Progress in the Analysis of Food Allergens through Molecular Biology Approaches
Abstract
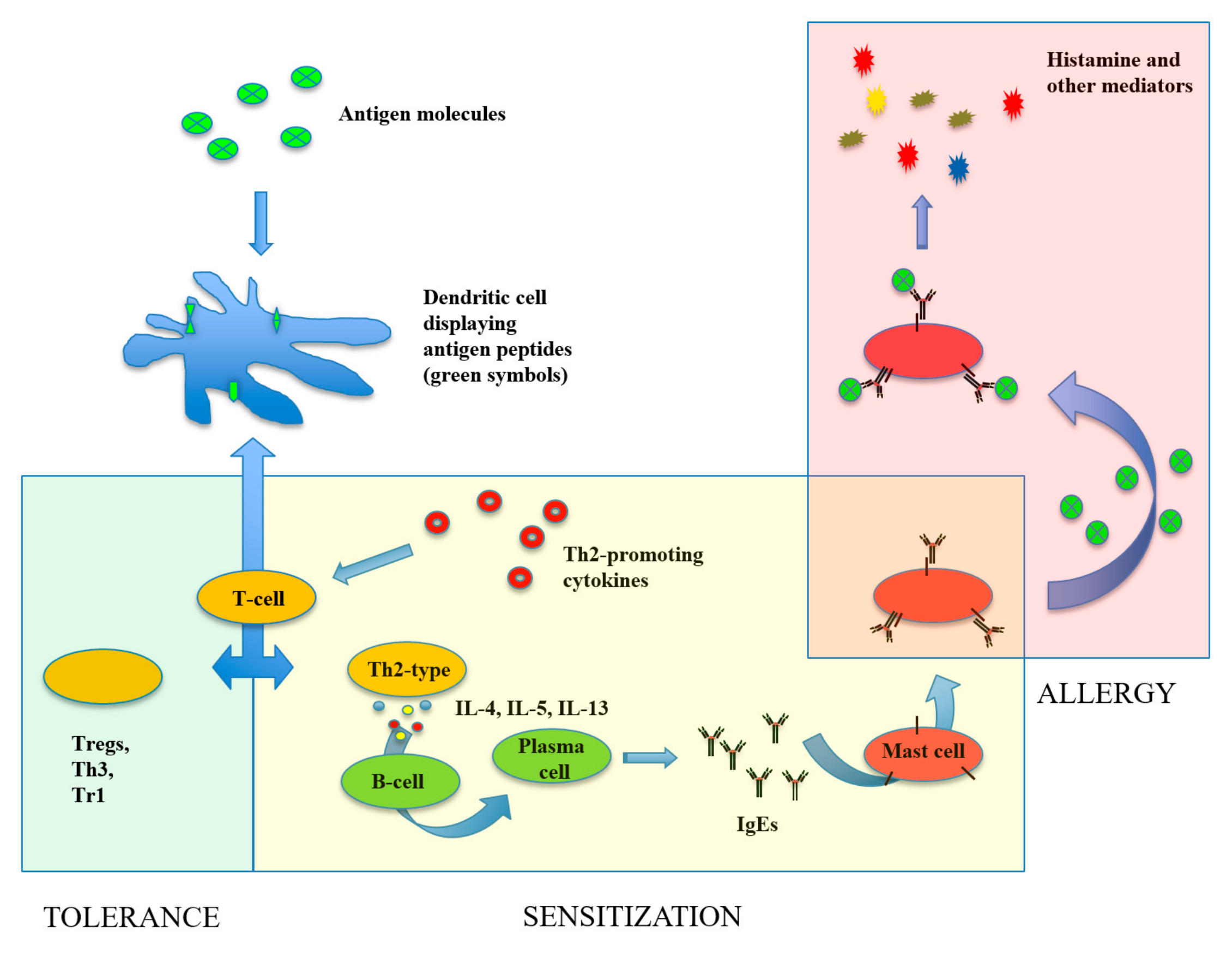
1. Introduction
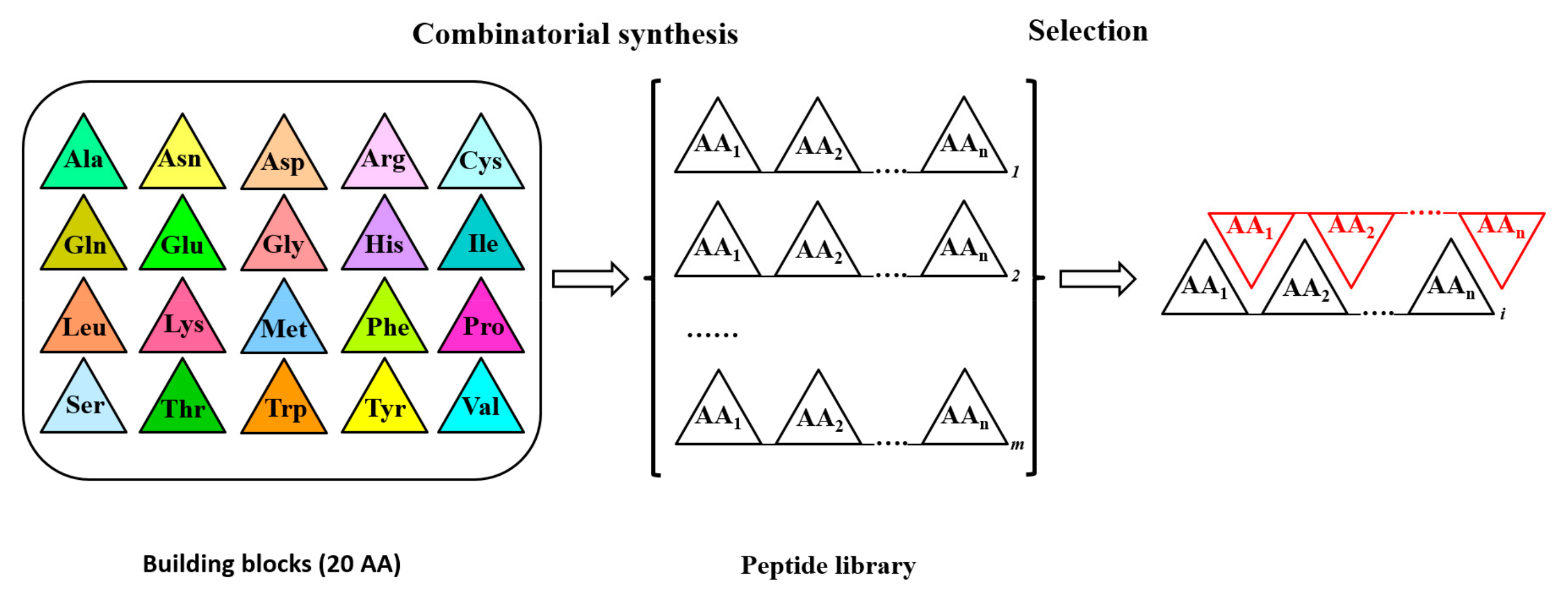
2. Epitope Mapping by Molecular Approaches
3. Epitope Prediction Analysis by Bioinformatic and Structural Tools
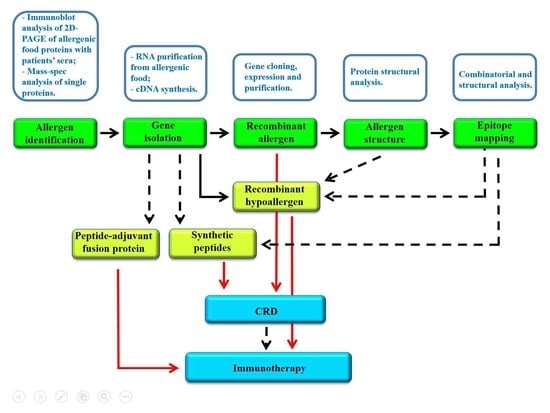
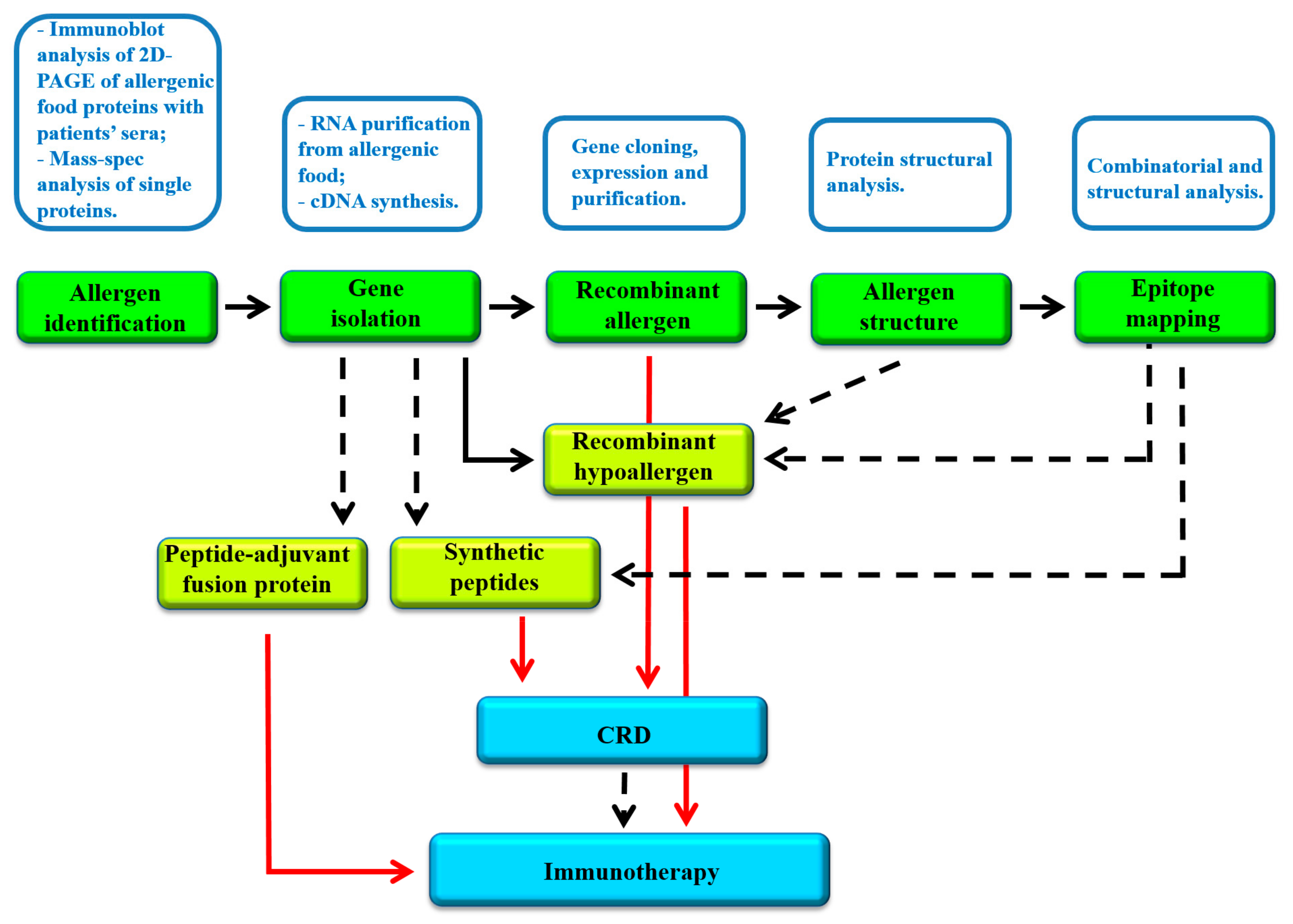
4. Recombinant Food Allergens Used for Component Resolved Diagnosis
5. Polypeptides and Recombinant Food Allergens for Immunotherapy
6. Food Allergen Databases and Web Resources
6.1. AllergenOnline
6.2. InformAll
6.3. Compare
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thomas, W.R.; Stewart, G.A.; Simpson, R.J.; Chua, K.Y.; Plozza, T.M.; Dilworth, R.J.; Nisbet, A.; Turner, K.J. Cloning and expression of DNA coding for the major house dust mite allergen Der p 1 in Escherichia coli. Int. Arch. Allergy Appl. Immunol. 1988, 85, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Cherayil, B.J.; Shi, H.; Wang, Y.; Zhu, Y. Food Allergy: From Molecular Mechanisms to Control Strategies; Springer Nature Singapore: Singapore, 2019; pp. 1–6. [Google Scholar]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Allergen Online. Available online: www.allergenonline.org (accessed on 1 June 2019).
- Sampson, H.A. Food allergy: Past, present and future. Allergol. Int. 2016, 65, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, P.S. Clinical aspects of food allergy. Biochem. Soc. Trans. 2002, 30, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Volpicella, M.; Leoni, C.; Fanizza, I.; Placido, A.; Pastorello, E.A.; Ceci, L.R. Overview of Plant Chitinases Identified as Food Allergens. J. Agric. Food Chem. 2014, 62, 5734–5742. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Ebner, C. Molecular and biochemical classification of plant-derived food allergens. J. Allergy Clin. Immunol. 2000, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Beaudoin, F.; Jenkins, J.; Griffiths-Jones, S.; Mills, E.N. Plant protein families and their relationships to food allergy. Biochem. Soc. Trans. 2002, 30, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Volpicella, M.; Dileo, M.C.G.; Gattulli, B.A.R.; Ceci, L.R. Chitinases as Food Allergens. Molecules 2019, 24, 2087. [Google Scholar] [CrossRef] [PubMed]
- De Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Palladino, C.; Breiteneder, H. Peanut allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Bohle, B.; Vieths, S. Improving diagnostic tests for food allergy with recombinant allergens. Methods 2004, 32, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Niespodziana, K.; Focke-Tejkl, M.; Marth, K.; Huber, H.; Neubauer, A.; Niederberger, V. Recombinant allergens: What does the future hold? J. Allergy Clin. Immunol. 2011, 127, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Ballmer-Weber, B.K.; Vieths, S. Potential, pitfalls, and prospects of food allergy diagnostics with recombinant allergens or synthetic sequential epitopes. J. Allergy Clin. Immunol. 2008, 121, 1323–1330. [Google Scholar] [CrossRef][Green Version]
- Borres, M.P.; Maruyama, N.; Sato, S.; Ebisawa, M. Recent advances in component resolved diagnosis in food allergy. Allergol. Int. 2016, 65, 378–387. [Google Scholar] [CrossRef]
- Dodig, S.; Čepelak, I. The potential of component-resolved diagnosis in laboratory diagnostics of allergy. Biochem. Medica 2018, 28. [Google Scholar] [CrossRef]
- Flores Kim, J.; McCleary, N.; Nwaru, B.I.; Stoddart, A.; Sheikh, A. Diagnostic accuracy, risk assessment, and cost-effectiveness of component-resolved diagnostics for food allergy: A systematic review. Allergy 2018, 73, 1609–1621. [Google Scholar] [CrossRef]
- Incorvaia, C.; Rapetti, A.; Aliani, M.; Castagneto, C.; Corso, N.; Landi, M.; Lietti, D.; Murante, N.; Muratore, L.; Russello, M.; et al. Food allergy as defined by component resolved diagnosis. Recent Pat. Inflam. Allergy Drug Discov. 2014, 8, 59–73. [Google Scholar] [CrossRef]
- Joo Chan, C.; Richardo, T.; Lim, R.L.H. Current Trend in Immunotherapy for Peanut Allergy. Int. Rev. Immunol. 2018, 37, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Wallner, M.; Breiteneder, H.; Hartl, A.; Thalhamer, J.; Ebner, C. Genetic engineering of allergens: Future therapeutic products. Int. Arch. Allergy Immunol. 2002, 128, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Niederberger, V. Recombinant allergens for immunotherapy. J. Allergy Clin. Immunol. 2007, 119, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, N.C.; Durham, S.R. New approaches to allergen immunotherapy. Ann. Allergy Asthma Immunol. 2018, 121, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Cook, Q.S.; Burks, A.W. Peptide and Recombinant Allergen Vaccines for Food Allergy. Clin. Rev. Allergy Immunol. 2018, 55, 162–171. [Google Scholar] [CrossRef]
- Valenta, R.; Linhart, B.; Swoboda, I.; Niederberger, V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens: Recombinant allergen-based immunotherapy. Allergy 2011, 66, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Lidholm, J.; Niederberger, V.; Hayek, B.; Kraft, D.; Grönlund, H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin. Exp. Allergy 1999, 29, 896–904. [Google Scholar] [CrossRef]
- Tscheppe, A.; Breiteneder, H. Recombinant Allergens in Structural Biology, Diagnosis, and Immunotherapy. Int. Arch. Allergy Immunol. 2017, 172, 187–202. [Google Scholar] [CrossRef]
- Valenta, R.; Ferreira, F.; Focke-Tejkl, M.; Linhart, B.; Niederberger, V.; Swoboda, I.; Vrtala, S. From Allergen Genes to Allergy Vaccines. Annu. Rev. Immunol. 2010, 28, 211–241. [Google Scholar] [CrossRef]
- Radauer, C. Navigating through the Jungle of Allergens: Features and Applications of Allergen Databases. Int. Arch. Allergy Immunol. 2017, 173, 1–11. [Google Scholar] [CrossRef]
- Pomés, A. Relevant B Cell Epitopes in Allergic Disease. Int. Arch. Allergy Immunol. 2010, 152, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.M.; Loomis-Price, L. B Cell Epitope Mapping Using Synthetic Peptides. In Current Protocols in Immunology; Coligan, J.E., Bierer, B.E., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 9.4.1–9.4.23. [Google Scholar]
- van’t Hof, W.; Driedijk, P.C.; van den Berg, M.; Beck-Sickinger, A.G.; Jung, G.; Aalberse, R.C. Epitope mapping of the Dermatophagoides pteronyssinus house dust mite major allergen Der p II using overlapping synthetic peptides. Mol. Immunol. 1991, 28, 1225–1232. [Google Scholar] [PubMed]
- Matsuura, T.; Ernst, A.; Zechel, D.L.; Plückthun, A. Combinatorial approaches to novel proteins. Chembiochem 2004, 5, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Volpicella, M.; Leoni, C.; Arnesano, F.; Gallerani, R.; Ceci, L.R. Analysis by phage display selection and site-directed retromutagenesis of the Mustard Trypsin Inhibitor 2 reactive site. J. Plant Physiol. 2010, 167, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Surface presentation of protein epitopes using bacteriophage expression systems. Curr. Opin. Biotechnol. 1991, 2, 668–673. [Google Scholar] [CrossRef]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef]
- Liu, C.; Sathe, S.K. Food Allergen Epitope Mapping. J. Agric. Food Chem. 2018, 66, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Lehrer, S.B.; Reese, G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int. Arch. Allergy Immunol. 2002, 127, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Mei, X.-J.; Hu, M.-J.; Yang, Y.; Liu, M.; Li, M.-S.; Zhang, M.-L.; Cao, M.-J.; Liu, G.-M. Analysis of the Allergenic Epitopes of Tropomyosin from Mud Crab Using Phage Display and Site-Directed Mutagenesis. J. Agric. Food Chem. 2018, 66, 9127–9137. [Google Scholar] [CrossRef] [PubMed]
- The Immune Epitope Database. Available online: www.iedb.org. (accessed on 25 March 2019).
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Gaur, S.N.; Lavasa, S.; Arora, N. In vitro assessment of allergenicity features and localization of probable IgE binding regions. Food Chem. Toxicol. 2015, 84, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.S.; Vitale, M.; Fehlner, P.; King, T.P. cDNA cloning and primary structure of a white-face hornet venom allergen, antigen 5. Proc. Nat. Acad. Sci. USA 1988, 85, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Duchene, M.; Vrtala, S.; Birkner, T.; Ebner, C.; Hirschwehr, R.; Breitenbach, M.; Rumpold, H.; Scheiner, O.; Kraft, D. Recombinant allergens for immunoblot diagnosis of tree-pollen allergy. J. Allergy Clin. Immunol. 1991, 88, 889–894. [Google Scholar] [CrossRef]
- Klemans, R.J.B.; Knol, E.F.; Bruijnzeel-Koomen, C.A.F.M.; Knulst, A.C. The diagnostic accuracy of specific IgE to Ara h 6 in adults is as good as Ara h 2. Allergy 2014, 69, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Prickett, S.R.; Rolland, J.M.; O’Hehir, R.E. Immunoregulatory T cell epitope peptides: The new frontier in allergy therapy. Clin. Exp. Allergy 2015, 45, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, C.; Mine, Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-β-associated mechanisms. Clin. Exp. Allergy 2010, 40, 668–678. [Google Scholar] [CrossRef]
- Simms, E.; Rudulier, C.; Wattie, J.; Kwok, W.W.; James, E.A.; Moldaver, D.M.; Jordana, M.; Larché, M. Ara h 1 Peptide Immunotherapy Ameliorates Peanut-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2015, 135. [Google Scholar] [CrossRef]
- Simms, E.; Wattie, J.; Waserman, S.; Jordana, M.; Larché, M. Ara h 1 Peptide Immunotherapy Protects Against Peanut-Induced Anaphylaxis in a Dose-Dependent Manner. J. Allergy Clin. Immunol. 2016, 137. [Google Scholar] [CrossRef]
- Anzengruber, J.; Bublin, M.; Bönisch, E.; Janesch, B.; Tscheppe, A.; Braun, M.L.; Varga, E.-M.; Hafner, C.; Breiteneder, H.; Schäffer, C. Lactobacillus buchneri S-layer as carrier for an Ara h 2-derived peptide for peanut allergen-specific immunotherapy. Mol. Immunol. 2017, 85, 81–88. [Google Scholar] [CrossRef]
- Cocco, R.R.; Järvinen, K.-M.; Sampson, H.A.; Beyer, K. Mutational analysis of major, sequential IgE-binding epitopes in alpha s1-casein, a major cow’s milk allergen. J. Allergy Clin. Immunol. 2003, 112, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Bannon, G.A.; Cockrell, G.; Connaughton, C.; West, C.M.; Helm, R.; Stanley, J.S.; King, N.; Rabjohn, P.; Sampson, H.A.; Burks, A.W. Engineering, Characterization and in vitro Efficacy of the Major Peanut Allergens for Use in Immunotherapy. Int. Arch. Allergy Immunol. 2001, 124, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Rabjohn, P.; West, C.M.; Connaughton, C.; Sampson, H.A.; Helm, R.M.; Burks, A.W.; Bannon, G.A. Modification of Peanut Allergen Ara h 3: Effects on IgE Binding and T Cell Stimulation. Int. Arch. Allergy Immunol. 2002, 128, 15–23. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Helm, R.; Stanley, J.S.; Vieths, S.; Lüttkopf, D.; Hatahet, L.; Sampson, H.; Pons, L.; Burks, W.; Bannon, G.A. Allergenic characteristics of a modified peanut allergen. Mol. Nutr. Food Res. 2005, 49, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Bolhaar, S.T.H.P.; Zuidmeer, L.; Ma, Y.; Ferreira, F.; Bruijnzeel-Koomen, C.A.F.M.; Hoffmann-Sommergruber, K.; Ree, R.; Knulst, A.C. A mutant of the major apple allergen, Mal d 1, demonstrating hypo-allergenicity in the target organ by double-blind placebo-controlled food challenge: Mal d 1 demonstrating hypo-allergenicity in target organ by DBPCFC. Clin. Exp. Allergy 2005, 35, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Mine, Y.; Sasaki, E.; Zhang, J.W. Reduction of antigenicity and allergenicity of genetically modified egg white allergen, ovomucoid third domain. Biochem. Biophys. Res. Commun. 2003, 302, 133–137. [Google Scholar] [CrossRef]
- Horn, J.R.; Ramaswamy, S.; Murphy, K.P. Structure and energetics of protein-protein interactions: The role of conformational heterogeneity in OMTKY3 binding to serine proteases. J. Mol. Biol. 2003, 331, 497–508. [Google Scholar] [CrossRef]
- Rupa, P.; Mine, Y. Engineered recombinant ovomucoid third domain can desensitize Balb/c mice of egg allergy. Allergy 2006, 61, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, P.; Withanage-Dona, D.; Tang, M.L.K.; Doran, T.; Suphioglu, C. Hypoallergenic Variant of the Major Egg White Allergen Gal d 1 Produced by Disruption of Cysteine Bridges. Nutrients 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, I.; Bugajska-Schretter, A.; Linhart, B.; Verdino, P.; Keller, W.; Schulmeister, U.; Sperr, W.R.; Valent, P.; Peltre, G.; Quirce, S.; et al. A recombinant hypoallergenic parvalbumin mutant for immunotherapy of IgE-mediated fish allergy. J. Immunol. 2007, 178, 6290–6296. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Casado, C.; Garrido-Arandia, M.; Gamboa, P.; Blanca-López, N.; Canto, G.; Varela, J.; Cuesta-Herranz, J.; Pacios, L.F.; Díaz-Perales, A.; Tordesillas, L. Allergenic characterization of new mutant forms of Pru p 3 as new immunotherapy vaccines. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.K.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.C.; Chu, K.H. Immunization with Hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.H.; Ivanciuc, O.; Braun, W. Bioinformatics approaches to classifying allergens and predicting cross-reactivity. Immunol. Allergy Clin. North Am. 2007, 27, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Rasi, C.; Palazzo, P.; Scala, E. Allergen databases: Current status and perspectives. Curr. Allergy Asthma Rep. 2009, 9, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Radauer, C.; Breiteneder, H. Allergen databases—a critical evaluation. Allergy 2019. [Google Scholar] [CrossRef] [PubMed]
- Sircar, G.; Sarkar, D.; Bhattacharya, S.G.; Saha, S. Allergen Databases. In Immunoinformatics; De, R.K., Tomar, N., Eds.; Springer: New York, NY, USA, 2014; Volume 1184, pp. 165–181. [Google Scholar]
- Mari, A.; Scala, E.; Palazzo, P.; Ridolfi, S.; Zennaro, D.; Carabella, G. Bioinformatics applied to allergy: Allergen databases, from collecting sequence information to data integration. The Allergome platform as a model. Cell. Immunol. 2006, 244, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Allergen Nomenclature. Available online: www.allergen.org (accessed on 27 March 2019).
- Pomés, A.; Davies, J.M.; Gadermaier, G.; Hilger, C.; Holzhauser, T.; Lidholm, J.; Lopata, A.L.; Mueller, G.A.; Nandy, A.; Radauer, C.; et al. WHO/IUIS Allergen Nomenclature: Providing a common language. Mol. Immunol. 2018, 100, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Structural Database of Allergenic Proteins. Available online: https://fermi.utmb.edu (accessed on 3 April 2019).
- Ivanciuc, O.; Schein, C.H.; Braun, W. SDAP: Database and computational tools for allergenic proteins. Nucleic Acids Res. 2003, 31, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Allergome. Available online: http://www.allergome.org/ (accessed on 6 April 2019).
- Mari, A.; Scala, E. Allergome: A unifying platform. Arbeiten aus dem Paul-Ehrlich-Institut (Bundesamt fur Sera und Impfstoffe) zu Frankfurt aM 2006, 95, 29–39. [Google Scholar] [PubMed]
- Goodman, R.E.; Ebisawa, M.; Ferreira, F.; Sampson, H.A.; van Ree, R.; Vieths, S.; Baumert, J.L.; Bohle, B.; Lalithambika, S.; Wise, J.; et al. AllergenOnline: A peer-reviewed, curated allergen database to assess novel food proteins for potential cross-reactivity. Mol. Nutr. Food Res. 2016, 60, 1183–1198. [Google Scholar] [CrossRef]
- InformAll Allergenic Food Database. Available online: http://research.bmh.manchester.ac.uk/informall/allergenic-foods/ (accessed on 2 April 2019).
- Mills, E.N.C.; Jenkins, J.A.; Sancho, A.I.; Miles, S.; Madsen, C.; Valovirta, E.; Frewer, L. Food allergy information resources for consumers, industry and regulators. Arb Paul Ehrlich Inst Bundesamt Sera Impfstoffe Frankf A M 2006, 95, 17–25. [Google Scholar] [PubMed]
- The Database of Allergen Families. Available online: http://www.meduniwien.ac.at/allfam/ (accessed on 9 April 2019).
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852. [Google Scholar] [CrossRef]
- The Compare Allergen Database. Available online: https://comparedatabase.org/ (accessed on 15 April 2019).
- AllerBase. Available online: http://196.1.114.46:1800/AllerBase/Home.html (accessed on 15 April 2019).
- Kadam, K.; Karbhal, R.; Jayaraman, V.K.; Sawant, S.; Kulkarni-Kale, U. AllerBase: A comprehensive allergen knowledgebase. Database 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- The Health and Environmental Sciences Institute. Available online: https://hesiglobal.org/ (accessed on 16 April 2019).
- Syed, A.; Garcia, M.A.; Lyu, S.-C.; Bucayu, R.; Kohli, A.; Ishida, S.; Berglund, J.P.; Tsai, M.; Maecker, H.; O’Riordan, G.; et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J. Allergy Clin. Immunol. 2014, 133, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Mondoulet, L.; Dioszeghy, V.; Busato, F.; Plaquet, C.; Dhelft, V.; Bethune, K.; Leclere, L.; Daviaud, C.; Ligouis, M.; Sampson, H.; et al. Gata3 hypermethylation and Foxp3 hypomethylation are associated with sustained protection and bystander effect following epicutaneous immunotherapy in peanut-sensitized mice. Allergy 2019, 74, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, Y.; Guo, R.; Deng, M.; Deng, K.; Wang, Z.; Zhang, Y.; Wang, F. Generation of beta-lactoglobulin knock-out goats using CRISPR/Cas9. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
| Database | URL | Note | Ref. |
|---|---|---|---|
| WHO/IUIS | www.allergen.org/ [72] | a, b | [73] |
| SDAP | https://fermi.utmb.edu [74] | c | [75] |
| Allergome | http://www.allergome.org/ [76] | d | [77] |
| AllergenOnline | http://www.allergenonline.org/ [6] | [78] | |
| InformAll | http://research.bmh.manchester.ac.uk/informall/allergenic-foods/ [79] | [80] | |
| IEDB | https://www.iedb.org/ [43] | [44] | |
| AllFam | http://www.meduniwien.ac.at/allfam/ [81] | e, f | [82] |
| COMPARE | https://comparedatabase.org/ [83] | -- | |
| AllerBase | http://196.1.114.46:1800/AllerBase/Home.html [84] | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpicella, M.; Leoni, C.; Dileo, M.C.G.; Ceci, L.R. Progress in the Analysis of Food Allergens through Molecular Biology Approaches. Cells 2019, 8, 1073. https://doi.org/10.3390/cells8091073
Volpicella M, Leoni C, Dileo MCG, Ceci LR. Progress in the Analysis of Food Allergens through Molecular Biology Approaches. Cells. 2019; 8(9):1073. https://doi.org/10.3390/cells8091073
Chicago/Turabian StyleVolpicella, Mariateresa, Claudia Leoni, Maria C.G. Dileo, and Luigi R. Ceci. 2019. "Progress in the Analysis of Food Allergens through Molecular Biology Approaches" Cells 8, no. 9: 1073. https://doi.org/10.3390/cells8091073
APA StyleVolpicella, M., Leoni, C., Dileo, M. C. G., & Ceci, L. R. (2019). Progress in the Analysis of Food Allergens through Molecular Biology Approaches. Cells, 8(9), 1073. https://doi.org/10.3390/cells8091073