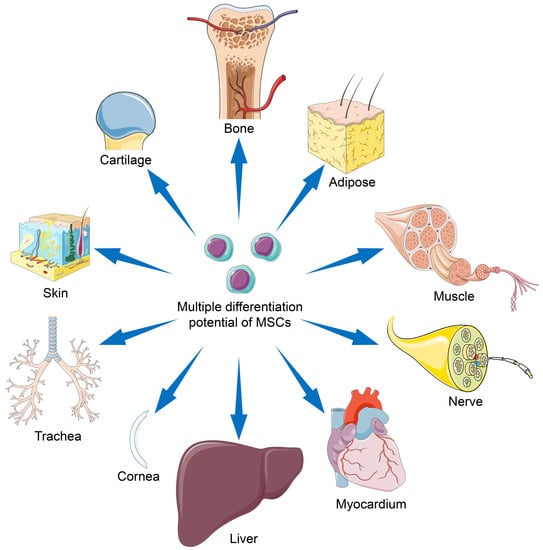
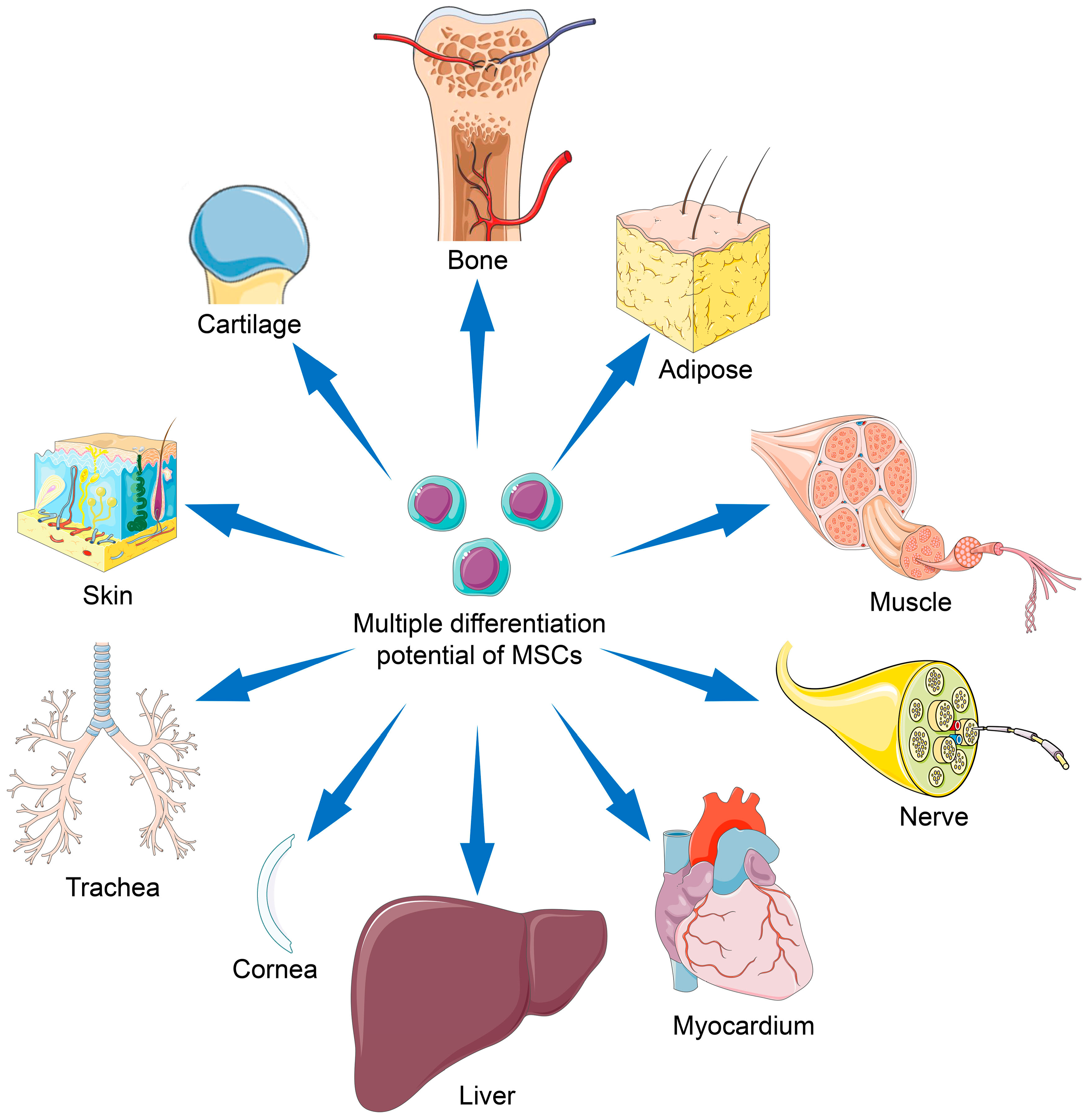
Mesenchymal Stem Cells for Regenerative Medicine
Abstract
:1. Introduction
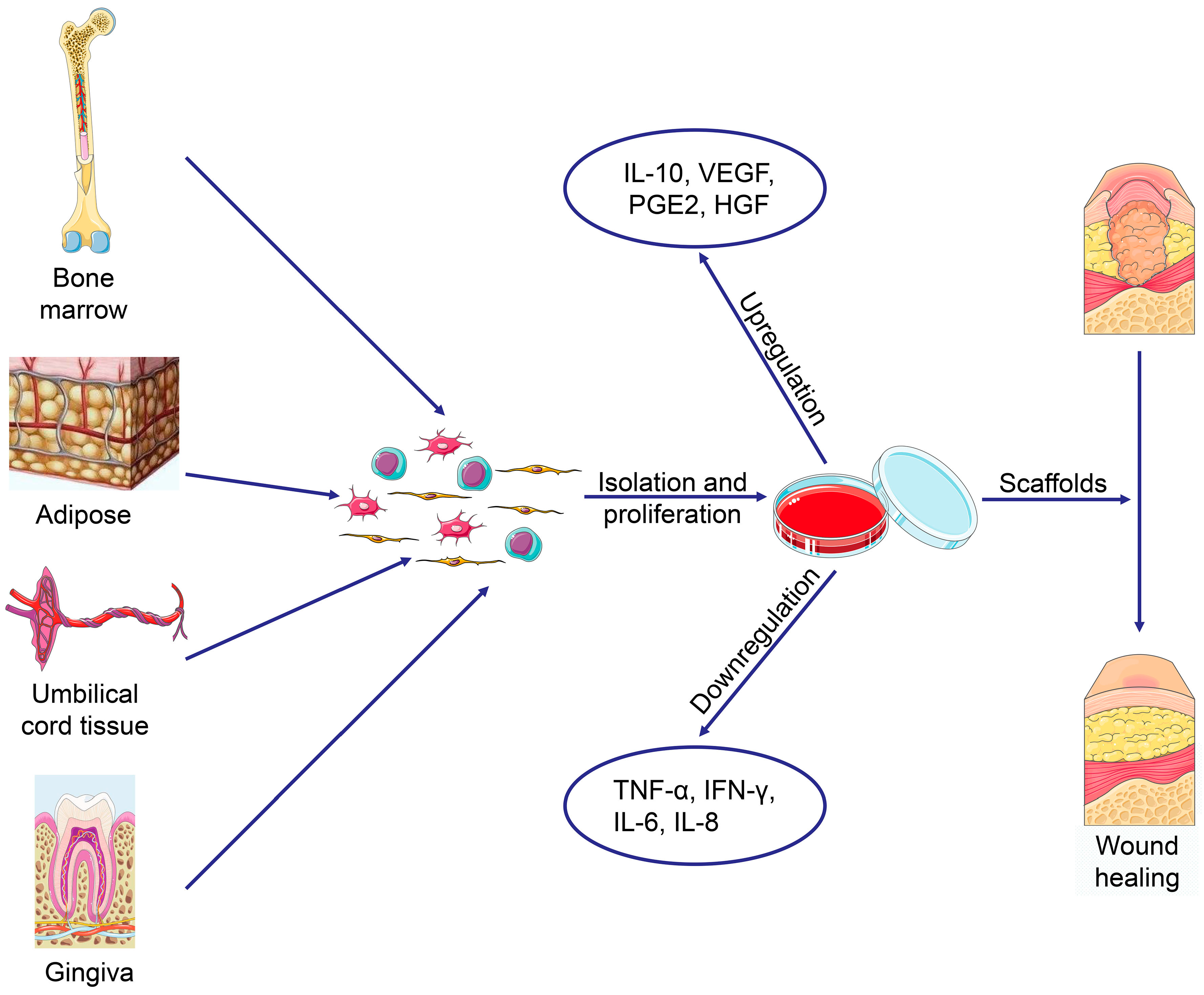
2. Discovery and Extraction of MSCs from Different Sources
3. Differentiation Potentials of MSC Types
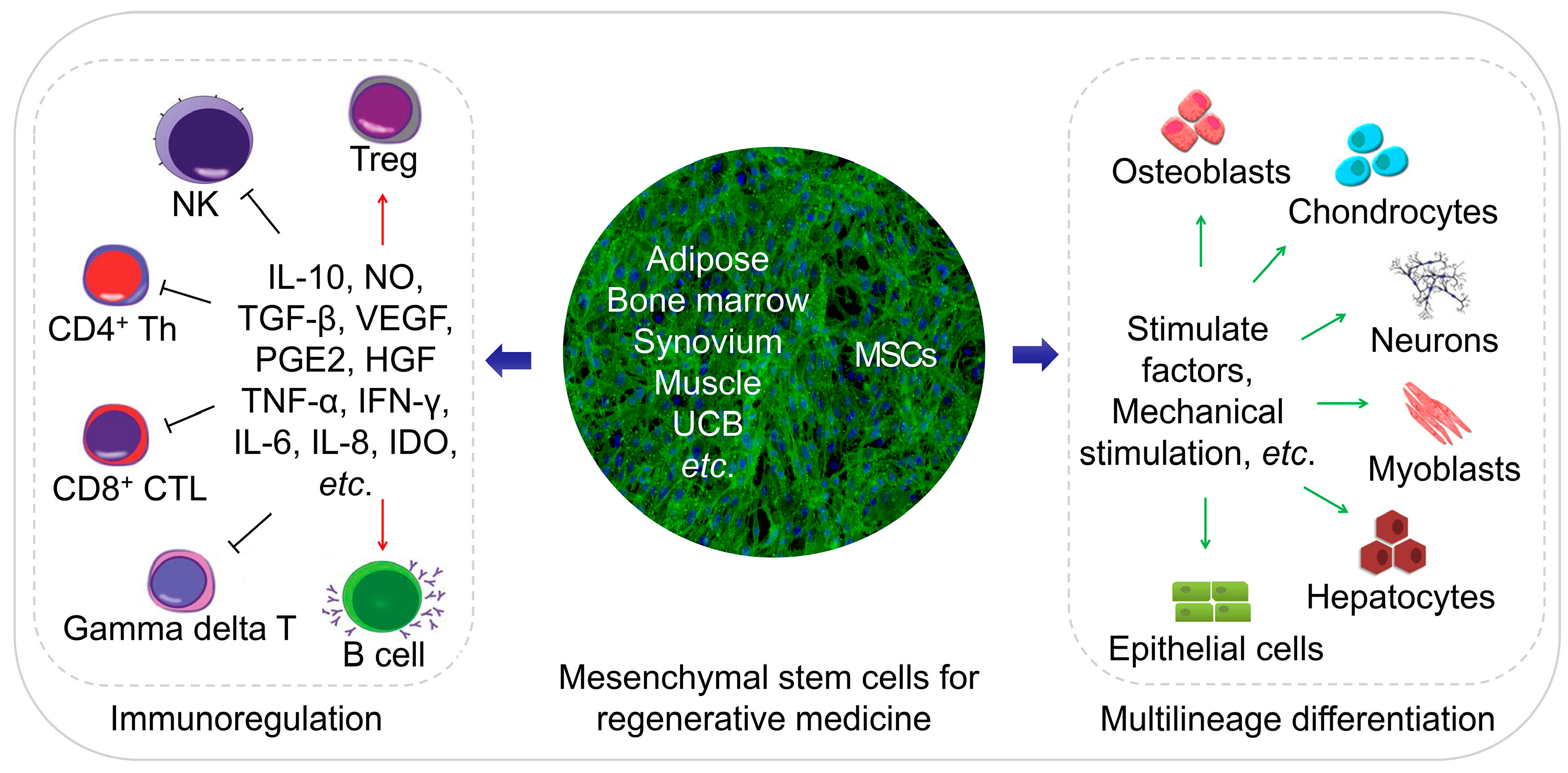
4. MSC-Based Regenerative Medicine
4.1. Bone Regeneration
4.2. Cartilage Repair
4.3. Regeneration of Other Musculoskeletal Tissues
4.4. Central Nervous System Rebuilding
4.5. Peripheral Nervous System Rebuilding
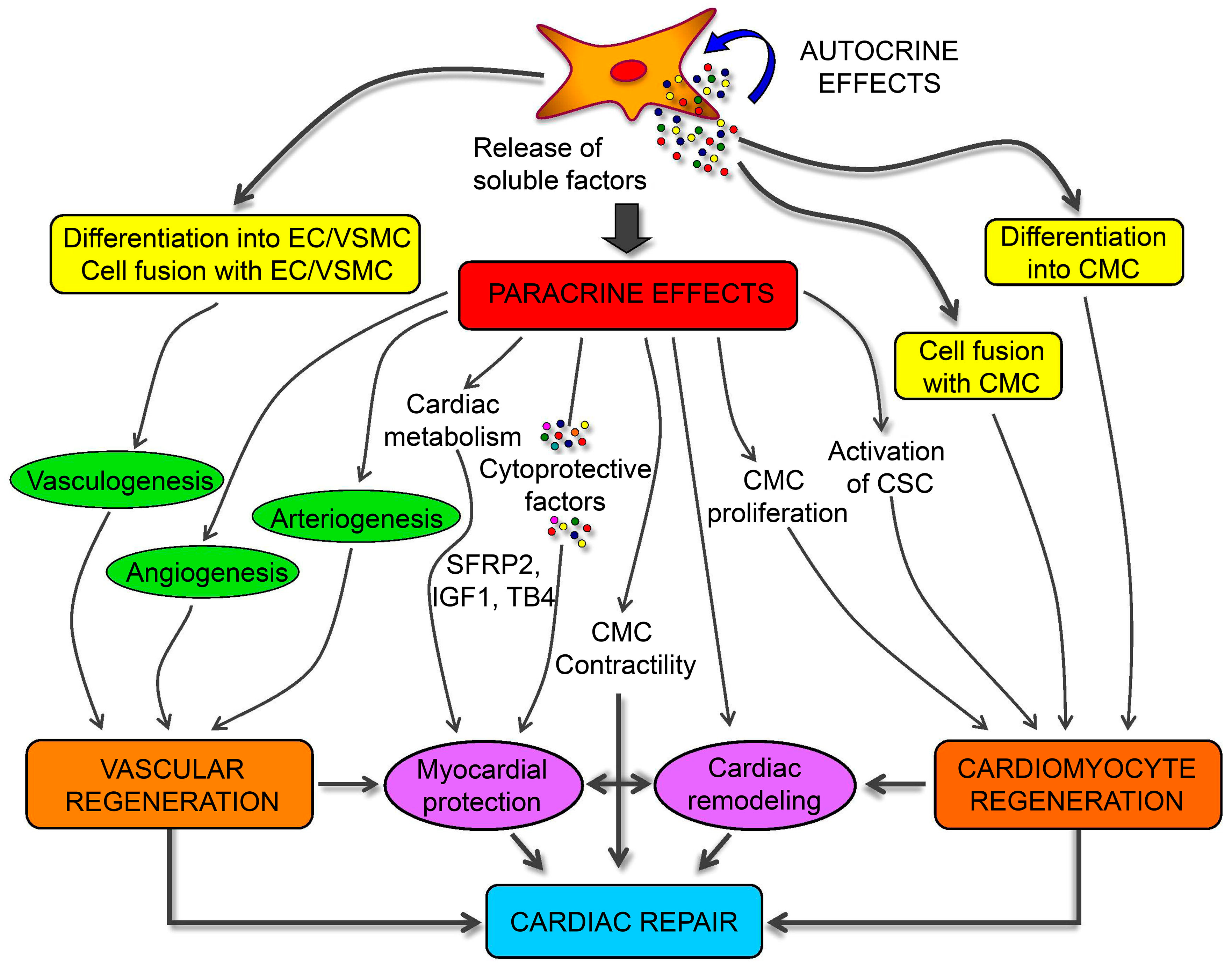
4.6. Myocardium Restoration
4.7. Liver Regeneration
4.8. Corneal Reconstruction
4.9. Tracheal Reconstruction
4.10. Skin Regeneration
4.11. Other Examples of MSC-Based Therapeutics
5. Potential Risk of Implanting MSCs
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Friedenstein, A.J.; Gorskaja, U.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Ashton, B.A.; Allen, T.D.; Howlett, C.; Eaglesom, C.; Hattori, A.; Owen, M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980, 151, 294–307. [Google Scholar] [CrossRef]
- Owen, M.; Friedenstein, A. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba. Found Symp. 1988, 136, 42–60. [Google Scholar] [PubMed]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine: Past, present, and future. Int. Rev. Neurobiol. 2013, 108, 1–33. [Google Scholar] [PubMed]
- Campagnoli, C.; Roberts, I.A.G.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shao, J.Z.; Xiang, L.X.; Dong, X.J.; Zhang, G.R. Mesenchymal stem cells: A promising candidate in regenerative medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Soleimani, M.; Nadri, S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009, 4, 102–106. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef]
- El-Ansary, M.; Abdel-Aziz, I.; Mogawer, S.; Abdel-Hamid, S.; Hammam, O.; Teaema, S.; Wahdan, M. Phase II trial: Undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. Rep. 2012, 8, 972–981. [Google Scholar] [CrossRef]
- Mao, P.; Joshi, K.; Li, J.F.; Kim, S.H.; Li, P.P.; Santana-Santos, L.; Luthra, S.; Chandran, U.R.; Benos, P.V.; Smith, L.; et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc. Natl. Acad. Sci. USA 2013, 110, 8644–8649. [Google Scholar] [CrossRef] [Green Version]
- Caniglia, C.J.; Schramme, M.C.; Smith, R.K. The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet. J. 2012, 44, 587–593. [Google Scholar] [CrossRef]
- Davies, L.C.; Lonnies, H.; Locke, M.; Sundberg, B.; Rosendahl, K.; Gotherstrom, C.; Le Blanc, K.; Stephens, P. Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev. 2012, 21, 1478–1487. [Google Scholar] [CrossRef]
- Herrmann, R.; Sturm, M.; Shaw, K.; Purtill, D.; Cooney, J.; Wright, M.; Phillips, M.; Cannell, P. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft versus host disease: A phase 1 study. Int. J. Hematol. 2012, 95, 182–188. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Smadja, D.M.; d’Audigier, C.; Guerin, C.L.; Mauge, L.; Dizier, B.; Silvestre, J.S.; Dal Cortivo, L.; Gaussem, P.; Emmerich, J. Angiogenic potential of BM MSCs derived from patients with critical leg ischemia. Bone Marrow Transplant. 2012, 47, 997–1000. [Google Scholar] [CrossRef]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.Q.; Luan, S.L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- Doorn, J.; Siddappa, R.; van Blitterswijk, C.A.; de Boer, J. Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A. 2012, 18, 558–567. [Google Scholar] [CrossRef]
- Higuera, G.A.; Schop, D.; Spitters, T.W.G.M.; van Dijkhuizen-Radersma, R.; Bracke, M.; de Bruijn, J.D.; Martens, D.; Karperien, M.; van Boxtel, A.; van Blitterswijk, C.A. Patterns of amino acid metabolism by proliferating human mesenchymal stem cells. Tissue Eng. Part A. 2012, 18, 654–664. [Google Scholar] [CrossRef]
- Zhao, D.W.; Cui, D.P.; Wang, B.J.; Tian, F.D.; Guo, L.; Yang, L.; Liu, B.Y.; Yu, X.B. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Futami, I.; Ishijima, M.; Kaneko, H.; Tsuji, K.; Ichikawa-Tomikawa, N.; Sadatsuki, R.; Muneta, T.; Arikawa-Hirasawa, E.; Sekiya, I.; Kaneko, K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PloS ONE 2012, 7, e45517. [Google Scholar] [CrossRef]
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64. [Google Scholar] [CrossRef]
- Marx, R.E.; Harrell, D.B. Translational research: The CD34+ cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int. J. Oral. Maxillofac. Implants 2014, 24, e201–209. [Google Scholar] [CrossRef]
- Sibov, T.T.; Severino, P.; Marti, L.C.; Pavon, L.F.; Oliveira, D.M.; Tobo, P.R.; Campos, A.H.; Paes, A.T.; Amaro, E.; Gamarra, L.F.; et al. Mesenchymal stem cells from umbilical cord blood: Parameters for isolation, characterization and adipogenic differentiation. Cytotechnology 2012, 64, 511–521. [Google Scholar] [CrossRef]
- Gao, L.R.; Pei, X.T.; Ding, Q.A.; Chen, Y.; Zhang, N.K.; Chen, H.Y.; Wang, Z.G.; Wang, Y.F.; Zhu, Z.M.; Li, T.C.; et al. A critical challenge: Dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int. J. Cardiol. 2013, 168, 3191–3199. [Google Scholar] [CrossRef]
- Dai, R.R.; Yu, Y.C.; Yan, G.F.; Hou, X.X.; Ni, Y.M.; Shi, G.C. Intratracheal administration of adipose derived mesenchymal stem cells alleviates chronic asthma in a mouse model. BMC Pulm. Med. 2018, 18, 131. [Google Scholar] [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 327, 449–462. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Harris, D.T. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 2013, 15, 330–343. [Google Scholar] [CrossRef]
- Park, J.S.; Shim, M.S.; Shim, S.H.; Yang, H.N.; Jeon, S.Y.; Woo, D.G.; Lee, D.R.; Yoon, T.K.; Park, K.H. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials 2011, 32, 8139–8149. [Google Scholar] [CrossRef]
- Pievani, A.; Scagliotti, V.; Russo, F.M.; Azario, I.; Rambaldi, B.; Sacchetti, B.; Marzorati, S.; Erba, E.; Giudici, G.; Riminucci, M.; et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy 2014, 16, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.S.; Fujita, N.; Kawahara, N.; Yui, S.; Nam, E.; Nishimura, R. A comparison of neurosphere differentiation potential of canine bone marrow-derived mesenchymal stem cells and adipose-derived mesenchymal stem cells. J. Vet. Med. Sci. 2013, 75, 879–886. [Google Scholar] [CrossRef]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei. Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef]
- Hou, L.L.; Cao, H.; Wang, D.M.; Wei, G.R.; Bai, C.X.; Zhang, Y.; Pei, X.T. Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. Int. J. Hematol. 2003, 78, 256–261. [Google Scholar] [CrossRef]
- Lei, H.; Yu, B.; Huang, Z.; Yang, X.; Liu, Z.; Mao, X.; Tian, G.; He, J.; Han, G.; Chen, H.; et al. Comparative analysis of mesenchymal stem cells from adult mouse adipose, muscle, and fetal muscle. Mol. Biol. Rep. 2013, 40, 885–892. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Qian, H.; Zhu, W.; Sun, X.; Hu, J.; Zhou, H.; Chen, Y. Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp. Biol. Med. 2004, 229, 623–631. [Google Scholar] [CrossRef]
- Yang, J.; Song, T.; Wu, P.; Chen, Y.; Fan, X.; Chen, H.; Zhang, J.; Huang, C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol. Med. Rep. 2012, 5, 108–113. [Google Scholar]
- Yin, L.B.; Zhu, Y.H.; Yang, J.G.; Ni, Y.J.; Zhou, Z.; Chen, Y.; Wen, L.X. Adipose tissue-derived mesenchymal stem cells differentiated into hepatocyte-like cells in vivo and in vitro. Mol. Med. Rep. 2015, 11, 1722–1732. [Google Scholar] [CrossRef]
- Banas, A.; Teratani, T.; Yamamoto, Y.; Tokuhara, M.; Takeshita, F.; Quinn, G.; Okochi, H.; Ochiya, T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 2007, 46, 219–228. [Google Scholar] [CrossRef]
- Zhou, R.P.; Li, Z.K.; He, C.Y.; Li, R.L.; Xia, H.B.; Li, C.Y.; Xiao, J.; Chen, Z.Y. Human umbilical cord mesenchymal stem cells and derived Hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model. PloS ONE 2014, 9, e104392. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, J.; Cho, K.J.; Lee, C.K.; Hwang, S.G.; Kim, G.J. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation 2012, 84, 223–231. [Google Scholar] [CrossRef]
- Katikireddy, K.R.; Dana, R.; Jurkunas, U.V. Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 2014, 32, 717–729. [Google Scholar] [CrossRef]
- Rohaina, C.M.; Then, K.Y.; Ng, A.M.H.; Halim, W.H.W.A.; Zahidin, A.Z.M.; Saim, A.; Idrus, R.B.H. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl. Res. 2014, 163, 200–210. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: from roots to boost. Stem Cells. 2019, 37, 855–864. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2018, 203, 96–110. [Google Scholar] [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma. 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Lozano-Calderon, S.A.; Swaim, S.O.; Federico, A.; Anderson, M.E.; Gebhardt, M.C. Predictors of soft-tissue complications and deep infection in allograft reconstruction of the proximal tibia. J. Surg. Oncol. 2016, 113, 811–817. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Milan, P.B.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. B 2018, 106, 61–72. [Google Scholar] [CrossRef]
- Yang, J.Z.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Bougioukli, S.; Sugiyama, O.; Pannell, W.; Ortega, B.; Tan, M.H.; Tang, A.H.; Yoho, R.; Oakes, D.A.; Lieberman, J.R. Genetherapy for bone repair using human cells: Superior osteogenic potential of bone morphogenetic protein 2-transduced mesenchymal stem cells derived from adipose tissue compared to bone marrow. Hum. Gene Ther. 2018, 29, 507–519. [Google Scholar] [CrossRef]
- Burrow, K.L.; Hoyland, J.A.; Richardson, S.M. Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017, 15, 2541275. [Google Scholar] [CrossRef]
- Ye, K.Q.; Liu, D.H.; Kuang, H.Z.; Cai, J.Y.; Chen, W.M.; Sun, B.B.; Xia, L.G.; Fang, B.; Morsi, Y.; Mo, X.M. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2019, 534, 625–636. [Google Scholar] [CrossRef]
- Aragon, J.; Salerno, S.; De Bartolo, L.; Irusta, S.; Mendoza, G. Polymeric electrospun scaffolds for bone morphogenetic protein 2 delivery in bone tissue engineering. J. Colloid Interface Sci. 2018, 531, 126–137. [Google Scholar] [CrossRef]
- Decambron, A.; Devriendt, N.; Larochette, N.; Manassero, M.; Bourguignon, M.; El-Hafci, H.; Petite, H.; Viateau, V.; Logeart-avramoglou, D. Effect of the bone morphogenetic protein-2 doses on the osteogenic potential of human multipotent stromal cells- containing tissue engineered constructs. Tissue Eng. Part A 2019, 25, 642–651. [Google Scholar] [CrossRef]
- Chen, F.; Bi, D.; Cheng, C.; Ma, S.; Liu, Y.; Cheng, K. Bone morphogenetic protein 7 enhances the osteogenic differentiation of human dermal-derived CD105+ fibroblast cells through the Smad and MAPK pathways. Int. J. Mol. Med. 2019, 43, 37–46. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liao, Y.P.; Li, F.S.; Hu, Y.; Li, Q.; Ma, Y.; Wang, H.; Zhou, Y.; He, B.C.; Su, Y.X. Wnt11 promotes BMP9-induced osteogenic differentiation through BMPs/Smads and p38 MAPK in mesenchymal stem cells. J. Cell Biochem. 2018, 119, 9462–9473. [Google Scholar] [CrossRef]
- Bizelli-Silveira, C.; Pullisaar, H.; Abildtrup, L.A.; Andersen, O.Z.; Spin-Neto, R.; Foss, M.; Kraft, D.C.E. Strontium enhances proliferation and osteogenic behavior of periodontal ligament cells in vitro. J. Periodont. Res. 2018, 53, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.; Tare, R.S.; Yang, L.Y.; Williams, D.F.; Ou, K.L.; Oreffo, R.O.C. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Westhauser, F.; Senger, A.S.; Reible, B.; Moghaddam, A. In vivo models for the evaluation of the osteogenic potency of bone substitutes seeded with mesenchymal stem cells of human origin: A concise review. Tissue Eng. Part C 2017, 23, 881–888. [Google Scholar] [CrossRef]
- Khojasteh, A.; Fahimipour, F.; Jafarian, M.; Sharifi, D.; Jahangir, S.; Khayyatan, F.; Eslaminejad, M.B. Bone engineering in dog mandible: Coculturing mesenchymal stem cells with endothelial progenitor cells in a composite scaffold containing vascular endothelial growth factor. J. Biomed. Mater. Res. B 2017, 105, 1767–1777. [Google Scholar] [CrossRef]
- Katagiri, W.; Watanabe, J.; Toyama, N.; Osugi, M.; Sakaguchi, K.; Hibi, H. Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant. Dent. 2017, 26, 607–612. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Liebergall, M.; Schroeder, J.; Mosheiff, R.; Gazit, Z.; Yoram, Z.; Rasooly, L.; Daskal, A.; Khoury, A.; Weil, Y.; Beyth, S. Stem cell-based therapy for prevention of delayed fracture union: A randomized and prospective preliminary study. Mol. Ther. 2013, 21, 1631–1638. [Google Scholar] [CrossRef]
- Giannotti, S.; Trombi, L.; Bottai, V.; Ghilardi, M.; D’Alessandro, D.; Danti, S.; Dell’Osso, G.; Guido, G.; Petrini, M. Use of autologous human mesenchymal stromal cell/fibrin clot constructs in upper limb non-unions: Long-term assessment. PloS ONE 2013, 8, e73893. [Google Scholar] [CrossRef]
- Honarpardaz, A.; Irani, S.; Pezeshki-Modaress, M.; Zandi, M.; Sadeghi, A. Enhanced chondrogenic differentiation of bone marrow mesenchymal stem cells on gelatin/glycosaminoglycan electrospun nanofibers with different amount of glycosaminoglycan. J. Biomed. Mater. Res. Part A 2019, 107, 38–48. [Google Scholar] [CrossRef]
- Fernandes, T.L.; Kimura, H.A.; Pinheiro, C.C.G.; Shimomura, K.; Nakamura, N.; Ferreira, J.R.M.; Gomoll, A.H.; Hernandez, A.J.; Bueno, D.F. Human synovial mesenchymal stem cells good manufacturing practices for articular cartilage regeneration. Tissue Eng. Part C 2018, 24, 709–716. [Google Scholar] [CrossRef]
- Zou, J.Y.; Bai, B.; Yao, Y.C. Progress of co-culture systems in cartilage regeneration. Expert. Opin. Biol. Ther. 2018, 18, 1151–1158. [Google Scholar] [CrossRef]
- Girao, A.F.; Semitela, A.; Ramalho, G.; Completo, A.; Marques, P. Mimicking nature: Fabrication of 3D anisotropic electrospun polycaprolactone scaffolds for cartilage tissue engineering applications. Composites, Part B 2018, 154, 99–107. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.B.; Li, S.; Deng, Y.; He, N.Y. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 20. [Google Scholar] [CrossRef]
- Legendre, F.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Gruchy, N.; Mallein-Gerin, F.; Leclercq, S.; Demoor, M.; Galera, P. Enhanced chondrogenesis of bone marrow-derived stem cells by using a combinatory cell therapy strategy with BMP-2/TGF-β1, hypoxia, and COL1A1/HtrA1 siRNAs. Sci. Rep. 2017, 7, 3406. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Borrajo, E.; Vidal, A.; Garcia-Fuentes, M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur. J. Pharm. Biopharm. 2017, 114, 69–78. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Abdelbaset-Ismail, A.; Aithal, H.P.; Kinjavdekar, P.; Pawde, A.M.; Kumar, G.S.; Sharma, G.T. Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for osteochondral defects in rabbits. Biomed. Pharmacother. 2017, 93, 1165–1174. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef]
- Wakitani, S.; Goto, T.; Pineda, S.J.; Young, R.G.; Mansour, J.M.; Caplan, A.I.; Goldberg, V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J. Bone Joint Surg. Am. 1994, 76, 579–592. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Slaper-Cortenbach, I.C.; van den Broek, M.P.; Nizak, R.; van Rijen, M.H.; de Weger, R.A.; Dhert, W.J.; Saris, D.B. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells 2017, 35, 256–264. [Google Scholar] [CrossRef]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr. Cartilage 2002, 10, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef]
- Sasaki, H.; Rothrauff, B.B.; Alexander, P.G.; Lin, H.; Gottardi, R.; Fu, F.H.; Tuan, R.S. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and TGF-β3. Am. J. Sports Med. 2018, 46, 2402–2413. [Google Scholar] [CrossRef]
- Shimomura, K.; Rothrauff, B.B.; Hart, D.A.; Hamamoto, S.; Kobayashi, M.; Yoshikawa, H.; Tuan, R.S.; Nakamura, N. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials 2018, 192, 346–354. [Google Scholar] [CrossRef]
- Liang, Y.; Idrees, E.; Szojka, A.R.A.; Andrews, S.H.J.; Kunze, M.; Mulet-Sierra, A.; Jomha, N.M.; Adesida, A.B. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomater. 2018, 80, 131–143. [Google Scholar] [CrossRef]
- Toratani, T.; Nakase, J.; Numata, H.; Oshima, T.; Takata, Y.; Nakayama, K.; Tsuchiya, H. Scaffold-free tissue-engineered allogenic adipose-derived stem cells promote meniscus healing. Arthroscopy 2017, 33, 346–354. [Google Scholar] [CrossRef]
- Tarafder, S.; Gulko, J.; Sim, K.H.; Yang, J.; Cook, J.L.; Lee, C.H. Engineered healing of avascular meniscus tears by stem cell recruitment. Sci. Rep. 2018, 8, 8150. [Google Scholar] [CrossRef]
- Chen, M.X.; Guo, W.M.; Gao, S.; Hao, C.X.; Shen, S.; Zhang, Z.Z.; Wang, Z.H.; Li, X.; Jing, X.G.; Zhang, X.L.; et al. Biomechanical stimulus based strategies for meniscus tissue engineering and regeneration. Tissue Eng. Part B 2018, 24, 392–402. [Google Scholar] [CrossRef]
- Chew, E.; Prakash, R.; Khan, W. Mesenchymal stem cells in human meniscal regeneration: A systematic review. Ann. Med. Surg. 2017, 24, 3–7. [Google Scholar] [CrossRef]
- Clanton, T.O.; Coupe, K.J. Hamstring strains in athletes: Diagnosis and treatment. J. Am. Acad. Orthop. Surg. 1998, 6, 237–248. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.H.; Lu, A.Q.; Tu, M.; Huang, W.; Huang, P. BMP14 induces tenogenic differentiation of bone marrow mesenchymal stem cells in vitro. Exp. Ther. Med. 2018, 16, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Aktas, E.; Chamberlain, C.S.; Saether, E.E.; Duenwald-Kuehl, S.E.; Kondratko-Mittnacht, J.; Stitgen, M.; Lee, J.S.; Clements, A.E.; Murphy, W.L.; Vanderby, R. Immune modulation with primed mesenchymal stem cells delivered via biodegradable scaffold to repair an Achilles tendon segmental defect. J. Orthop. Res. 2017, 35, 269–280. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, Y.J.; Moon, S.W.; Lee, B.H.; Shim, J.H.; Cho, D.W.; Wang, J.H. Three-dimensional bio-printed scaffold sleeves with mesenchymal stem cells for enhancement of tendon-to-bone healing in anterior cruciate ligament reconstruction using soft-tissue tendon graft. Arthroscopy 2018, 34, 166–179. [Google Scholar] [CrossRef]
- Wang, T.; Thien, C.; Wang, C.; Ni, M.; Gao, J.J.; Wang, A.; Jiang, Q.; Tuan, R.S.; Zheng, Q.J.; Zheng, M.H. 3D uniaxial mechanical stimulation induces tenogenic differentiation of tendon-derived stem cells through a PI3K/AKT signaling pathway. FASEB J. 2018, 32, 4804–4814. [Google Scholar] [CrossRef]
- Gulecyuz, M.F.; Macha, K.; Pietschmann, M.F.; Ficklscherer, A.; Sievers, B.; Rossbach, B.P.; Jansson, V.; Muller, P.E. Allogenic myocytes and mesenchymal stem cells partially improve fatty rotator cuff degeneration in a rat model. Stem Cell Rev. Rep. 2018, 14, 847–859. [Google Scholar] [CrossRef]
- Bertolo, A.; Mehr, M.; Aebli, N.; Baur, M.; Ferguson, S.J.; Stoyanov, J.V. Influence of different commercial scaffolds on the in vitro differentiation of human mesenchymal stem cells to nucleus pulposus-like cells. Eur. Spine J. 2012, 21 Suppl 6, S826–838. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Huang, X.; Fang, W.; Tao, Y.; Zhao, T.; Liang, C.; Hua, J.; Chen, Q.; Li, F. Injectable decellularized nucleus pulposus-based cell delivery system for differentiation of adipose-derived stem cells and nucleus pulposus regeneration. Acta Biomater. 2018, 81, 115–128. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, L.; Shi, G.D.; Shi, J.G.; Sun, J.C.; Xu, G.H.; Guo, Y.F.; Yuan, W. In vivo evaluation of the adenovirus-mediated Sox9 and BMP2 double gene transduction on treatment of intervertebral disc degeneration in rabbit annular puncture model. J. Biomater. Tissue Eng. 2018, 8, 1091–1099. [Google Scholar] [CrossRef]
- Varma, D.M.; DiNicolas, M.S.; Nicoll, S.B. Injectable, redox-polymerized carboxymethylcellulose hydrogels promote nucleus pulposus-like extracellular matrix elaboration by human MSCs in a cell density-dependent manner. J. Biomater. Appl. 2018, 33, 576–589. [Google Scholar] [CrossRef] [Green Version]
- Kraus, P.; Lufkin, T. Implications for a stem cell regenerative medicine based approach to human intervertebral disk degeneration. Front. Cell Dev. Biol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sanchez, A.; Garcia-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef]
- Pettine, K.A.; Suzuki, R.K.; Sand, T.T.; Murphy, M.B. Autologous bone marrow concentrate intradiscal injection for the treatment of degenerative disc disease with three-year follow-up. Int. Orthop. 2017, 41, 2097–2103. [Google Scholar] [CrossRef]
- Kumar, H.; Ha, D.H.; Lee, E.J.; Park, J.H.; Shim, J.H.; Ahn, T.K.; Kim, K.T.; Ropper, A.E.; Sohn, S.; Kim, C.H.; et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017, 8, 262. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.H.; Song, S.U.; Yi, T.G.; Yoon, S.H.; Park, S.R.; Choi, B.H. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J. Tissue Eng. Regen. Med. 2018, 12, E1034–E1045. [Google Scholar] [CrossRef]
- Jahan-Abad, A.J.; Negah, S.S.; Ravandi, H.H.; Ghasemi, S.; Borhani-Haghighi, M.; Stummer, W.; Gorji, A.; Ghadiri, M.K. Human neural stem/progenitor cells derived from epileptic human brain in a self-assembling peptide nanoscaffold improve traumatic brain injury in rats. Mol. Neurobiol. 2018, 55, 9122–9138. [Google Scholar] [CrossRef]
- Luo, L.H.; He, Y.; Wang, X.Y.; Key, B.; Lee, B.H.; Li, H.Q.; Ye, Q.S. Potential roles of dental pulp stem cells in neural regeneration and repair. Stem Cells Int. 2018, 2018, 1731289. [Google Scholar] [CrossRef]
- Fesharaki, M.; Razavi, S.; Ghasemi-Mobarakeh, L.; Behjati, M.; Yarahmadian, R.; Kazemi, M.; Hejazi, H. Differentiation of human scalp adipose-derived mesenchymal stem cells into mature neural cells on electrospun nanofibrous scaffolds for nerve tissue engineering applications. Cell J. 2018, 20, 168–176. [Google Scholar]
- Ma, Y.H.; Zeng, X.; Qiu, X.C.; Wei, Q.S.; Che, M.T.; Ding, Y.; Liu, Z.; Wu, G.H.; Sun, J.H.; Pang, M.; et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials 2018, 160, 37–55. [Google Scholar] [CrossRef]
- Comar, M.; Delbue, S.; Zanotta, N.; Valencic, E.; Piscianz, E.; Del Savio, R.; Tesser, A.; Tommasini, A.; Ferrante, P. In vivo detection of polyomaviruses JCV and SV40 in mesenchymal stem cells from human umbilical cords. Pediatr. Blood Cancer 2014, 61, 1347–1349. [Google Scholar] [CrossRef]
- van Velthoven, C.T.J.; Kavelaars, A.; Heijnen, C.J. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr. Res. 2012, 71, 474–481. [Google Scholar] [CrossRef]
- Shi, B.; Ding, J.X.; Liu, Y.; Zhuang, X.M.; Zhuang, X.L.; Chen, X.S.; Fu, C.F. ERK1/2 pathway-mediated differentiation of IGF-1-transfected spinal cord-derived neural stem cells into oligodendrocytes. PloS ONE 2014, 9, e106038. [Google Scholar] [CrossRef]
- de Araujo Farias, V.; Carrillo-Galvez, A.B.; Martin, F.; Anderson, P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018, 43, 25–37. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.Q.; Qu, C.S.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Oliveri, R.S.; Bello, S.; Biering-Sorensen, F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: Systematic review with meta-analyses of rat models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery 2016, 78, 436–447. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, H.B.; Dai, G.H.; Wang, X.D.; Hua, R.R.; Liu, X.B.; Wang, P.S.; Chen, G.M.; Yue, W.; An, Y.H. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef]
- Hu, C.X.; Li, L.J. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef]
- Sykova, E.; Rychmach, P.; Drahoradova, I.; Konradova, S.; Ruzickova, K.; Vorisek, I.; Forostyak, S.; Homola, A.; Bojar, M. Transplantation of mesenchymal stromal cells in patients with amyotrophic lateral sclerosis: Results of phase I/IIa clinical trial. Cell Transplant. 2017, 26, 647–658. [Google Scholar] [CrossRef]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): A study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Venkatesh, K.; Sen, D. Mesenchymal stem cells as a source of dopaminergic neurons: A potential cell based therapy for Parkinson’s disease. Curr. Stem Cell Res. T. 2017, 12, 326–347. [Google Scholar] [CrossRef]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; StrauSs, S.; Vogt, P.M.; Radtke, C. Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Y.; Yin, H.Y.; Guo, Z.Y.; Xu, F.; Xiao, B.; Jiang, W.L.; Guo, W.M.; Meng, H.Y.; Lu, S.B.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef]
- Fan, L.H.; Yu, Z.F.; Li, J.; Dang, X.Q.; Wang, K.Z. Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC Musculoskel. Dis. 2014, 15, 165. [Google Scholar] [CrossRef]
- Hu, F.H.; Zhang, X.F.; Liu, H.X.; Xu, P.; Doulathunnisa; Teng, G.J.; Xiao, Z.D. Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 171–178. [Google Scholar] [CrossRef]
- Papa, S.; Vismara, I.; Mariani, A.; Barilani, M.; Rimondo, S.; De Paola, M.; Panini, N.; Erba, E.; Mauri, E.; Rossi, F.; et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J. Controlled Release 2018, 278, 49–56. [Google Scholar] [CrossRef]
- Lin, T.; Liu, S.; Chen, S.H.; Qiu, S.; Rao, Z.L.; Liu, J.H.; Zhu, S.; Yan, L.W.; Mao, H.Q.; Zhu, Q.T.; et al. Hydrogel derived from porcine decellularized nerve tissue as a promising biomaterial for repairing peripheral nerve defects. Acta Biomater. 2018, 73, 326–338. [Google Scholar] [CrossRef]
- Lee, S.J.; Esworthy, T.; Stake, S.; Miao, S.; Zuo, Y.Y.; Harris, B.T.; Zhang, L.G. Advances in 3D bioprinting for neural tissue engineering. Adv. Biosyst. 2018, 2, 18. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, C.; Day, A.G.E.; Murray-Dunning, C.; Thanabalasundaram, L.; Cowan, J.; Stevanato, L.; Grace, N.; Cameron, G.; Drake, R.A.L.; Sinden, J.; et al. An allogeneic ‘off the shelf’ therapeutic strategy for peripheral nerve tissue engineering using clinical grade human neural stem cells. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef]
- Thomson, A.A.; Cunha, G.R. Prostatic growth and development are regulated by FGF10. Development 1999, 126, 3693–3701. [Google Scholar]
- Matthes, S.M.; Reimers, K.; Janssen, I.; Liebsch, C.; Kocsis, J.D.; Vogt, P.M.; Radtke, C. Intravenous transplantation of mesenchymal stromal cells to enhance peripheral nerve regeneration. Biomed. Res. Int. 2013, 2013, 573169. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Mori, D.; Miyagawa, S.; Yajima, S.; Saito, S.; Fukushima, S.; Ueno, T.; Toda, K.; Kawai, K.; Kurata, H.; Nishida, H.; et al. Cell spray transplantation of adipose-derived mesenchymal stem cell recovers ischemic cardiomyopathy in a porcine model. Transplantation 2018, 102, 2012–2024. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Cervio, E. Mesenchymal stem cell therapy for heart disease. Vascul. Pharmacol. 2012, 57, 48–55. [Google Scholar] [CrossRef]
- Butler, J.; Epstein, S.E.; Greene, S.J.; Quyyumi, A.A.; Sikora, S.; Kim, R.J.; Anderson, A.S.; Wilcox, J.E.; Tankovich, N.I.; Lipinski, M.J.; et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy safety and efficacy results of a phase II-a randomized trial. Circ. Res. 2017, 120, 332–340. [Google Scholar] [CrossRef]
- Choe, G.; Park, J.; Jo, H.; Kim, Y.S.; Ahn, Y.; Lee, J.Y. Studies on the effects of microencapsulated human mesenchymal stem cells in RGD-modified alginate on cardiomyocytes under oxidative stress conditions using in vitro biomimetic co-culture system. Int. J. Biol. Macromol. 2018, 123, 512–520. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, S. Differentiation of cardiomyocytes from amniotic fluid-derived mesenchymal stem cells by combined induction with transforming growth factor β1 and 5-azacytidine. Mol. Med. Rep. 2017, 16, 5887–5893. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.H.; Yim, C.; Lee, M.; Kang, H.J.; Choi, D. Therapeutic effects of a mesenchymal stem cell-based insulin-like growth factor-1/enhanced green fluorescent protein dual gene sorting system in a myocardial infarction rat model. Mol. Med. Rep. 2018, 18, 5563–5571. [Google Scholar] [CrossRef]
- Haneef, K.; Ali, A.; Khan, I.; Naeem, N.; Jamall, S.; Salim, A. Role of IL-7 in fusion of rat bone marrow mesenchymal stem cells with cardiomyocytes in vitro and improvement of cardiac function in vivo. Cardiovasc. Ther. 2018, 36, e12479. [Google Scholar] [CrossRef]
- Fujita, Y.; Kawamoto, A. Stem cell-based peripheral vascular regeneration. Adv. Drug Deliv. Rev. 2017, 120, 25–40. [Google Scholar] [CrossRef]
- Shafei, A.E.; Ali, M.A.; Ghanem, H.G.; Shehata, A.I.; Abdelgawad, A.A.; Handal, H.R.; Talaat, K.A.; Ashaal, A.E.; El-Shal, A.S. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J. Gene. Med. 2017, 19, e2995. [Google Scholar] [CrossRef]
- Tse, H.F.; Kwong, Y.L.; Chan, J.K.; Lo, G.; Ho, C.L.; Lau, C.P. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003, 361, 47–49. [Google Scholar] [CrossRef]
- Eng, G.; Lee, B.W.; Parsa, H.; Chin, C.D.; Schneider, J.; Linkov, G.; Sia, S.K.; Vunjak-Novakovic, G. Assembly of complex cell microenvironments using geometrically docked hydrogel shapes. Proc. Natl. Acad. Sci. USA 2013, 110, 4551–4556. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.S.; Wang, X.Y.; Li, Y.X.; He, L. Let-7a-transfected mesenchymal stem cells ameliorate monocrotaline-induced pulmonary hypertension by suppressing pulmonary artery smooth muscle cell growth through STAT3-BMPR2 signaling. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2013, 369, 2525–2534. [Google Scholar] [CrossRef]
- Petersen, B.E.; Bowen, W.C.; Patrene, K.D.; Mars, W.M.; Sullivan, A.K.; Murase, N.; Boggs, S.S.; Greenberger, J.S.; Goff, J.P. Bone marrow as a potential source of hepatic oval cells. Science 1999, 284, 1168–1170. [Google Scholar] [CrossRef]
- Shi, D.Y.; Zhang, J.M.; Zhou, Q.; Xin, J.J.; Jiang, J.; Jiang, L.Y.; Wu, T.Z.; Li, J.; Ding, W.C.; Li, J.; et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut 2017, 66, 955–964. [Google Scholar] [CrossRef]
- Higashiyama, R.; Inagaki, Y.; Hong, Y.Y.; Kushida, M.; Nakao, S.; Niioka, M.; Watanabe, T.; Okano, H.; Matsuzaki, Y.; Shiota, G.; et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology 2007, 45, 213–222. [Google Scholar] [CrossRef]
- Liu, W.H.; Song, F.Q.; Ren, L.N.; Guo, W.Q.; Wang, T.; Feng, Y.X.; Tang, L.J.; Li, K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J. Cell Mol. Med. 2015, 19, 511–520. [Google Scholar] [CrossRef]
- Lou, G.H.; Yang, Y.; Liu, F.F.; Ye, B.J.; Chen, Z.; Zheng, M.; Liu, Y.N. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell Mol. Med. 2017, 21, 2963–2973. [Google Scholar] [CrossRef]
- Suk, K.T.; Yoon, J.H.; Kim, M.Y.; Kim, C.W.; Kim, J.K.; Park, H.; Hwang, S.G.; Kim, D.J.; Lee, B.S.; Lee, S.H.; et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology 2016, 64, 2185–2197. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhao, C.; Wang, D.; Ma, X.; Zhao, S.; Wang, S.; Niu, L.; Sun, L. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. Int. J. Rheum. Dis. 2017, 20, 1219–1226. [Google Scholar] [CrossRef]
- Fang, X.Q.; Zhang, J.F.; Song, H.Y.; Chen, Z.L.; Dong, J.; Chen, X.; Pan, J.J.; Liu, B.; Chen, C.X. Effect of umbilical cord mesenchymal stem cell transplantation on immune function and prognosis of patients with decompensated hepatitis B cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2016, 24, 907–910. [Google Scholar]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.H.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.S.; Verfaillie, C.M. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J. Clin. Invest. 2002, 109, 1291–1302. [Google Scholar] [CrossRef]
- Lee, K.D.; Kuo, T.K.C.; Whang-Peng, J.; Chung, Y.F.; Lin, C.T.; Chou, S.H.; Chen, J.R.; Chen, Y.P.; Lee, O.K.S. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology 2004, 40, 1275–1284. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Takahashi, K.; Matsuda, A.; Patel, T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells improve survival from lethal hepatic failure in mice. Stem Cell Transl. Med. 2017, 6, 1262–1272. [Google Scholar] [CrossRef]
- Papanikolaou, I.G.; Katselis, C.; Apostolou, K.; Feretis, T.; Lymperi, M.; Konstadoulakis, M.M.; Papalois, A.E.; Zografos, G.C. Mesenchymal stem cells transplantation following partial hepatectomy: A new concept to promote liver regeneration-systematic review of the literature focused on experimental studies in rodent models. Stem Cells Int. 2017, 22, 7567958. [Google Scholar] [CrossRef]
- Di Bonzo, L.V.; Ferrero, I.; Cravanzola, C.; Mareschi, K.; Rustichell, D.; Novo, E.; Sanavio, F.; Cannito, S.; Zamara, E.; Bertero, M.; et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: Engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008, 57, 223–231. [Google Scholar] [CrossRef]
- Decker, M.; Martinez-Morentin, L.; Wang, G.N.; Lee, Y.J.; Liu, Q.X.; Leslie, J.; Ding, L. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat. Cell Biol. 2017, 19, 677–688. [Google Scholar] [CrossRef]
- Detry, O.; Vandermeulen, M.; Delbouille, M.H.; Somja, J.; Bletard, N.; Briquet, A.; Lechanteur, C.; Giet, O.; Baudoux, E.; Hannon, M.; et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J. Hepatol. 2017, 67, 47–55. [Google Scholar] [CrossRef]
- Tsubota, K.; Satake, Y.; Kaido, M.; Shinozaki, N.; Shimmura, S.; Bissen-Miyajima, H.; Shimazaki, J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N. Engl. J. Med. 1999, 340, 1697–1703. [Google Scholar] [CrossRef]
- Navas, A.; Magana-Guerrero, F.S.; Dominguez-Lopez, A.; Chavez-Garcia, C.; Partido, G.; Graue-Hernandez, E.O.; Sanchez-Garcia, F.J.; Garfias, Y. Anti-inflammatory and anti-fibrotic effects of human amniotic membrane mesenchymal stem cells and their potential in corneal repair. Stem Cell Transl Med. 2018, 7, 906–917. [Google Scholar] [CrossRef]
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ni Dhubhghaill, S.; Koppen, C.; Tassignon, M.J.; Zakaria, N. Limbal stem cell deficiency: Current treatment options and emerging therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef]
- Shen, T.; Shen, J.; Zheng, Q.Q.; Li, Q.S.; Zhao, H.L.; Cui, L.; Hong, C.Y. Cell viability and extracellular matrix synthesis in a co-culture system of corneal stromal cells and adipose-derived mesenchymal stem cells. Int. J. Ophthalmol. 2017, 10, 670–678. [Google Scholar]
- Yamashita, K.; Inagaki, E.; Hatou, S.; Higa, K.; Ogawa, A.; Miyashita, H.; Tsubota, K.; Shimmura, S. Corneal endothelial regeneration using mesenchymal stem cell derived from human umbilical cord. Stem Cells Dev. 2018, 27, 1097–1108. [Google Scholar] [CrossRef]
- Lohan, P.; Murphy, N.; Treacy, O.; Lynch, K.; Morcos, M.; Chen, B.L.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Third-party allogeneic mesenchymal stromal cells prevent rejection in a pre-sensitized high-risk model of corneal transplantation. Front Immunol. 2018, 9, 2666. [Google Scholar] [CrossRef]
- Ma, Y.L.; Xu, Y.S.; Xiao, Z.F.; Yang, W.; Zhang, C.; Song, E.; Du, Y.Q.; Li, L.S. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells 2006, 24, 315–321. [Google Scholar] [CrossRef]
- Gu, S.F.; Xing, C.Z.; Han, J.Y.; Tso, M.O.M.; Hong, J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol. Vis. 2009, 15, 99–107. [Google Scholar]
- Lee, H.J.; Ko, J.H.; Ko, A.Y.; Kim, M.K.; Wee, W.R.; Oh, J.Y. Intravenous infusion of mesenchymal stem/stromal cells decreased CCR7+ antigen presenting cells in mice with corneal allotransplantation. Curr. Eye. Res. 2014, 39, 780–789. [Google Scholar] [CrossRef]
- Yao, L.; Li, Z.R.; Su, W.R.; Li, Y.P.; Lin, M.L.; Zhang, W.X.; Liu, Y.; Wan, Q.; Liang, D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PloS ONE 2012, 7, e30842. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, M.K.; Shin, M.S.; Lee, H.J.; Ko, J.H.; Wee, W.R.; Lee, J.H. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells 2008, 26, 1047–1055. [Google Scholar] [CrossRef]
- Bray, L.J.; Heazlewood, C.F.; Munster, D.J.; Hutmacher, D.W.; Atkinson, K.; Harkin, D.G. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 2014, 16, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.Y.; Lee, R.H.; Yu, J.M.; Ko, J.H.; Lee, H.J.; Ko, A.Y.; Roddy, G.W.; Prockop, D.J. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol. Ther. 2012, 20, 2143–2152. [Google Scholar] [CrossRef]
- Den Hondt, M.; Vranckx, J.J. Reconstruction of defects of the trachea. J. Mater. Sci.: Mater. Med. 2017, 28, 11. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Gong, X.; Su, H.; Han, X. Secretion of rat tracheal epithelial cells induces mesenchymal stem cells to differentiate into epithelial cells. Cell Biol. Int. 2012, 36, 169–175. [Google Scholar] [CrossRef]
- Paunescu, V.; Deak, E.; Herman, D.; Siska, I.R.; Tanasie, G.; Bunu, C.; Anghel, S.; Tatu, C.A.; Oprea, T.I.; Henschler, R.; et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J. Cell Mol. Med. 2007, 11, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Li, T.Y.; Tsang, L.L.; Fok, K.L.; Lo, P.S.; Zhu, J.X.; Ho, L.S.; Chung, Y.W.; Chan, H.C. Differentiation of adult rat bone marrow stem cells into epithelial progenitor cells in culture. Cell Biol. Int. 2006, 30, 823–828. [Google Scholar] [CrossRef]
- Pan, S.; Zhong, Y.; Shan, Y.; Liu, X.; Xiao, Y.; Shi, H. Selection of the optimum 3D-printed pore and the surface modification techniques for tissue engineering tracheal scaffold in vivo reconstruction. J. Biomed. Mater. Res. A 2019, 107, 360–370. [Google Scholar] [CrossRef]
- Ghorbani, F.; Moradi, L.; Shadmehr, M.B.; Bonakdar, S.; Droodinia, A.; Safshekan, F. In-vivo characterization of a 3D hybrid scaffold based on PCL/decellularized aorta for tracheal tissue engineering. Mater. Sci. Eng. C 2017, 81, 74–83. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Shi, H. The biocompatibility and immunogenicity study of decellularized tracheal matrix. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 441–447. [Google Scholar]
- Chang, J.W.; Park, S.A.; Park, J.K.; Choi, J.W.; Kim, Y.S.; Shin, Y.S.; Kim, C.H. Tissue-engineered tracheal reconstruction using three-dimensionally printed artificial tracheal graft: Preliminary report. Artif. Organs 2014, 38, E95–E105. [Google Scholar] [CrossRef]
- Siddiqi, S.; de Wit, R.; van der Heide, S.; Oosterwijk, E.; Verhagen, A. Aortic allografts: Final destination?-a summary of clinical tracheal substitutes. J. Thorac. Dis. 2018, 10, 5149–5153. [Google Scholar] [CrossRef]
- Jorge, L.F.; Francisco, J.C.; Bergonse, N.; Baena, C.; Carvalho, K.A.T.; Abdelwahid, E.; Neto, J.R.F.; Moreira, L.F.P.; Guarita-Souza, L.C. Tracheal repair with acellular human amniotic membrane in a rabbit model. J. Tissue Eng. Regen. Med. 2018, 12, E1525–E1530. [Google Scholar] [CrossRef]
- Xu, Y.; Li, D.; Yin, Z.Q.; He, A.J.; Lin, M.M.; Jiang, G.N.; Song, X.; Hu, X.F.; Liu, Y.; Wang, J.P.; et al. Tissue-engineered trachea regeneration using decellularized trachea matrix treated with laser micropore technique. Acta Biomater. 2017, 58, 113–121. [Google Scholar] [CrossRef]
- Jing, H.; Gao, B.; Gao, M.; Yin, H.; Mo, X.; Zhang, X.; Luo, K.; Feng, B.; Fu, W.; Wang, J.; et al. Restoring tracheal defects in a rabbit model with tissue engineered patches based on TGF-β3-encapsulating electrospun poly(L-lactic acid-co-ε-caprolactone)/collagen scaffolds. Artif. Cells, Nanomed., Biotechnol. 2018, 46, 985–995. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Tian, L.; He, X.; Gao, Q.; Wu, T.; Ramakrishna, S.; Zheng, J.; Mo, X. Evaluation of the potential of rhTGF-β3 encapsulated P(LLA-CL)/collagen nanofibers for tracheal cartilage regeneration using mesenchymal stems cells derived from Wharton’s jelly of human umbilical cord. Mater. Sci. Eng. C 2017, 70, 637–645. [Google Scholar] [CrossRef]
- Bae, S.W.; Lee, K.W.; Park, J.H.; Lee, J.; Jung, C.R.; Yu, J.; Kim, H.Y.; Kim, D.H. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 14. [Google Scholar] [CrossRef]
- Maughan, E.F.; Hynds, R.E.; Proctor, T.J.; Janes, S.M.; Elliott, M.; Birchall, M.A.; Lowdell, M.W.; De Coppi, P. Autologous cell seeding in tracheal tissue engineering. Curr. Stem Cell Rep. 2017, 3, 279–289. [Google Scholar] [CrossRef]
- Macchiarini, P.; Jungebluth, P.; Go, T.; Asnaghi, M.A.; Rees, L.E.; Cogan, T.A.; Dodson, A.; Martorell, J.; Bellini, S.; Parnigotto, P.P.; et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008, 372, 2023–2030. [Google Scholar] [CrossRef]
- Gonfiotti, A.; Jaus, M.O.; Barale, D.; Baiguera, S.; Comin, C.; Lavorini, F.; Fontana, G.; Sibila, O.; Rombola, G.; Jungebluth, P.; et al. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet 2014, 383, 238–244. [Google Scholar] [CrossRef]
- Elliott, M.J.; De Coppi, P.; Speggiorin, S.; Roebuck, D.; Butler, C.R.; Samuel, E.; Crowley, C.; McLaren, C.; Fierens, A.; Vondrys, D.; et al. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet 2012, 380, 994–1000. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Laverdet, B.; Micallef, L.; Lebreton, C.; Mollard, J.; Lataillade, J.J.; Coulomb, B.; Desmouliere, A. Use of mesenchymal stem cells for cutaneous repair and skin substitute elaboration. Pathol. Biol. 2014, 62, 108–117. [Google Scholar] [CrossRef]
- Feldman, D.S.; McCauley, J.F. Mesenchymal stem cells and transforming growth factor-β3 (TGF-β3) to enhance the regenerative ability of an albumin scaffold in full thickness wound healing. J. Funct. Biomater. 2018, 9, 65. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.S.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Beken, S.V.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Invest. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Ong, H.T.; Dilley, R.J. Novel non-angiogenic role for mesenchymal stem cell-derived vascular endothelial growth factor on keratinocytes during wound healing. Cytokine Growth Factor Rev. 2018, 44, 69–79. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, F.; Xu, Y.; Yan, J.X.; Wu, Y.D.; Li, S.H.; Liao, X.; Liang, J.X.; Li, Z.H.; Liu, H.W. A novel role of angiotensin II in epidermal cell lineage determination: Angiotensin II promotes the differentiation of mesenchymal stem cells into keratinocytes through the p38 MAPK, JNK and JAK2 signalling pathways. Exp. Dermatol. 2019, 28, 59–65. [Google Scholar] [CrossRef]
- Kolakshyapati, P.; Li, X.Y.; Chen, C.Y.; Zhang, M.X.; Tan, W.Q.; Ma, L.E.; Gao, C.Y. Gene-activated matrix/bone marrow-derived mesenchymal stem cells constructs regenerate sweat glands-like structure in vivo. Sci. Rep. 2017, 7, 13. [Google Scholar] [CrossRef]
- Yang, D.; Sun, S.; Wang, Z.; Zhu, P.; Yang, Z.; Zhang, B. Stromal cell-derived factor-1 receptor CXCR4-overexpressing bone marrow mesenchymal stem cells accelerate wound healing by migrating into skin injury areas. Cell Reprogram. 2013, 15, 206–215. [Google Scholar] [CrossRef]
- Kisseleva, T.; Uchinami, H.; Feirt, N.; Quintana-Bustamante, O.; Segovia, J.C.; Schwabe, R.F.; Brenner, D.A. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006, 45, 429–438. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ishikawa, H.; Kawai, K.; Tabata, Y.; Suzuki, S. Enhanced wound healing by topical administration of mesenchymal stem cells transfected with stromal cell-derived factor-1. Biomaterials 2013, 34, 9393–9400. [Google Scholar] [CrossRef] [Green Version]
- Cai, S.; Pan, Y.; Han, B.; Sun, T.Z.; Sheng, Z.Y.; Fu, X.B. Transplantation of human bone marrow-derived mesenchymal stem cells transfected with ectodysplasin for regeneration of sweat glands. Chin. Med. J. 2011, 124, 2260–2268. [Google Scholar]
- Solmaz, H.; Ulgen, Y.; Gulsoy, M. Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med. Sci. 2017, 32, 903–910. [Google Scholar] [CrossRef]
- Murphy, K.C.; Whitehead, J.; Zhou, D.J.; Ho, S.S.; Leach, J.K. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017, 64, 176–186. [Google Scholar] [CrossRef]
- Tavakol, D.N.; Jeffries, L.A.; Schwager, S.C.; Kelly-Goss, M.R.; Peirce, S.M. Design of a 3D-bioprinted mesenchymal stem-cell laden construct for accelerating angiogenesis in diabetic wounds. FASEB J. 2017, 31, 2. [Google Scholar]
- Shokrgozar, M.A.; Fattahi, M.; Bonakdar, S.; Ragerdi Kashani, I.; Majidi, M.; Haghighipour, N.; Bayati, V.; Sanati, H.; Saeedi, S.N. Healing potential of mesenchymal stem cells cultured on a collagen-based scaffold for skin regeneration. Iran. Biomed. J. 2012, 16, 68–76. [Google Scholar]
- Rodrigues, C.; de Assis, A.M.; Moura, D.J.; Halmenschlager, G.; Saffi, J.; Xavier, L.L.; Fernandes Mda, C.; Wink, M.R. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE 2014, 9, e96241. [Google Scholar] [CrossRef]
- Navone, S.E.; Pascucci, L.; Dossena, M.; Ferri, A.; Invernici, G.; Acerbi, F.; Cristini, S.; Bedini, G.; Tosetti, V.; Ceserani, V.; et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.M.; Deng, Y.; Wang, G.B.; Wang, Z.; Wang, L. Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 13. [Google Scholar] [CrossRef]
- Pelizzo, G.; Avanzini, M.A.; Mantelli, M.; Croce, S.; Maltese, A.; Vestri, E.; De Silvestri, A.; Percivalle, E.; Calcaterra, V. Granulation tissue-derived mesenchymal stromal cells: A potential application for burn wound healing in pediatric patients. J. Stem Cells Regen. Med. 2018, 14, 53–58. [Google Scholar]
- Lightner, A.L.; Wang, Z.; Zubair, A.C.; Dozois, E.J. A systematic review and meta-analysis of mesenchymal stem cell injections for the treatment of perianal Crohn’s disease: Progress made and future directions. Dis. Colon Rectum. 2018, 61, 629–640. [Google Scholar] [CrossRef]
- Wu, Q.N.; Lei, X.T.; Chen, L.; Zheng, Y.L.; Huang, H.M.; Qian, C.; Liang, Z.W. Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: A case report. Ann. Transl. Med. 2018, 6, 7. [Google Scholar] [CrossRef]
- Kuhl, T.; Mezger, M.; Hausser, I.; Handgretinger, R.; Bruckner-Tuderman, L.; Nystrom, A. High local concentrations of intradermal MSCs restore skin integrity and facilitate wound healing in dystrophic epidermolysis bullosa. Mol. Ther. 2015, 23, 1368–1379. [Google Scholar] [CrossRef]
- Portas, M.; Mansilla, E.; Drago, H.; Dubner, D.; Radl, A.; Coppola, A.; Di Giorgio, M. Use of human cadaveric mesenchymal stem cells for cell therapy of a chronic radiation-induced skin lesion: A case report. Radiat. Prot. Dosim. 2016, 171, 99–106. [Google Scholar] [CrossRef]
- Zhuge, Y.; Regueiro, M.M.; Tian, R.; Li, Y.; Xia, X.; Vazquez-Padron, R.; Elliot, S.; Thaller, S.R.; Liu, Z.J.; Velazquez, O.C. The effect of estrogen on diabetic wound healing is mediated through increasing the function of various bone marrow-derived progenitor cells. J. Vasc. Surg. 2018, 68, 127S–135S. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Fu, X.; Cai, S.; Lei, Y.; Sun, T.; Bai, X.; Chen, M. Regeneration of functional sweat gland-like structures by transplanted differentiated bone marrow mesenchymal stem cells. Wound Repair Regen. 2009, 17, 427–435. [Google Scholar] [CrossRef]
- Xia, Y.; You, X.E.; Chen, H.; Yan, Y.J.; He, Y.C.; Ding, S.Z. Epidermal growth factor promotes mesenchymal stem cell-mediated wound healing and hair follicle regeneration. Int. J. Clin. Exp. Pathol. 2017, 10, 7390–7400. [Google Scholar]
- Jiang, M.H.; Li, G.L.; Liu, J.F.; Liu, L.S.; Wu, B.Y.; Huang, W.J.; He, W.; Deng, C.H.; Wang, D.; Li, C.L.; et al. Nestin+ kidney resident mesenchymal stem cells for the treatment of acute kidney ischemia injury. Biomaterials 2015, 50, 56–66. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, H.; Liu, Y.D.; Xiao, D.D.; Li, W.; Wang, Q.; Zhao, Y.; Sun, K.; Zhang, M.; Lu, M.J. Adipose-derived stem-cell-implanted poly(ε-caprolactone)/chitosan scaffold improves bladder regeneration in a rat model. Regen. Med. 2018, 13, 331–342. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.P.; Wang, Y.; Lozano, G. Mesenchymal stem cells and the origin of Ewing’s sarcoma. Sarcoma 2011, 2011, 276463. [Google Scholar] [CrossRef]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, e1000029. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Popova, A.P.; Bozyk, P.D.; Goldsmith, A.M.; Linn, M.J.; Lei, J.; Bentley, J.K.; Hershenson, M.B. Autocrine production of TGF-β1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2010, 298, L735–743. [Google Scholar] [CrossRef]
- Collins, J.J.; Thebaud, B. Lung mesenchymal stromal cells in development and disease: To serve and protect? Antioxid. Redox. Signal. 2014, 21, 1849–1862. [Google Scholar] [CrossRef]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E.; Takagishi, K.; Kato, Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem. Biophys. Res. Commun. 2001, 288, 413–419. [Google Scholar] [CrossRef]
- Shahdadfar, A.; Fronsdal, K.; Haug, T.; Reinholt, F.P.; Brinchmann, J.E. In vitro expansion of human mesenchymal stem cells: Choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells 2005, 23, 1357–1366. [Google Scholar] [CrossRef]
- Berniakovich, I.; Giorgio, M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int. J. Mol. Sci. 2013, 14, 2119–2134. [Google Scholar] [CrossRef]
- Holubova, M.; Lysak, D.; Vlas, T.; Vannucci, L.; Jindra, P. Expanded cryopreserved mesenchymal stromal cells as an optimal source for graft-versus-host disease treatment. Biologicals. 2014, 42, 139–144. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing. Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
| MSC Type | Source | Extraction Approach | Culture Medium | Marker | Reference |
|---|---|---|---|---|---|
| BMSCs | Human: tubular bones and iliac crest bone marrow | 1. Aspirate 1 mL of bone marrow for bone canal; 2. Extraction is diluted in PBS (1:1) and centrifuged for 30 min at 3000 rpm; 3. The obtained buffy coat is isolated, washed, and plated on culture flasks for incubation | LG-DMEM with 1% (W/V) antibiotic/antimycotic, 10% (V/V) FBS | CD29+, CD44+, CD73+, CD90+, CD105+, Sca-1+, CD14−, CD34−, CD45−, CD19−, CD11b−, CD31−, CD86−, Ia−, and HLA-DR− | [13,14,15] |
| Mouse, rat, and rabbit: tubular bones, e.g., femurs and tibias | 1. Collect femurs and tibias, cleanse the tissue with scissors, and wash the bones with 70% (V/V) ethanol and then PBS; 2. Cut off the proximal and distal parts of bones, and flush out bone marrow from bone canal by a spring to culture flasks for incubation; 3. At days 3–5, non-adherent cells are removed | Mouse: CD29+, CD44+, CD73+, CD90+, CD105+, Sca-1+, CD14−, CD34−, CD45−, CD11b−, CD31−, Vcam-1−, C-Kit−, CD135−, CD11b−, Ia−, and CD86− | [12,13,14,16] | ||
| Rat: CD29+, CD44+, CD54+, CD73+, CD90+, CD105+, CD106+, Sca-1+, CD14−, CD34−, CD45−, and CD11b− | [17,31] | ||||
| Rabbit: CD29+, CD44+, CD73+, CD81+, CD90+, CD166+, CD14−, CD34−, CD45−, CD117−, and HLD-DR− | [15] | ||||
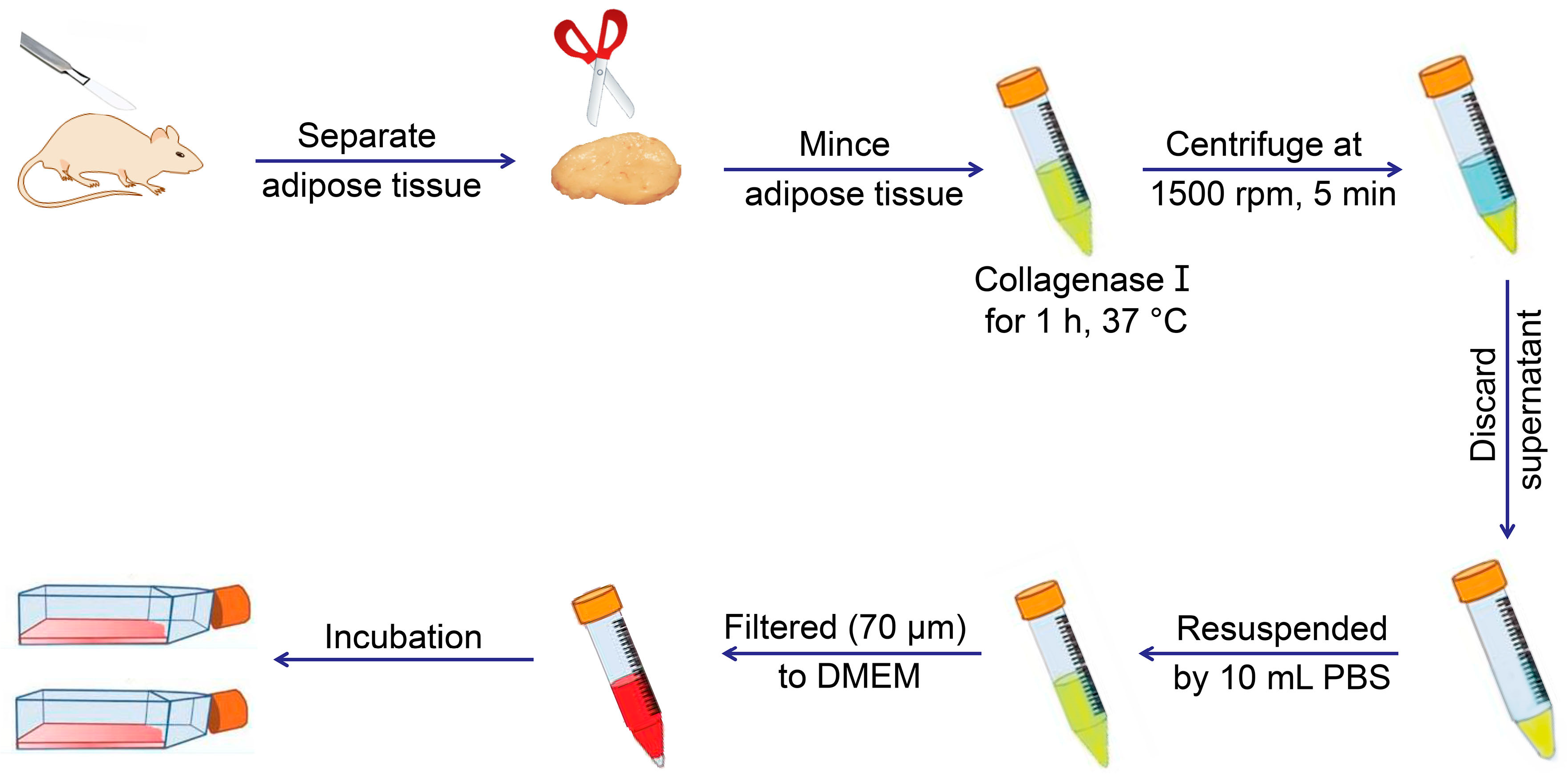
| ADSCs | Human: subcutaneous adipose in abdomen, buttocks, and abdominal zone | 1. Separate adipose from host body, and mince it with scissors or scalpel; 2. Digested by collagenase type I for 1 h at 37 °C gently shaking in a water bath; 3. Centrifuge the sample and discard the superior lipid layer; 4. Filtered through 100 and 40, or 70 μm filters; 5. Washed by 10 mL PBS and centrifuged again; 6. Discard the supernatant, resuspend the cells, and transfer them to culture flask for incubation | DMEM with 1% (W/V) P/S, 10% (V/V) FBS | Human: CD29+, CD44+, CD73+, CD90+, CD105+, CD146+, CD166+, MHC-I+, CD31−, CD45−, and HLA-DR− | [18,19,20,21] |
| Mouse, rat, and rabbit: subcutaneous adipose | Mouse: CD34+, CD44+, CD45+, CD90+, MHC-I+, MHC-II+, and CD117−. | [30] | |||
| Rat: CD44+, CD73+, CD90+, MHC-I+, CD31−, and CD45− | |||||
| Rabbit: CD44+, CD105+, NG2+, CD34−, and CD45− | [22,23] | ||||
| SMSCs | Synovium, especially in knee joints, of human, mouse, rat, rabbit, pig, etc. | 1. Separate synovium from host knee joint, and mince it with scissors or scalpel; 2. Digested by collagenase type II, D or P at 37 °C, and filtered through a 70 μm nylon filter; 3. The released cells are washed and resuspended in a culture medium for incubation | DMEM or αMEM with 1% (W/V) P/S, 250 ng mL−1 amphotericin B, and 10% (V/V) FBS | Human: CD10+, CD13+, CD49+, CD44+, CD73+, CD90+, CD105+, CD147+, CD166+, CD14−, CD20−, CD31−, CD34−, CD45−, CD62−, CD68−, CD113−, CD117−, HLA-DR−, and ALP− | [24] |
| Mouse: CD29+, CD44+, CD90+, CD34−, CD45−, and CD107− | [25] | ||||
| Rat: CD90+, CD11b−, and CD45− | [31] | ||||
| Rabbit: CD44+, CD90+, and CD105+ | [26,27] | ||||
| UCB-MSCs | Umbilical cord blood of human | 1. Harvest of human umbilical cord blood; 2. Mononuclear cells (MNC) are isolated from the buffy coat layer; 3. Seed into 25 cm2 flask, and non-adherent cells are removed after 48 h | LG-DMEM, 1% P/S, 250 ng mL−1 amphotericin B, and 10% (V/V) FBS | CD29+, CD44+, CD73+, CD90+, CD105+, CD166+, CD14−, CD31−, CD34−, CD45−, CD106−, and HLA-DR− | [28] |
| Differentiation Direction * | Preferred MSC Type | Basic Induction Medium | Identify Methods | Application Field | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Basic Medium | Induce Agents | Staining | IHC | RT-PCR | Others | ||||
| Osteoblast | BMSCs | LG-DMEM, 10% (V/V) FBS, 1% (W/V) antibiotic/antimycotic (In some studies, the osteogenic medium used HG-DMEM solution) | 10.0 mM β-glycerophosphate, 50.0 μg mL−1 ascorbic acid, and 100 nM dexamethasone | Alizarin red staining, Von Kossa Staining | Col I, OCN, OPN | Col I, OCN, OPN, ALP, BSP, Osterix, RUNX2 | ALP activity, Calcium assay kit | Bone regeneration | [32] |
| Chondrocyte | 50.0 μM ascorbic acid, 100 nM dexamethasone, 10.0 ng mL−1 TGF-β1/TGF-β3 | Alcian blue staining, Toluidine blue staining | Col II | Col II, SOX-9, Aggrecan, SOX-5, SOX-6, NOX 4, Col X, Chondroitin 4-sulfotransferase | GAG assay kit | Cartilage regeneration | [33,34] | ||
| Neurocyte | BMSCs, ADSCs | 10.0 ng mL−1 EGF, 20 ng mL−1 HGF, 20 ng mL−1 VEGF; 8 days later, 200 µM BHA, 5.0 mM KCl, 2.0 µM valproic acid, 10 µM forskolin, 1.0 µM hydrocortisone, and 5.0 µM insulin are added to the medium | — | Enolase, Tubulin-βIII, GFAP, S100, MBP, MAP2, NF | Tubulin-βIII, GFAP, Enolase, NeuN, NCAM, Glial cell marker, NANOG, OCT4 and SOX-2, MAP2, NF-M, GAP 43 | — | Nerve regeneration | [35,36,37] | |
| Cardiomyocyte | ADSCs | 10.0 µg L−1 bFGF, 10.0 µM 5-azacytidine; one day later, the medium maintained in the same conditions without 5-azacytidine for four weeks | — | Desmin, M-cadherin, MHC, α-cardiac actin, cTnI | Desmin, MYOD1, MYOG, MHC, α-cardiac actin, cTnT, MYF5/6, MEF2C, TNNI1/2, CKM, Myosin2, HCN2, HCN4 | Heterotypic Cell Fusion Assay | Myocardial regeneration | [38,39,40] | |
| Hepatocyte | PDSCs | 1X ITS, 10−8 M dexamethasone, 20.0 ng mL−1 EGF, 20.0 ng mL−1 FGF, 40.0 ng mL−1 OsM, 40 ng mL−1 HGF; After two weeks, the medium is replaced with hepatic differentiation medium with an increased concentration of dexamethasone at 10−5 M and/or 1.0 µM TSA | PAS staining | ALB, AFP, CK-18, PanCK, CK 19, Transthyretin | ALB, AFP, β-actin, CK-18, HNF-4α, Transthyretin, TDO2, and CYP7A1 | LDL/CM-Dil uptake assay; Cell morphology; Ammonia clearance; Albumin production; ELISA assay | Liver regeneration | [41,42,43,44] | |
| Keratocyte | No comparative studies | LG-DMEM: F-12 3:1, 5% FBS, 1% (W/V) antibiotic/antimycotic | Induction medium: without pyruvate, 25.0 ng mL−1 BMP-4, 1.0 mM all-trans retinoic acid, and 10.0 ng mL−1 EGF; Differentiation medium: 5.0 µg mL−1 insulin, 2.0 nM tri-iodothyronine, 2.0 nM adenine, and 10.0 ng mL−1 EGF | H&E staining | CK3, β1-integrin, and E-cadherin, p63, CK12, CK8, CK14, CK15 | ABCG2, β1-integrin, CEBPδ, CK3, and p63, Oct4, Sox2, Nanog, Rex1, DSC1, and DSG1 | Transepithelial electrical resistance | Corneal regeneration | [45,46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. https://doi.org/10.3390/cells8080886
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019; 8(8):886. https://doi.org/10.3390/cells8080886
Chicago/Turabian StyleHan, Yu, Xuezhou Li, Yanbo Zhang, Yuping Han, Fei Chang, and Jianxun Ding. 2019. "Mesenchymal Stem Cells for Regenerative Medicine" Cells 8, no. 8: 886. https://doi.org/10.3390/cells8080886
APA StyleHan, Y., Li, X., Zhang, Y., Han, Y., Chang, F., & Ding, J. (2019). Mesenchymal Stem Cells for Regenerative Medicine. Cells, 8(8), 886. https://doi.org/10.3390/cells8080886