Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight
Abstract
:1. Introduction
2. Materials and Methods
3. Review
3.1. Physiology of Osteopontin in Humans and Experimental Animals
3.1.1. Osteopontin Genes, Transcripts, and Proteins
3.1.2. Tissue-Restricted Patterns of Osteopontin Expression and Processing
3.1.3. Regulation of Osteopontin Expression
3.1.4. Osteopontin as a Matricellular Component
3.1.5. Osteopontin as a Secreted Protein
3.1.6. Osteopontin as an Intracellular Molecule
3.1.7. Osteopontin in Non-Malignant Diseases
3.2. Osteopontin Signaling in Inflammation
3.2.1. Inflammatory Signaling Pathways Initiated by Osteopontin
3.2.2. Chronic Lung Diseases and Osteopontin
3.3. Impact of Osteopontin on Cancer Development and Progression
3.3.1. Impact of Osteopontin on Thoracic Cancers
3.3.2. Osteopontin in Lung Cancer
3.3.3. Malignant Pleural Effusion Promotion by Osteopontin
3.3.4. Osteopontin as a Pro-Metastatic Molecule
3.4. Potential Clinical Implications for Osteopontin in Cancer
3.4.1. Osteopontin as a Biomarker
3.4.2. Osteopontin as a Therapeutic Target
3.4.3. Tools for Future Research on Osteopontin
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Young, M.F.; Kerr, J.M.; Termine, J.D.; Wewer, U.M.; Wang, M.G.; McBride, O.W.; Fisher, L.W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics 1990, 7, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Fet, V.; Dickinson, M.E.; Hogan, B.L.M. Localization of the mouse gene for secreted phosphoprotein 1 (Spp-1) (2ar, osteopontin, bone sialoprotein 1, 44-kDa bone phosphoprotein, tumor-secreted phosphoprotein) to chromosome 5, closely linked to Ric (Rickettsia resistance). Genomics 1989, 5, 375–377. [Google Scholar] [CrossRef]
- Chae, S.; Jun, H.-O.; Lee, E.G.; Yang, S.-J.; Lee, D.C.; Jung, J.K.; Park, K.C.; Yeom, Y.I.; Kim, K.-W. Osteopontin splice variants differentially modulate the migratory activity of hepatocellular carcinoma cell lines. Int. J. Oncol. 2009, 35, 1409–1416. [Google Scholar] [Green Version]
- Standal, T.; Borset, M.; Sundan, A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp. Oncol. 2004, 26, 179–184. [Google Scholar]
- Inoue, M.; Shinohara, M.L. Intracellular osteopontin (iOPN) and immunity. Immunol. Res. 2011, 49, 160–172. [Google Scholar] [CrossRef]
- Christensen, B.; Nielsen, M.S.; Haselmann, K.F.; Petersen, T.E.; Sørensen, E.S. Post-translationally modified residues of native human osteopontin are located in clusters: Identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem. J. 2005, 390, 285–292. [Google Scholar] [CrossRef]
- Li, H.; Shen, H.; Yan, G.; Zhang, Y.; Liu, M.; Fang, P.; Yu, H.; Yang, P. Site-specific structural characterization of O-glycosylation and identification of phosphorylation sites of recombinant osteopontin. Biochimica Biophysica Acta (BBA)—Proteins Proteom. 2015, 1854, 581–591. [Google Scholar] [CrossRef]
- Maeda, N.; Maenaka, K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2198. [Google Scholar] [CrossRef]
- McKee, M.D.; Pedraza, C.E.; Kaartinen, M.T. Osteopontin and wound healing in bone. Cells Tissues Organs (Print) 2011, 194, 313–319. [Google Scholar] [CrossRef]
- Rittling, S.R.; Singh, R. Osteopontin in Immune-mediated Diseases. J. Dent. Res. 2015, 94, 1638–1645. [Google Scholar] [CrossRef] [Green Version]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef]
- Lin, Q.; Guo, L.; Lin, G.; Chen, Z.; Chen, T.; Lin, J.; Zhang, B.; Gu, X. Clinical and prognostic significance of OPN and VEGF expression in patients with non-small-cell lung cancer. Cancer Epidemiol. 2015, 39, 539–544. [Google Scholar] [CrossRef]
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, O.; Kida, S.; Matsushita, Y.; Murase, S.; Hattori, T.; Kubohara, Y.; Kikuchi, H.; Oshima, Y. Identification of brefelamide as a novel inhibitor of osteopontin that suppresses invasion of A549 lung cancer cells. Oncol. Rep. 2016, 36, 2357–2364. [Google Scholar] [CrossRef]
- Senger, D.R.; Wirth, D.F.; Hynes, R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Franzén, A.; Heinegård, D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem. J. 1985, 232, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Oldberg, A.; Franzén, A.; Heinegård, D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc. Natl. Acad. Sci. USA 1986, 83, 8819–8823. [Google Scholar] [CrossRef]
- Fisher, L.W.; Torchia, D.A.; Fohr, B.; Young, M.F.; Fedarko, N.S. Flexible Structures of SIBLING Proteins, Bone Sialoprotein, and Osteopontin. Biochem. Biophys. Res. Commun. 2001, 280, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.-K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 93–108. [Google Scholar] [CrossRef]
- Hijiya, N.; Setoguchi, M.; Matsuura, K.; Higuchi, Y.; Akizuki, S.; Yamamoto, S. Cloning and characterization of the human osteopontin gene and its promoter. Biochem. J. 1994, 303, 255–262. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Mirza, M.; Weber, G.F. An osteopontin splice variant induces anchorage independence in human breast cancer cells. Oncogene 2006, 25, 2192–2202. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.; da Silva, C.L.; Vashishth, D. Synergistic effect of extracellularly supplemented osteopontin and osteocalcin on stem cell proliferation, osteogenic differentiation, and angiogenic properties. J. Cell. Biochem. 2018, 120, 6555–6569. [Google Scholar] [CrossRef]
- SPP1 (Secreted Phosphoprotein 1). Available online: http://atlasgeneticsoncology.org/Genes/SPP1ID42379ch4q22 (accessed on 5 December 2018).
- Sodek, J.; Chen, J.; Nagata, T.; Kasugai, S.; Todescan, R.; Li, I.W.; Kim, R.H. Regulation of osteopontin expression in osteoblasts. Ann. N. Y. Acad. Sci. 1995, 760, 223–241. [Google Scholar] [CrossRef]
- Zohar, R.; Lee, W.; Arora, P.; Cheifetz, S.; McCulloch, C.; Sodek, J. Single cell analysis of intracellular osteopontin in osteogenic cultures of fetal rat calvarial cells. J. Cell. Physiol. 1997, 170, 88–100. [Google Scholar] [CrossRef]
- Ikegame, M.; Ejiri, S.; Okamura, H. Expression of Noncollagenous Bone Matrix Proteins in Osteoblasts Stimulated by Mechanical Stretching in the Cranial Suture of Neonatal Mice—PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30113872 (accessed on 2 January 2019).
- Teixeira, L.N.; de Castro Raucci, L.M.S.; Alonso, G.C.; Coletta, R.D.; Rosa, A.L.; de Oliveira, P.T. Osteopontin expression in co-cultures of human squamous cell carcinoma-derived cells and osteoblastic cells and its effects on the neoplastic cell phenotype and osteoclastic activation. Tumour Biol. 2016, 37, 12371–12385. [Google Scholar] [CrossRef]
- Qian, J.; Xu, L.; Sun, X.; Wang, Y.; Xuan, W.; Zhang, Q.; Zhao, P.; Wu, Q.; Liu, R.; Che, N.; et al. Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 26. [Google Scholar] [CrossRef] [Green Version]
- Pestell, T.G.; Jiao, X.; Kumar, M.; Peck, A.R.; Prisco, M.; Deng, S.; Li, Z.; Ertel, A.; Casimiro, M.C.; Ju, X.; et al. Stromal cyclin D1 promotes heterotypic immune signaling and breast cancer growth. Oncotarget 2017, 8, 81754–81775. [Google Scholar] [CrossRef]
- Kaleta, B.; Boguska, A. Sildenafil, a Phosphodiesterase Type 5 Inhibitor, Downregulates Osteopontin in Human Peripheral Blood Mononuclear Cells. Arch. Immunol. Ther. Exp. (Warsz.) 2017, 65, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Chen, J.; He, J.; Lu, C.; Wei, Y.; Wang, L.; Xu, X.; Li, L.; Uede, T.; Diao, H. Osteopontin promotes dendritic cell maturation and function in response to HBV antigens. Drug Des. Dev. Ther. 2015, 9, 3003–3016. [Google Scholar]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of osteopontin with IL-18 in obese individuals: Implications for insulin resistance. PLoS ONE 2013, 8, e63944. [Google Scholar] [CrossRef]
- Shinohara, M.L.; Jansson, M.; Hwang, E.S.; Werneck, M.B.F.; Glimcher, L.H.; Cantor, H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 2005, 102, 17101–17106. [Google Scholar] [CrossRef] [Green Version]
- Vaschetto, R.; Nicola, S.; Olivieri, C.; Boggio, E.; Piccolella, F.; Mesturini, R.; Damnotti, F.; Colombo, D.; Navalesi, P.; Della Corte, F.; et al. Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 2008, 34, 2176–2184. [Google Scholar] [CrossRef]
- Schack, L.; Lange, A.; Kelsen, J.; Agnholt, J.; Christensen, B.; Petersen, T.E.; Sørensen, E.S. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J. Dairy Sci. 2009, 92, 5378–5385. [Google Scholar] [CrossRef]
- Kolbach, A.M.; Afzal, O.; Halligan, B.; Sorokina, E.; Kleinman, J.G.; Wesson, J.A. Relative deficiency of acidic isoforms of osteopontin from stone former urine. Urol. Res. 2012, 40, 447–454. [Google Scholar] [CrossRef]
- Kanayama, M.; Xu, S.; Danzaki, K.; Gibson, J.R.; Inoue, M.; Gregory, S.G.; Shinohara, M.L. Skewing of the population balance of lymphoid and myeloid cells by secreted and intracellular osteopontin. Nat. Immunol. 2017, 18, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Arikawa, T.; Chen, Y.-H.; Moriwaki, Y.; Price, M.; Brown, M.; Perfect, J.R.; Shinohara, M.L. T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 5295–5300. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, M.L.; Kim, H.-J.; Kim, J.-H.; Garcia, V.A.; Cantor, H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 2008, 105, 7235–7239. [Google Scholar] [CrossRef] [Green Version]
- Jovic, S.; Shikhagaie, M.; Mörgelin, M.; Erjefält, J.S.; Kjellström, S.; Egesten, A. Osteopontin is increased in cystic fibrosis and can skew the functional balance between ELR-positive and ELR-negative CXC-chemokines. J. Cyst. Fibros. 2015, 14, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Samitas, K.; Zervas, E.; Vittorakis, S.; Semitekolou, M.; Alissafi, T.; Bossios, A.; Gogos, H.; Economidou, E.; Lötvall, J.; Xanthou, G.; et al. Osteopontin expression and relation to disease severity in human asthma. Eur. Respir. J. 2011, 37, 331–341. [Google Scholar] [CrossRef]
- Kasetty, G.; Papareddy, P.; Bhongir, R.K.V.; Ali, M.N.; Mori, M.; Wygrecka, M.; Erjefält, J.S.; Hultgårdh-Nilsson, A.; Palmberg, L.; Herwald, H.; et al. Osteopontin protects against lung injury caused by extracellular histones. Mucosal Immunol. 2019, 12, 39. [Google Scholar] [CrossRef]
- Minai-Tehrani, A.; Chang, S.-H.; Park, S.B.; Cho, M.-H. The O-glycosylation mutant osteopontin alters lung cancer cell growth and migration in vitro and in vivo. Int. J. Mol. Med. 2013, 32, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-N.; Kang, B.-B.; Chen, J.H. Transcriptional regulation of human osteopontin promoter by C/EBPalpha and AML-1 in metastatic cancer cells. Oncogene 2004, 23, 278–288. [Google Scholar] [CrossRef]
- Takami, Y.; Russell, M.B.; Gao, C.; Mi, Z.; Guo, H.; Mantyh, C.R.; Kuo, P.C. Sp1 regulates osteopontin expression in SW480 human colon adenocarcinoma cells. Surgery 2007, 142, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, Q.; Li, D.; Li, J.; Aki, D.; Liu, Y.-C. The E3 ligase VHL controls alveolar macrophage function via metabolic-epigenetic regulation. J. Exp. Med. 2018, 215, 3180–3193. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, O.; Kida, S.; Matsushita, Y.; Hattori, T. Down-regulation of osteopontin mediates a novel mechanism underlying the cytostatic activity of TGF-β. Cell. Oncol. 2016, 39, 119–128. [Google Scholar] [CrossRef]
- Schultz, J.; Lorenz, P.; Ibrahim, S.M.; Kundt, G.; Gross, G.; Kunz, M. The functional-443T/C osteopontin promoter polymorphism influences osteopontin gene expression in melanoma cells via binding of c-Myb transcription factor. Mol. Carcinog. 2009, 48, 14–23. [Google Scholar] [CrossRef]
- Flajollet, S.; Tian, T.V.; Flourens, A.; Tomavo, N.; Villers, A.; Bonnelye, E.; Aubert, S.; Leroy, X.; Duterque-Coquillaud, M. Abnormal expression of the ERG transcription factor in prostate cancer cells activates osteopontin. Mol. Cancer Res. 2011, 9, 914–924. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Li, X.; Cui, L.; Fu, M.; Rabie, A.B.; Zhang, D. Functional analysis of core binding factor a1 and its relationship with related genes expressed by human periodontal ligament cells exposed to mechanical stress. Eur. J. Orthod. 2010, 32, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, C.; Bevelacqua, V.; Polesel, J.; Falzone, L.; Cannavò, P.S.; Spandidos, D.A.; Malaponte, G.; Libra, M. NF-κB inhibition is associated with OPN/MMP-9 downregulation in cutaneous melanoma. Oncol. Rep. 2017, 37, 737–746. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, H.; Gao, H.; Liu, Y.; Yan, T.; Zou, L.; Chen, L. IFN-γ inhibits osteopontin expression in human decidual stromal cells and can be attenuated by 1α,25-dihydroxyvitamin D3. Am. J. Reprod. Immunol. 2012, 68, 353–361. [Google Scholar] [CrossRef]
- Cai, M.; Bompada, P.; Salehi, A.; Acosta, J.R.; Prasad, R.B.; Atac, D.; Laakso, M.; Groop, L.; De Marinis, Y. Role of osteopontin and its regulation in pancreatic islet. Biochem. Biophys. Res. Commun. 2018, 495, 1426–1431. [Google Scholar] [CrossRef]
- Liu, W.-L.; Zhang, H.; Zheng, Y.; Wang, H.-T.; Chen, F.-H.; Xu, L.; Wei, Y.; Sun, Y.-Q.; Shi, J.-B.; Li, H.-B. Expression and regulation of osteopontin in chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy 2015, 45, 414–422. [Google Scholar] [CrossRef]
- Chen, M.; Chen, G.; Nie, H.; Zhang, X.; Niu, X.; Zang, Y.C.Q.; Skinner, S.M.; Zhang, J.Z.; Killian, J.M.; Hong, J. Regulatory effects of IFN-beta on production of osteopontin and IL-17 by CD4+ T Cells in MS. Eur. J. Immunol. 2009, 39, 2525–2536. [Google Scholar] [CrossRef]
- Scutera, S.; Salvi, V.; Lorenzi, L.; Piersigilli, G.; Lonardi, S.; Alotto, D.; Casarin, S.; Castagnoli, C.; Dander, E.; D’Amico, G.; et al. Adaptive Regulation of Osteopontin Production by Dendritic Cells Through the Bidirectional Interaction with Mesenchymal Stromal Cells. Front. Immunol. 2018, 9, 1207. [Google Scholar] [CrossRef]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef]
- Labudzynskyi, D.; Shymanskyy, I.; Veliky, M. Role of vitamin D3 in regulation of interleukin-6 and osteopontin expression in liver of diabetic mice. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2916–2919. [Google Scholar]
- Zhuang, S.; Hua, X.; He, K.; Zhou, T.; Zhang, J.; Wu, H.; Ma, X.; Xia, Q.; Zhang, J. Inhibition of GSK-3β induces AP-1-mediated osteopontin expression to promote cholestatic liver fibrosis. FASEB J. 2018, 32, 4494–4503. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biol. 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Agnihotri, R.; Crawford, H.C.; Haro, H.; Matrisian, L.M.; Havrda, M.C.; Liaw, L. Osteopontin, a Novel Substrate for Matrix Metalloproteinase-3 (Stromelysin-1) and Matrix Metalloproteinase-7 (Matrilysin). J. Biol. Chem. 2001, 276, 28261–28267. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-J.; Wu, C.-C.; Sheu, G.-T.; Chang, H.-Y.; Chen, M.-Y.; Lin, Y.-Y.; Chuang, C.-Y.; Hsu, S.-L.; Chang, J.T. Integrin β3 and CD44 levels determine the effects of the OPN-a splicing variant on lung cancer cell growth. Oncotarget 2016, 7, 55572–55584. [Google Scholar] [CrossRef]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lv, G.; Wang, B.; Kuang, L. XIST/miR-376c-5p/OPN axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Ge, Q.; Ruan, C.-C.; Ma, Y.; Tang, X.-F.; Wu, Q.-H.; Wang, J.-G.; Zhu, D.-L.; Gao, P.-J. Osteopontin regulates macrophage activation and osteoclast formation in hypertensive patients with vascular calcification. Sci. Rep. 2017, 7, 40253. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Du, W.; Chen, Z.; Xiang, C. Upregulation of PD-L1 by SPP1 mediates macrophage polarization and facilitates immune escape in lung adenocarcinoma. Exp. Cell Res. 2017, 359, 449–457. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Waller, A.H.; Sanchez-Ross, M.; Kaluski, E.; Klapholz, M. Osteopontin in cardiovascular disease: A potential therapeutic target. Cardiol. Rev. 2010, 18, 125–131. [Google Scholar] [CrossRef]
- Kahles, F.; Findeisen, H.M.; Bruemmer, D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014, 3, 384–393. [Google Scholar] [CrossRef]
- Lund, S.A.; Wilson, C.L.; Raines, E.W.; Tang, J.; Giachelli, C.M.; Scatena, M. Osteopontin mediates macrophage chemotaxis via α4 and α9 integrins and survival via the α4 integrin. J. Cell. Biochem. 2013, 114, 1194–1202. [Google Scholar] [CrossRef]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization After Myocardial Infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Chen, Y.; Zhang, G.; Fan, Z. Controlled release of osteopontin and interleukin-10 from modified endovascular coil promote cerebral aneurysm healing. J. Neurol. Sci. 2016, 360, 13–17. [Google Scholar] [CrossRef]
- Santamaría, M.H.; Corral, R.S. Osteopontin-dependent regulation of Th1 and Th17 cytokine responses in Trypanosoma cruzi-infected C57BL/6 mice. Cytokine 2013, 61, 491–498. [Google Scholar] [CrossRef]
- Hirano, Y.; Aziz, M.; Yang, W.-L.; Wang, Z.; Zhou, M.; Ochani, M.; Khader, A.; Wang, P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit. Care 2015, 19, 53. [Google Scholar] [CrossRef]
- Leavenworth, J.W.; Verbinnen, B.; Wang, Q.; Shen, E.; Cantor, H. Intracellular osteopontin regulates homeostasis and function of natural killer cells. Proc. Natl. Acad. Sci. USA 2015, 112, 494–499. [Google Scholar] [CrossRef]
- Kasetty, G.; Bhongir, R.K.V.; Papareddy, P.; Tufvesson, E.; Stenberg, H.; Bjermer, L.; Hultgårdh-Nilsson, A.; Herwald, H.; Egesten, A. Osteopontin protects against pneumococcal infection in a murine model of allergic airway inflammation. Allergy 2018, 74, 663–674. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, Q.; Chen, Y.; Luo, R.Z. Role of Leptin/Osteopontin Axis in the Function of Eosinophils in Allergic Rhinitis with Obesity. Mediat. Inflamm. 2018, 2018, 9138904. [Google Scholar] [CrossRef]
- Goncalves DaSilva, A.; Liaw, L.; Yong, V.W. Cleavage of Osteopontin by Matrix Metalloproteinase-12 Modulates Experimental Autoimmune Encephalomyelitis Disease in C57BL/6 Mice. Am. J. Pathol. 2010, 177, 1448–1458. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150. [Google Scholar] [CrossRef]
- Arjomandi, M.; Frelinger, J.; Donde, A.; Wong, H.; Yellamilli, A.; Raymond, W. Secreted Osteopontin Is Highly Polymerized in Human Airways and Fragmented in Asthmatic Airway Secretions. PLoS ONE 2011, 6, e25678. [Google Scholar] [CrossRef]
- Gela, A.; Bhongir, R.K.V.; Mori, M.; Keenan, P.; Mörgelin, M.; Erjefält, J.S.; Herwald, H.; Egesten, A.; Kasetty, G. Osteopontin That Is Elevated in the Airways during COPD Impairs the Antibacterial Activity of Common Innate Antibiotics. PLoS ONE 2016, 11, e0146192. [Google Scholar] [CrossRef]
- Papaporfyriou, A.; Loukides, S.; Kostikas, K.; Simoes, D.C.M.; Papatheodorou, G.; Konstantellou, E.; Hillas, G.; Papiris, S.; Koulouris, N.; Bakakos, P. Increased levels of osteopontin in sputum supernatant in patients with COPD. Chest 2014, 146, 951–958. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.H.; Kim, W.; Lim, S.; Lee, S.H.; Kim, Y.E.; Cho, Y.J.; Jeong, Y.Y.; Kim, H.C.; Lee, J.D.; et al. Increased plasma osteopontin in frequent exacerbator and acute exacerbation of COPD. Clin. Respir. J. 2014, 8, 305–311. [Google Scholar] [CrossRef]
- Hetman, O.; Krakhmalova, E.; Radzishevska, Y. The diagnostic value of osteopontin as an early marker of pilmonary hypertension affected by chronic obstructive pulmonary disease and concomitant ischemic heart disease. Georgian Med. News 2018, 41–447. [Google Scholar]
- Saker, M.; Lipskaia, L.; Marcos, E.; Abid, S.; Parpaleix, A.; Houssaini, A.; Validire, P.; Girard, P.; Noureddine, H.; Boyer, L.; et al. Osteopontin, a Key Mediator Expressed by Senescent Pulmonary Vascular Cells in Pulmonary Hypertension. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1879–1890. [Google Scholar] [CrossRef] [Green Version]
- Maneechotesuwan, K.; Kasetsinsombat, K.; Wongkajornsilp, A.; Barnes, P.J. Simvastatin up-regulates adenosine deaminase and suppresses osteopontin expression in COPD patients through an IL-13-dependent mechanism. Respir. Res. 2016, 17, 104. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Myofibroblasts and lung fibrosis induced by carbon nanotube exposure. Part. Fibre Toxicol. 2016, 13, 60. [Google Scholar] [CrossRef]
- Dong, J.; Porter, D.W.; Batteli, L.A.; Wolfarth, M.G.; Richardson, D.L.; Ma, Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch. Toxicol. 2015, 89, 621–633. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.F.; Hubbs, A.F.; Battelli, L.A.; McKinney, W.; Friend, S.; Wolfarth, M.G.; Andrew, M.; Castranova, V.; Porter, D.W. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef]
- Dong, J.; Ma, Q. Osteopontin enhances multi-walled carbon nanotube-triggered lung fibrosis by promoting TGF-β1 activation and myofibroblast differentiation. Part. Fibre Toxicol. 2017, 14, 18. [Google Scholar] [CrossRef]
- Dadrich, M.; Nicolay, N.H.; Flechsig, P.; Bickelhaupt, S.; Hoeltgen, L.; Roeder, F.; Hauser, K.; Tietz, A.; Jenne, J.; Lopez, R.; et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology 2015, 5, e1123366. [Google Scholar] [CrossRef]
- Luo, Y.; Grötsch, B.; Hannemann, N.; Jimenez, M.; Ipseiz, N.; Uluckan, O.; Lin, N.; Schett, G.; Wagner, E.F.; Bozec, A. Fra-2 Expression in Osteoblasts Regulates Systemic Inflammation and Lung Injury through Osteopontin. Mol. Cell. Biol. 2018, 38, e00022-18. [Google Scholar] [CrossRef]
- Pardo, A.; Gibson, K.; Cisneros, J.; Richards, T.J.; Yang, Y.; Becerril, C.; Yousem, S.; Herrera, I.; Ruiz, V.; Selman, M.; et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005, 2, e251. [Google Scholar] [CrossRef]
- White, E.S.; Xia, M.; Murray, S.; Dyal, R.; Flaherty, C.M.; Flaherty, K.R.; Moore, B.B.; Cheng, L.; Doyle, T.J.; Villalba, J.; et al. Plasma Surfactant Protein-D, Matrix Metalloproteinase-7, and Osteopontin Index Distinguishes Idiopathic Pulmonary Fibrosis from Other Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2016, 194, 1242–1251. [Google Scholar] [CrossRef]
- Foster, M.W.; Morrison, L.D.; Todd, J.L.; Snyder, L.D.; Thompson, J.W.; Soderblom, E.J.; Plonk, K.; Weinhold, K.J.; Townsend, R.; Minnich, A.; et al. Quantitative proteomics of bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. J. Proteome Res. 2015, 14, 1238–1249. [Google Scholar] [CrossRef]
- Li, S.; Yang, R.; Sun, X.; Miao, S.; Lu, T.; Wang, Y.; Wo, Y.; Jiao, W. Identification of SPP1 as a promising biomarker to predict clinical outcome of lung adenocarcinoma individuals. Gene 2018, 679, 398–404. [Google Scholar] [CrossRef]
- He, F.; Ai, B.; Tian, L. Identification of genes and pathways in esophageal adenocarcinoma using bioinformatics analysis. Biomed. Rep. 2018, 9, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Wisniewski, T.; Zyromska, A.; Makarewicz, R.; Zekanowska, E. Osteopontin And Angiogenic Factors as New Biomarkers of Prostate Cancer. Urol. J. 2018, 16, 134–140. [Google Scholar]
- Hauptman, N.; Boštjančič, E.; Žlajpah, M.; Ranković, B.; Zidar, N. Bioinformatics Analysis Reveals Most Prominent Gene Candidates to Distinguish Colorectal Adenoma from Adenocarcinoma. BioMed Res. Int. 2018, 2018, 9416515. [Google Scholar] [CrossRef]
- Tu, Y.; Fan, G.; Xi, H.; Zeng, T.; Sun, H.; Cai, X.; Kong, W. Identification of candidate aberrantly methylated and differentially expressed genes in thyroid cancer. J. Cell. Biochem. 2018, 119, 8797–8806. [Google Scholar] [CrossRef]
- Anborgh, P.H.; Lee, D.J.; Stam, P.F.; Tuck, A.B.; Chambers, A.F. Role of osteopontin as a predictive biomarker for anti-EGFR therapy in triple-negative breast cancer. Expert Opin. Ther. Targets 2018, 22, 727–734. [Google Scholar] [CrossRef]
- Treskova, I.; Topolcan, O.; Windrichova, J.; Simanek, V.; Slouka, D.; Treska, V.; Kucera, R. OPG, OPN, EGF and VEGF Levels at Individual Breslow Score Stages in Malignant Melanoma. Anticancer Res. 2018, 38, 4907–4911. [Google Scholar] [CrossRef]
- Ferreira, L.; Lima, R.; Bastos, A.; Silva, A.; Tavares, C.; Pestana, A.; Rios, E.; Eloy, C.; Sobrinho-Simões, M.; Gimba, E.; et al. OPNa Overexpression Is Associated with Matrix Calcification in Thyroid Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 2990. [Google Scholar] [CrossRef]
- Hao, C.; Wang, Z.; Gu, Y.; Jiang, W.G.; Cheng, S. Prognostic Value of Osteopontin Splice Variant-c Expression in Breast Cancers: A Meta-Analysis. BioMed Res. Int. 2016, 2016, 7310694. [Google Scholar] [CrossRef]
- Cabiati, M.; Gaggini, M.; Cesare, M.M.; Caselli, C.; De Simone, P.; Filipponi, F.; Basta, G.; Gastaldelli, A.; Del Ry, S. Osteopontin in hepatocellular carcinoma: A possible biomarker for diagnosis and follow-up. Cytokine 2017, 99, 59–65. [Google Scholar] [CrossRef]
- Goparaju, C.M.V.; Pass, H.I.; Blasberg, J.D.; Hirsch, N.; Donington, J.S. Functional heterogeneity of osteopontin isoforms in non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1516–1523. [Google Scholar] [CrossRef]
- Hao, C.; Cui, Y.; Hu, M.U.; Zhi, X.; Zhang, L.; Li, W.; Wu, W.; Cheng, S.; Jiang, W.G. OPN-a Splicing Variant Expression in Non-small Cell Lung Cancer and its Effects on the Bone Metastatic Abilities of Lung Cancer Cells In Vitro. Anticancer Res. 2017, 37, 2245–2254. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Sun, T.; Meng, F.; Qu, A.; Li, C.; Shen, H.; Jin, Y.; Li, W. Osteopontin as a potential biomarker of proliferation and invasiveness for lung cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 1061–1070. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Wang, P.; Wang, F.; Yu, Z.; Li, M.; Chen, S.; Ning, F. OPN Polymorphism Is Related to the Chemotherapy Response and Prognosis in Advanced NSCLC. Int. J. Genom. 2014, 2014, 846142. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Wu, W.; Li, Y.; Li, J. Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J. Exp. Clin. Cancer Res. 2013, 32, 45. [Google Scholar] [CrossRef]
- Chang, Y.S.; Kim, H.J.; Chang, J.; Ahn, C.M.; Kim, S.K.; Kim, S.K. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer 2007, 57, 373–380. [Google Scholar] [CrossRef]
- Sun, B.; Li, Y.; Zhang, Z.; You, J.; Wang, C. Osteopontin Combined with CD44v6, a Novel Prognostic Biomarker in Non-Small Cell Lung Cancer Undergoing Curative Resection. Ann. Thorac. Surg. 2013, 96, 1943–1951. [Google Scholar] [CrossRef]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef]
- Liu, H.; Wei, S.; Zhang, L.; Yuan, C.; Duan, Y.; Wang, Q. Secreted Phosphoprotein 1 Promotes the Development of Small Cell Lung Cancer Cells by Inhibiting Autophagy and Apoptosis. Pathol. Oncol. Res. 2018. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, Y.; Jin, X.; Zhao, W.; Hu, T.; Wu, F.; Huang, J. Osteopontin promotes cancer cell drug resistance, invasion, and lactate production and is associated with poor outcome of patients with advanced non-small-cell lung cancer. Onco Targets Ther. 2018, 11, 5933–5941. [Google Scholar] [CrossRef]
- Saijo, A.; Goto, H.; Nakano, M.; Mitsuhashi, A.; Aono, Y.; Hanibuchi, M.; Ogawa, H.; Uehara, H.; Kondo, K.; Nishioka, Y. Bone marrow-derived fibrocytes promote stem cell-like properties of lung cancer cells. Cancer Lett. 2018, 421, 17–27. [Google Scholar] [CrossRef]
- Liu, F.; Bai, C.; Guo, Z. The prognostic value of osteopontin in limited-stage small cell lung cancer patients and its mechanism. Oncotarget 2017, 8, 70084–70096. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.I.; Kim, S.Y.; Lee, J.H.; Kim, J.Y.; Cho, E.W.; Kim, I.-G. Osteopontin production by TM4SF4 signaling drives a positive feedback autocrine loop with the STAT3 pathway to maintain cancer stem cell-like properties in lung cancer cells. Oncotarget 2017, 8, 101284–101297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Fan, J.; Chen, Q.; Lei, C.; Qiao, B.; Liu, Q. SPP1 and AGER as potential prognostic biomarkers for lung adenocarcinoma. Oncol. Lett. 2018, 15, 7028–7036. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Yuan, D.; Wang, Z.; Wang, Y.; Song, Y. Prognostic value of secreted phosphoprotein-1 in pleural effusion associated with non-small cell lung cancer. BMC Cancer 2014, 14, 280. [Google Scholar] [CrossRef]
- Moschos, C.; Porfiridis, I.; Psallidas, I.; Kollintza, A.; Stathopoulos, G.T.; Papiris, S.A.; Roussos, C.; Kalomenidis, I. Osteopontin is upregulated in malignant and inflammatory pleural effusions. Respirology 2009, 14, 716–722. [Google Scholar] [CrossRef]
- Hsu, L.-H.; Hsu, P.-C.; Liao, T.-L.; Feng, A.-C.; Chu, N.-M.; Kao, S.-H. Pleural fluid osteopontin, vascular endothelial growth factor, and urokinase-type plasminogen activator levels as predictors of pleurodesis outcome and prognosticators in patients with malignant pleural effusion: A prospective cohort study. BMC Cancer 2016, 16, 463. [Google Scholar] [CrossRef]
- Giannou, A.D.; Marazioti, A.; Spella, M.; Kanellakis, N.I.; Apostolopoulou, H.; Psallidas, I.; Prijovich, Z.M.; Vreka, M.; Zazara, D.E.; Lilis, I.; et al. Mast cells mediate malignant pleural effusion formation. J. Clin. Investig. 2015, 125, 2317–2334. [Google Scholar] [CrossRef]
- Psallidas, I.; Stathopoulos, G.T.; Maniatis, N.A.; Magkouta, S.; Moschos, C.; Karabela, S.P.; Kollintza, A.; Simoes, D.C.M.; Kardara, M.; Vassiliou, S.; et al. Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene 2013, 32, 528–535. [Google Scholar] [CrossRef]
- Felten, M.K.; Khatab, K.; Knoll, L.; Schettgen, T.; Müller-Berndorff, H.; Kraus, T. Changes of mesothelin and osteopontin levels over time in formerly asbestos-exposed power industry workers. Int. Arch. Occup. Environ. Health 2014, 87, 195–204. [Google Scholar] [CrossRef]
- Bayram, M.; Dongel, I.; Akbaş, A.; Benli, İ.; Akkoyunlu, M.E.; Bakan, N.D. Serum Biomarkers in Patients with Mesothelioma and Pleural Plaques and Healthy Subjects Exposed to Naturally Occurring Asbestos. Lung 2014, 192, 197–203. [Google Scholar] [CrossRef]
- Bonotti, A.; Simonini, S.; Pantani, E.; Giusti, L.; Donadio, E.; Mazzoni, M.R.; Chella, A.; Marconi, L.; Ambrosino, N.; Lucchi, M.; et al. Serum mesothelin, osteopontin and vimentin: Useful markers for clinical monitoring of malignant pleural mesothelioma. Int. J. Biol. Markers 2017, 32, e126–e131. [Google Scholar] [CrossRef]
- Jamil, M.O.; Jerome, M.S.; Miley, D.; Selander, K.S.; Robert, F. A pilot study of zoledronic acid in the treatment of patients with advanced malignant pleural mesothelioma. Lung Cancer (Auckl) 2017, 8, 39–44. [Google Scholar] [CrossRef]
- Takeuchi, S.; Seike, M.; Noro, R.; Soeno, C.; Sugano, T.; Zou, F.; Uesaka, H.; Nishijima, N.; Matsumoto, M.; Minegishi, Y.; et al. Significance of osteopontin in the sensitivity of malignant pleural mesothelioma to pemetrexed. Int. J. Oncol. 2014, 44, 1886–1894. [Google Scholar] [CrossRef]
- Sun, B.; You, J.; Li, Y.; Zhang, Z.; Wang, C. Osteopontin knockdown suppresses non-small cell lung cancer cell invasion and metastasis. Chin. Med. J. 2013, 126, 1683–1688. [Google Scholar]
- Polat, B.; Wohlleben, G.; Katzer, A.; Djuzenova, C.S.; Technau, A.; Flentje, M. Influence of osteopontin silencing on survival and migration of lung cancer cells. Strahlenther. Onkol. 2013, 189, 62–67. [Google Scholar] [CrossRef]
- Ng, L.; Wan, T.M.-H.; Lam, C.S.-C.; Chow, A.K.-M.; Wong, S.K.-M.; Man, J.H.-W.; Li, H.-S.; Cheng, N.S.-M.; Pak, R.C.-H.; Cheung, A.H.-K.; et al. Post-operative plasma osteopontin predicts distant metastasis in human colorectal cancer. PLoS ONE 2015, 10, e0126219. [Google Scholar] [CrossRef]
- Kothari, A.N.; Arffa, M.L.; Chang, V.; Blackwell, R.H.; Syn, W.-K.; Zhang, J.; Mi, Z.; Kuo, P.C. Osteopontin-A Master Regulator of Epithelial-Mesenchymal Transition. J. Clin. Med. 2016, 5, 39. [Google Scholar] [CrossRef]
- Li, N.Y.; Weber, C.E.; Mi, Z.; Wai, P.Y.; Cuevas, B.D.; Kuo, P.C. Osteopontin up-regulates critical epithelial-mesenchymal transition transcription factors to induce an aggressive breast cancer phenotype. J. Am. Coll. Surg. 2013, 217, 17–26. [Google Scholar] [CrossRef]
- Raja, R.; Kale, S.; Thorat, D.; Soundararajan, G.; Lohite, K.; Mane, A.; Karnik, S.; Kundu, G.C. Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis. Oncogene 2014, 33, 2053–2064. [Google Scholar] [CrossRef]
- Dong, Q.; Zhu, X.; Dai, C.; Zhang, X.; Gao, X.; Wei, J.; Sheng, Y.; Zheng, Y.; Yu, J.; Xie, L.; et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget 2016, 7, 12997–13012. [Google Scholar] [CrossRef]
- Jia, R.; Liang, Y.; Chen, R.; Liu, G.; Wang, H.; Tang, M.; Zhou, X.; Wang, H.; Yang, Y.; Wei, H.; et al. Osteopontin facilitates tumor metastasis by regulating epithelial–mesenchymal plasticity. Cell Death Dis. 2016, 7, e2564. [Google Scholar] [CrossRef]
- Shi, L.; Wang, X. Role of osteopontin in lung cancer evolution and heterogeneity. Semin. Cell Dev. Biol. 2017, 64, 40–47. [Google Scholar] [CrossRef]
- Giopanou, I.; Lilis, I.; Papaleonidopoulos, V.; Agalioti, T.; Kanellakis, N.I.; Spiropoulou, N.; Spella, M.; Stathopoulos, G.T. Tumor-derived osteopontin isoforms cooperate with TRP53 and CCL2 to promote lung metastasis. Oncoimmunology 2016, 6, e1256528. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.; Zhang, B.; Wang, L.; Wu, L.; Kan, Q.; Fan, K. MMP-9-cleaved osteopontin isoform mediates tumor immune escape by inducing expansion of myeloid-derived suppressor cells. Biochem. Biophys. Res. Commun. 2017, 493, 1478–1484. [Google Scholar] [CrossRef]
- Kang, C.G.; Han, H.J.; Lee, H.-J.; Kim, S.-H.; Lee, E.-O. Rho-associated kinase signaling is required for osteopontin-induced cell invasion through inactivating cofilin in human non-small cell lung cancer cell lines. Bioorg. Med. Chem. Lett. 2015, 25, 1956–1960. [Google Scholar] [CrossRef]
- Li, Y.; Du, W.; Han, J.; Ge, J. LAMP3 promotes the invasion of osteosarcoma cells via SPP1 signaling. Mol. Med. Rep. 2017, 16, 5947–5953. [Google Scholar] [CrossRef]
- Kamioka, Y.; Takakura, K.; Sumiyama, K.; Matsuda, M. Intravital Förster resonance energy transfer imaging reveals osteopontin-mediated polymorphonuclear leukocyte activation by tumor cell emboli. Cancer Sci. 2017, 108, 226–235. [Google Scholar] [CrossRef]
- Turgut Cosan, D.; Oner, C.; Mutlu Sahin, F. Micro RNA-126 coordinates cell behavior and signaling cascades according to characteristics of breast cancer cells. Bratislavske lekarske listy 2016, 117, 639–647. [Google Scholar] [CrossRef]
- Coppola, D.; Szabo, M.; Boulware, D.; Muraca, P.; Alsarraj, M.; Chambers, A.F.; Yeatman, T.J. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin. Cancer Res. 2004, 10, 184–190. [Google Scholar] [CrossRef]
- Rouanne, M.; Adam, J.; Goubar, A.; Robin, A.; Ohana, C.; Louvet, E.; Cormier, J.; Mercier, O.; Dorfmüller, P.; Fattal, S.; et al. Osteopontin and thrombospondin-1 play opposite roles in promoting tumor aggressiveness of primary resected non-small cell lung cancer. BMC Cancer 2016, 16, 483. [Google Scholar] [CrossRef]
- El-Tanani, M.K.; Yuen, H.-F.; Shi, Z.; Platt-Higgins, A.; Buckley, N.E.; Mullan, P.B.; Harkin, D.P.; Johnston, P.G.; Rudland, P.S. Osteopontin can act as an effector for a germline mutation of BRCA1 in malignant transformation of breast cancer-related cells. Cancer Sci. 2010, 101, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Pass, H.I.; Lott, D.; Lonardo, F.; Harbut, M.; Liu, Z.; Tang, N.; Carbone, M.; Webb, C.; Wali, A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 2005, 353, 1564–1573. [Google Scholar] [CrossRef]
- Paleari, L.; Rotolo, N.; Imperatori, A.; Puzone, R.; Sessa, F.; Franzi, F.; Meacci, E.; Camplese, P.; Cesario, A.; Paganuzzi, M. Osteopontin is not a specific marker in malignant pleural mesothelioma. Int. J. Biol. Markers 2009, 24, 112–117. [Google Scholar] [CrossRef]
- Hu, Z.-D.; Liu, X.-F.; Liu, X.-C.; Ding, C.-M.; Hu, C.-J. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: A systematic review and meta-analysis. Clin. Chim. Acta 2014, 433, 44–48. [Google Scholar] [CrossRef]
- Creaney, J.; Yeoman, D.; Musk, A.W.; de Klerk, N.; Skates, S.J.; Robinson, B.W.S. Plasma versus serum levels of osteopontin and mesothelin in patients with malignant mesothelioma--which is best? Lung Cancer 2011, 74, 55–60. [Google Scholar] [CrossRef]
- Cristaudo, A.; Foddis, R.; Bonotti, A.; Simonini, S.; Vivaldi, A.; Guglielmi, G.; Ambrosino, N.; Canessa, P.A.; Chella, A.; Lucchi, M.; et al. Comparison between plasma and serum osteopontin levels: Usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int. J. Biol. Markers 2010, 25, 164–170. [Google Scholar] [CrossRef]
- Cappia, S.; Righi, L.; Mirabelli, D.; Ceppi, P.; Bacillo, E.; Ardissone, F.; Molinaro, L.; Scagliotti, G.V.; Papotti, M. Prognostic role of osteopontin expression in malignant pleural mesothelioma. Am. J. Clin. Pathol. 2008, 130, 58–64. [Google Scholar] [CrossRef]
- Rud, A.K.; Boye, K.; Oijordsbakken, M.; Lund-Iversen, M.; Halvorsen, A.R.; Solberg, S.K.; Berge, G.; Helland, A.; Brustugun, O.T.; Mælandsmo, G.M. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer 2013, 13, 540. [Google Scholar] [CrossRef]
- Petta, V.; Loukides, S.; Kostikas, K.; Papaioannou, A.I.; Papatheodorou, G.; Cholidou, K.; Tomos, I.; Papiris, S.; Koulouris, N.G.; Bakakos, P. Serum osteopontin in patients with lung cancer and chronic obstructive pulmonary disease: Does the co-existence make the difference? J. Thorac. Dis. 2018, 10, 740–748. [Google Scholar] [CrossRef]
- Liaw, L.; Birk, D.E.; Ballas, C.B.; Whitsitt, J.S.; Davidson, J.M.; Hogan, B.L. Altered wound healing in mice lacking a functional osteopontin gene (spp1). J. Clin. Investig. 1998, 101, 1468–1478. [Google Scholar] [CrossRef]
- Berger, A.H.; Brooks, A.N.; Wu, X.; Shrestha, Y.; Chouinard, C.; Piccioni, F.; Bagul, M.; Kamburov, A.; Imielinski, M.; Hogstrom, L.; et al. High-throughput Phenotyping of Lung Cancer Somatic Mutations. Cancer Cell 2016, 30, 214–228. [Google Scholar] [CrossRef] [Green Version]
- Takafuji, V.; Forgues, M.; Unsworth, E.; Goldsmith, P.; Wang, X.W. An osteopontin fragment is essential for tumor cell invasion in hepatocellular carcinoma. Oncogene 2007, 26, 6361–6371. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Weber, G.F. The osteopontin-c splice junction is important for anchorage-independent growth. Mol. Carcinog. 2014, 53, 480–487. [Google Scholar] [CrossRef]
- Zhang, G.; He, B.; Weber, G.F. Growth factor signaling induces metastasis genes in transformed cells: Molecular connection between Akt kinase and osteopontin in breast cancer. Mol. Cell. Biol. 2003, 23, 6507–6519. [Google Scholar] [CrossRef]
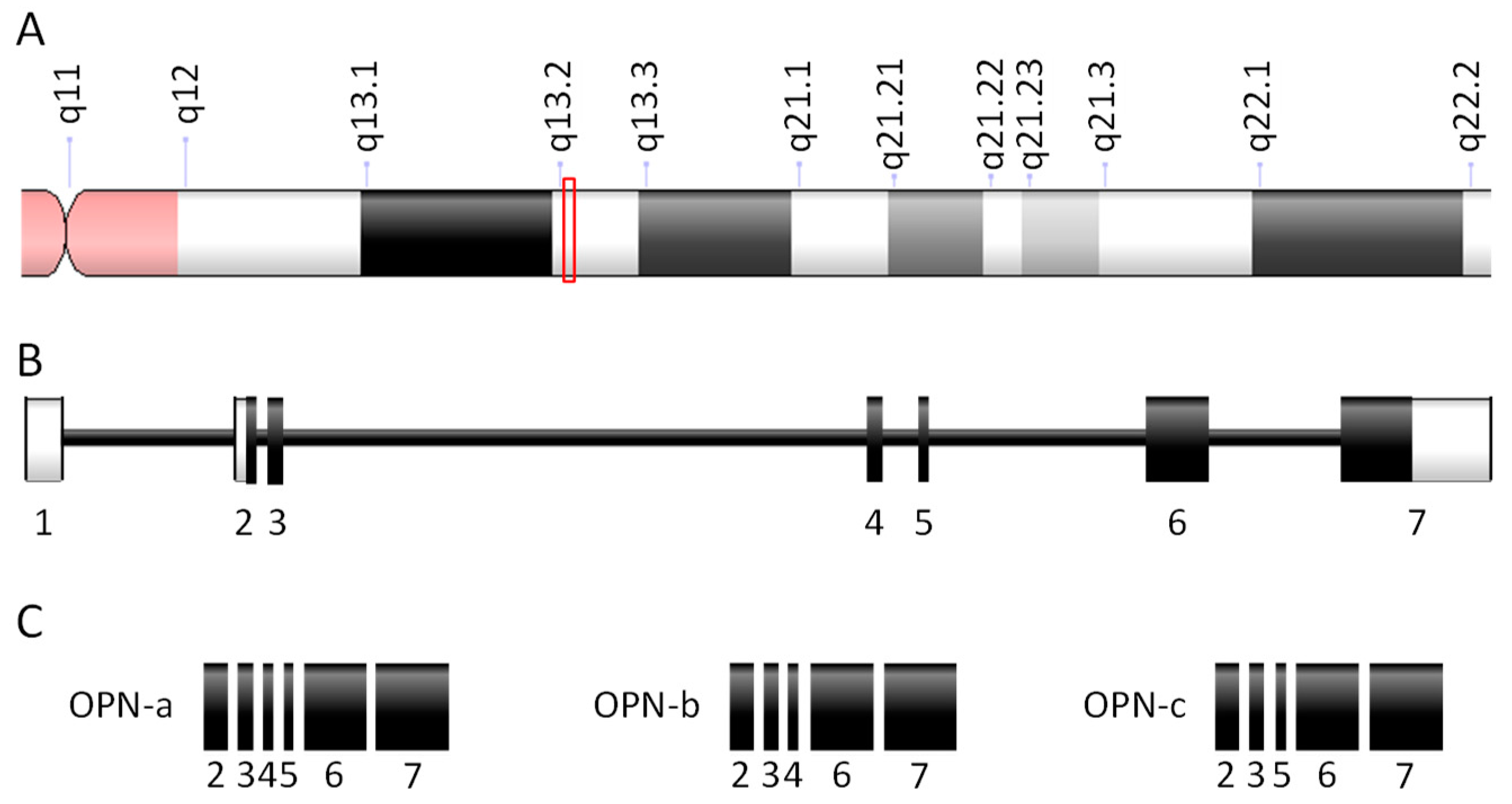
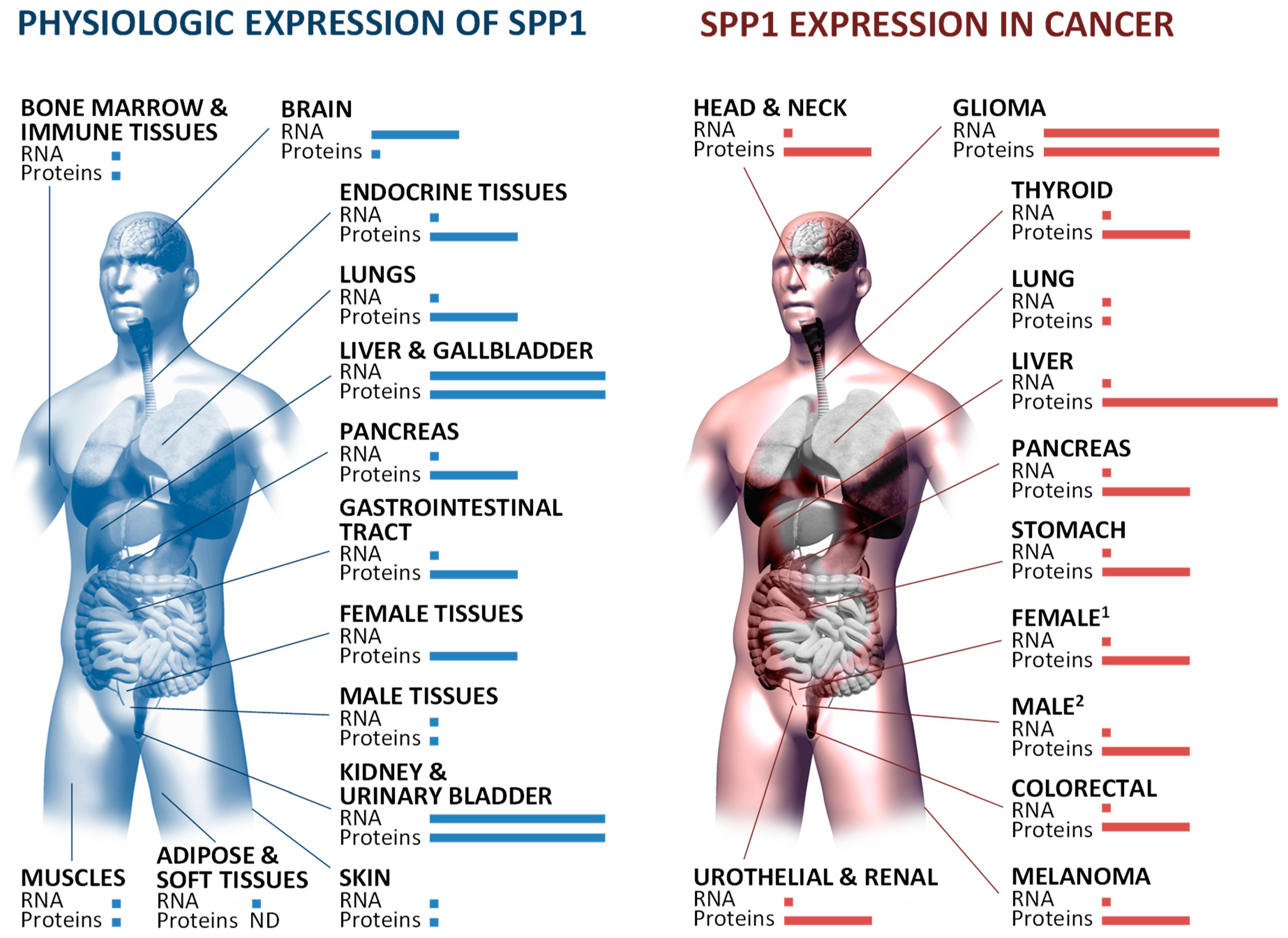
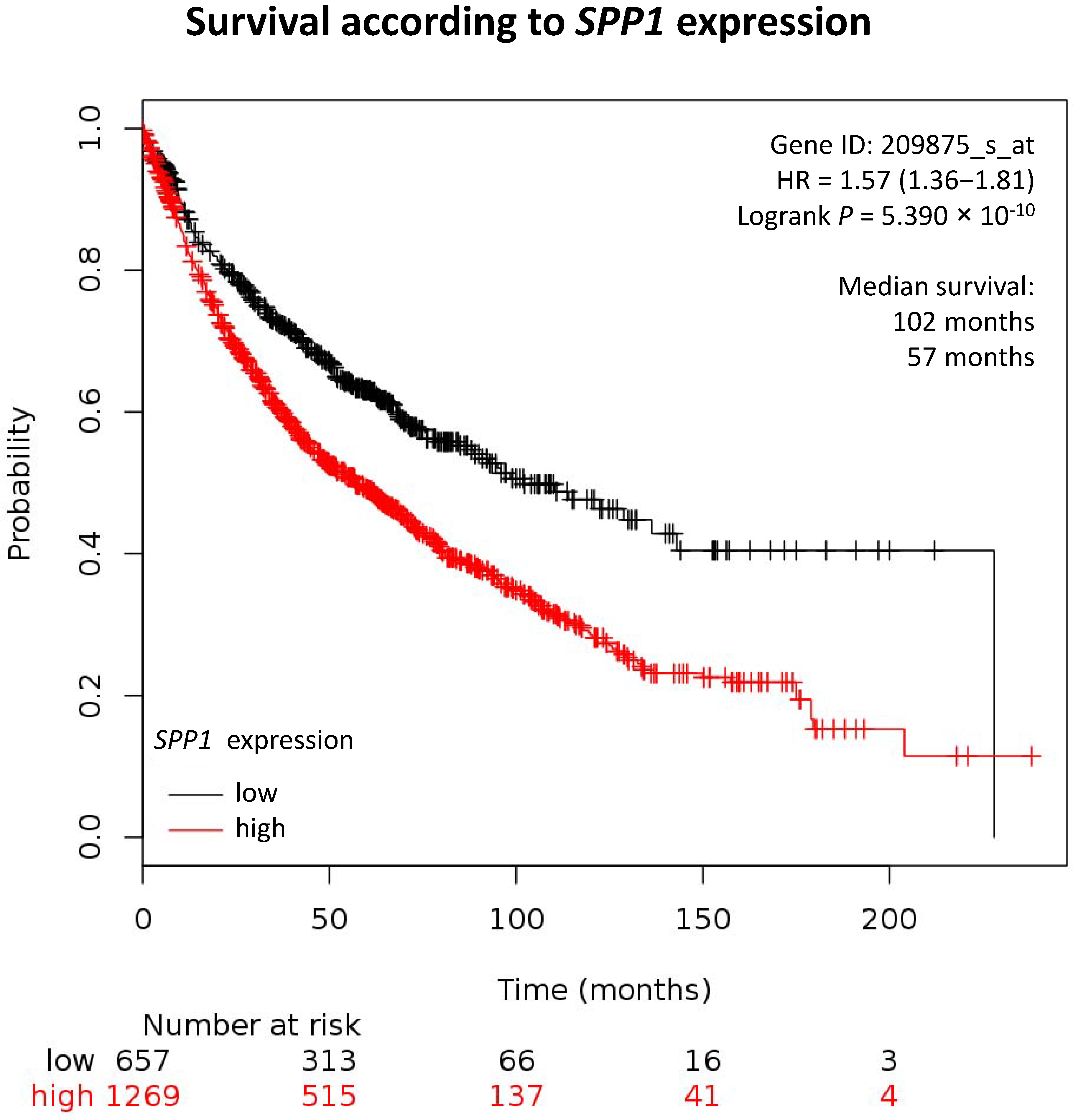
| Molecule | Activation | Inhibition |
|---|---|---|
| Transcription factors | c-Myb [48] | Smad4 [47] |
| ERG [49] | ||
| AML-1 [44] | ||
| C/EBPα [44] | ||
| SP-1 [45] CBFA1 [50] NF-κB [51] | ||
| Hormones | 1,25-dihydroxyvitamin D3 [52] Incretins [53] | |
| Inflammatory mediators | INFγ 1 [54] | INFγ 1 [52] |
| TNFα [54] | INFβ [55] | |
| IL-6 1 [54] | PGE2 [56] | |
| TGFβ [54] | ||
| IL-17a [54] | ||
| Other | Inorganic phosphate [57] VHL [46] | |
| Glucose [53] |
| Cancer Type | Patient Number | Cut-off | Overall Survival in Months | |||
|---|---|---|---|---|---|---|
| Low SPP1 Expression | High SPP1 Expression | Log-rank P Value | SPP1 Effect on Overall Survival | |||
| Bladder 1 | 405 | 929 | 46 | 30 | 5. 80 × 10−2 | ↘ |
| Breast 2 | 3951 | 3485 | 217 | 163 | 1. 00 × 10−16 | ↘ |
| Cervical 1 | 304 | 788 | NA | NA | 9. 50 × 10−4 | ↘ |
| Esophageal 3 | 161 | 1911 | 45 | 23 | 6. 30 × 10−2 | ↘ |
| Gastric | 876 | 2289 | 31 | 27 | 2. 90 × 10−1 | ↘ |
| Head and neck 1 | 500 | 5017 | 59 | 31 | 1. 00 × 10−2 | ↘ |
| Kidney renal 1 | 530 | 42,547 | 118 | 77 | 1. 10 × 10−1 | ↘ |
| Kidney renal papillary cell 3 | 288 | 140,810 | 87 | 50 | 1. 80 × 10−1 | ↘ |
| Liver | 364 | 5242 | 84 | 28 | 3. 50 × 10−6 | ↘ |
| Lung 2 | 1926 | 4151 | 102 | 57 | 5. 30 × 10−10 | ↘ |
| Lung adenocarcinoma | 720 | 4305 | 136 | 75 | 1. 30 × 10−7 | ↘ |
| Lung squamous-cell carcinoma | 524 | 14,780 | 64 | 33 | 3. 70 × 10−2 | ↘ |
| Ovarian | 1435 | 8960 | 23 | 18 | 7. 70 × 10−7 | ↘ |
| Pancreatic ductal 4 | 177 | 5866 | 73 | 18 | 5. 90 × 10−4 | ↘ |
| Pheochromocytoma and Paraganglioma | 178 | 223 | NA | NA | 4. 80 × 10−1 | = |
| Rectum 4 | 165 | 2620 | 52 | 37 | 2. 10 × 10−2 | ↘ |
| Sarcoma | 259 | 516 | 87 | 62 | 5. 40 × 10−2 | ↘ |
| Testicular Germ Cell | 134 | 470 | NA | NA | 2. 20 × 10−1 | = |
| Thymoma | 119 | 208 | NA | NA | 6. 70 × 10−2 | ↘ |
| Thyroid 3 | 502 | 308 | NA | NA | 3. 50 × 10−1 | = |
| Uterine 3 | 405 | 929 | 104 | 52 | 2. 20 × 10−1 | ↘ |
| Organism | Mutation | Isoform | Tag | Name |
|---|---|---|---|---|
| Human | OPN-a | pDONR223_SPP1_WT_V5 [157] | ||
| OPN-a | GST | pGEX-6P1-OPNa-delta S [21] | ||
| OPN-a | pCR3. 1-OPNa [21] | |||
| OPN-b | pCR3. 1-OPNb [21] | |||
| OPN-b | GST | pGEX-6P1-OPNb-delta S [21] | ||
| OPN-c | GST | pGEX-6P1-OPNc-delta S [21] | ||
| OPN-c | pCR3. 1-OPNc [21] | |||
| Base pairs 49–942 | OPN-a | flag | pDest490-OPN-a [158] | |
| Base pairs 1–175, 217–942 | OPN-b | flag | pDest490-OPN-b [158] | |
| EEKQ-->AAAA | OPN-c | pCR3. 1-OPNc M1 [159] | ||
| EEKQNAV-->AAAAAAA | OPN-c | pCR3. 1-OPNc M3 [159] | ||
| EEKQNA-->EEKNA | OPN-c | pCR3. 1-OPNc M4 [159] | ||
| EEKQNA-->EEKQANA | OPN-c | pCR3. 1-OPNc M5 [159] | ||
| SGSSEEKQNAVSSEET-->AGAAEEKQNAVAAEEA | OPN-c | pCR3. 1-OPNc PSM1 [159] | ||
| SGSSEEKQNAVSSEET-->AGAAEEKQNAVSSEET | OPN-c | pCR3. 1-OPNc PSM2 [159] | ||
| SGSSEEKQNAVSSEET-->SGSSEEKQNAVAAEEA | OPN-c | pCR3. 1-OPNc PSM3 [159] | ||
| SGSSEEKQNAVSSEET-->SGAAEEKQNAVSSEET | OPN-c | pCR3. 1-OPNc PSM4 [159] | ||
| SGSSEEKQNAVSSEET-->SGSSEEKQNAVAAEET | OPN-c | pCR3. 1-OPNc PSM5 [159] | ||
| Base pairs 1–93, 175–942 | OPN-c | pDest490-OPN-c [158] | ||
| Base pairs 499–630 | flag | pDest490-OPN-10 kDa [158] | ||
| Base pairs 49–498 | flag | pDest490-OPN-NT [158] | ||
| Base pairs 631–942 | flag | pDest490-OPN-CT [158] | ||
| Mouse | opn-1 | mOPN-pcDNA [160] | ||
| opn-1 | mOPN-PB0 [160] | |||
| opn-2 | EGFP | pSpp1-is2 [123] | ||
| opn-3 | EGFP | pSpp1-is3 [123] | ||
| antisense | as-mOPN-PB0 [160] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. https://doi.org/10.3390/cells8080815
Lamort A-S, Giopanou I, Psallidas I, Stathopoulos GT. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells. 2019; 8(8):815. https://doi.org/10.3390/cells8080815
Chicago/Turabian StyleLamort, Anne-Sophie, Ioanna Giopanou, Ioannis Psallidas, and Georgios T. Stathopoulos. 2019. "Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight" Cells 8, no. 8: 815. https://doi.org/10.3390/cells8080815
APA StyleLamort, A.-S., Giopanou, I., Psallidas, I., & Stathopoulos, G. T. (2019). Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells, 8(8), 815. https://doi.org/10.3390/cells8080815





