733–2739. [Google Scholar] [CrossRef]
- Canzonieri, C.; Centenara, L.; Ornati, F.; Pagella, F.; Matti, E.; Alvisi, C.; Danesino, C.; Perego, M.; Olivieri, C. Endoscopic evaluation of gastrointestinal tract in patients with hereditary hemorrhagic telangiectasia and correlation with their genotypes. Genet. Med. 2014, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Colorado, P.C.; Torre, A.; Kamphaus, G.; Maeshima, Y.; Hopfer, H.; Takahashi, K.; Volk, R.; Zamborsky, E.D.; Herman, S.; Sarkar, P.K.; et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000, 60, 2520–2526. [Google Scholar] [PubMed]
- Wu, Y.; Ge, G. Complexity of type IV collagens: From network assembly to function. Biol. Chem. 2019, 400, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Sund, M.; Xie, L.; Kalluri, R. The contribution of vascular basement membranes and extracellular matrix to the mechanics of tumor angiogenesis. APMIS 2004, 112, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Bignon, M.; Pichol-Thievend, C.; Hardouin, J.; Malbouyres, M.; Brechot, N.; Nasciutti, L.; Barret, A.; Teillon, J.; Guillon, E.; Etienne, E.; et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 2011, 118, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.S. Cutaneous collagenous vasculopathy: A new case series with clinicopathologic and ultrastructural correlation, literature review, and insight into the pathogenesis. Am. J. Dermatopathol. 2015, 37, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Toda-Brito, H.; Resende, C.; Catorze, G.; Viana, I. Cutaneous collagenous vasculopathy: A rare cause of generalised cutaneous telangiectasia. BMJ Case Rep. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Pece-Barbara, N.; Vera, S.; Kathirkamathamby, K.; Liebner, S.; Di Guglielmo, G.M.; Dejana, E.; Wrana, J.L.; Letarte, M. Endoglin null endothelial cells proliferate faster and are more responsive to transforming growth factor beta1 with higher affinity receptors and an activated Alk1 pathway. J. Biol. Chem. 2005, 280, 27800–27808. [Google Scholar] [CrossRef] [PubMed]
- Larrivée, B.; Prahst, C.; Gordon, E.; del Toro, R.; Mathivet, T.; Duarte, A.; Simons, M.; Eichmann, A. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 2012, 22, 489–500. [Google Scholar] [CrossRef]
- Moya, I.M.; Umans, L.; Maas, E.; Pereira, P.N.; Beets, K.; Francis, A.; Sents, W.; Robertson, E.J.; Mummery, C.L.; Huylebroeck, D.; et al. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev. Cell 2012, 22, 501–514. [Google Scholar] [CrossRef]
- Castillo, S.D.; Tzouanacou, E.; Zaw-Thin, M.; Berenjeno, I.M.; Parker, V.E.; Chivite, I.; Milà-Guasch, M.; Pearce, W.; Solomon, I.; Angulo-Urarte, A.; et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, M.; Rahim, M.S.; Dames, S.A. Regulation of the Target of Rapamycin and Other Phosphatidylinositol 3-Kinase-Related Kinases by Membrane Targeting. Membranes 2015, 5, 553–575. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.; Seront, E.; Duez, S.; Dupont, S.; Van Damme, A.; Schmitz, S.; Hoyoux, C.; Chopinet, C.; Clapuyt, P.; Hammer, F.; et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: A monocentric prospective phase II study. Orphanet J. Rare Dis. 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Skaro, A.I.; Anton, I.; Marotta, P.J.; Paul, J.; McAlister, V.C. Regression of cutaneous and gastrointestinal telangiectasia with sirolimus and aspirin in a patient with hereditary hemorrhagic telangiectasia. Ann. Intern Med. 2006, 144, 226–227. [Google Scholar] [PubMed]
- Geisthoff, U.W.; Nguyen, H.L.; Hess, D. Improvement in hereditary hemorrhagic telangiectasia after treatment with the phosphoinositide 3-kinase inhibitor BKM120. Ann. Hematol. 2014, 93, 703–704. [Google Scholar] [CrossRef]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Chandakkar, P.; Zhao, H.; Papoin, J.; Chatterjee, P.K.; Christen, E.; Metz, C.N.; Blanc, L.; Campagne, F.; Marambaud, P. Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Hum. Mol. Genet. 2017, 26, 4786–4798. [Google Scholar] [CrossRef]


| No. | Age, Years | M/F | TTE | Thoracic CT | Abdominal CT | GI Telangiectasia | CI, L/min/m2 | ESS | Mutations |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | 2 | Pulmonary AVM RUL, LUL, LLL (embolized) | Pancreatic telangiectasias | Esophagus–duodenum | 3.06 | 4.75 | ENG: Exon 6: p.(Tyr258*) (c.774C > A) NONSENSE |
| 2 * | 52 | F | 1 | Pulmonary AVM LLL (embolized) | No pathological findings | Gastroduodenal, proximal–middle jejunum | 2.47 | 3.04 | ENG: Exon 7: p.(Val323Leufs*10) (c.967_968delGT) FRAMESHIFT |
| 3 | 59 | F | 2 | Pulmonary AVM LUL, RLL, RUL (embolized) | Ileal-jejunum AVM | Stomach–proximal–middle jejunum | 2.32 | 7.68 | ENG: Exon 3: p.(Arg93*) (c.277C > T) NONSENSE |
| 4 | 38 | M | 3 | Pulmonary AVM LLL, RUL (embolized) | Intrahepatic telangiectasias Hepatic AP shunt | Not performed | 3.4 | 3.84 | ENG: Exon 9: p.(Cys412Tyr) (c.1235G > A) MISSENSE |
| 5 * | 43 | M | 3 | Pulmonary AVM LUL, RUL (embolized) | No pathological findings | Not performed | 2.44 | 4.42 | ENG: Exon 7: p.(Val323Leufs*10) (c.967_968delGT) FRAMESHIFT |
| 6 | 42 | M | 1 | No pathological findings | Intrahepatic telangiectasias | Stomach–ascending colon | 2.77 | 3.33 | ENG: Exon 1–3: (c.-3659_361-537del) CNV (COPY NUMBER VARIANT) |
| 7 * | 56 | F | 2 | No pathological findings | No pathological findings | Gastroduodenal–Ileocecal valve | 3.36 | 5.47 | ENG: Exon 7: p (Val323Leufs*10) (c.967_968delGT) FRAMESHIFT |
| 8 + | 62 | F | 1 | No pathological findings | Intrahepatic telangiectasias Hepatic AV shunt Hepatic artery enlargement FNH Intrapancreatic AVM Ileal AVM Cecal AVM Uterine AVM | Not performed | 3.74 | 2.83 | ACVRL1: Exon 3: p.(Thr82del) (c.244_246delACC) IN-FRAME DELETION |
| 9 | 49 | M | 1 | No pathological findings | No pathological findings | Not performed | 2.28 | 6.38 | ACVRL1: Exon 3: p.(Cys77Arg) (c.229T > C) MISSENSE |
| 10 ** | 41 | F | 3 | Pulmonary AVM LLL | Intrahepatic telangiectasias Hepatic AV shunt Hepatic artery enlargement NRH Uterine AVM | Not performed | 3.12 | 2.93 | ACVRL1: Exon 10: p.(Arg479Pro) (c.1436G > C) MISSENSE |
| 11 **,+ | 70 | F | 0 | No pathological findings | Hepatomegaly Hepatic AV shunt Hepatic artery enlargement Intrapancreatic AVM Left renal artery aneurysm | Not performed | 3.7 | 6.59 | ACVRL1: Exon 10: p.(Arg479Pro) (c.1436G > C) MISSENSE |
| 12 | 49 | M | 0 | Not performed | Intrahepatic telangiectasias Intrapancreatic AVM Gastro-omental artery aneurysms | Not performed | 4.57 | 13.5 | ACVRL1: Exon 10: p.(Arg479Pro) (c.1436G > C) MISSENSE |
| 13 + | 51 | M | 1 | No pathological findings | Hepatomegaly Hepatic AP shunt Hepatic artery enlargement | Not performed | 3.3 | 6.05 | ACVRL1: Exon 10: p.(Arg484Trp) (c.1450C > T) MISSENSE |
| 14 | 51 | F | 1 | No pathological findings | Hepatic telangiectasias Hepatic AP shunt Hepatic AV shunt | Not performed | 3.2 | 1.41 | ACVRL1: Exon 3: p.(Pro23Leufs*2) (c.68delC) FRAMESHIFT |
| 15 | 60 | M | 0 | No pathological findings | Hepatic telangiectasias Hepatic AP shunt Hepatic AV shunt Intrapancreatic AVM | Gastroduodenal | 2.9 | 6.09 | ACVRL1: Exon 3: p.(Arg67Gln) (c.200G > A) MISSENSE |
| Conventional Pattern n (%) or Mean (SD) | Angiokeratoma-Like Pattern n (%) or Mean (SD) | P | |
|---|---|---|---|
| Patients | 7 (46.6) | 8 (53.3) | NA |
| Female gender | 3 (42.8) | 5 (62.5) | 0.619 |
| Age, years | 47.2 (9.5) | 57.2 (7.0) | 0.042 * |
| Mutation | |||
| ENG | 5 (87.5) | 2 (25) | 0.132 |
| ACVRL1 | 2 (28.5) | 6 (75) | |
| ESS | 3.9 (0.6) | 5.4 (1.5) | 0.049 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iriarte, A.; Figueras, A.; Cerdà, P.; Mora, J.M.; Jucglà, A.; Penín, R.; Viñals, F.; Riera-Mestre, A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells 2019, 8, 971. https://doi.org/10.3390/cells8090971
Iriarte A, Figueras A, Cerdà P, Mora JM, Jucglà A, Penín R, Viñals F, Riera-Mestre A. PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells. 2019; 8(9):971. https://doi.org/10.3390/cells8090971
Chicago/Turabian StyleIriarte, Adriana, Agnes Figueras, Pau Cerdà, José María Mora, Anna Jucglà, Rosa Penín, Francesc Viñals, and Antoni Riera-Mestre. 2019. "PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1" Cells 8, no. 9: 971. https://doi.org/10.3390/cells8090971
APA StyleIriarte, A., Figueras, A., Cerdà, P., Mora, J. M., Jucglà, A., Penín, R., Viñals, F., & Riera-Mestre, A. (2019). PI3K (Phosphatidylinositol 3-Kinase) Activation and Endothelial Cell Proliferation in Patients with Hemorrhagic Hereditary Telangiectasia Type 1. Cells, 8(9), 971. https://doi.org/10.3390/cells8090971





