Protective to a T: The Role of T Cells during Zika Virus Infection
Abstract
1. Introduction
2. Profiling the T Cell Response to ZIKV Infection
2.1. T Cell Responses in Mice
2.2. T Cell Responses in Humans
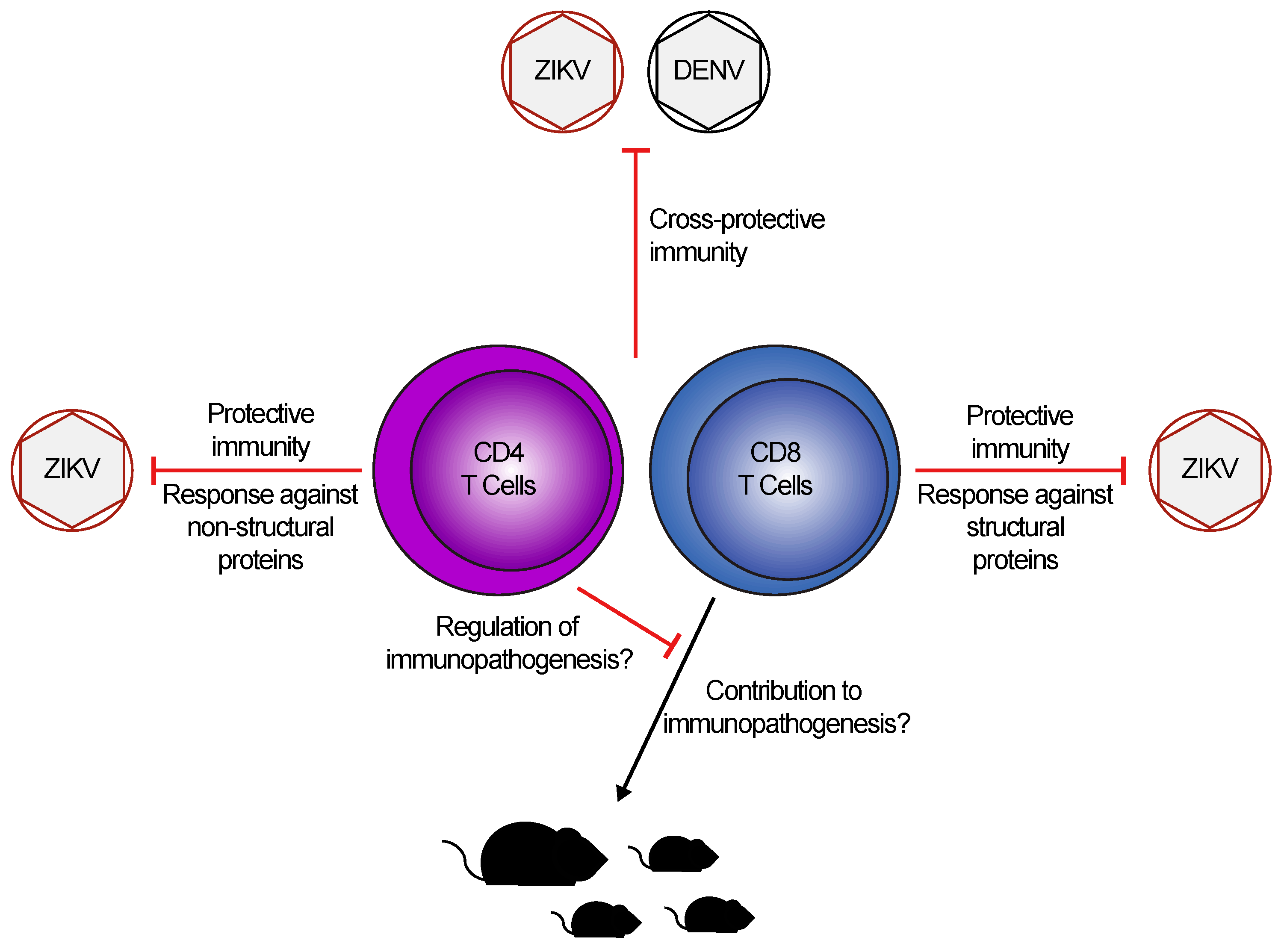
3. T Cell Responses to DENV and ZIKV: Cross-Protective or Pathogenic?
4. Role of T Cells in ZIKV Pathogenesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Wilson, N.S.; Waithman, J.; Villadangos, J.A.; Carbone, F.R.; Heath, W.R.; Belz, G.T. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol 2004, 5, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Valbon, S.F.; Condotta, S.A.; Richer, M.J. Regulation of effector and memory CD8+ T cell function by inflammatory cytokines. Cytokine 2016, 82, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.R.; Kongsgaard, M.; Steffensen, M.A.; Fenger, C.; Rasmussen, M.; Skjodt, K.; Finsen, B.; Stryhn, A.; Buus, S.; Christensen, J.P.; et al. CD8+ T cells complement antibodies in protecting against yellow fever virus. J. Immunol. 2015, 194, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lobigs, M.; Lee, E.; Mullbacher, A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 2003, 77, 13323–13334. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Rajah, M.M.; Pardy, R.D.; Condotta, S.A.; Richer, M.J.; Sagan, S.M. Zika Virus: Emergence, Phylogenetics, Challenges, and Opportunities. ACS Infect. Dis. 2016, 2, 763–772. [Google Scholar] [CrossRef]
- Kindhauser, M.K.; Allen, T.; Frank, V.; Santhana, R.S.; Dye, C. Zika: The origin and spread of a mosquito-borne virus. Bull. World Health Organ. 2016, 94, 675–686. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef]
- The History of Zika Virus. Available online: http://www.who.int/emergencies/zika-virus/history/en/ (accessed on 16 May 2019).
- Swati, G. Zika Spreads Rapidly in India, with 94 Cases Confirmed; CNN: Atlanta, GA, USA, 2018. [Google Scholar]
- Slater, J. India Wrestles with First Significant Outbreak of Zika Virus; The Washington Post: Washington, DC, USA, 2018. [Google Scholar]
- Krauer, F.; Riesen, M.; Reveiz, L.; Oladapo, O.T.; Martinez-Vega, R.; Porgo, T.V.; Haefliger, A.; Broutet, N.J.; Low, N.; Group, W.H.O.Z.C.W. Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS Med. 2017, 14, e1002203. [Google Scholar] [CrossRef]
- Dick, G.W. Zika virus. II. Pathogenicity and physical properties. Trans. R Soc. Trop. Med. Hyg. 1952, 46, 521–534. [Google Scholar] [CrossRef]
- Simpson, D.I. Zika Virus Infection in Man. Trans. R. Soc. Trop. Med. Hyg. 1964, 58, 335–338. [Google Scholar] [CrossRef]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef]
- Elong Ngono, A.; Young, M.P.; Bunz, M.; Xu, Z.; Hattakam, S.; Vizcarra, E.; Regla-Nava, J.A.; Tang, W.W.; Yamabhai, M.; Wen, J.; et al. CD4+ T cells promote humoral immunity and viral control during Zika virus infection. PLoS Pathog. 2019, 15, e1007474. [Google Scholar] [CrossRef]
- Hassert, M.; Wolf, K.J.; Schwetye, K.E.; DiPaolo, R.J.; Brien, J.D.; Pinto, A.K. CD4+ T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog 2018, 14, e1007237. [Google Scholar] [CrossRef]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4+ T cells and IFNgamma signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun 2018, 9, 3136. [Google Scholar] [CrossRef]
- Scott, J.M.; Lebratti, T.J.; Richner, J.M.; Jiang, X.; Fernandez, E.; Zhao, H.; Fremont, D.H.; Diamond, M.S.; Shin, H. Cellular and Humoral Immunity Protect against Vaginal Zika Virus Infection in Mice. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Kolumam, G.A.; Thomas, S.; Thompson, L.J.; Sprent, J.; Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005, 202, 637–650. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef]
- Richer, M.J.; Nolz, J.C.; Harty, J.T. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8+ T cells by enhancing T cell receptor signaling. Immunity 2013, 38, 140–152. [Google Scholar] [CrossRef]
- Longhi, M.P.; Trumpfheller, C.; Idoyaga, J.; Caskey, M.; Matos, I.; Kluger, C.; Salazar, A.M.; Colonna, M.; Steinman, R.M. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 2009, 206, 1589–1602. [Google Scholar] [CrossRef]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Zhang, Y.; Han, X.; Jia, B.; Liu, H.; Liu, D.; Tan, S.; Wang, Q.; Bi, Y.; et al. CD8+ T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Nazerai, L.; Scholler, A.S.; Rasmussen, P.O.S.; Buus, S.; Stryhn, A.; Christensen, J.P.; Thomsen, A.R. A New In Vivo Model to Study Protective Immunity to Zika Virus Infection in Mice With Intact Type I Interferon Signaling. Front. Immunol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016. [Google Scholar] [CrossRef]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a Novel Murine Model to Study Zika Virus. Am. J. Trop Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef]
- Winkler, C.W.; Myers, L.M.; Woods, T.A.; Messer, R.J.; Carmody, A.B.; McNally, K.L.; Scott, D.P.; Hasenkrug, K.J.; Best, S.M.; Peterson, K.E. Adaptive Immune Responses to Zika Virus Are Important for Controlling Virus Infection and Preventing Infection in Brain and Testes. J. Immunol. 2017, 198, 3526–3535. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Gorchakov, R.; Lai, L.; Natrajan, M.S.; Patel, S.M.; Atmar, R.L.; Keitel, W.A.; Hoft, D.F.; Barrett, J.; Bailey, J.; et al. Clinical, Virologic, and Immunologic Characteristics of Zika Virus Infection in a Cohort of US Patients: Prolonged RNA Detection in Whole Blood. Open Forum Infect. Dis. 2019, 6, ofy352. [Google Scholar] [CrossRef]
- Ricciardi, M.J.; Magnani, D.M.; Grifoni, A.; Kwon, Y.C.; Gutman, M.J.; Grubaugh, N.D.; Gangavarapu, K.; Sharkey, M.; Silveira, C.G.T.; Bailey, V.K.; et al. Ontogeny of the B- and T-cell response in a primary Zika virus infection of a dengue-naive individual during the 2016 outbreak in Miami, FL. PLoS Negl. Trop. Dis. 2017, 11, e0006000. [Google Scholar] [CrossRef]
- Lai, L.; Rouphael, N.; Xu, Y.; Natrajan, M.S.; Beck, A.; Hart, M.; Feldhammer, M.; Feldpausch, A.; Hill, C.; Wu, H.; et al. Innate, T-, and B-Cell Responses in Acute Human Zika Patients. Clin. Infect. Dis. 2018, 66, 1–10. [Google Scholar] [CrossRef]
- Edupuganti, S.; Natrajan, M.S.; Rouphael, N.; Lai, L.; Xu, Y.; Feldhammer, M.; Hill, C.; Patel, S.M.; Johnson, S.J.; Bower, M.; et al. Biphasic Zika Illness With Rash and Joint Pain. Open Forum Infect. Dis. 2017, 4, ofx133. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Rouphael, N.; Xu, Y.; Natrajan, M.; Lai, L.; Patel, S.M.; Levit, R.D.; Edupuganti, S.; Mulligan, M.J. Pericarditis Associated With Acute Zika Virus Infection in a Returning Traveler. Open Forum Infect. Dis. 2017, 4, ofx103. [Google Scholar] [CrossRef]
- Lum, F.M.; Lye, D.C.B.; Tan, J.J.L.; Lee, B.; Chia, P.Y.; Chua, T.K.; Amrun, S.N.; Kam, Y.W.; Yee, W.X.; Ling, W.P.; et al. Longitudinal Study of Cellular and Systemic Cytokine Signatures to Define the Dynamics of a Balanced Immune Environment During Disease Manifestation in Zika Virus-Infected Patients. J. Infect. Dis. 2018, 218, 814–824. [Google Scholar] [CrossRef]
- Cimini, E.; Castilletti, C.; Sacchi, A.; Casetti, R.; Bordoni, V.; Romanelli, A.; Turchi, F.; Martini, F.; Tumino, N.; Nicastri, E.; et al. Human Zika infection induces a reduction of IFN-gamma producing CD4 T-cells and a parallel expansion of effector Vdelta2 T-cells. Sci. Rep. 2017, 7, 6313. [Google Scholar] [CrossRef]
- Halstead, S.B. Pathogenic exploitation of Fc activity. In Antibody Fc: Linking Adaptive and Innate Immunity; Ackerman, M.E., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 333–350. [Google Scholar]
- Klenerman, P.; Zinkernagel, R.M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 1998, 394, 482–485. [Google Scholar] [CrossRef]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-Reactivity and Anti-viral Function of Dengue Capsid and NS3-Specific Memory T Cells Toward Zika Virus. Front. Immunol. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Koblischke, M.; Stiasny, K.; Aberle, S.W.; Malafa, S.; Tschouchnikas, G.; Schwaiger, J.; Kundi, M.; Heinz, F.X.; Aberle, J.H. Structural Influence on the Dominance of Virus-Specific CD4 T Cell Epitopes in Zika Virus Infection. Front. Immunol. 2018, 9, 1196. [Google Scholar] [CrossRef]
- Grifoni, A.; Pham, J.; Sidney, J.; O'Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Paquin-Proulx, D.; Leal, F.E.; Terrassani Silveira, C.G.; Maestri, A.; Brockmeyer, C.; Kitchen, S.M.; Cabido, V.D.; Kallas, E.G.; Nixon, D.F. T-cell Responses in Individuals Infected with Zika Virus and in Those Vaccinated Against Dengue Virus. Pathog. Immun. 2017, 2, 274–292. [Google Scholar] [CrossRef]
- Herrera, B.B.; Tsai, W.Y.; Brites, C.; Luz, E.; Pedroso, C.; Drexler, J.F.; Wang, W.K.; Kanki, P.J. T Cell Responses to Nonstructural Protein 3 Distinguish Infections by Dengue and Zika Viruses. MBio 2018, 9. [Google Scholar] [CrossRef]
- Badolato-Correa, J.; Sanchez-Arcila, J.C.; Alves de Souza, T.M.; Santos Barbosa, L.; Conrado Guerra Nunes, P.; da Rocha Queiroz Lima, M.; Gandini, M.; Bispo de Filippis, A.M.; Venancio da Cunha, R.; Leal de Azeredo, E.; et al. Human T cell responses to Dengue and Zika virus infection compared to Dengue/Zika coinfection. Immun. Inflamm. Dis. 2018, 6, 194–206. [Google Scholar] [CrossRef]
- Grifoni, A.; Costa-Ramos, P.; Pham, J.; Tian, Y.; Rosales, S.L.; Seumois, G.; Sidney, J.; de Silva, A.D.; Premkumar, L.; Collins, M.H.; et al. Cutting Edge: Transcriptional Profiling Reveals Multifunctional and Cytotoxic Antiviral Responses of Zika Virus-Specific CD8+ T Cells. J. Immunol. 2018, 201, 3487–3491. [Google Scholar] [CrossRef]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol 2017, 2, 17036. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Suleyman, O.M.; Ortega-Prieto, A.M.; Skelton, J.K.; Bonnesoeur, P.; Blohm, A.; Carregaro, V.; Silva, J.S.; James, E.A.; Maillere, B.; et al. T cell immunity to Zika virus targets immunodominant epitopes that show cross-reactivity with other Flaviviruses. Sci. Rep. 2018, 8, 672. [Google Scholar] [CrossRef]
- Wen, J.; Elong Ngono, A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8+ T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Elong Ngono, A.; Viramontes, K.M.; Huynh, A.T.; Wang, Y.T.; Nguyen, A.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat. Commun. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Peiris, J.S.; Hui, K.P.; Yen, H.L. Host response to influenza virus: Protection versus immunopathology. Curr. Opin. Immunol. 2010, 22, 475–481. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Knudson, C.J.; Hartwig, S.M.; Pewe, L.L.; Meyerholz, D.K.; Langlois, R.A.; Harty, J.T.; Varga, S.M. Memory CD8 T cells mediate severe immunopathology following respiratory syncytial virus infection. PLoS Pathog. 2018, 14, e1006810. [Google Scholar] [CrossRef]
- Gelpi, E.; Preusser, M.; Laggner, U.; Garzuly, F.; Holzmann, H.; Heinz, F.X.; Budka, H. Inflammatory response in human tick-borne encephalitis: Analysis of postmortem brain tissue. J. Neurovirol. 2006, 12, 322–327. [Google Scholar] [CrossRef]
- Ruzek, D.; Salat, J.; Palus, M.; Gritsun, T.S.; Gould, E.A.; Dykova, I.; Skallova, A.; Jelinek, J.; Kopecky, J.; Grubhoffer, L. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 2009, 384, 1–6. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Ireland, D.D.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and Neurodegeneration in CNS of Immunocompetent Neonatal C57Bl/6 Mice. PLoS Pathog 2016, 12, e1006004. [Google Scholar] [CrossRef]
- Manangeeswaran, M.; Kielczewski, J.L.; Sen, H.N.; Xu, B.C.; Ireland, D.D.C.; McWilliams, I.L.; Chan, C.C.; Caspi, R.R.; Verthelyi, D. ZIKA virus infection causes persistent chorioretinal lesions. Emerg. Microbes. Infect. 2018, 7, 96. [Google Scholar] [CrossRef]
- Li, S.; Armstrong, N.; Zhao, H.; Hou, W.; Liu, J.; Chen, C.; Wan, J.; Wang, W.; Zhong, C.; Liu, C.; et al. Zika Virus Fatally Infects Wild Type Neonatal Mice and Replicates in Central Nervous System. Viruses 2018, 10, 49. [Google Scholar] [CrossRef]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Ruckert, C.; Govero, J.; et al. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef]
- Wu, Y.H.; Tseng, C.K.; Lin, C.K.; Wei, C.K.; Lee, J.C.; Young, K.C. ICR suckling mouse model of Zika virus infection for disease modeling and drug validation. PLoS Negl. Trop. Dis. 2018, 12, e0006848. [Google Scholar] [CrossRef]
- Couderc, T.; Chretien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Pedras-Vasconcelos, J.A.; Puig, M.; Sauder, C.; Wolbert, C.; Ovanesov, M.; Goucher, D.; Verthelyi, D. Immunotherapy with CpG oligonucleotides and antibodies to TNF-alpha rescues neonatal mice from lethal arenavirus-induced meningoencephalitis. J. Immunol. 2008, 180, 8231–8240. [Google Scholar] [CrossRef]
- Jurado, K.A.; Yockey, L.J.; Wong, P.W.; Lee, S.; Huttner, A.J.; Iwasaki, A. Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice. Nat. Microbiol. 2018, 3, 141–147. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardy, R.D.; Richer, M.J. Protective to a T: The Role of T Cells during Zika Virus Infection. Cells 2019, 8, 820. https://doi.org/10.3390/cells8080820
Pardy RD, Richer MJ. Protective to a T: The Role of T Cells during Zika Virus Infection. Cells. 2019; 8(8):820. https://doi.org/10.3390/cells8080820
Chicago/Turabian StylePardy, Ryan D., and Martin J. Richer. 2019. "Protective to a T: The Role of T Cells during Zika Virus Infection" Cells 8, no. 8: 820. https://doi.org/10.3390/cells8080820
APA StylePardy, R. D., & Richer, M. J. (2019). Protective to a T: The Role of T Cells during Zika Virus Infection. Cells, 8(8), 820. https://doi.org/10.3390/cells8080820




