An RNA Metabolism and Surveillance Quartet in the Major Histocompatibility Complex
Abstract
1. Introduction
2. NELF-E or RD
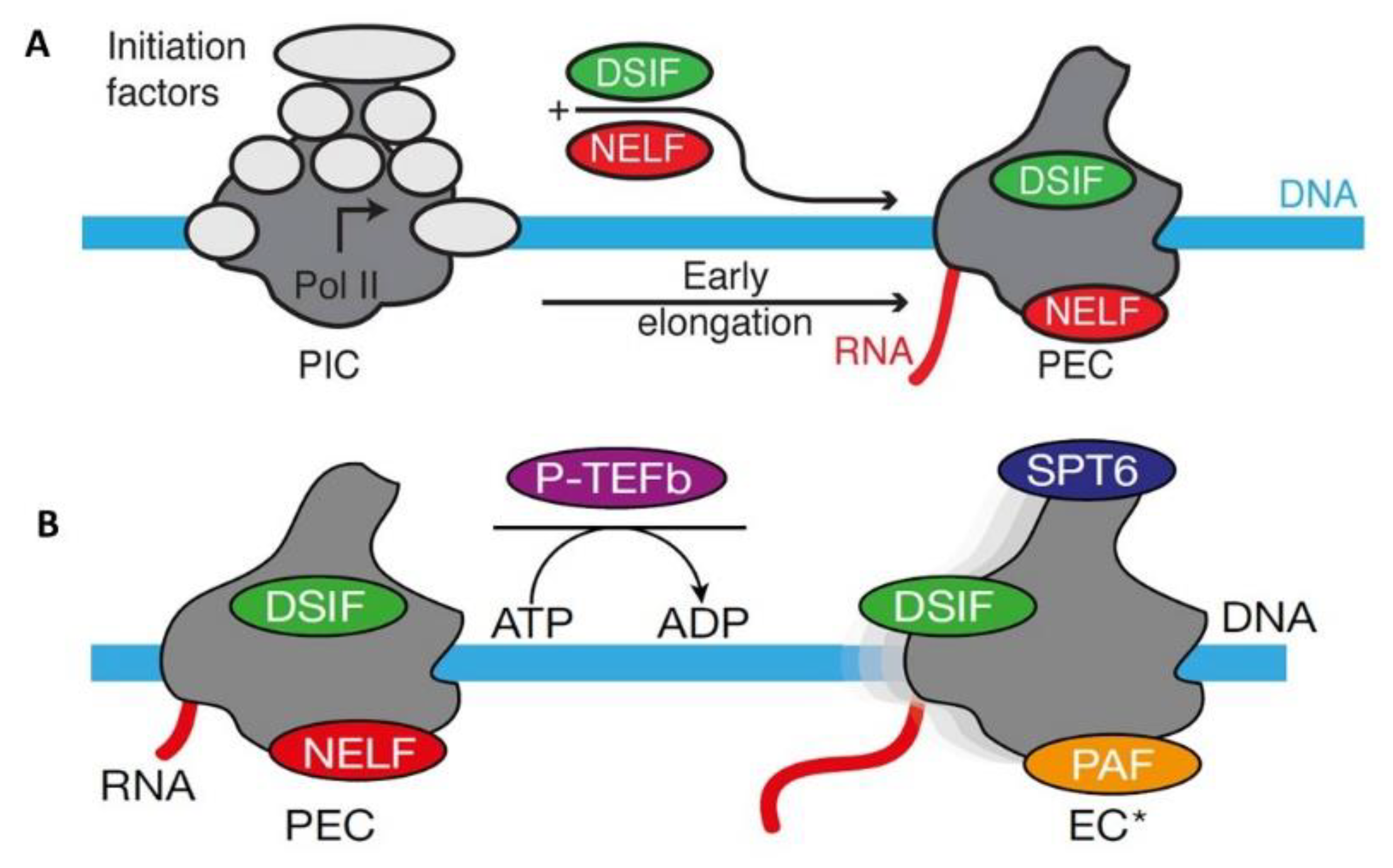
To Start a Long Journey, Get Prepared, Do a Test Run, Pause to Check Things Out, Modify and Let Go: The Amazing RD or Negative Elongation Factor Subunit E (NELF-E) in Gene Transcription
A Gene Within a Gene: miR-1236 in Intron 3 of NELF-E
3. SKIV2L or SKI2W
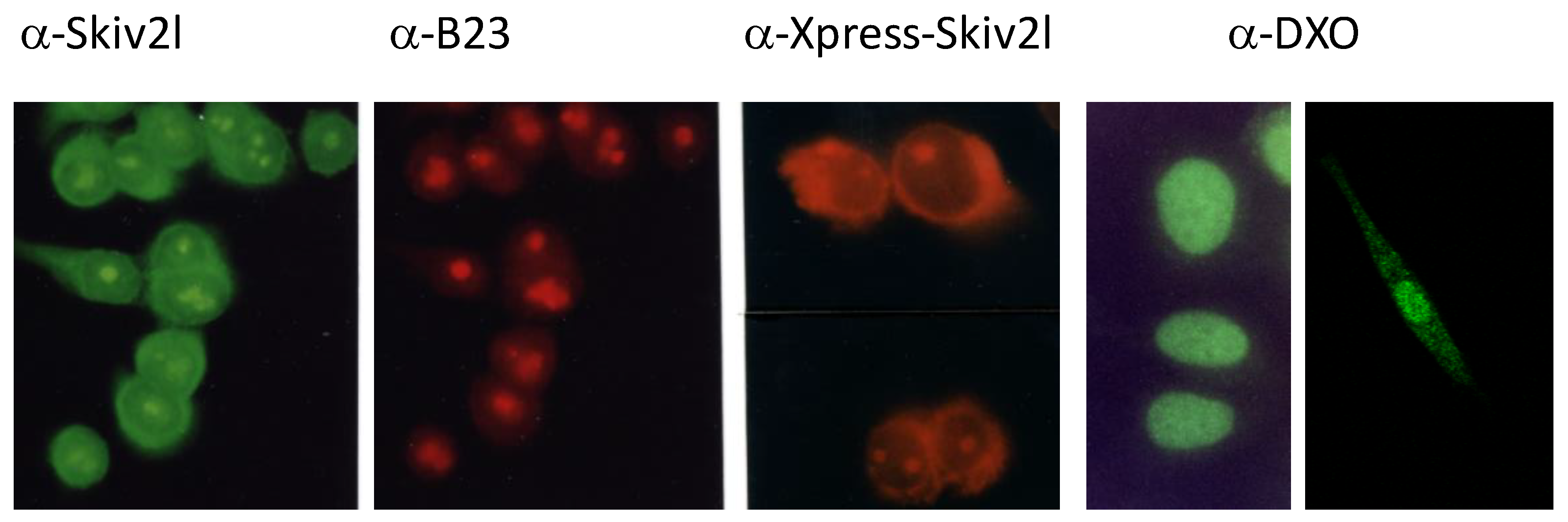
Quality Controls I—Clearing Broken, Bad, Used and Viral RNA Products in the Cell Requires An Engine to Unwind Helical structures of RNA for Degradation—The Helicase for Cytoplasmic Exosomes SKIV2L (Ski2w or DDX13)
4. DXO or DOM3Z
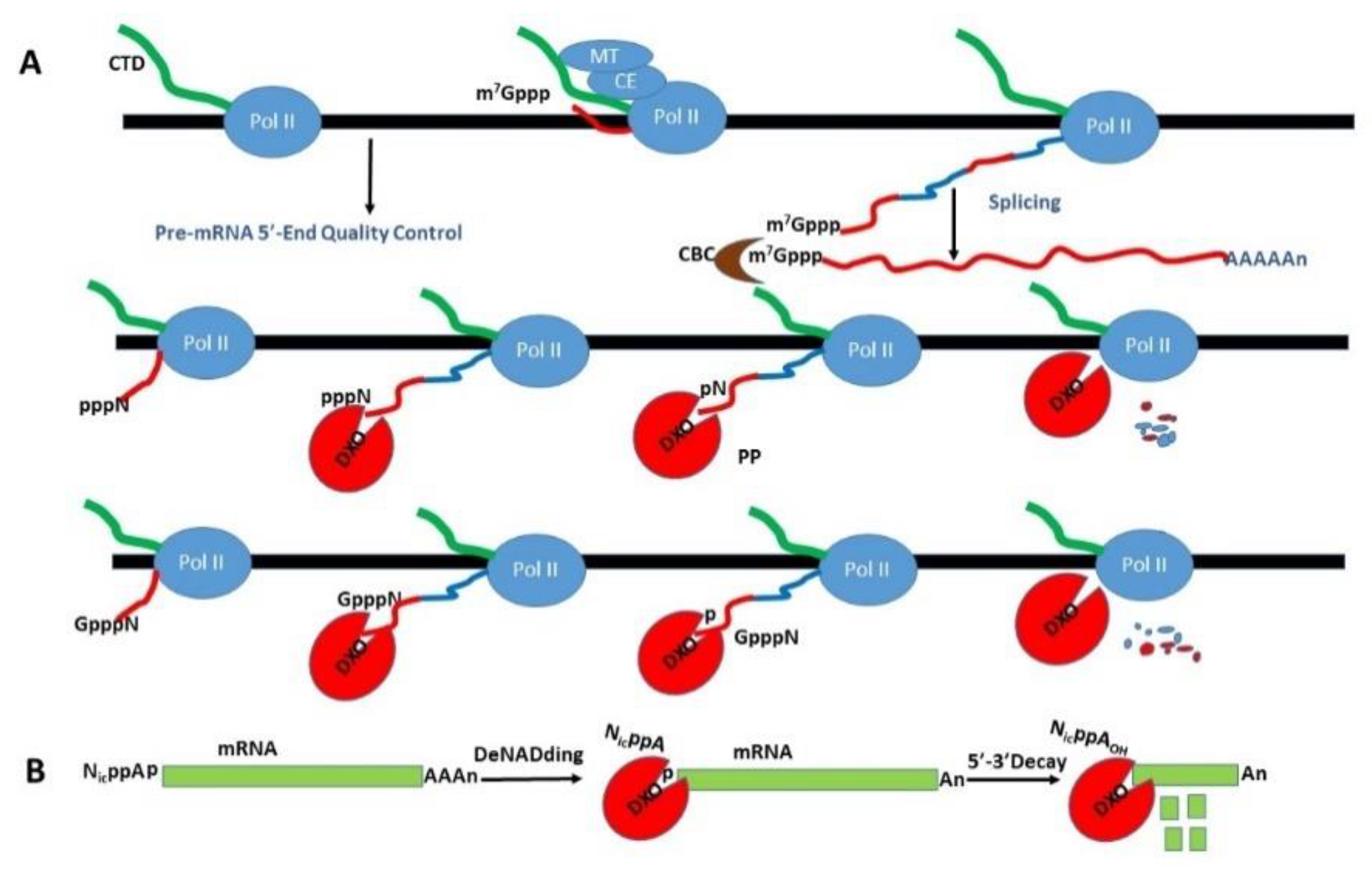
Quality Controls II—Destroy Disqualified Products—DXO (DOM3Z) to Degrade Miscapped or Uncapped RNA from the Start to the End
5. STK19 (RP1/G11)
An On-and-Off Switch—The Nuclear Kinase STK19 (RP1 or G11) Whose Malfunction Can Cause Melanoma
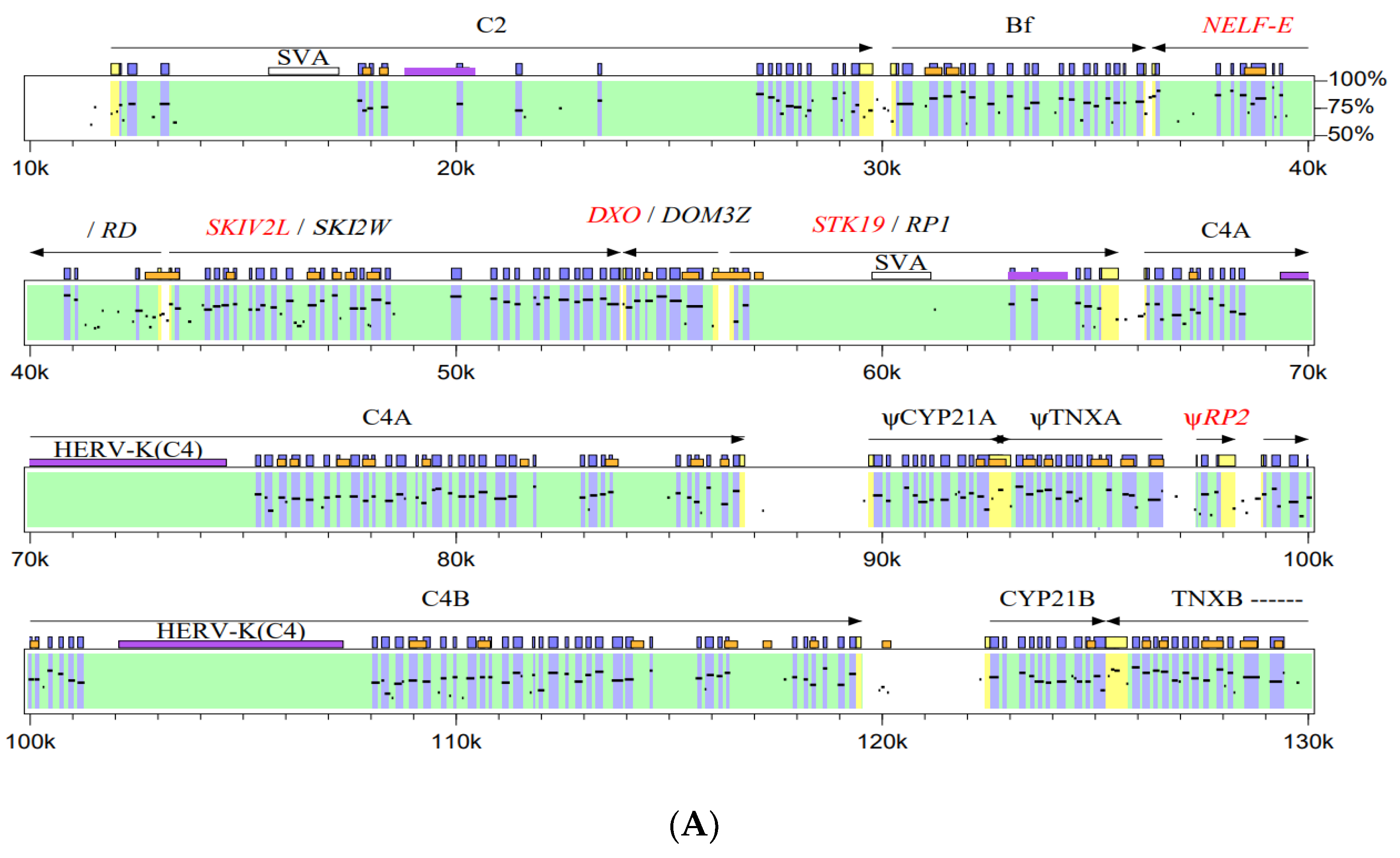
6. The Physical Linkage of NELF-E and SKIV2L (RD and SKI2W), DXO and STK19 (DOM3Z and RP1) and the Emergence of NSDK Quartet in the MHC
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARS2 | arsenite resistance protein 2; |
| CBC | cap-binding complex; |
| CDK9 | cyclin dependent kinase 9; |
| CSB | Cockayne syndrome B protein (also named ERCC6); |
| DRB | 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole; |
| DSIF | DRB sensitivity inducing factor; |
| DXO | decapping exoribonuclease; |
| EC * | activated elongation complex; |
| EMT | epithelial–mesenchymal transition; |
| HCC | hepatocellular carcinoma; |
| IRE-1 | Inositol-requiring enzyme 1; |
| MDA5 | melanoma differentiation-associated protein 5; |
| MK2 | MAPK-activated protein kinase II; |
| NSDK | NELF-E (RD), SKIV2L (SKI2W), DXO (DOM3Z) and STK19; |
| NELF | negative elongation factor; |
| P-TEFb | positive transcription elongation factor b; |
| PAF | Pol II associated factor; |
| PARP-1 | poly-ADP-ribose polymerase 1; |
| PEC | paused elongation complex; |
| PHAX | phosphorylated adaptor for RNA export; |
| RD | a gene with Arg-Asp repeats, also known as NELF-E; |
| RIG-I | retinoic acid-inducible gene 1; |
| RLRS | RIG-I-like receptors; |
| RNAPII | RNA polymerase II; |
| RP | the gene upstream of complement C4, also known as STK19; |
| RRM | RNA recognition and binding domain; |
| SKI | yeast genes by which mutations lead to superkilling phenotypes after viral infection; |
| snRNA | small nuclear RNA; |
| snoRNA | small nucleolar RNA; |
| SPT6 | yeast homolog of suppressor for transposon Ty insertion, 6; |
| UPR | unfolded protein response; |
| TAR | transactivation response element in HIV; |
| ZEB1 | zinc-finger E-box binding homeobox 1 |
References
- Carroll, M.C.; Campbell, R.D.; Bentley, D.R.; Porter, R.R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature 1984, 307, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Horton, R.; Wilming, L.; Rand, V.; Lovering, R.C.; Bruford, E.A.; Khodiyar, V.K.; Lush, M.J.; Povey, S.; Talbot, C.C., Jr.; Wright, M.W.; et al. Gene map of the extended human MHC. Nat. Rev. Genet. 2004, 5, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.W.; Cooper, R.G.; Vencovsky, J.; Rider, L.G.; Danko, K.; Wedderburn, L.R.; Lundberg, I.E.; Pachman, L.M.; Reed, A.M.; Ytterberg, S.R.; et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 2013, 65, 3239–3247. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R.; Leelayuwat, C.; Gaudieri, S.; Tay, G.; Hui, J.; Cattley, S.; Martinez, P.; Kulski, J. Genomics of the major histocompatibility complex: haplotypes, duplication, retroviruses and disease. Immunol. Rev. 1999, 167, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, G.; Bahram, S. Genetics of the central MHC. Curr. Opin. Immunol. 2004, 16, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K.E.; Wu, Y.L.; Yang, Y.; Spencer, C.H.; Hauptmann, G.; Hebert, L.A.; Atkinson, J.P.; Yu, C.Y. Early Components of the Complement Classical Activation Pathway in Human Systemic Autoimmune Diseases. Front. Immunol. 2016, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Savelli, S.L.; Roubey, R.A.S.; Kitzmiller, K.J.; Zhou, D.; Nagaraja, H.N.; Mulvihill, E.; Barbar-Smiley, F.; Ardoin, S.P.; Wu, Y.L.; Yu, C.Y. Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) Among Patients With Antiphospholipid Antibodies (aPL). Front. Immunol. 2019, 10, 885. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Wu, L.C.; Morley, B.J.; Campbell, R.D. Cell-specific expression of the human complement protein factor B gene: evidence for the role of two distinct 5’-flanking elements. Cell 1987, 48, 331–342. [Google Scholar] [CrossRef]
- Shen, L.; Wu, L.C.; Sanlioglu, S.; Chen, R.; Mendoza, A.R.; Dangel, A.W.; Carroll, M.C.; Zipf, W.B.; Yu, C.Y. Structure and genetics of the partially duplicated gene RP located immediately upstream of the complement C4A and the C4B genes in the HLA class III region. Molecular cloning, exon-intron structure, composite retroposon, and breakpoint of gene duplication. J. Biol. Chem. 1994, 269, 8466–8476. [Google Scholar]
- Yu, C.Y. The complete exon-intron structure of a human complement component C4A gene. DNA sequences, polymorphism, and linkage to the 21-hydroxylase gene. J. Immunol. 1991, 146, 1057–1066. [Google Scholar] [PubMed]
- Levi-Strauss, M.; Carroll, M.C.; Steinmetz, M.; Meo, T. A previously undetected MHC gene with an unusual periodic structure. Science 1988, 240, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Dangel, A.W.; Shen, L.; Mendoza, A.R.; Wu, L.C.; Yu, C.Y. Human helicase gene SKI2W in the HLA class III region exhibits striking structural similarities to the yeast antiviral gene SKI2 and to the human gene KIAA0052: emergence of a new gene family. Nucleic Acids Res. 1995, 23, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shen, L.; Dangel, A.W.; Wu, L.C.; Yu, C.Y. Four ubiquitously expressed genes, RD (D6S45)-SKI2W (SKIV2L)-DOM3Z-RP1 (D6S60E), are present between complement component genes factor B and C4 in the class III region of the HLA. Genomics 1998, 53, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, C.Y. Organizations and gene duplications of the human and mouse MHC complement gene clusters. Exp. Clin Immunogenet. 2000, 17, 1–17. [Google Scholar] [CrossRef]
- Yu, C.Y.; Yang, Z.; Blanchong, C.A.; Miller, W. The human and mouse MHC class III region: a parade of 21 genes at the centromeric segment. Immunol. Today 2000, 21, 320–328. [Google Scholar]
- Yang, Z.; Qu, X.; Yu, C.Y. Features of the two gene pairs RD-SKI2W and DOM3Z-RP1 located between complement component genes factor B and C4 at the MHC class III region. Front. Biosci. 2001, 6, D927–D935. [Google Scholar] [CrossRef]
- Cheng, J.; Macon, K.J.; Volanakis, J.E. cDNA cloning and characterization of the protein encoded by RD, a gene located in the class III region of the human major histocompatibility complex. Biochem. J. 1993, 294, 589–593. [Google Scholar] [CrossRef]
- Surowy, C.S.; Hoganson, G.; Gosink, J.; Strunk, K.; Spritz, R.A. The human RD protein is closely related to nuclear RNA-binding proteins and has been highly conserved. Gene 1990, 90, 299–302. [Google Scholar] [CrossRef]
- Speiser, P.W.; White, P.C. Structure of the human RD gene: A highly conserved gene in the class III region of the major histocompatibility complex. DNA 1989, 8, 745–751. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Takagi, T.; Wada, T.; Yano, K.; Furuya, A.; Sugimoto, S.; Hasegawa, J.; Handa, H. NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 1999, 97, 41–51. [Google Scholar] [CrossRef]
- Fujinaga, K.; Irwin, D.; Huang, Y.; Taube, R.; Kurosu, T.; Peterlin, B.M. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol. Cell Biol. 2004, 24, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Inukai, N.; Narita, T.; Wada, T.; Handa, H. Evidence that negative elongation factor represses transcription elongation through binding to a DRB sensitivity-inducing factor/RNA polymerase II complex and RNA. Mol. Cell Biol. 2002, 22, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Yamaguchi, Y.; Yano, K.; Sugimoto, S.; Chanarat, S.; Wada, T.; Kim, D.K.; Hasegawa, J.; Omori, M.; Inukai, N.; et al. Human transcription elongation factor NELF: identification of novel subunits and reconstitution of the functionally active complex. Mol. Cell Biol. 2003, 23, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.; Yung, T.M.; Yamamoto, J.; Tsuboi, Y.; Tanabe, H.; Tanaka, K.; Yamaguchi, Y.; Handa, H. NELF interacts with CBC and participates in 3’ end processing of replication-dependent histone mRNAs. Mol. Cell 2007, 26, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Schulze, W.M.; Cusack, S. Structural basis for mutually exclusive co-transcriptional nuclear cap-binding complexes with either NELF-E or ARS2. Nat. Commun. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N.; Neumann, L.; Wenzel, S.; Schweimer, K.; Rosch, P.; Wohrl, B.M. Structural studies on the RNA-recognition motif of NELF E, a cellular negative transcription elongation factor involved in the regulation of HIV transcription. Biochem. J. 2006, 400, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Pagano, J.M.; Kwak, H.; Waters, C.T.; Sprouse, R.O.; White, B.S.; Ozer, A.; Szeto, K.; Shalloway, D.; Craighead, H.G.; Lis, J.T. Defining NELF-E RNA binding in HIV-1 and promoter-proximal pause regions. PLoS Genet. 2014, 10, e1004090. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.A.; Zhang, Y.; Jiang, H.; Hussey, K.M.; Shrimp, J.H.; Lin, H.; Schwede, F.; Yu, Y.; Kraus, W.L. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science 2016, 353, 45–50. [Google Scholar] [CrossRef]
- Guo, J.; Price, D.H. RNA polymerase II transcription elongation control. Chem. Rev. 2013, 113, 8583–8603. [Google Scholar] [CrossRef]
- Awwad, S.W.; Abu-Zhayia, E.R.; Guttmann-Raviv, N.; Ayoub, N. NELF-E is recruited to DNA double-strand break sites to promote transcriptional repression and repair. EMBO Rep. 2017, 18, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.E.; Voigt, A.; Tollenaere, M.A.X.; Sahu, S.K.; Juretschke, T.; Kreim, N.; Mailand, N.; Choudhary, C.; Bekker-Jensen, S.; Akutsu, M.; et al. p38-MK2 signaling axis regulates RNA metabolism after UV-light-induced DNA damage. Nat. Commun. 2018, 9, 1017. [Google Scholar] [CrossRef]
- Vos, S.M.; Farnung, L.; Urlaub, H.; Cramer, P. Structure of paused transcription complex Pol II-DSIF-NELF. Nature 2018, 560, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.M.; Farnung, L.; Boehning, M.; Wigge, C.; Linden, A.; Urlaub, H.; Cramer, P. Structure of activated transcription complex Pol II-DSIF-PAF-SPT6. Nature 2018, 560, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Iida, M.; Iizuka, N.; Tsunedomi, R.; Tsutsui, M.; Yoshida, S.; Maeda, Y.; Tokuhisa, Y.; Sakamoto, K.; Yoshimura, K.; Tamesa, T.; et al. Overexpression of the RD RNA binding protein in hepatitis C virus-related hepatocellular carcinoma. Oncol. Rep. 2012, 28, 728–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dang, H.; Takai, A.; Forgues, M.; Pomyen, Y.; Mou, H.; Xue, W.; Ray, D.; Ha, K.C.H.; Morris, Q.D.; Hughes, T.R.; et al. Oncogenic Activation of the RNA Binding Protein NELFE and MYC Signaling in Hepatocellular Carcinoma. Cancer Cell 2017, 32, 101–114.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Teng, S.C.; Cheng, T.H.; Wu, K.J. miR-1236 regulates hypoxia-induced epithelial-mesenchymal transition and cell migration/invasion through repressing SENP1 and HDAC3. Cancer Lett. 2016, 378, 59–67. [Google Scholar] [CrossRef]
- Butkyte, S.; Ciupas, L.; Jakubauskiene, E.; Vilys, L.; Mocevicius, P.; Kanopka, A.; Vilkaitis, G. Splicing-dependent expression of microRNAs of mirtron origin in human digestive and excretory system cancer cells. Clin. Epigenetics 2016, 8, 33. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Zhang, W.; Li, Y.; Dong, R.; Zhang, Q.; Yang, Q.; Yuan, C.; Shen, K.; et al. miR-1236-3p represses the cell migration and invasion abilities by targeting ZEB1 in high-grade serous ovarian carcinoma. Oncol. Rep. 2014, 31, 1905–1910. [Google Scholar] [CrossRef]
- An, J.X.; Ma, M.H.; Zhang, C.D.; Shao, S.; Zhou, N.M.; Dai, D.Q. miR-1236-3p inhibits invasion and metastasis in gastric cancer by targeting MTA2. Cancer Cell Int. 2018, 18, 66. [Google Scholar] [CrossRef]
- Wang, C.; Tang, K.; Li, Z.; Chen, Z.; Xu, H.; Ye, Z. Targeted p21(WAF1/CIP1) activation by miR-1236 inhibits cell proliferation and correlates with favorable survival in renal cell carcinoma. Urol. Oncol. 2016, 34, 59.e23–59.e34. [Google Scholar] [CrossRef] [PubMed]
- Synowsky, S.A.; Heck, A.J. The yeast Ski complex is a hetero-tetramer. Protein Sci. 2008, 17, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Widner, W.R.; Wickner, R.B. Evidence that the SKI antiviral system of Saccharomyces cerevisiae acts by blocking expression of viral mRNA. Mol. Cell Biol. 1993, 13, 4331–4341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ridley, S.P.; Sommer, S.S.; Wickner, R.B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol. Cell Biol. 1984, 4, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Bai, X.; Johnson, A.W. The yeast antiviral proteins Ski2p, Ski3p, and Ski8p exist as a complex in vivo. RNA 2000, 6, 449–457. [Google Scholar] [CrossRef]
- Schneider, C.; Tollervey, D. Threading the barrel of the RNA exosome. Trends Biochem. Sci. 2013, 38, 485–493. [Google Scholar] [CrossRef]
- Halbach, F.; Reichelt, P.; Rode, M.; Conti, E. The yeast ski complex: crystal structure and RNA channeling to the exosome complex. Cell 2013, 154, 814–826. [Google Scholar] [CrossRef]
- Anderson, J.S.; Parker, R.P. The 3’ to 5’ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3’ to 5’ exonucleases of the exosome complex. EMBO J. 1998, 17, 1497–1506. [Google Scholar] [CrossRef]
- Weick, E.M.; Puno, M.R.; Januszyk, K.; Zinder, J.C.; DiMattia, M.A.; Lima, C.D. Helicase-Dependent RNA Decay Illuminated by a Cryo-EM Structure of a Human Nuclear RNA Exosome-MTR4 Complex. Cell 2018, 173, 1663–1677.e21. [Google Scholar] [CrossRef]
- Thoms, M.; Thomson, E.; Bassler, J.; Gnadig, M.; Griesel, S.; Hurt, E. The Exosome Is Recruited to RNA Substrates through Specific Adaptor Proteins. Cell 2015, 162, 1029–1038. [Google Scholar] [CrossRef]
- Lee, S.G.; Song, K. Identification and characterization of a bidirectional promoter from the intergenic region between the human DDX13 and RD genes. Mol. Cells 2000, 10, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yang, Z.; Zhang, S.; Shen, L.; Dangel, A.W.; Hughes, J.H.; Redman, K.L.; Wu, L.C.; Yu, C.Y. The human DEVH-box protein Ski2w from the HLA is localized in nucleoli and ribosomes. Nucleic Acids Res. 1998, 26, 4068–4077. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Pace, F.V. RNA helicases: modulators of RNA structure. Trends Cell Biol. 1994, 4, 271–274. [Google Scholar] [CrossRef]
- Lee, S.G.; Song, K. Genomic organization of the human DDX13 gene located between RD and RP1 in the class III MHC complex. Mol. Cells 1997, 7, 414–418. [Google Scholar] [PubMed]
- Yang, Z. Molecular genetic and biochemical studies of the human and mouse MHC complement gene clusters. PhD Dissertation, The Ohio State University, Columbus, OH, USA, 1999. [Google Scholar]
- Eckard, S.C.; Rice, G.I.; Fabre, A.; Badens, C.; Gray, E.E.; Hartley, J.L.; Crow, Y.J.; Stetson, D.B. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 2014, 15, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Ly, H. Comparative Structure and Function Analysis of the RIG-I-Like Receptors: RIG-I and MDA5. Front. Immunol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Charroux, B.; Martinez-Vinson, C.; Roquelaure, B.; Odul, E.; Sayar, E.; Smith, H.; Colomb, V.; Andre, N.; Hugot, J.P.; et al. SKIV2L mutations cause syndromic diarrhea, or trichohepatoenteric syndrome. Am. J. Hum. Genet. 2012, 90, 689–692. [Google Scholar] [CrossRef]
- Hartley, J.L.; Zachos, N.C.; Dawood, B.; Donowitz, M.; Forman, J.; Pollitt, R.J.; Morgan, N.V.; Tee, L.; Gissen, P.; Kahr, W.H.; et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy). Gastroenterology 2010, 138, 2388–2398. [Google Scholar] [CrossRef]
- Avery, G.B.; Villavicencio, O.; Lilly, J.R.; Randolph, J.G. Intractable diarrhea in early infancy. Pediatrics 1968, 41, 712–722. [Google Scholar]
- Stankler, L.; Lloyd, D.; Pollitt, R.J.; Gray, E.S.; Thom, H.; Russell, G. Unexplained diarrhoea and failure to thrive in 2 siblings with unusual facies and abnormal scalp hair shafts: a new syndrome. Arch. Dis. Child. 1982, 57, 212–216. [Google Scholar] [CrossRef]
- Girault, D.; Goulet, O.; Le Deist, F.; Brousse, N.; Colomb, V.; Cesarini, J.P.; de Potter, S.; Canioni, D.; Griscelli, C.; Fischer, A.; et al. Intractable infant diarrhea associated with phenotypic abnormalities and immunodeficiency. J. Pediatr. 1994, 125, 36–42. [Google Scholar] [CrossRef]
- Dweikat, I.; Sultan, M.; Maraqa, N.; Hindi, T.; Abu-Rmeileh, S.; Abu-Libdeh, B. Tricho-hepato-enteric syndrome: a case of hemochromatosis with intractable diarrhea, dysmorphic features, and hair abnormality. Am. J. Med. Genet. A 2007, 143A, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.M.; Stevens, C.R.; Sabeti, P.C.; Walsh, E.C.; McWhinnie, A.J.; Shah, A.; Green, T.; Rioux, J.D.; Vyse, T.J. Identification of two independent risk factors for lupus within the MHC in United Kingdom families. PLoS Genet. 2007, 3, e192. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.J.; Silvestri, G.; Patterson, C.C.; Hogg, R.E.; Chakravarthy, U.; Hughes, A.E. Further assessment of the complement component 2 and factor B region associated with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2009, 50, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kopplin, L.J.; Igo, R.P., Jr.; Wang, Y.; Sivakumaran, T.A.; Hagstrom, S.A.; Peachey, N.S.; Francis, P.J.; Klein, M.L.; SanGiovanni, J.P.; Chew, E.Y.; et al. Genome-wide association identifies SKIV2L and MYRIP as protective factors for age-related macular degeneration. Genes Immun. 2010, 11, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Shi, Y.; Qu, C.; Zhao, P.; Liu, X.; Gong, B.; Ma, S.; Zhou, Y.; Zhang, Q.; Fei, P.; et al. A genetic variant in the SKIV2L gene is significantly associated with age-related macular degeneration in a Han Chinese population. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, S.; Sakurada, Y.; Mabuchi, F.; Sugiyama, A.; Kubota, T.; Iijima, H. Genetic variants in the SKIV2L gene in exudative age-related macular degeneration in the Japanese population. Ophthalmic Genet. 2014, 35, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, L.J.; Tam, P.O.; Shi, Y.; Lai, T.Y.; Liu, D.T.; Chiang, S.W.; Yang, M.; Yang, Z.; Pang, C.P. Associations of the C2-CFB-RDBP-SKIV2L locus with age-related macular degeneration and polypoidal choroidal vasculopathy. Ophthalmology 2013, 120, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Honda, S.; Kuno, S.; Negi, A. Role of RDBP and SKIV2L variants in the major histocompatibility complex class III region in polypoidal choroidal vasculopathy etiology. Ophthalmology 2009, 116, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Fabre, A.; Martinez-Vinson, C.; Goulet, O.; Badens, C. Syndromic diarrhea/Tricho-hepato-enteric syndrome. Orphanet. J. Rare Dis. 2013, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Teo, K.M.; Ng, R.T.; Chong, S.Y.; Kee, B.P.; Chua, K.H. Novel mutations in SKIV2L and TTC37 genes in Malaysian children with trichohepatoenteric syndrome. Gene 2016, 586, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Pan, J.; Jin, Y.; Wang, C.; Liu, Z. Targeted next-generation sequencing identification of a novel missense mutation of the SKIV2L gene in a patient with trichohepatoenteric syndrome. Mol. Med. Rep. 2016, 14, 2107–2110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiejima, E.; Yasumi, T.; Nakase, H.; Matsuura, M.; Honzawa, Y.; Higuchi, H.; Okafuji, I.; Yorifuji, T.; Tanaka, T.; Izawa, K.; et al. Tricho-hepato-enteric syndrome with novel SKIV2L gene mutations: A case report. Medicine (Baltimore) 2017, 96, e8601. [Google Scholar] [CrossRef] [PubMed]
- Xinias, I.; Mavroudi, A.; Mouselimis, D.; Tsarouchas, A.; Vasilaki, K.; Roilides, I.; Lacaille, F.; Giouleme, O. Trichohepatoenteric syndrome: A rare mutation in SKIV2L gene in the first Balkan reported case. SAGE Open Med. Case Rep. 2018, 6, 2050313X18807795. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.J.; Andreoletti, G.; Coelho, T.; Haggarty, R.; Batra, A.; Afzal, N.A.; Beattie, R.M.; Ennis, S. Identification of Variants in Genes Associated with Single-gene Inflammatory Bowel Disease by Whole-exome Sequencing. Inflamm. Bowel Dis. 2016, 22, 2317–2327. [Google Scholar] [CrossRef]
- Vely, F.; Barlogis, V.; Marinier, E.; Coste, M.E.; Dubern, B.; Dugelay, E.; Lemale, J.; Martinez-Vinson, C.; Peretti, N.; Perry, A.; et al. Combined Immunodeficiency in Patients With Trichohepatoenteric Syndrome. Front. Immunol. 2018, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, P.; Esteve, C.; Chaix, C.; Beroud, C.; Levy, N.; consortium, T.c.; Fabre, A.; Badens, C. Tricho-Hepato-Enteric Syndrome mutation update: Mutations spectrum of TTC37 and SKIV2L, clinical analysis and future prospects. Hum. Mutat. 2018, 39, 774–789. [Google Scholar] [CrossRef]
- Verloes, A.; Lombet, J.; Lambert, Y.; Hubert, A.F.; Deprez, M.; Fridman, V.; Gosseye, S.; Rigo, J.; Sokal, E. Tricho-hepato-enteric syndrome: Further delineation of a distinct syndrome with neonatal hemochromatosis phenotype, intractable diarrhea, and hair anomalies. Am. J. Med. Genet. 1997, 68, 391–395. [Google Scholar] [CrossRef]
- Poulton, C.; Pathak, G.; Mina, K.; Lassman, T.; Azmanov, D.N.; McCormack, E.; Broley, S.; Dreyer, L.; Gration, D.; Taylor, E.; et al. Tricho-hepatic-enteric syndrome (THES) without intractable diarrhoea. Gene 2019, 699, 110–114. [Google Scholar] [CrossRef]
- Monies, D.M.; Rahbeeni, Z.; Abouelhoda, M.; Naim, E.A.; Al-Younes, B.; Meyer, B.F.; Al-Mehaidib, A. Expanding phenotypic and allelic heterogeneity of tricho-hepato-enteric syndrome. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 352–356. [Google Scholar] [CrossRef]
- Lintner, K.E.; Patwardhan, A.; Rider, L.G.; Abdul-Aziz, R.; Wu, Y.L.; Lundstrom, E.; Padyukov, L.; Zhou, B.; Alhomosh, A.; Newsom, D.; et al. Gene copy-number variations (CNVs) of complement C4 and C4A deficiency in genetic risk and pathogenesis of juvenile dermatomyositis. Ann. Rheum. Dis. 2016, 75, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Ronnblom, L.; Pascual, V. The innate immune system in SLE: type I interferons and dendritic cells. Lupus 2008, 17, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Bai, X.; Lee, I.; Kallstrom, G.; Ho, J.; Brown, J.; Stevens, A.; Johnson, A.W. Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Mol. Cell Biol. 2000, 20, 4006–4015. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Cooper-Morgan, A.; Jiao, X.; Kiledjian, M.; Manley, J.L.; Tong, L. Structure and function of the 5’-->3’ exoribonuclease Rat1 and its activating partner Rai1. Nature 2009, 458, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Xiang, S.; Oh, C.; Martin, C.E.; Tong, L.; Kiledjian, M. Identification of a quality-control mechanism for mRNA 5’-end capping. Nature 2010, 467, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Jiao, X.; Chiba, K.; Oh, C.; Martin, C.E.; Kiledjian, M.; Tong, L. Dxo1 is a new type of eukaryotic enzyme with both decapping and 5’-3’ exoribonuclease activity. Nat. Struct. Mol. Biol. 2012, 19, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Chang, J.H.; Kilic, T.; Tong, L.; Kiledjian, M. A mammalian pre-mRNA 5’ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing. Mol. Cell 2013, 50, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.T.; Xiang, S. mRNA quality control at the 5’ end. J. Zhejiang Univ. Sci. B 2014, 15, 438–443. [Google Scholar] [CrossRef]
- Kiledjian, M. Eukaryotic RNA 5’-End NAD(+) Capping and DeNADding. Trends Cell Biol 2018, 28, 454–464. [Google Scholar] [CrossRef]
- Picard-Jean, F.; Brand, C.; Tremblay-Letourneau, M.; Allaire, A.; Beaudoin, M.C.; Boudreault, S.; Duval, C.; Rainville-Sirois, J.; Robert, F.; Pelletier, J.; et al. Correction: 2’-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS ONE 2018, 13, e0202308. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Doamekpor, S.K.; Bird, J.G.; Nickels, B.E.; Tong, L.; Hart, R.P.; Kiledjian, M. 5’ End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding. Cell 2017, 168, 1015–1027.e10. [Google Scholar] [CrossRef] [PubMed]
- Belt, K.T.; Yu, C.Y.; Carroll, M.C.; Porter, R.R. Polymorphism of human complement component C4. Immunogenetics 1985, 21, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mendoza, A.R.; Welch, T.R.; Zipf, W.B.; Yu, C.Y. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J. Biol. Chem. 1999, 274, 12147–12156. [Google Scholar] [PubMed]
- Yu, C.Y.; Chung, E.K.; Yang, Y.; Blanchong, C.A.; Jacobsen, N.; Saxena, K.; Yang, Z.; Miller, W.; Varga, L.; Fust, G. Dancing with complement C4 and the RP-C4-CYP21-TNX (RCCX) modules of the major histocompatibility complex. Prog. Nucleic Acid Res. Mol. Biol. 2003, 75, 217–292. [Google Scholar] [PubMed]
- Sargent, C.A.; Anderson, M.J.; Hsieh, S.L.; Kendall, E.; Gomez-Escobar, N.; Campbell, R.D. Characterisation of the novel gene G11 lying adjacent to the complement C4A gene in the human major histocompatibility complex. Hum. Mol. Genet. 1994, 3, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Escobar, N.; Chou, C.F.; Lin, W.W.; Hsieh, S.L.; Campbell, R.D. The G11 gene located in the major histocompatibility complex encodes a novel nuclear serine/threonine protein kinase. J. Biol. Chem. 1998, 273, 30954–30960. [Google Scholar] [CrossRef] [PubMed]
- Blanchong, C.A.; Zhou, B.; Rupert, K.L.; Chung, E.K.; Jones, K.N.; Sotos, J.F.; Zipf, W.B.; Rennebohm, R.M.; Yu, C.Y. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J. Exp. Med. 2000, 191, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, E.M.; Goodier, J.L.; Zhang, Y.; Kazazian, H.H., Jr. SVA elements are nonautonomous retrotransposons that cause disease in humans. Am. J. Hum. Genet. 2003, 73, 1444–1451. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H., Jr. SVA retrotransposons: Evolution and genetic instability. Semin Cancer Biol 2010, 20, 234–245. [Google Scholar] [CrossRef]
- Savage, A.L.; Bubb, V.J.; Breen, G.; Quinn, J.P. Characterisation of the potential function of SVA retrotransposons to modulate gene expression patterns. BMC Evol. Biol. 2013, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Lehner, B.; Semple, J.I.; Brown, S.E.; Counsell, D.; Campbell, R.D.; Sanderson, C.M. Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region. Genomics 2004, 83, 153–167. [Google Scholar] [CrossRef]
- STK19 serine/threonine kinase 19 [Homo sapiens (human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/8859 (accessed on 1 June 2019).
- Boeing, S.; Williamson, L.; Encheva, V.; Gori, I.; Saunders, R.E.; Instrell, R.; Aygun, O.; Rodriguez-Martinez, M.; Weems, J.C.; Kelly, G.P.; et al. Multiomic Analysis of the UV-Induced DNA Damage Response. Cell Rep. 2016, 15, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A landscape of driver mutations in melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Shitara, D.; Tell-Marti, G.; Badenas, C.; Enokihara, M.M.; Alos, L.; Larque, A.B.; Michalany, N.; Puig-Butille, J.A.; Carrera, C.; Malvehy, J.; et al. Mutational status of naevus-associated melanomas. Br. J. Dermatol. 2015, 173, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhu, B.; Zhang, T.; Liu, T.; Chen, S.; Liu, Y.; Li, X.; Miao, X.; Li, S.; Mi, X.; et al. Pharmacological Targeting of STK19 Inhibits Oncogenic NRAS-Driven Melanomagenesis. Cell 2019, 176, 1113–1127.e16. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Next-generation sequencing traces human induced pluripotent stem cell lines clonally generated from heterogeneous cancer tissue. World J. Stem Cells 2017, 9, 77–88. [Google Scholar] [CrossRef]
- Kraja, A.T.; Chasman, D.I.; North, K.E.; Reiner, A.P.; Yanek, L.R.; Kilpelainen, T.O.; Smith, J.A.; Dehghan, A.; Dupuis, J.; Johnson, A.D.; et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol. Genet. Metab. 2014, 112, 317–338. [Google Scholar] [CrossRef]
- Amare, A.T.; Vaez, A.; Hsu, Y.H.; Direk, N.; Kamali, Z.; Howard, D.M.; McIntosh, A.M.; Tiemeier, H.; Bultmann, U.; Snieder, H.; et al. Bivariate genome-wide association analyses of the broad depression phenotype combined with major depressive disorder, bipolar disorder or schizophrenia reveal eight novel genetic loci for depression. Mol. Psychiatry 2019. [Google Scholar] [CrossRef]
- Negative elongation factor E [Drosophila melanogaster]. Available online: https://www.ncbi.nlm.nih.gov/protein/NP_648241.1 (accessed on 1 June 2019).
- Negative elongation factor E isoform X2 [Danio rerio]. Available online: https://www.ncbi.nlm.nih.gov/protein/XP_005170084.1 (accessed on 1 June 2019).
- Paulsen, J.E.; Capowski, E.E.; Strome, S. Phenotypic and molecular analysis of mes-3, a maternal-effect gene required for proliferation and viability of the germ line in C. elegans. Genetics 1995, 141, 1383–1398. [Google Scholar] [PubMed]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.M.; Campbell, R.D. Genetic organization of the human MHC class III region. Front. Biosci. 2001, 6, D914–D926. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C., Jr. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Note added at update: A recent review that is highly relevant and provides comprehensive background knowledge to this article: Cramer, P. Organization and regulation of gene transcription. Nature 2019, 573, 45–54.
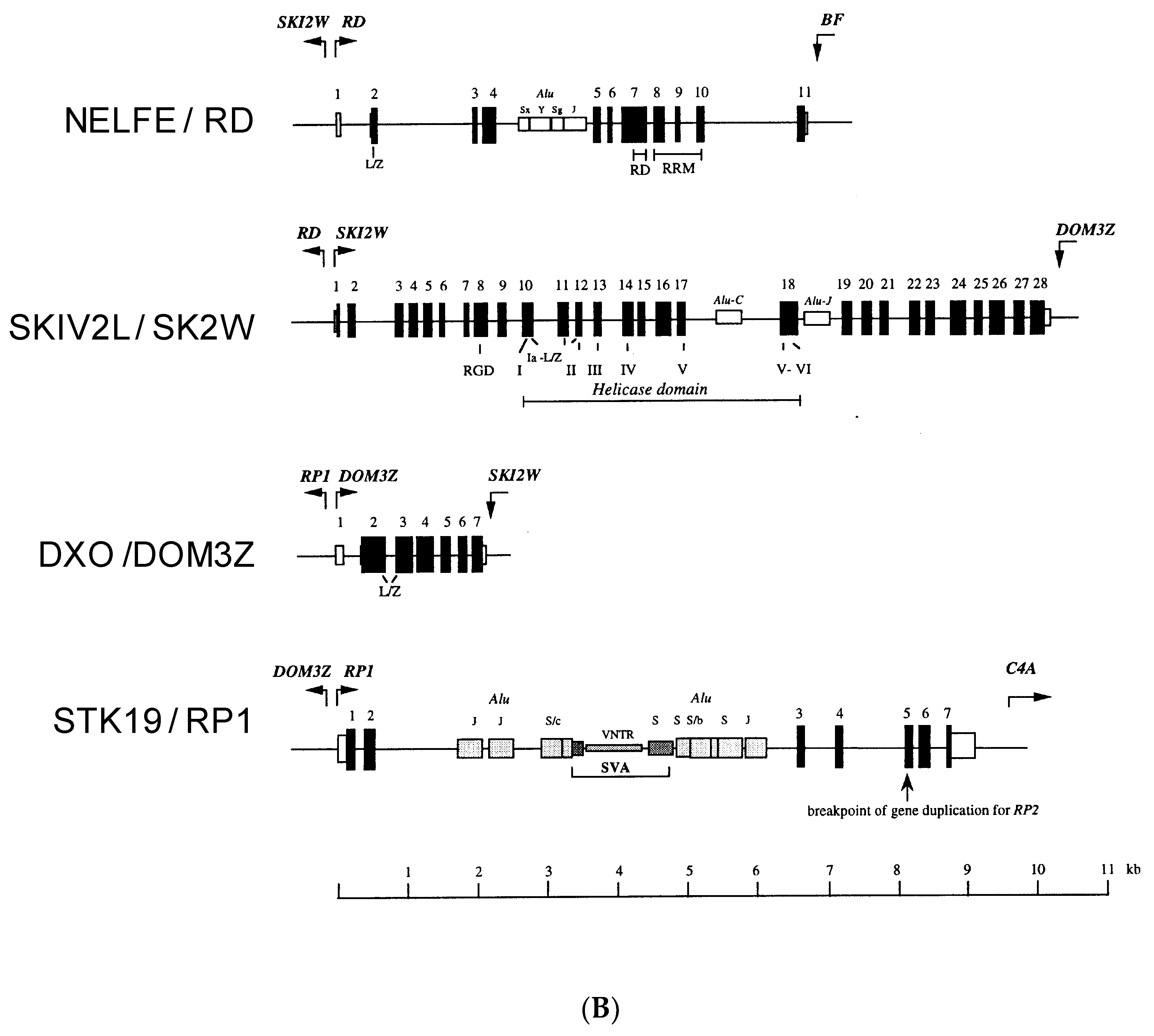
| Official Symbol | Original Name; Other Earlier Names | Gene, Transcripts and Proteins (Location, cDNA, aa) | Expression | Specific Features | Function | Website |
|---|---|---|---|---|---|---|
| NELF-E | RD RDP; RDBP; D6S45 | 11 exons 6.7 kb 380 aa | Ubiquitous; highest in testis | CpG rich, leucine zipper motif, RD repeats, RNA recognition motif (RRM); miR-1236 in intron 3 | A subunit of the negative RNA elongation factor that represses transcriptional elongation by RNA polymerase II | https://www.ncbi.nlm.nih.gov/gene/7936 |
| SKIV2L | SKI2W HLP, SKI2, 170A, DDX13, DDX49, SKIV2, SKIV2L1, THES2 | 28 exons 11 kb 1246 aa | Ubiquitous; highest in spleen | CpG rich, leucine zipper motif, helicase domain, RGD and Alu element | Unwinds RNA secondary structures, RNA turnover, antiviral defense, modulates type 1 interferon expression | https://www.ncbi.nlm.nih.gov/gene/6499 |
| DXO | DOM3Z DOM3L, NG6, RAI1 | 7 exons 2.2 kb 396 aa | Ubiquitous; highest in testis and adrenal gland | CpG rich; leucine zipper motif | RNA quality control, decapping and 5′→3′ RNA decay | https://www.ncbi.nlm.nih.gov/gene/1797 |
| STK19 | RP1 and RP2 HLA-RP1, G11, D6S60, D6S60E | 9 exons 11 kb 364 aa or 7 exons 9.1 kb 254 aa | Ubiquitous; highest in adrenal gland | Partial gene duplication in the RCCX modules; SVA (SINE - 21 CpG rich VNTRs-Alu) in intron; 8 Alu elements | Nuclear Serine/Threonine kinase | https://www.ncbi.nlm.nih.gov/gene/8859 |
| Disease | SNP | Relationship to Disease | Remarks |
|---|---|---|---|
| SLE (Systemic Lupus Erythematosus) | T allele of rs419788 in intron 6 | Confer disease susceptibility in additive pattern | One copy confers a low risk of disease and two copies result in greater susceptibility [64] |
| AMD (Age-related macular degeneration) | R151Q (rs438999) | May exert a functional effect in AMD | Strong LD (linkage disequilibrium) with Bf R32Q (rs641153) [65] |
| Intronic SNP (rs429608) | AMD genetic protective factors | Protective effect for AMD [66] in Han Chinese and Japanese populations [67,68] | |
| rs429608 and rs453821 | Significantly associated with neovascular AMD | Not associated significantly with PCV [69] | |
| PCV (Polypoidal Choroidal Vasculopathy) | 3′UTR (rs2075702) | Significant association with PCV | Decreased risk of developing PCV [70] |
| SD/THES (Syndromic diarrhea/tricho-hepato-enteric syndrome) | c.1635_1636insA (p.Gly546Argfs*35) c.2266C>T (p.Arg756*) c.2442G>A (p.Trp814*) c.848G>A (p.Trp283*) c.1022T>G (p.Val341Gly) c.2572del (p.Val858*) c.2662_2663del (p.Arg888Glyfs*12) c.1434del (p.Ser479Alafs*3) | Deleterious mutations detected in six individuals with typical SD/THES | Molecular defects in SKIV2L cause SD/THES [58,71] |
| c.1891G>A p.Gly631Ser c.3187C>T p.Arg1063* | Two new mutations found in a trichohepatoenteric syndrome patient | The patient was a Malaysian child [72] | |
| (c.1891G>A) (c.1120C>T) | Two variants identified in THES patients | These two mutations can cause THES [73] | |
| c.1420G>T (p.Q474*) c.3262G>T (p.E1088*) | Novel compound heterozygous nonsense mutations were identified in THES patient | Decreased levels of SKIV2L protein expression in blood mononuclear cells [74] | |
| 25 exons (p.Glu1038 fs*7 (c.3112_3140del)) | A rare mutation in THES patient | This patient died at three year old [75] | |
| IBD (Inflammatory Bowel Disease) | c.354+5G>A | Identified a novel splicing mutation in a patient with IBD (ulcerative colitis) | This mutation was related to Inflammatory Bowel Disease [76] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Lai, M.; Luo, A.; Yu, C.-Y. An RNA Metabolism and Surveillance Quartet in the Major Histocompatibility Complex. Cells 2019, 8, 1008. https://doi.org/10.3390/cells8091008
Zhou D, Lai M, Luo A, Yu C-Y. An RNA Metabolism and Surveillance Quartet in the Major Histocompatibility Complex. Cells. 2019; 8(9):1008. https://doi.org/10.3390/cells8091008
Chicago/Turabian StyleZhou, Danlei, Michalea Lai, Aiqin Luo, and Chack-Yung Yu. 2019. "An RNA Metabolism and Surveillance Quartet in the Major Histocompatibility Complex" Cells 8, no. 9: 1008. https://doi.org/10.3390/cells8091008
APA StyleZhou, D., Lai, M., Luo, A., & Yu, C.-Y. (2019). An RNA Metabolism and Surveillance Quartet in the Major Histocompatibility Complex. Cells, 8(9), 1008. https://doi.org/10.3390/cells8091008





