Myeloid Krüppel-Like Factor 2 Critically Regulates K/BxN Serum-Induced Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Monocytes from Murine Bone Marrow
2.2. Isolation of Human Monocytes
2.3. Real Time RT-PCR Analysis
2.4. Osteoclast Differentiation and TRAP Staining
2.5. Osteoclast Immunostaining
2.6. Induction of Arthritis in Mice
2.7. Histological Assessment of Arthritis
2.8. Western Blotting
2.9. Immunohistochemistry for Human Samples
2.10. Overexpression and Knockdown of KLF2
2.11. Chromatin Immunoprecipitation (ChIP) and Quantitative RT-PCR
2.12. Statistical Analysis
3. Results
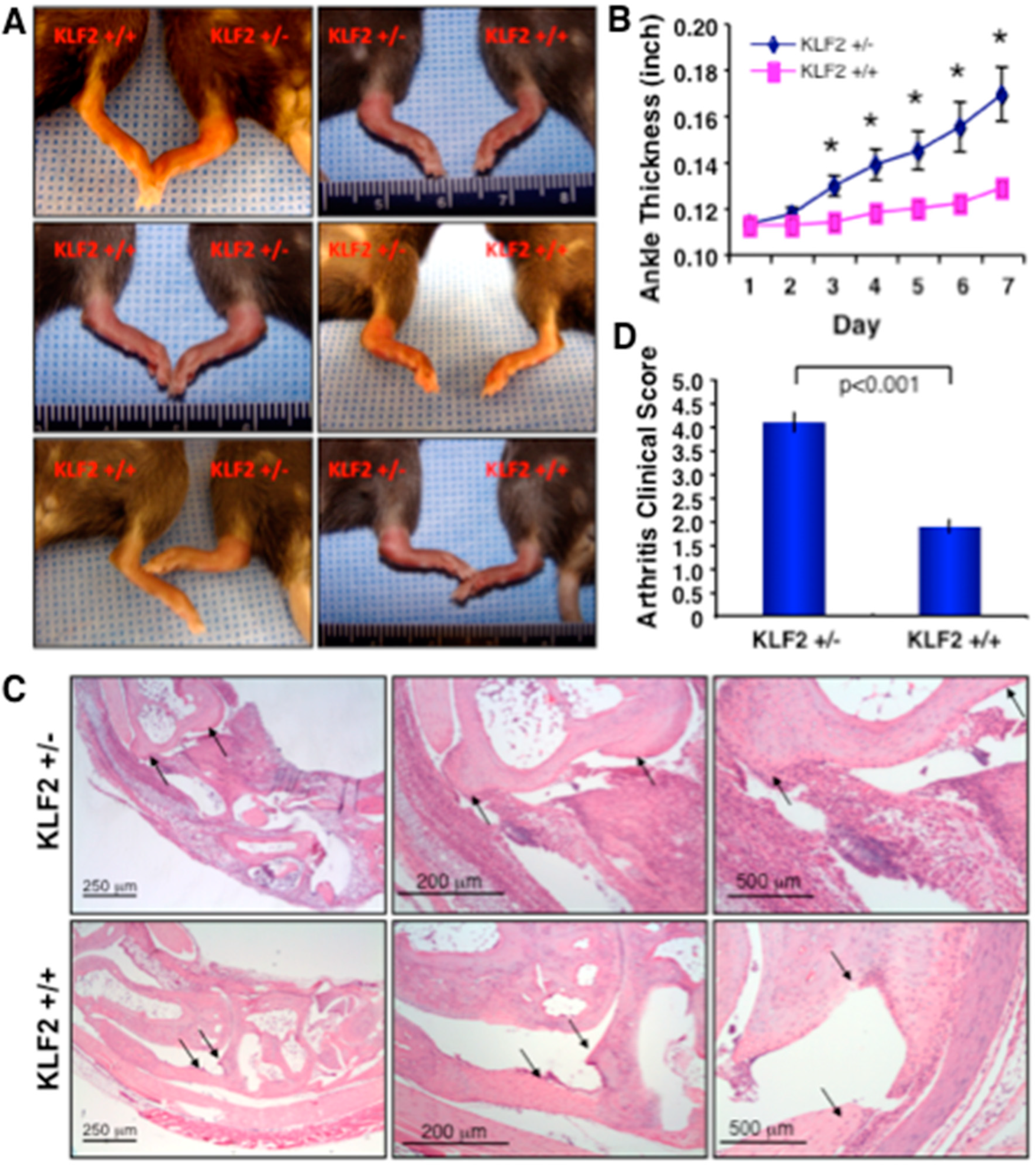
3.1. Effect of K/BxN Serum-Induced Arthritis in KLF2 Hemizygous Mice
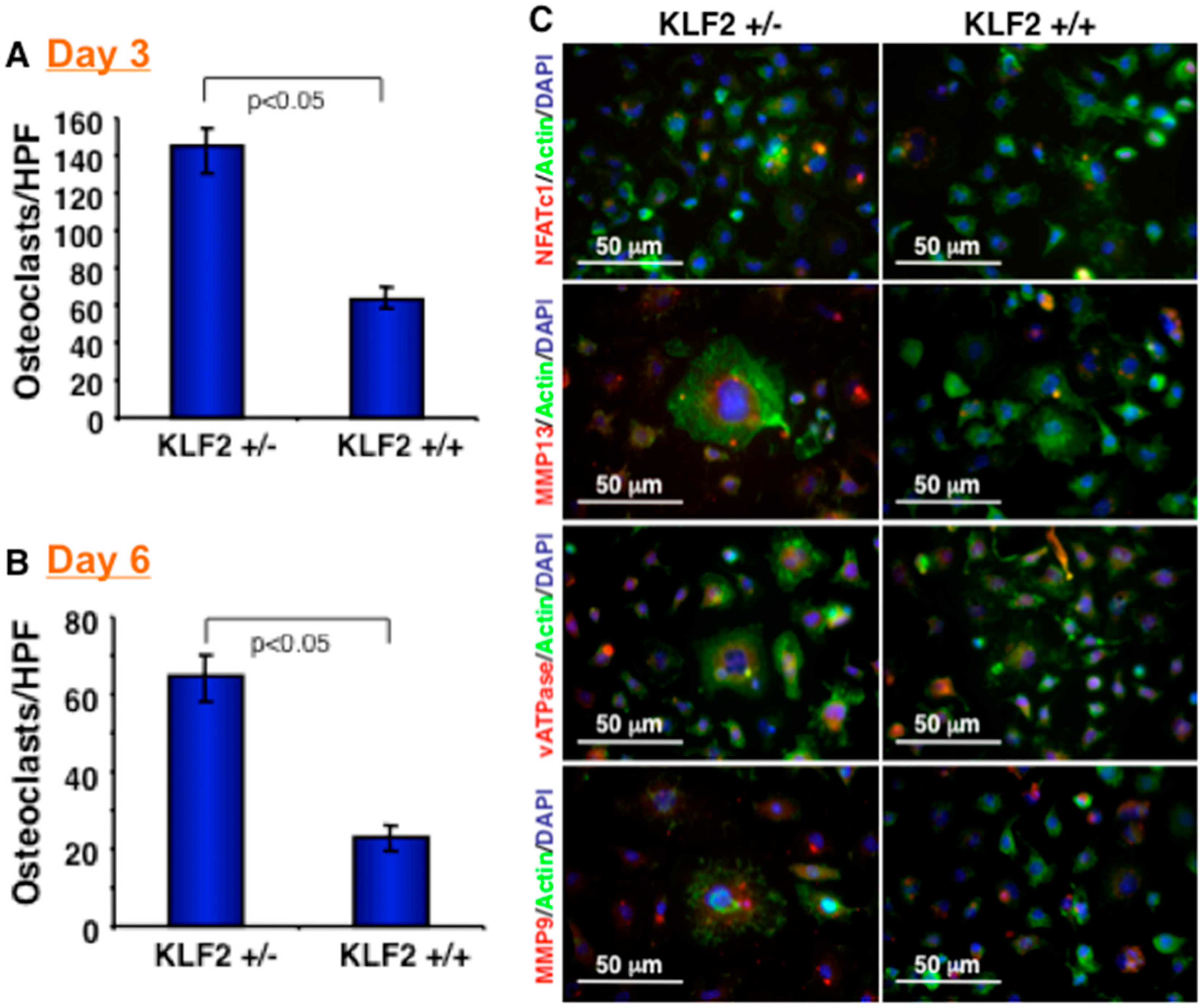
3.2. Effect of KLF2 Hemizygosity on Osteoclast Differentiation and Maturation
3.3. Expression of Molecules in Bone Marrow after Induced Arthritis
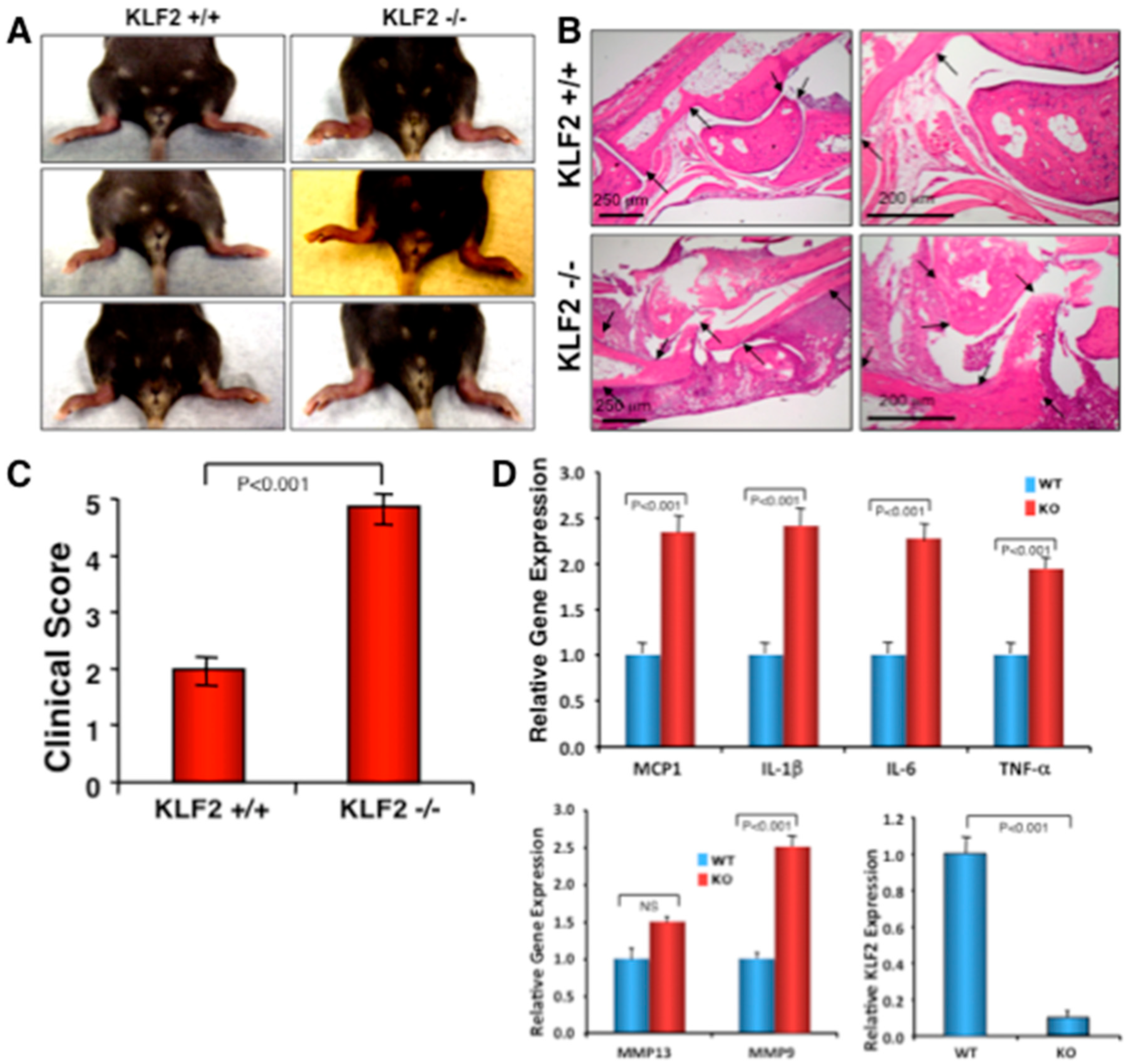
3.4. Effect of Monocyte-Specific Conditional KLF2 Knock Out on K/BxN Serum-Induced Arthritic Pathogenesis
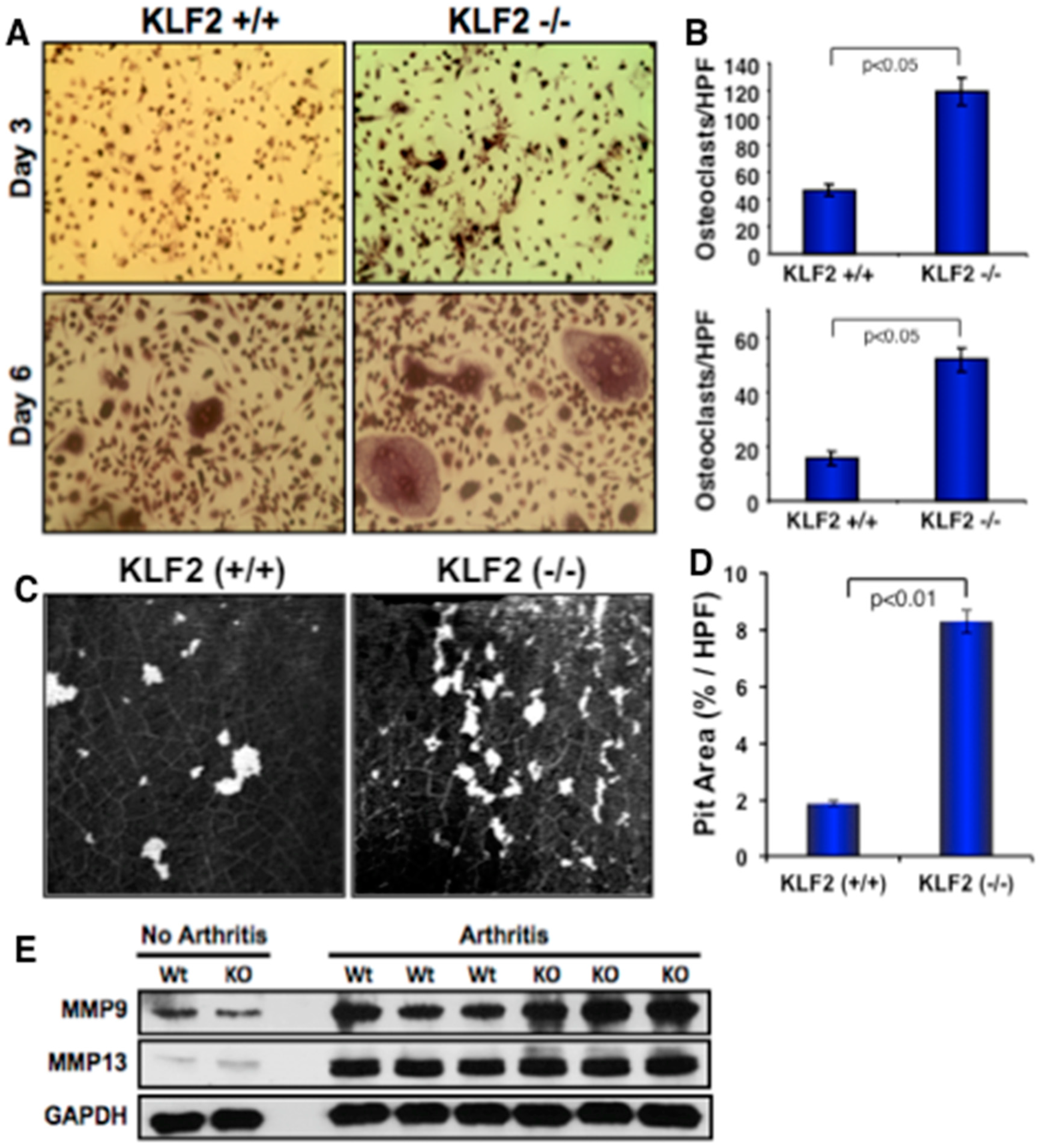
3.5. Effect of Monocyte-Specific KLF2 Deficiency on Osteoclast Differentiation and Function
3.6. Expression of KLF2 in Monocytes after Arthritis Development in Mice
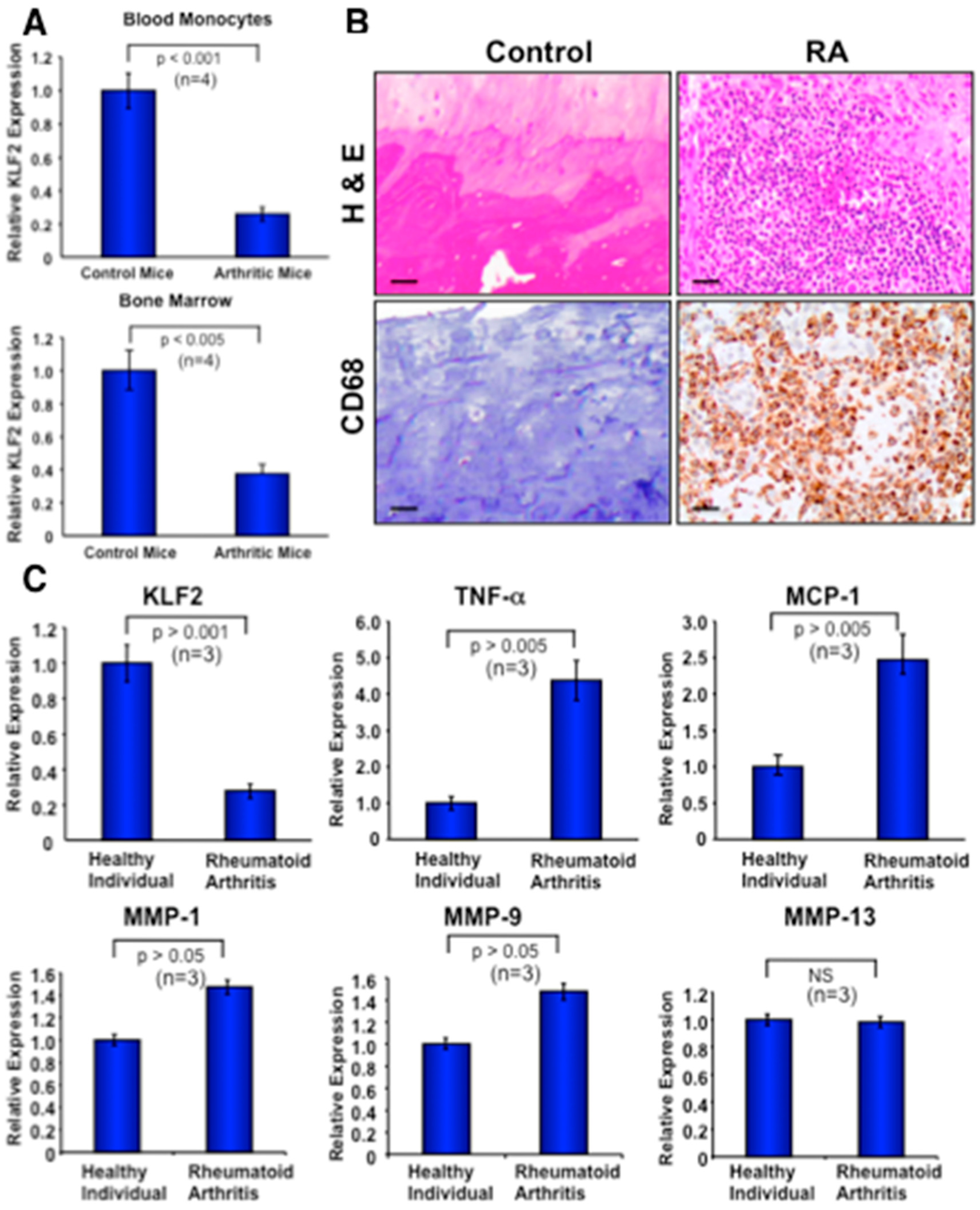
3.7. Recruitment of Activated Monocytes in Human Arthritic Joints and Expression of Molecules in Peripheral Blood Monocytes
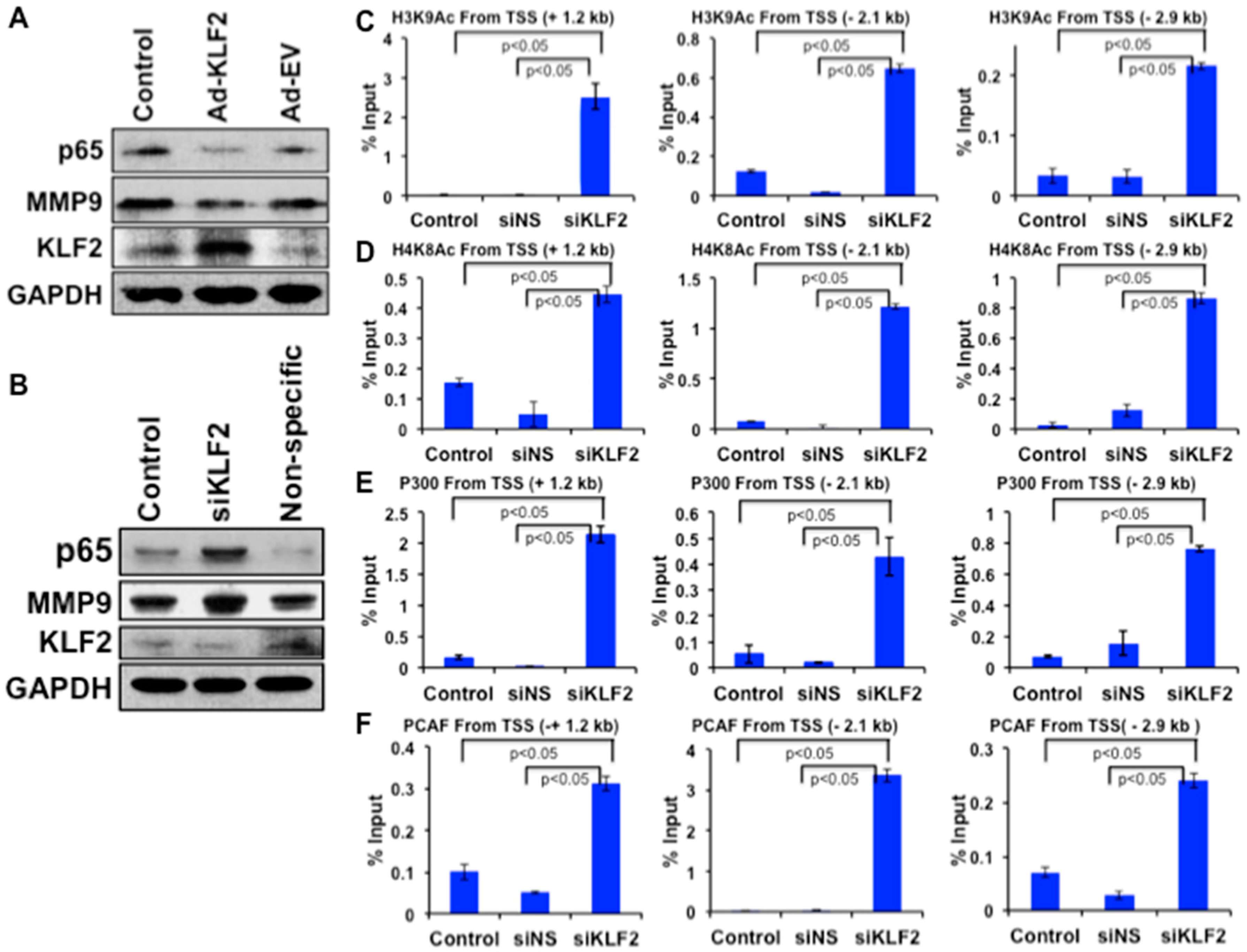
3.8. Mechanistic Regulation of MMP9 by KLF2 in Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sokka, T. Long-term outcomes of rheumatoid arthritis. Curr. Opin. Rheumatol. 2009, 21, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Therneau, T.M.; Gabriel, S.E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010, 62, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arend, W.P.; Firestein, G.S. Pre-rheumatoid arthritis: Predisposition and transition to clinical synovitis. Nat. Rev. Rheumatol. 2012, 8, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. The disease formerly known as rheumatoid arthritis. Arthritis Res. Ther. 2014, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Catrina, A.I.; Joshua, V.; Klareskog, L.; Malmstrom, V. Mechanisms involved in triggering rheumatoid arthritis. Immunol. Rev. 2016, 269, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Smolen, J.S. Historical observations contributing insights on etiopathogenesis of rheumatoid arthritis and role of rheumatoid factor. J. Exp. Med. 2016, 213, 1937–1950. [Google Scholar] [CrossRef] [Green Version]
- Fox, D.A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: New perspectives. Arthritis Rheum. 1997, 40, 598–609. [Google Scholar] [CrossRef]
- Imhof, B.A.; Aurrand-Lions, M. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 2004, 4, 432–444. [Google Scholar] [CrossRef]
- Bennink, R.J.; Thurlings, R.M.; van Hemert, F.J.; Voermans, C.; Dohmen, S.E.; van Eck-Smit, B.L.; Tak, P.P.; Busemann-Sokole, E. Biodistribution and radiation dosimetry of 99mTc-HMPAO-labeled monocytes in patients with rheumatoid arthritis. J. Nucl. Med. 2008, 49, 1380–1385. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Brenner, M.B. Role of adhesion molecules in synovial inflammation. Curr. Opin. Rheumatol. 2006, 18, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; Koch, A.E. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007, 19, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Komano, Y.; Nanki, T.; Hayashida, K.; Taniguchi, K.; Miyasaka, N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res. Ther. 2006, 8, R152. [Google Scholar] [CrossRef] [PubMed]
- Kinne, R.W.; Stuhlmuller, B.; Burmester, G.R. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res. Ther. 2007, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Ishiguro, N.; Ito, T.; Oguchi, T.; Kojima, T.; Iwata, H.; Ionescu, M.; Poole, A.R. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 2001, 44, 2503–2511. [Google Scholar] [CrossRef]
- Das, H.; Kumar, A.; Lin, Z.; Patino, W.D.; Hwang, P.M.; Feinberg, M.W.; Majumder, P.K.; Jain, M.K. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc. Natl. Acad. Sci. USA 2006, 103, 6653–6658. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Lu, J.; Joseph, M.; Aggarwal, R.; Kanji, S.; McMichael, B.K.; Lee, B.S.; Agarwal, S.; Ray-Chaudhury, A.; Iwenofu, O.H.; et al. Kruppel-like factor 2 (KLF2) regulates monocyte differentiation and functions in mBSA and IL-1beta-induced arthritis. Curr. Mol. Med. 2012, 12, 113–125. [Google Scholar] [CrossRef]
- Das, M.; Laha, D.; Kanji, S.; Joseph, M.; Aggarwal, R.; Iwenofu, O.H.; Pompili, V.J.; Jain, M.K.; Das, H. Induction of Kruppel-like factor 2 reduces K/BxN serum-induced arthritis. J. Cell. Mol. Med. 2019, 23, 1386–1395. [Google Scholar] [CrossRef]
- Laha, D.; Deb, M.; Das, H. KLF2 (kruppel like factor 2 [lung]) regulates osteoclastogenesis by modulating autophagy. Autophagy 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mahabeleshwar, G.H.; Kawanami, D.; Sharma, N.; Takami, Y.; Zhou, G.; Shi, H.; Nayak, L.; Jeyaraj, D.; Grealy, R.; White, M.; et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity 2011, 34, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.P.; Kern, C.B.; Crable, S.C.; Lingrel, J.B. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: Identification of a new multigene family. Mol. Cell. Biol. 1995, 15, 5957–5965. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Morrisey, E.E.; Anandappa, R.; Sigrist, K.; Lu, M.M.; Parmacek, M.S.; Soudais, C.; Leiden, J.M. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997, 11, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.F.; Kuo, C.T.; Leiden, J.M. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nat. Immunol. 2001, 2, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Veselits, M.L.; Barton, K.P.; Lu, M.M.; Clendenin, C.; Leiden, J.M. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997, 11, 2996–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone acetyltransferases. Annu. Rev. Biochem. 2001, 70, 81–120. [Google Scholar] [CrossRef]
- Hargreaves, D.C.; Horng, T.; Medzhitov, R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell 2009, 138, 129–145. [Google Scholar] [CrossRef]
- Escoubet-Lozach, L.; Benner, C.; Kaikkonen, M.U.; Lozach, J.; Heinz, S.; Spann, N.J.; Crotti, A.; Stender, J.; Ghisletti, S.; Reichart, D.; et al. Mechanisms establishing TLR4-responsive activation states of inflammatory response genes. PLoS Genet. 2011, 7, e1002401. [Google Scholar] [CrossRef]
- Kouskoff, V.; Korganow, A.S.; Duchatelle, V.; Degott, C.; Benoist, C.; Mathis, D. Organ-specific disease provoked by systemic autoimmunity. Cell 1996, 87, 811–822. [Google Scholar] [CrossRef]
- Das, H.; Groh, V.; Kuijl, C.; Sugita, M.; Morita, C.T.; Spies, T.; Bukowski, J.F. MICA engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 2001, 15, 83–93. [Google Scholar] [CrossRef]
- McMichael, B.K.; Cheney, R.E.; Lee, B.S. Myosin X regulates sealing zone patterning in osteoclasts through linkage of podosomes and microtubules. J. Biol. Chem. 2010, 285, 9506–9515. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Ortiz-Lopez, A.; Campbell, J.J.; Gerard, C.J.; Mathis, D.; Benoist, C. Deficiency of CXCR2, but not other chemokine receptors, attenuates autoantibody-mediated arthritis in a murine model. Arthritis Rheum. 2010, 62, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Staite, N.D.; Richard, K.A.; Aspar, D.G.; Franz, K.A.; Galinet, L.A.; Dunn, C.J. Induction of an acute erosive monarticular arthritis in mice by interleukin-1 and methylated bovine serum albumin. Arthritis Rheum. 1990, 33, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Bischof, R.J.; Zafiropoulos, D.; Hamilton, J.A.; Campbell, I.K. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and granulocyte macrophage (GM)-CSF: Evidence of macrophage infiltration and local proliferation. Clin. Exp. Immunol. 2000, 119, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Pivetta, E.; Scapolan, M.; Pecolo, M.; Wassermann, B.; Abu-Rumeileh, I.; Balestreri, L.; Borsatti, E.; Tripodo, C.; Colombatti, A.; Spessotto, P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011, 13, R105. [Google Scholar] [CrossRef]
- Nannuru, K.C.; Futakuchi, M.; Varney, M.L.; Vincent, T.M.; Marcusson, E.G.; Singh, R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010, 70, 3494–3504. [Google Scholar] [CrossRef]
- Qin, A.; Cheng, T.S.; Pavlos, N.J.; Lin, Z.; Dai, K.R.; Zheng, M.H. V-ATPases in osteoclasts: Structure, function and potential inhibitors of bone resorption. Int. J. Biochem. Cell Biol. 2012, 44, 1422–1435. [Google Scholar] [CrossRef]
- Voronov, I.; Ochotny, N.; Jaumouille, V.; Owen, C.; Manolson, M.F.; Aubin, J.E. The R740S mutation in the V-ATPase a3 subunit increases lysosomal pH, impairs NFATc1 translocation, and decreases in vitro osteoclastogenesis. J. Bone Miner. Res. 2013, 28, 108–118. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 2 (Suppl. 8), S3. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H.; Yudoh, K.; Katayama, R.; Nakazawa, F.; Uzuki, M.; Sawai, T.; Yonezawa, T.; Saeki, Y.; Panayi, G.S.; Pitzalis, C.; et al. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 2002, 41, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Couper, K.N.; Blount, D.G.; Riley, E.M. IL-10: The master regulator of immunity to infection. J. Immunol. 2008, 180, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Okamoto, H.; Toyama, Y.; Momohara, S. Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS J. 2008, 275, 4448–4455. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Hara, M.; Yoshimura, T.; Leonard, E.J.; Inoue, K.; Kashiwazaki, S. Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin. Immunol. Immunopathol. 1993, 69, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef]
- Roberts, C.A.; Dickinson, A.K.; Taams, L.S. The Interplay Between Monocytes/Macrophages and CD4(+) T Cell Subsets in Rheumatoid Arthritis. Front. Immunol. 2015, 6, 571. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, J.S.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J. Ethnopharmacol. 2010, 127, 589–595. [Google Scholar] [CrossRef]
- Davignon, J.L.; Hayder, M.; Baron, M.; Boyer, J.F.; Constantin, A.; Apparailly, F.; Poupot, R.; Cantagrel, A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013, 52, 590–598. [Google Scholar] [CrossRef]
- Seitz, M.; Hunstein, W. Enhanced prostanoid release from monocytes of patients with rheumatoid arthritis and active systemic lupus erythematosus. Ann. Rheum. Dis. 1985, 44, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Shore, A.; Jaglal, S.; Keystone, E.C. Enhanced interleukin 1 generation by monocytes in vitro is temporally linked to an early event in the onset or exacerbation of rheumatoid arthritis. Clin. Exp. Immunol. 1986, 65, 293–302. [Google Scholar] [PubMed]
- Chambers, T.J.; McSheehy, P.M.; Thomson, B.M.; Fuller, K. The effect of calcium-regulating hormones and prostaglandins on bone resorption by osteoclasts disaggregated from neonatal rabbit bones. Endocrinology 1985, 116, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Heymann, D.; Rousselle, A.V. gp130 Cytokine family and bone cells. Cytokine 2000, 12, 1455–1468. [Google Scholar] [CrossRef]
- Cavender, D.E.; Haskard, D.O.; Joseph, B.; Ziff, M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J. Immunol. 1986, 136, 203–207. [Google Scholar]
- Fujii, I.; Shingu, M.; Nobunaga, M. Monocyte activation in early onset rheumatoid arthritis. Ann. Rheum. Dis. 1990, 49, 497–503. [Google Scholar] [CrossRef]
- CHolness, L.; Simmons, D.L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 1993, 81, 1607–1613. [Google Scholar] [Green Version]
- Zhao, X.; Benveniste, E.N. Transcriptional activation of human matrix metalloproteinase-9 gene expression by multiple co-activators. J. Mol. Biol. 2008, 383, 945–956. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, M.; Deb, M.; Laha, D.; Joseph, M.; Kanji, S.; Aggarwal, R.; Iwenofu, O.H.; Pompili, V.J.; Jarjour, W.; Das, H. Myeloid Krüppel-Like Factor 2 Critically Regulates K/BxN Serum-Induced Arthritis. Cells 2019, 8, 908. https://doi.org/10.3390/cells8080908
Das M, Deb M, Laha D, Joseph M, Kanji S, Aggarwal R, Iwenofu OH, Pompili VJ, Jarjour W, Das H. Myeloid Krüppel-Like Factor 2 Critically Regulates K/BxN Serum-Induced Arthritis. Cells. 2019; 8(8):908. https://doi.org/10.3390/cells8080908
Chicago/Turabian StyleDas, Manjusri, Moonmoon Deb, Dipranjan Laha, Matthew Joseph, Suman Kanji, Reeva Aggarwal, O. Hans Iwenofu, Vincent J. Pompili, Wael Jarjour, and Hiranmoy Das. 2019. "Myeloid Krüppel-Like Factor 2 Critically Regulates K/BxN Serum-Induced Arthritis" Cells 8, no. 8: 908. https://doi.org/10.3390/cells8080908
APA StyleDas, M., Deb, M., Laha, D., Joseph, M., Kanji, S., Aggarwal, R., Iwenofu, O. H., Pompili, V. J., Jarjour, W., & Das, H. (2019). Myeloid Krüppel-Like Factor 2 Critically Regulates K/BxN Serum-Induced Arthritis. Cells, 8(8), 908. https://doi.org/10.3390/cells8080908






