The Progress of Investigating the CD137-CD137L Axis as a Potential Target for Systemic Lupus Erythematosus
Abstract
1. The Molecular Structure and Physiology of CD137 and CD137 Ligand
1.1. Genetics and Molecular Structure of CD137 and CD137 Ligand
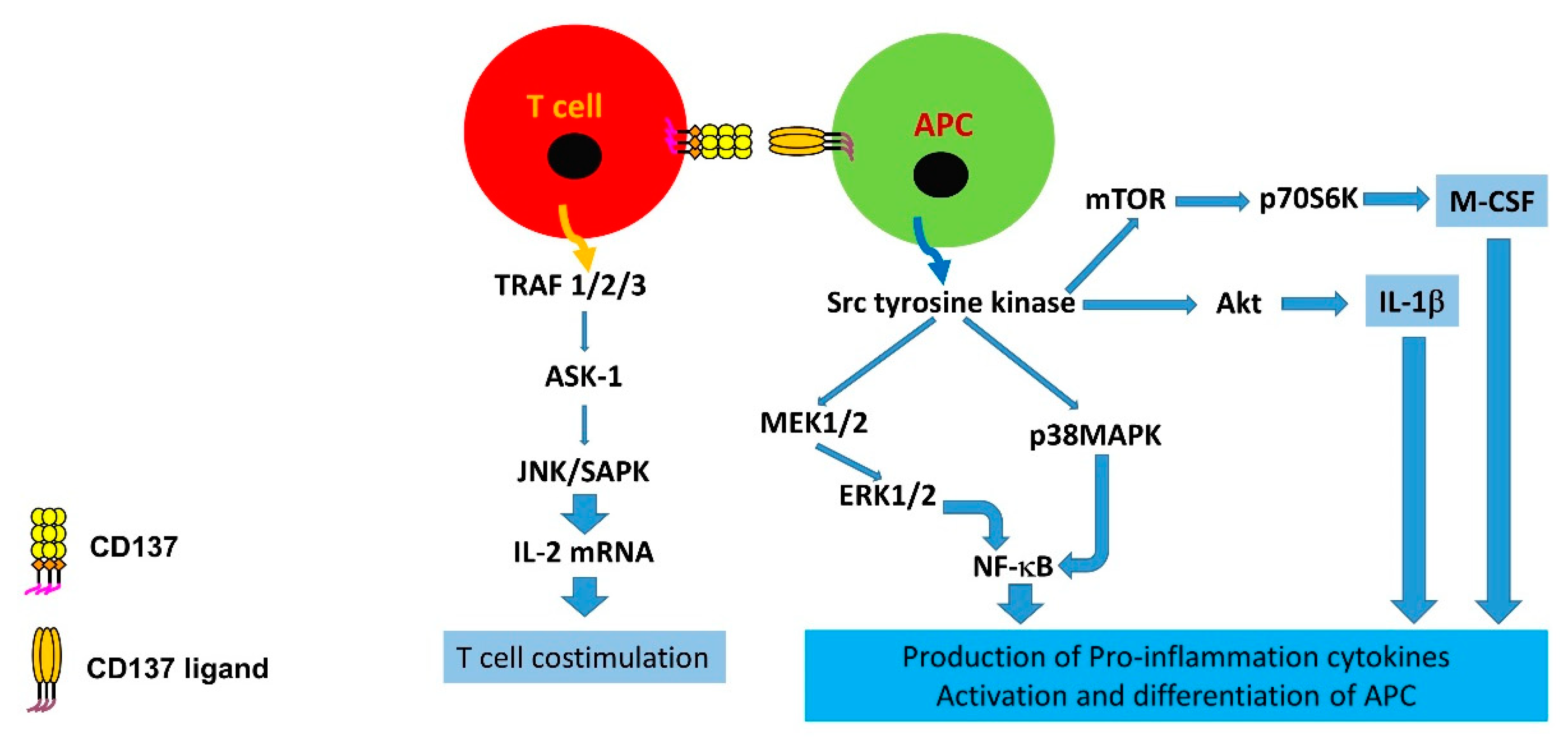
1.2. Immunological Alterations and Downstream Signalling of CD137 and CD137L
2. CD137 and CD137L in Various Autoimmune Conditions
3. CD137 and Systemic Lupus Erythematosus
3.1. Animal Studies
3.2. Human Studies
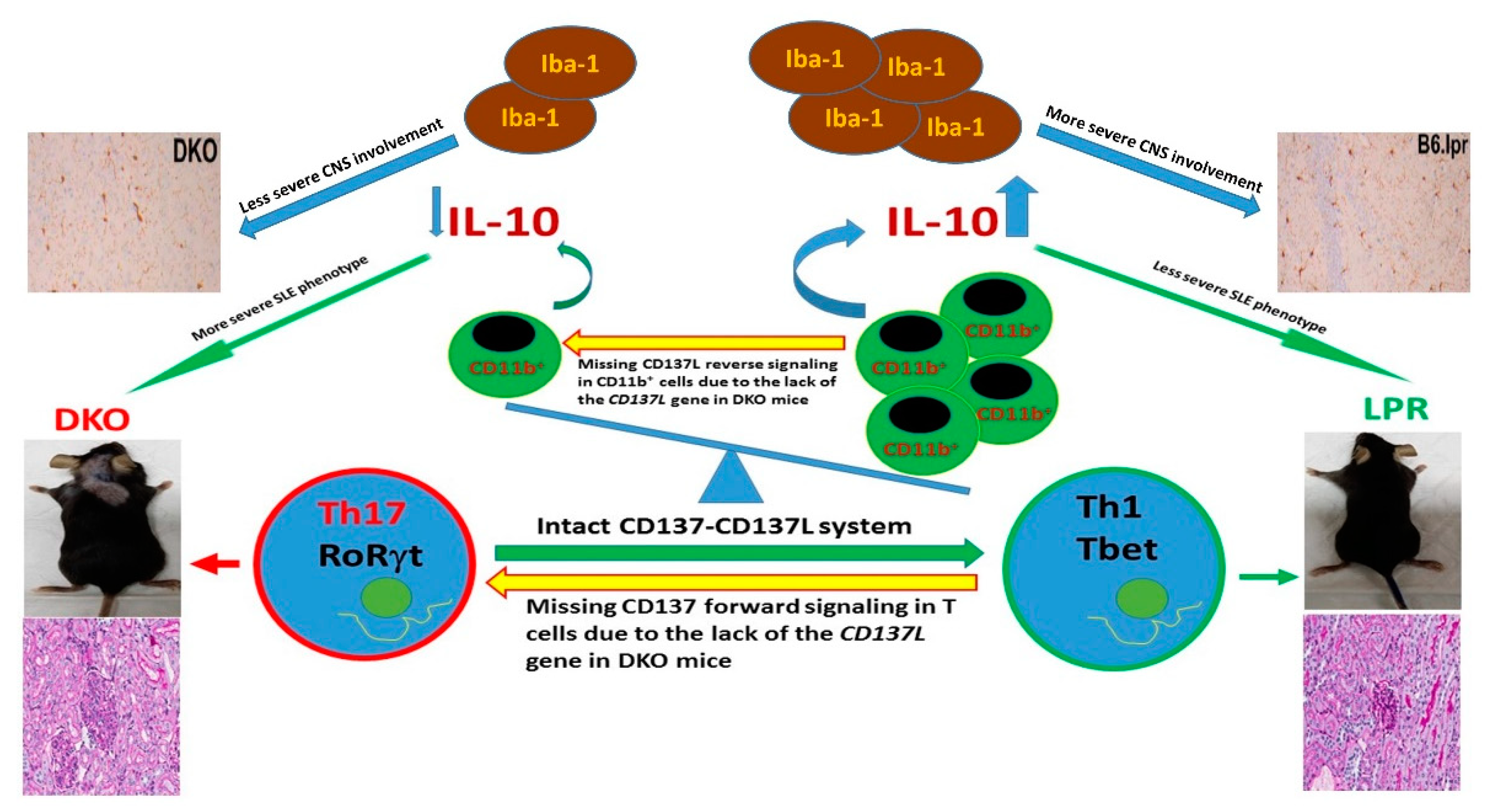
4. What Is the Impact of Knocking out the CD137L Gene on SLE?
5. Conclusions and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Schwarz, H.; Tuckwell, J.; Lotz, M. A receptor induced by lymphocyte activation (ILA): A new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene 1993, 134, 295–298. [Google Scholar] [CrossRef]
- Schwarz, H.; Arden, K.; Lotz, M. CD137, a member of the tumor necrosis factor receptor family is located on chromosome 1p36, in a cluster of related genes, and colocalizes with several malignancies. Biochem. Biophys. Res. Commun. 1997, 235, 699–703. [Google Scholar] [CrossRef]
- Gruss, H.J.; Dower, S.K. Tumor necrosis factor ligand superfamily: Involvement in the pathology of malignant lymphomas. Blood 1995, 85, 3378–3404. [Google Scholar]
- DeBenedette, M.A.; Randall Chu, N.; Pollok, K.E.; Hurtado, J.; Wade, W.F.; Kwon, B.S.; Watts, T.H. Role of 4-1BB ligand in costimulation of T lymphocyte growth and its upregulation on M12 B lymphomas by camp. J. Exp. Med. 1995, 181, 985–992. [Google Scholar] [CrossRef]
- Hurtado, J.C.; Kim, S.H.; Pollok, K.E.; Lee, Z.H.; Kwon, B.S. Potential role of 4-1BB in T cell activation: Comparison with the costimulatory molecule CD28. J. Immunol. 1995, 155, 3360–3367. [Google Scholar]
- Setareh, M.; Schwarz, H.; Lotz, M. A mRNA variant encoding a soluble form of 4-1BB, a member of the murine NGF/TNF receptor family. Gene 1995, 164, 311–315. [Google Scholar] [CrossRef]
- Michel, J.; Langstein, J.; Hofstädter, F.; Schwarz, H. A soluble form of CD137 (ILA/4-1BB) is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur. J. Immunol. 1998, 28, 290–295. [Google Scholar] [CrossRef]
- Alderson, M.R.; Smith, C.A.; Tough, T.W.; Davis-Smith, T.; Armitage, R.J.; Falk, B.; Roux, E.; Baker, E.; Sutherland, G.R.; Din, W.S.; et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 1994, 24, 2219–2227. [Google Scholar] [CrossRef]
- Kwon, B.S.; Weissman, S.M. cDNA sequences of two inducible T-cell genes. Proc. Natl. Acad. Sci. USA 1989, 86, 1963–1967. [Google Scholar] [CrossRef]
- Shuford, W.W.; Klussman, K.; Tritchler, D.D.; Loo, D.T.; Chalupny, J.; Siadak, A.W.; Brown, T.J.; Emswiler, J.; Raecho, H.; Larsen, C.P.; et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997, 186, 47–55. [Google Scholar] [CrossRef]
- Mak, A.; Kow, N.Y. The pathology of t cells in systemic lupus erythematosus. J. Immunol. Res. 2014, 2014, 419029. [Google Scholar] [CrossRef]
- Kwon, B.; Kim, B.S.; Cho, H.R.; Park, J.E.; Kwon, B.S. Involvement of tumor necrosis factor receptor superfamily (TNFRSF) members in the pathogenesis of inflammatory diseases. Exp. Mol. Med. 2003, 35, 8–16. [Google Scholar] [CrossRef]
- Watts, T.H. Tnf/Tnfr family members in costimulation of t cell responses. Annu. Rev. Immunol. 2005, 23, 23–68. [Google Scholar] [CrossRef]
- Bukczynski, J.; Wen, T.; Watts, T.H. Costimulation of human CD28- T cells by 4-1BB ligand. Eur. J. Immunol. 2003, 33, 446–454. [Google Scholar] [CrossRef][Green Version]
- Langstein, J.; Michel, J.; Fritsche, J.; Kreutz, M.; Andreesen, R.; Schwarz, H. CD137, (ILA/4-1BB), a member of the TNF receptor family regulates monocyte activation via reverse signaling. J. Immunol. 1998, 160, 2488–2494. [Google Scholar]
- Shao, Z.; Schwarz, H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J. Leukoc. Biol. 2011, 89, 21–29. [Google Scholar] [CrossRef]
- Cannons, J.L.; Hoeflich, K.P.; Woodgett, J.R.; Watts, T.H. Role of the stress kinase pathway in signaling via the T cell costimulatory receptor 4-1BB. J. Immunol. 1999, 163, 2990–2998. [Google Scholar]
- Söllner, L.; Shaqireen Kwajah, M.M.; Wu, J.T.; Schwarz, H. Signal transduction mechanisms of CD137 ligand in human monocytes. Cell. Signal. 2007, 19, 1899–1908. [Google Scholar] [CrossRef]
- Kim, D.K.; Sang, C.L.; Lee, H.W. CD137 ligand-mediated reverse signals increase cell viability and cytokine expression in murine myeloid cells: Involvement of mTOR/p70S6 kinase and Akt. Eur. J. Immunol. 2009, 39, 2617–2628. [Google Scholar] [CrossRef]
- Zeng, Q.; Mallilankaraman, K.; Schwarz, H. Increased akt-driven glycolysis is the basis for the higher potency of CD137L-DCs. Front. Immunol. 2019, 10, 868. [Google Scholar] [CrossRef]
- Kwajah, M.M.S.; Schwarz, H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur. J. Immunol. 2010, 40, 1938–1949. [Google Scholar] [CrossRef]
- Harfuddin, Z.; Kwajah, S.; Chong Nyi Sim, A.; MacAry, P.A.; Schwarz, H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. OncoImmunology 2013, 2, e26859. [Google Scholar] [CrossRef]
- Dharmadhikari, B.; Nickles, E.; Harfuddin, Z.; Ishak, N.D.B.; Zeng, Q.; Bertoletti, A.; Schwarz, H. CD137L-dendritic cells induce potent responses against cancer-associated viruses and polarise human CD8+ T cells to a Tc1 phenotype. Cancer Immunol. Immunother. 2018, 67, 893–905. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, D.; Shao, Z.; Martinez Gómez, J.M.; Schwarz, H. Species difference of CD137 ligand signalling in human and murine monocytes. PLoS ONE 2011, 6, e16129. [Google Scholar] [CrossRef]
- Holmdahl, R.; Bockermann, R.; Bäcklund, J.; Yamada, H. The molecular pathogenesis of collagen-induced arthritis in mice—A model for rheumatoid arthritis. Ageing Res. Rev. 2002, 1, 135–147. [Google Scholar] [CrossRef]
- Choi, B.K.; Asai, T.; Vinay, D.S.; Kim, Y.H.; Kwon, B.S. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine 2006, 34, 233–242. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, B.K.; Shin, S.M.; Kim, C.H.; Oh, H.S.; Park, S.H.; Lee, D.G.; Lee, M.J.; Kim, K.H.; Vinay, D.S.; et al. 4-1BB triggering ameliorates experimental autoimmune encephalomyelitis by modulating the balance between Th17 and regulatory t cells. J. Immunol. 2011, 187, 1120–1128. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Dharmadhikari, B.; Wu, M.; Abdullah, N.S.; Rajendran, S.; Ishak, N.D.; Nickles, E.; Harfuddin, Z.; Schwarz, H. CD137 and CD137L signals are main drivers of type 1, cell-mediated immune responses. OncoImmunology 2016, 5, e1113367. [Google Scholar] [CrossRef]
- Xu, M.M.; Ménoret, A.; Nicholas, S.A.E.; Günther, S.; Sundberg, E.J.; Zhou, B.; Rodriguez, A.; Murphy, P.A.; Vella, A.T. Direct CD137 costimulation of CD8 T cells promotes retention and innate-like function within nascent atherogenic foci. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1480–H1494. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, B.K.; Kang, W.J.; Kim, K.H.; Kang, S.W.; Mellor, A.L.; Munn, D.H.; Kwon, B.S. IFN-γ-indoleamine-2,3 dioxygenase acts as a major suppressive factor in 4-1BB-mediated immune suppression in vivo. J. Leukoc. Biol. 2009, 85, 817–825. [Google Scholar] [CrossRef]
- Vinay, D.S.; Choi, J.H.; Kim, J.D.; Choi, B.K.; Kwon, B.S. Role of endogenous 4-1BB in the development of systemic lupus erythematosus. Immunology 2007, 122, 394–400. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.M.; Subudhi, S.K.; Chen, J.; Koka, R.; Chen, L.; Fu, Y.X. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat. Med. 2002, 8, 1405–1413. [Google Scholar] [CrossRef]
- Foell, J.; Strahotin, S.; O’Neil, S.P.; McCausland, M.M.; Suwyn, C.; Haber, M.; Chander, P.N.; Bapat, A.S.; Yan, X.J.; Chiorazzi, N.; et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. J. Clin. Investig. 2003, 111, 1505–1518. [Google Scholar] [CrossRef]
- Shao, Z.; Sun, F.; Koh, D.R.; Schwarz, H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol. Immunol. 2008, 45, 3990–3999. [Google Scholar] [CrossRef]
- Jung, H.W.; Choi, S.W.; Choi, J.I.L.; Kwon, B.S. Serum concentrations of soluble 4-1BB, and 4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp. Mol. Med. 2004, 36, 13–22. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Zordan, T.; Tsokos, G.C.; Datta, S.K. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: Isolation of CD4-8- T helper cell lines that express the γδ T-cell antigen receptor. Proc. Natl. Acad. Sci. USA 1990, 87, 7020–7024. [Google Scholar] [CrossRef]
- Busser, B.W.; Adair, B.S.; Erikson, J.; Laufer, T.M. Activation of diverse repertoires of autoreactive T cells enhances the loss of anti-dsDNA B cell tolerance. J. Clin. Investig. 2003, 112, 1361–1371. [Google Scholar] [CrossRef]
- Kim, E.C.; Moon, J.H.; Kang, S.W.; Kwon, B.; Lee, H.W. TMEM126A, a CD137 ligand binding protein, couples with the TLR4 signal transduction pathway in macrophages. Mol. Immunol. 2015, 64, 244–251. [Google Scholar] [CrossRef]
- Mak, A.; Dharmadhikari, B.; Kow, N.Y.; Thamboo, T.P.; Tang, Q.; Wong, L.W.; Sajikumar, S.; Wong, H.Y.; Schwarz, H. Deletion of CD137 ligand exacerbates renal and cutaneous but alleviates cerebral manifestations in Lupus. Front. Immunol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Krebs, C.F.; Schmidt, T.; Riedel, J.H.; Panzer, U. T helper type 17 cells in immune-mediated glomerular disease. Nat. Rev. Nephrol. 2017, 13, 647–659. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 73. [Google Scholar] [CrossRef]
- Baglaenko, Y.; Manion, K.P.; Chang, N.H.; Gracey, E.; Loh, C.; Wither, J.E. IL-10 production is critical for sustaining the expansion of CD5+ B and NKT cells and restraining autoantibody production in congenic lupus-prone mice. PLoS ONE 2016, 11, e0150515. [Google Scholar] [CrossRef]
- Kow, N.Y.; Mak, A. Costimulatory pathways: Physiology and potential therapeutic manipulation in systemic lupus Erythematosus. Clin. Dev. Immunol. 2013, 2013, 245928. [Google Scholar] [CrossRef]
| Mouse Model/Human (First Author, Year) | Intervention | Phenotypic Changes | CD4 | CD8 | DNTC or B Cells | AutoAb | Treg | sCD137 |
|---|---|---|---|---|---|---|---|---|
| MRLlpr (Vinay et al., 2007) | Knocking out CD137 gene | Exacerbation of SLE, ↓survival | ↑ | ↔ | ↑/↑ in function | ↑ | ND | ND |
| MRLlpr (Sun et al., 2002) | Agonistic anti-CD137Ab | Amelioration of SLE, ↑survival | ↓ | ↑ | ↓/↓ | ↓ | ND | ND |
| NZB/W F1 (Foell et al., 2003) | Agonistic anti-CD137Ab | Amelioration of SLE, ↑survival | ↔ | ↔ | ND/↔ | ↓ | ↑ | ND |
| BALB/c and B6/lpr or gld −/− (Shao et al., 2008) | nil | ND | ND | ND | ND | ND | ND | Increase in lpr−/− and gld−/− mice regardless of background |
| B6.MRLlpr (Mak et al., 2019) | Knocking out CD137L gene | Exacerbation of nephritis & dermatitis, ↓survival but amelioration of CNS inflammation | ↔, but ↑Th17 | ↓activated CD8+ T cells | ↔ | ND | ↔ | ND |
| Human (Jung et al., 2004) | nil | ND | ND | ND | ND | ND | ND | sCD137 and sCD137L increase in SLE and they correlate with each other |
 unchanged; ↓ decrease.
unchanged; ↓ decrease.© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mak, A.; Schwarz, H. The Progress of Investigating the CD137-CD137L Axis as a Potential Target for Systemic Lupus Erythematosus. Cells 2019, 8, 1044. https://doi.org/10.3390/cells8091044
Mak A, Schwarz H. The Progress of Investigating the CD137-CD137L Axis as a Potential Target for Systemic Lupus Erythematosus. Cells. 2019; 8(9):1044. https://doi.org/10.3390/cells8091044
Chicago/Turabian StyleMak, Anselm, and Herbert Schwarz. 2019. "The Progress of Investigating the CD137-CD137L Axis as a Potential Target for Systemic Lupus Erythematosus" Cells 8, no. 9: 1044. https://doi.org/10.3390/cells8091044
APA StyleMak, A., & Schwarz, H. (2019). The Progress of Investigating the CD137-CD137L Axis as a Potential Target for Systemic Lupus Erythematosus. Cells, 8(9), 1044. https://doi.org/10.3390/cells8091044






