A Ciliary View of the Immunological Synapse
Abstract
:1. Introduction
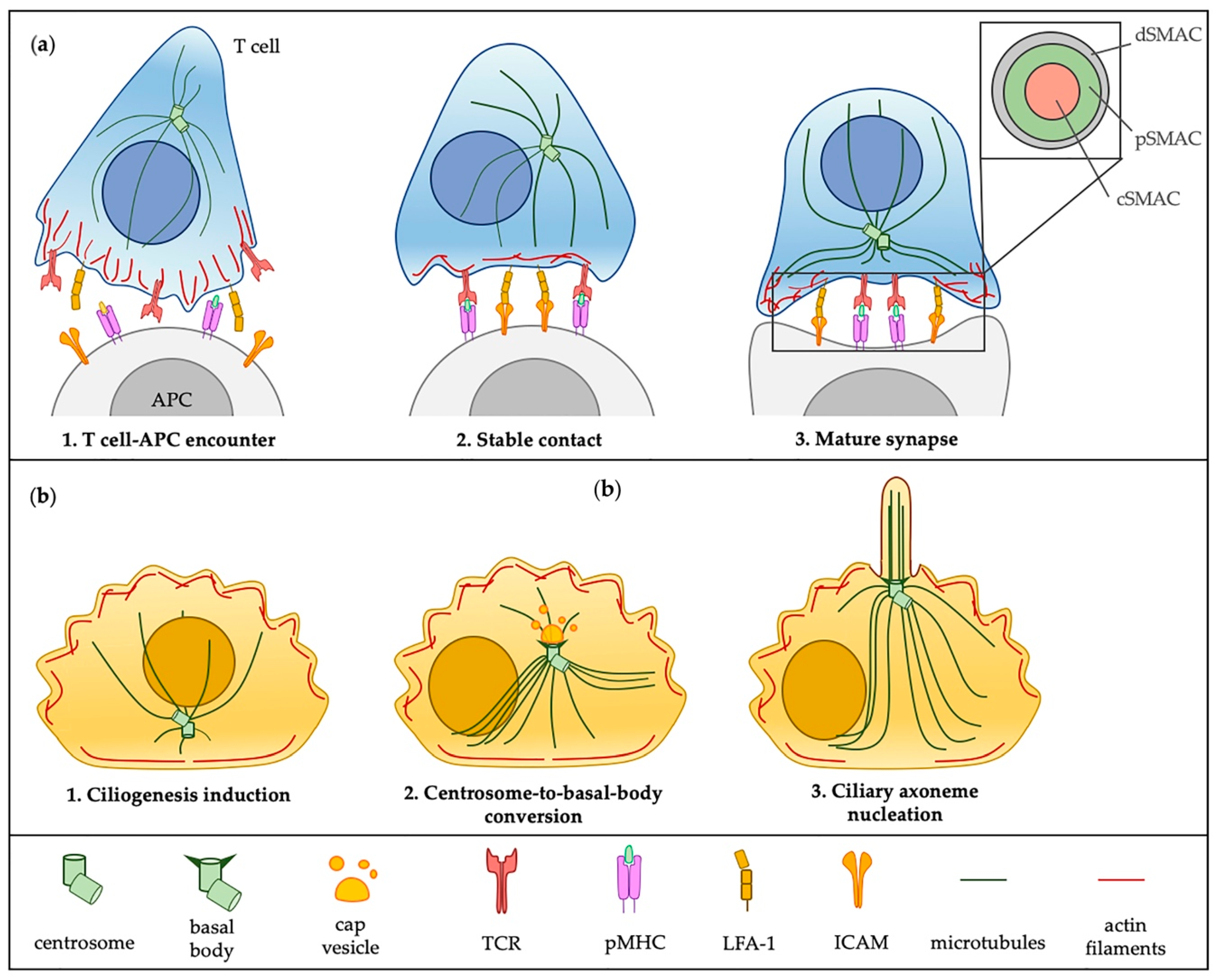
2. The Immunological Synapse and the Primary Cilium at a Glance: More Similarities Than Differences
2.1. Similarities in the Architectural Framework of the IS and the Primary Cilium
2.2. Functions Shared by the IS and the Primary Cilium
3. A “Ciliary” View of the Immunological Synapse
3.1. Cytoskeleton Regulates Assembly and Function of the IS and the Primary Cilium
3.1.1. Pushing or Pulling: How the Centrosome Moves toward the Apical Membrane
3.1.2. Dropping Anchor at the Plasma Membrane
3.1.3. The Actin Cytoskeleton Contributes to Ciliogenesis
3.1.4. The Actin Cytoskeleton Controls Mechanical Communication at the IS
3.1.5. Emerging Implications of Septins in the Assembly of Polarized Structures
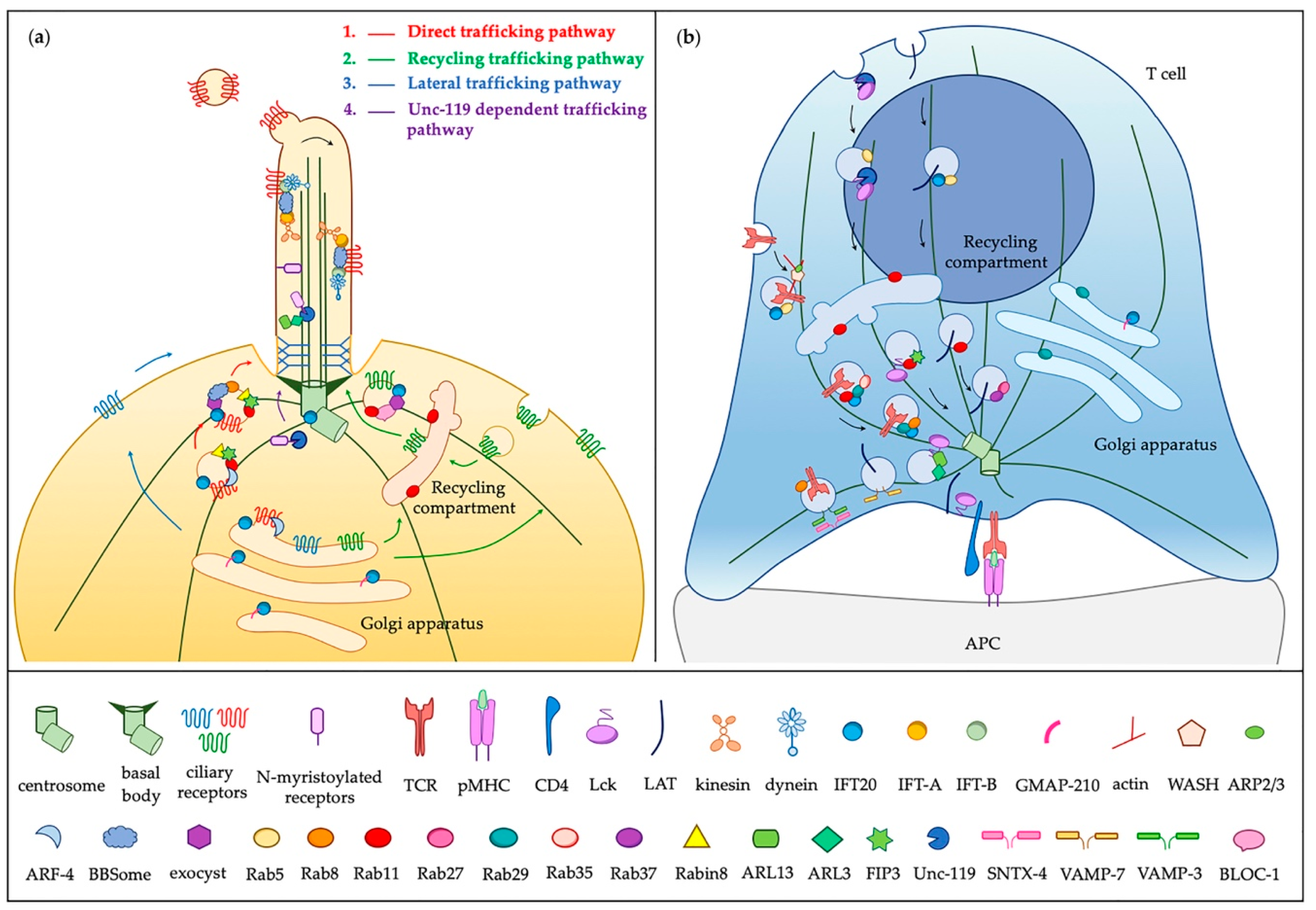
3.2. Vesicular Trafficking to the Primary Cilium and the Immunological Synapse
3.2.1. An Overview of Membrane-Associated Protein Trafficking to and into the Cilium
3.2.2. On the Way to the IS: Ciliary Regulators of Conserved Trafficking Machinery
3.3. Breaking the Phospholipid Code
3.4. Front-to-Rear Polarization during Early Ciliogenesis and IS Assembly
4. Investigating Extraciliary Functions of Ciliary Proteins Has Opened New Scenarios
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Carrion, A.; Vicente-Manzanares, M. Concerning immune synapses: A spatiotemporal timeline. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tato, C.M.; Davis, M.M. How the immune system talks to itself: The varied role of synapses. Immunol. Rev. 2013, 251, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L.; Chakraborty, A.K.; Shaw, A.S. Understanding the structure and function of the immunological synapse. Cold Spring Harb. Perspect. Biol. 2010, 2, a002311. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L. Modular design of immunological synapses and kinapses. Cold Spring Harb. Perspect. Biol. 2009, 1, a002873. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, B.A.; Kupfer, H.; Maslanik, W.; Delli, J.; Kappler, J.; Zaller, D.M.; Kupfer, A. Staging and resetting T cell activation in SMACs. Nat. Immunol. 2002, 3, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Delon, J.; Kaibuchi, K.; Germain, R.N. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity 2001, 15, 691–701. [Google Scholar] [CrossRef]
- Johnson, K.G.; Bromley, S.K.; Dustin, M.L.; Thomas, M.L. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc. Natl. Acad. Sci. USA 2000, 97, 10138–10143. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, S.P. Centriole formation and ciliogenesis. Aspen Emphysema Conf. 1968, 11, 213–216. [Google Scholar] [PubMed]
- Pazour, G.J.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.B.; Cole, D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.L.; Morrison, C.G. Centrin2 regulates CP110 removal in primary cilium formation. J. Cell Biol. 2015, 208, 693–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinchcombe, J.C.; Randzavola, L.O.; Angus, K.L.; Mantell, J.M.; Verkade, P.; Griffiths, G.M. Mother Centriole Distal Appendages Mediate Centrosome Docking at the Immunological Synapse and Reveal Mechanistic Parallels with Ciliogenesis. Curr. Biol. 2015, 25, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, C.J.; Thomas, J.A. The mechanism of T cell mediated cytotoxicity. III. Changes in target cell susceptibility during the cell cycle. Proc. R. Soc. Lond. B Biol. Sci. 1976, 194, 417–429. [Google Scholar] [PubMed]
- Pitaval, A.; Senger, F.; Letort, G.; Gidrol, X.; Guyon, L.; Sillibourne, J.; Thery, M. Microtubule stabilization drives 3D centrosome migration to initiate primary ciliogenesis. J. Cell Biol. 2017, 216, 3713–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, G.M.; Tsun, A.; Stinchcombe, J.C. The immunological synapse: A focal point for endocytosis and exocytosis. J. Cell Biol. 2010, 189, 399–406. [Google Scholar] [CrossRef]
- Poole, C.A.; Flint, M.H.; Beaumont, B.W. Analysis of the morphology and function of primary cilia in connective tissues: A cellular cybernetic probe? Cell Motil. 1985, 5, 175–193. [Google Scholar] [CrossRef]
- Kupfer, A.; Dennert, G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J. Immunol. 1984, 133, 2762–2766. [Google Scholar]
- Follit, J.A.; San Agustin, J.T.; Xu, F.; Jonassen, J.A.; Samtani, R.; Lo, C.W.; Pazour, G.J. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008, 4, e1000315. [Google Scholar] [CrossRef]
- Finetti, F.; Paccani, S.R.; Riparbelli, M.G.; Giacomello, E.; Perinetti, G.; Pazour, G.J.; Rosenbaum, J.L.; Baldari, C.T. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 2009, 11, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galgano, D.; Onnis, A.; Pappalardo, E.; Galvagni, F.; Acuto, O.; Baldari, C.T. The T cell IFT20 interactome reveals new players in immune synapse assembly. J. Cell Sci. 2017, 130, 1110–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, R.; Huse, M. Mechanical Communication at the Immunological Synapse. Trends Cell Biol. 2017, 27, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Cemerski, S.; Shaw, A. Immune synapses in T-cell activation. Curr. Opin. Immunol. 2006, 18, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernandez-Delgado, I.; Latorre-Pellicer, A.; Acin-Perez, R.; Martin-Cofreces, N.B.; Jaso-Tamame, A.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018, 9, 2658. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the Primary Cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Campi, G.; Yokosuka, T.; Saito, T.; Dustin, M.L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 2006, 25, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.; Choudhuri, K.; Varma, R.; Dustin, M.L. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity 2010, 32, 531–540. [Google Scholar] [CrossRef]
- Onnis, A.; Baldari, C.T. Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Front. Cell Dev. Biol. 2019, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Batista, A.; Millan, J.; Mittelbrunn, M.; Sanchez-Madrid, F.; Alonso, M.A. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J. Immunol. 2004, 172, 6709–6714. [Google Scholar] [CrossRef] [PubMed]
- Das, V.; Nal, B.; Dujeancourt, A.; Thoulouze, M.I.; Galli, T.; Roux, P.; Dautry-Varsat, A.; Alcover, A. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 2004, 20, 577–588. [Google Scholar] [CrossRef]
- Finetti, F.; Patrussi, L.; Masi, G.; Onnis, A.; Galgano, D.; Lucherini, O.M.; Pazour, G.J.; Baldari, C.T. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J. Cell Sci. 2014, 127, 1924–1937. [Google Scholar] [CrossRef] [PubMed]
- Bonello, G.; Blanchard, N.; Montoya, M.C.; Aguado, E.; Langlet, C.; He, H.T.; Nunez-Cruz, S.; Malissen, M.; Sanchez-Madrid, F.; Olive, D.; et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 2004, 117, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, L.I.; Ebert, P.J.; Krummel, M.F.; Weiss, A.; Davis, M.M. Dynamics of p56lck translocation to the T cell immunological synapse following agonist and antagonist stimulation. Immunity 2002, 17, 809–822. [Google Scholar] [CrossRef]
- Sung, C.H.; Leroux, M.R. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat. Cell Biol. 2013, 15, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmerah, A. The ciliary pocket. Curr. Opin. Cell Biol. 2013, 25, 78–84. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Mogensen, J.B.; Christensen, S.T. Endocytic Control of Cellular Signaling at the Primary Cilium. Trends Biochem. Sci. 2016, 41, 784–797. [Google Scholar] [CrossRef]
- Finetti, F.; Cassioli, C.; Baldari, C.T. Transcellular communication at the immunological synapse: A vesicular traffic-mediated mutual exchange. F1000Research 2017, 6, 1880. [Google Scholar] [CrossRef]
- Wang, J.; Barr, M.M. Ciliary Extracellular Vesicles: Txt Msg Organelles. Cell Mol. Neurobiol. 2016, 36, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Maguire, J.E.; Silva, M.; Nguyen, K.C.; Hellen, E.; Kern, A.D.; Hall, D.H.; Barr, M.M. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol. Biol. Cell 2015, 26, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Silva, M.; Haas, L.A.; Morsci, N.S.; Nguyen, K.C.; Hall, D.H.; Barr, M.M. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol. 2014, 24, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Nager, A.R.; Goldstein, J.S.; Herranz-Perez, V.; Portran, D.; Ye, F.; Garcia-Verdugo, J.M.; Nachury, M.V. An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2017, 168, 252.e214–263.e214. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, K.; Llodra, J.; Roth, E.W.; Tsai, J.; Gordo, S.; Wucherpfennig, K.W.; Kam, L.C.; Stokes, D.L.; Dustin, M.L. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 2014, 507, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lorenzo, M.J.; Anel, A.; Gamen, S.; Monle n, I.; Lasierra, P.; Larrad, L.; Pineiro, A.; Alava, M.A.; Naval, J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J. Immunol. 1999, 163, 1274–1281. [Google Scholar] [PubMed]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, K.; Ferland, R.J. Primary cilia proteins: Ciliary and extraciliary sites and functions. Cell Mol. Life Sci. 2018, 75, 1521–1540. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cofreces, N.B.; Baixauli, F.; Lopez, M.J.; Gil, D.; Monjas, A.; Alarcon, B.; Sanchez-Madrid, F. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J. 2012, 31, 4140–4152. [Google Scholar] [CrossRef]
- Kim, S.; Dynlacht, B.D. Assembling a primary cilium. Curr. Opin. Cell Biol. 2013, 25, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Blacque, O.E.; Scheidel, N.; Kuhns, S. Rab GTPases in cilium formation and function. Small GTPases 2018, 9, 76–94. [Google Scholar] [CrossRef]
- Zucchetti, A.E.; Bataille, L.; Carpier, J.M.; Dogniaux, S.; San Roman-Jouve, M.; Maurin, M.; Stuck, M.W.; Rios, R.M.; Baldari, C.T.; Pazour, G.J.; et al. The golgin GMAP210 organizes the transfer of signaling vesicles to the immune synapse: Parallel between vesicular trafficking at the immune synapse and at the cilium. Nat. Commun. 2019, 10, 2864. [Google Scholar] [CrossRef]
- Yi, J.; Wu, X.; Chung, A.H.; Chen, J.K.; Kapoor, T.M.; Hammer, J.A. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J. Cell Biol. 2013, 202, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, N.; Di Bartolo, V.; Hivroz, C. In the immune synapse, ZAP-70 controls T cell polarization and recruitment of signaling proteins but not formation of the synaptic pattern. Immunity 2002, 17, 389–399. [Google Scholar] [CrossRef]
- Kuhne, M.R.; Lin, J.; Yablonski, D.; Mollenauer, M.N.; Ehrlich, L.I.; Huppa, J.; Davis, M.M.; Weiss, A. Linker for activation of T cells, zeta-associated protein-70, and Src homology 2 domain-containing leukocyte protein-76 are required for TCR-induced microtubule-organizing center polarization. J. Immunol. 2003, 171, 860–866. [Google Scholar] [CrossRef]
- Lowin-Kropf, B.; Shapiro, V.S.; Weiss, A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J. Cell Biol. 1998, 140, 861–871. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Sancho, D.; Fernandez, E.; Vicente-Manzanares, M.; Gordon-Alonso, M.; Montoya, M.C.; Michel, F.; Acuto, O.; Alarcon, B.; Sanchez-Madrid, F. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J. Immunol. 2006, 176, 4201–4207. [Google Scholar] [CrossRef]
- Tsun, A.; Qureshi, I.; Stinchcombe, J.C.; Jenkins, M.R.; de la Roche, M.; Kleczkowska, J.; Zamoyska, R.; Griffiths, G.M. Centrosome docking at the immunological synapse is controlled by Lck signaling. J. Cell Biol. 2011, 192, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Quann, E.J.; Liu, X.; Altan-Bonnet, G.; Huse, M. A cascade of protein kinase C isozymes promotes cytoskeletal polarization in T cells. Nat. Immunol. 2011, 12, 647–654. [Google Scholar] [CrossRef]
- Quann, E.J.; Merino, E.; Furuta, T.; Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 2009, 10, 627–635. [Google Scholar] [CrossRef]
- Combs, J.; Kim, S.J.; Tan, S.; Ligon, L.A.; Holzbaur, E.L.; Kuhn, J.; Poenie, M. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl. Acad. Sci. USA 2006, 103, 14883–14888. [Google Scholar] [CrossRef] [Green Version]
- Kotak, S.; Busso, C.; Gonczy, P. NuMA interacts with phosphoinositides and links the mitotic spindle with the plasma membrane. EMBO J. 2014, 33, 1815–1830. [Google Scholar] [CrossRef] [Green Version]
- Merdes, A.; Ramyar, K.; Vechio, J.D.; Cleveland, D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 1996, 87, 447–458. [Google Scholar] [CrossRef]
- Lasserre, R.; Charrin, S.; Cuche, C.; Danckaert, A.; Thoulouze, M.I.; de Chaumont, F.; Duong, T.; Perrault, N.; Varin-Blank, N.; Olivo-Marin, J.C.; et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010, 29, 2301–2314. [Google Scholar] [CrossRef] [Green Version]
- Filbert, E.L.; Le Borgne, M.; Lin, J.; Heuser, J.E.; Shaw, A.S. Stathmin regulates microtubule dynamics and microtubule organizing center polarization in activated T cells. J. Immunol. 2012, 188, 5421–5427. [Google Scholar] [CrossRef]
- Zyss, D.; Ebrahimi, H.; Gergely, F. Casein kinase I delta controls centrosome positioning during T cell activation. J. Cell Biol. 2011, 195, 781–797. [Google Scholar] [CrossRef]
- Greer, Y.E.; Westlake, C.J.; Gao, B.; Bharti, K.; Shiba, Y.; Xavier, C.P.; Pazour, G.J.; Yang, Y.; Rubin, J.S. Casein kinase 1delta functions at the centrosome and Golgi to promote ciliogenesis. Mol. Biol. Cell 2014, 25, 1629–1640. [Google Scholar] [CrossRef]
- Schroder, J.M.; Larsen, J.; Komarova, Y.; Akhmanova, A.; Thorsteinsson, R.I.; Grigoriev, I.; Manguso, R.; Christensen, S.T.; Pedersen, S.F.; Geimer, S.; et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. J. Cell Sci. 2011, 124, 2539–2551. [Google Scholar] [CrossRef] [Green Version]
- Poole, C.A.; Jensen, C.G.; Snyder, J.A.; Gray, C.G.; Hermanutz, V.L.; Wheatley, D.N. Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol. Int. 1997, 21, 483–494. [Google Scholar] [CrossRef]
- Nguyen, A.M.; Young, Y.N.; Jacobs, C.R. The primary cilium is a self-adaptable, integrating nexus for mechanical stimuli and cellular signaling. Biol. Open 2015, 4, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Pugacheva, E.N.; Jablonski, S.A.; Hartman, T.R.; Henske, E.P.; Golemis, E.A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 2007, 129, 1351–1363. [Google Scholar] [CrossRef]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef]
- Nunez-Andrade, N.; Iborra, S.; Trullo, A.; Moreno-Gonzalo, O.; Calvo, E.; Catalan, E.; Menasche, G.; Sancho, D.; Vazquez, J.; Yao, T.P.; et al. HDAC6 regulates the dynamics of lytic granules in cytotoxic T lymphocytes. J. Cell Sci. 2016, 129, 1305–1311. [Google Scholar] [CrossRef] [Green Version]
- Serrador, J.M.; Cabrero, J.R.; Sancho, D.; Mittelbrunn, M.; Urzainqui, A.; Sanchez-Madrid, F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity 2004, 20, 417–428. [Google Scholar] [CrossRef]
- Andres-Delgado, L.; Anton, O.M.; Bartolini, F.; Ruiz-Saenz, A.; Correas, I.; Gundersen, G.G.; Alonso, M.A. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J. Cell Biol. 2012, 198, 1025–1037. [Google Scholar] [CrossRef] [Green Version]
- Bustos-Moran, E.; Blas-Rus, N.; Martin-Cofreces, N.B.; Sanchez-Madrid, F. Microtubule-associated protein-4 controls nanovesicle dynamics and T cell activation. J. Cell Sci. 2017, 130, 1217–1223. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Robles-Valero, J.; Cabrero, J.R.; Mittelbrunn, M.; Gordon-Alonso, M.; Sung, C.H.; Alarcon, B.; Vazquez, J.; Sanchez-Madrid, F. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol. 2008, 182, 951–962. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto-Tane, A.; Yokosuka, T.; Sakata-Sogawa, K.; Sakuma, M.; Ishihara, C.; Tokunaga, M.; Saito, T. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity 2011, 34, 919–931. [Google Scholar] [CrossRef]
- Lim, W.M.; Ito, Y.; Sakata-Sogawa, K.; Tokunaga, M. CLIP-170 is essential for MTOC repositioning during T cell activation by regulating dynein localisation on the cell surface. Sci. Rep. 2018, 8, 17447. [Google Scholar] [CrossRef]
- McKenney, R.J.; Huynh, W.; Vale, R.D.; Sirajuddin, M. Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 2016, 35, 1175–1185. [Google Scholar] [CrossRef]
- Graser, S.; Stierhof, Y.D.; Lavoie, S.B.; Gassner, O.S.; Lamla, S.; Le Clech, M.; Nigg, E.A. Cep164, a novel centriole appendage protein required for primary cilium formation. J. Cell Biol. 2007, 179, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.; Kubo, A.; Tsukita, S.; Tsukita, S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 2005, 7, 517–524. [Google Scholar] [CrossRef]
- Joo, K.; Kim, C.G.; Lee, M.S.; Moon, H.Y.; Lee, S.H.; Kim, M.J.; Kweon, H.S.; Park, W.Y.; Kim, C.H.; Gleeson, J.G.; et al. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proc. Natl. Acad. Sci. USA 2013, 110, 5987–5992. [Google Scholar] [CrossRef] [Green Version]
- Chaitin, M.H.; Carlsen, R.B.; Samara, G.J. Immunogold localization of actin in developing photoreceptor cilia of normal and rds mutant mice. Exp. Eye Res. 1988, 47, 437–446. [Google Scholar] [CrossRef]
- Kohli, P.; Hohne, M.; Jungst, C.; Bertsch, S.; Ebert, L.K.; Schauss, A.C.; Benzing, T.; Rinschen, M.M.; Schermer, B. The ciliary membrane-associated proteome reveals actin-binding proteins as key components of cilia. EMBO Rep. 2017, 18, 1521–1535. [Google Scholar] [CrossRef]
- Pitaval, A.; Tseng, Q.; Bornens, M.; Thery, M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J. Cell Biol. 2010, 191, 303–312. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.E.; Heynen-Genel, S.; Suyama, E.; Ono, K.; Lee, K.; Ideker, T.; Aza-Blanc, P.; Gleeson, J.G. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010, 464, 1048–1051. [Google Scholar] [CrossRef] [Green Version]
- Dawe, H.R.; Adams, M.; Wheway, G.; Szymanska, K.; Logan, C.V.; Noegel, A.A.; Gull, K.; Johnson, C.A. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J. Cell Sci. 2009, 122, 2716–2726. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.T.; Chen, H.Y.; Tang, T.K. Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat. Cell Biol. 2018, 20, 175–185. [Google Scholar] [CrossRef]
- Ran, J.; Yang, Y.; Li, D.; Liu, M.; Zhou, J. Deacetylation of alpha-tubulin and cortactin is required for HDAC6 to trigger ciliary disassembly. Sci. Rep. 2015, 5, 12917. [Google Scholar] [CrossRef]
- Phua, S.C.; Chiba, S.; Suzuki, M.; Su, E.; Roberson, E.C.; Pusapati, G.V.; Setou, M.; Rohatgi, R.; Reiter, J.F.; Ikegami, K.; et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 2017, 168, 264.e215–279.e215. [Google Scholar] [CrossRef]
- Drummond, M.L.; Li, M.; Tarapore, E.; Nguyen, T.T.L.; Barouni, B.J.; Cruz, S.; Tan, K.C.; Oro, A.E.; Atwood, S.X. Actin polymerization controls cilia-mediated signaling. J. Cell Biol. 2018, 217, 3255–3266. [Google Scholar] [CrossRef]
- Dustin, M.L.; Cooper, J.A. The immunological synapse and the actin cytoskeleton: Molecular hardware for T cell signaling. Nat. Immunol. 2000, 1, 23–29. [Google Scholar] [CrossRef]
- Ritter, A.T.; Asano, Y.; Stinchcombe, J.C.; Dieckmann, N.M.; Chen, B.C.; Gawden-Bone, C.; van Engelenburg, S.; Legant, W.; Gao, L.; Davidson, M.W.; et al. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity 2015, 42, 864–876. [Google Scholar] [CrossRef]
- Ritter, A.T.; Kapnick, S.M.; Murugesan, S.; Schwartzberg, P.L.; Griffiths, G.M.; Lippincott-Schwartz, J. Cortical actin recovery at the immunological synapse leads to termination of lytic granule secretion in cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2017, 114, E6585–E6594. [Google Scholar] [CrossRef] [Green Version]
- Gawden-Bone, C.M.; Frazer, G.L.; Richard, A.C.; Ma, C.Y.; Strege, K.; Griffiths, G.M. PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427.e424–437.e424. [Google Scholar] [CrossRef]
- Babich, A.; Li, S.; O’Connor, R.S.; Milone, M.C.; Freedman, B.D.; Burkhardt, J.K. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J. Cell Biol. 2012, 197, 775–787. [Google Scholar] [CrossRef]
- Yi, J.; Wu, X.S.; Crites, T.; Hammer, J.A., 3rd. Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol. Biol. Cell 2012, 23, 834–852. [Google Scholar] [CrossRef]
- Comrie, W.A.; Burkhardt, J.K. Action and Traction: Cytoskeletal Control of Receptor Triggering at the Immunological Synapse. Front. Immunol. 2016, 7, 68. [Google Scholar] [CrossRef]
- Cannon, J.L.; Burkhardt, J.K. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 2004, 173, 1658–1662. [Google Scholar] [CrossRef]
- Nolz, J.C.; Gomez, T.S.; Zhu, P.; Li, S.; Medeiros, R.B.; Shimizu, Y.; Burkhardt, J.K.; Freedman, B.D.; Billadeau, D.D. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr. Biol. 2006, 16, 24–34. [Google Scholar] [CrossRef]
- Nolz, J.C.; Medeiros, R.B.; Mitchell, J.S.; Zhu, P.; Freedman, B.D.; Shimizu, Y.; Billadeau, D.D. WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse. Mol. Cell Biol. 2007, 27, 5986–6000. [Google Scholar] [CrossRef]
- Cai, E.; Marchuk, K.; Beemiller, P.; Beppler, C.; Rubashkin, M.G.; Weaver, V.M.; Gerard, A.; Liu, T.L.; Chen, B.C.; Betzig, E.; et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science 2017, 356, eaal3118. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Depoil, D.; Martinelli, R.; Judokusumo, E.; Carmona, G.; Gertler, F.B.; Kam, L.C.; Carman, C.V.; Burkhardt, J.K.; Irvine, D.J.; et al. Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. eLife 2015, 4, e04953. [Google Scholar] [CrossRef]
- Murugesan, S.; Hong, J.; Yi, J.; Li, D.; Beach, J.R.; Shao, L.; Meinhardt, J.; Madison, G.; Wu, X.; Betzig, E.; et al. Formin-generated actomyosin arcs propel T cell receptor microcluster movement at the immune synapse. J. Cell Biol. 2016, 215, 383–399. [Google Scholar] [CrossRef]
- Gomez, T.S.; Kumar, K.; Medeiros, R.B.; Shimizu, Y.; Leibson, P.J.; Billadeau, D.D. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity 2007, 26, 177–190. [Google Scholar] [CrossRef]
- Das, D.K.; Feng, Y.; Mallis, R.J.; Li, X.; Keskin, D.B.; Hussey, R.E.; Brady, S.K.; Wang, J.H.; Wagner, G.; Reinherz, E.L.; et al. Force-dependent transition in the T-cell receptor beta-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl. Acad. Sci. USA 2015, 112, 1517–1522. [Google Scholar] [CrossRef]
- Feng, Y.; Brazin, K.N.; Kobayashi, E.; Mallis, R.J.; Reinherz, E.L.; Lang, M.J. Mechanosensing drives acuity of alphabeta T-cell recognition. Proc. Natl. Acad. Sci. USA 2017, 114, E8204–E8213. [Google Scholar] [CrossRef]
- Kim, S.T.; Takeuchi, K.; Sun, Z.Y.; Touma, M.; Castro, C.E.; Fahmy, A.; Lang, M.J.; Wagner, G.; Reinherz, E.L. The alphabeta T cell receptor is an anisotropic mechanosensor. J. Biol. Chem. 2009, 284, 31028–31037. [Google Scholar] [CrossRef]
- Liu, B.; Chen, W.; Evavold, B.D.; Zhu, C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell 2014, 157, 357–368. [Google Scholar] [CrossRef]
- Hanaoka, K.; Qian, F.; Boletta, A.; Bhunia, A.K.; Piontek, K.; Tsiokas, L.; Sukhatme, V.P.; Guggino, W.B.; Germino, G.G. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 2000, 408, 990–994. [Google Scholar] [CrossRef]
- Beise, N.; Trimble, W. Septins at a glance. J. Cell Sci. 2011, 124, 4141–4146. [Google Scholar] [CrossRef] [Green Version]
- Mostowy, S.; Cossart, P. Septins: The fourth component of the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2012, 13, 183–194. [Google Scholar] [CrossRef]
- Dash, S.N.; Lehtonen, E.; Wasik, A.A.; Schepis, A.; Paavola, J.; Panula, P.; Nelson, W.J.; Lehtonen, S. Sept7b is essential for pronephric function and development of left-right asymmetry in zebrafish embryogenesis. J. Cell Sci. 2014, 127, 1476–1486. [Google Scholar] [CrossRef]
- Ghossoub, R.; Hu, Q.; Failler, M.; Rouyez, M.C.; Spitzbarth, B.; Mostowy, S.; Wolfrum, U.; Saunier, S.; Cossart, P.; Jamesnelson, W.; et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 2013, 126, 2583–2594. [Google Scholar] [CrossRef]
- Hu, Q.; Milenkovic, L.; Jin, H.; Scott, M.P.; Nachury, M.V.; Spiliotis, E.T.; Nelson, W.J. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 2010, 329, 436–439. [Google Scholar] [CrossRef]
- Kim, S.K.; Shindo, A.; Park, T.J.; Oh, E.C.; Ghosh, S.; Gray, R.S.; Lewis, R.A.; Johnson, C.A.; Attie-Bittach, T.; Katsanis, N.; et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 2010, 329, 1337–1340. [Google Scholar] [CrossRef]
- Mujal, A.M.; Gilden, J.K.; Gerard, A.; Kinoshita, M.; Krummel, M.F. A septin requirement differentiates autonomous and contact-facilitated T cell proliferation. Nat. Immunol. 2016, 17, 315–322. [Google Scholar] [CrossRef]
- Maleth, J.; Choi, S.; Muallem, S.; Ahuja, M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat. Commun. 2014, 5, 5843. [Google Scholar] [CrossRef]
- Sharma, S.; Quintana, A.; Findlay, G.M.; Mettlen, M.; Baust, B.; Jain, M.; Nilsson, R.; Rao, A.; Hogan, P.G. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature 2013, 499, 238–242. [Google Scholar] [CrossRef]
- Mazelova, J.; Astuto-Gribble, L.; Inoue, H.; Tam, B.M.; Schonteich, E.; Prekeris, R.; Moritz, O.L.; Randazzo, P.A.; Deretic, D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009, 28, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Morita, Y.; Mazelova, J.; Deretic, D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012, 31, 4057–4071. [Google Scholar] [CrossRef] [Green Version]
- Knodler, A.; Feng, S.; Zhang, J.; Zhang, X.; Das, A.; Peranen, J.; Guo, W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 6346–6351. [Google Scholar] [CrossRef] [Green Version]
- Nachury, M.V.; Loktev, A.V.; Zhang, Q.; Westlake, C.J.; Peranen, J.; Merdes, A.; Slusarski, D.C.; Scheller, R.H.; Bazan, J.F.; Sheffield, V.C.; et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007, 129, 1201–1213. [Google Scholar] [CrossRef]
- Westlake, C.J.; Baye, L.M.; Nachury, M.V.; Wright, K.J.; Ervin, K.E.; Phu, L.; Chalouni, C.; Beck, J.S.; Kirkpatrick, D.S.; Slusarski, D.C.; et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc. Natl. Acad. Sci. USA 2011, 108, 2759–2764. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, S.; Egerer, J.; Fuchs, E.; Haas, A.K.; Barr, F.A. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 2007, 178, 363–369. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, W.; Lipschutz, J.H. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol. Biol. Cell 2009, 20, 2522–2529. [Google Scholar] [CrossRef]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef]
- Keady, B.T.; Le, Y.Z.; Pazour, G.J. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol. Biol. Cell 2011, 22, 921–930. [Google Scholar] [CrossRef]
- Monis, W.J.; Faundez, V.; Pazour, G.J. BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J. Cell Biol. 2017, 216, 2131–2150. [Google Scholar] [CrossRef] [Green Version]
- Milenkovic, L.; Scott, M.P.; Rohatgi, R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J. Cell Biol. 2009, 187, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Wright, K.J.; Baye, L.M.; Olivier-Mason, A.; Mukhopadhyay, S.; Sang, L.; Kwong, M.; Wang, W.; Pretorius, P.R.; Sheffield, V.C.; Sengupta, P.; et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011, 25, 2347–2360. [Google Scholar] [CrossRef]
- Soares, H.; Henriques, R.; Sachse, M.; Ventimiglia, L.; Alonso, M.A.; Zimmer, C.; Thoulouze, M.I.; Alcover, A. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J. Exp. Med. 2013, 210, 2415–2433. [Google Scholar] [CrossRef] [Green Version]
- Finetti, F.; Patrussi, L.; Galgano, D.; Cassioli, C.; Perinetti, G.; Pazour, G.J.; Baldari, C.T. The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling T-cell receptors to the immune synapse. J. Cell Sci. 2015, 128, 2541–2552. [Google Scholar] [CrossRef]
- Bouchet, J.; Del Rio-Iniguez, I.; Lasserre, R.; Aguera-Gonzalez, S.; Cuche, C.; Danckaert, A.; McCaffrey, M.W.; Di Bartolo, V.; Alcover, A. Rac1-Rab11-FIP3 regulatory hub coordinates vesicle traffic with actin remodeling and T-cell activation. EMBO J. 2016, 35, 1160–1174. [Google Scholar] [CrossRef]
- Bouchet, J.; Del Rio-Iniguez, I.; Vazquez-Chavez, E.; Lasserre, R.; Aguera-Gonzalez, S.; Cuche, C.; McCaffrey, M.W.; Di Bartolo, V.; Alcover, A. Rab11-FIP3 Regulation of Lck Endosomal Traffic Controls TCR Signal Transduction. J. Immunol. 2017, 198, 2967–2978. [Google Scholar] [CrossRef]
- Onnis, A.; Finetti, F.; Patrussi, L.; Gottardo, M.; Cassioli, C.; Spano, S.; Baldari, C.T. The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ. 2015, 22, 1687–1699. [Google Scholar] [CrossRef] [Green Version]
- Patino-Lopez, G.; Dong, X.; Ben-Aissa, K.; Bernot, K.M.; Itoh, T.; Fukuda, M.; Kruhlak, M.J.; Samelson, L.E.; Shaw, S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J. Biol. Chem. 2008, 283, 18323–18330. [Google Scholar] [CrossRef]
- Stephen, L.A.; ElMaghloob, Y.; McIlwraith, M.J.; Yelland, T.; Castro Sanchez, P.; Roda-Navarro, P.; Ismail, S. The Ciliary Machinery Is Repurposed for T Cell Immune Synapse Trafficking of LCK. Dev. Cell 2018, 47, 122.e124–132.e124. [Google Scholar] [CrossRef]
- Kaplan, O.I.; Doroquez, D.B.; Cevik, S.; Bowie, R.V.; Clarke, L.; Sanders, A.A.; Kida, K.; Rappoport, J.Z.; Sengupta, P.; Blacque, O.E. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr. Biol. 2012, 22, 451–460. [Google Scholar] [CrossRef]
- Ward, H.H.; Brown-Glaberman, U.; Wang, J.; Morita, Y.; Alper, S.L.; Bedrick, E.J.; Gattone, V.H., 2nd; Deretic, D.; Wandinger-Ness, A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol. Biol. Cell 2011, 22, 3289–3305. [Google Scholar] [CrossRef]
- Babbey, C.M.; Bacallao, R.L.; Dunn, K.W. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal. Physiol. 2010, 299, F495–F506. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, I.; Dynlacht, B.D. Cilium assembly and disassembly. Nat. Cell Biol. 2016, 18, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Deretic, D. The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J. Cell Sci. 2015, 128, 1375–1385. [Google Scholar] [CrossRef] [Green Version]
- Boehlke, C.; Bashkurov, M.; Buescher, A.; Krick, T.; John, A.K.; Nitschke, R.; Walz, G.; Kuehn, E.W. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J. Cell Sci. 2010, 123, 1460–1467. [Google Scholar] [CrossRef]
- Schwarz, N.; Hardcastle, A.J.; Cheetham, M.E. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vis. Res. 2012, 75, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; White, S.R.; Shida, T.; Schulz, S.; Aguiar, M.; Gygi, S.P.; Bazan, J.F.; Nachury, M.V. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 2010, 141, 1208–1219. [Google Scholar] [CrossRef]
- Evans, R.J.; Schwarz, N.; Nagel-Wolfrum, K.; Wolfrum, U.; Hardcastle, A.J.; Cheetham, M.E. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 2010, 19, 1358–1367. [Google Scholar] [CrossRef] [Green Version]
- Omori, Y.; Zhao, C.; Saras, A.; Mukhopadhyay, S.; Kim, W.; Furukawa, T.; Sengupta, P.; Veraksa, A.; Malicki, J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 2008, 10, 437–444. [Google Scholar] [CrossRef]
- Barral, D.C.; Garg, S.; Casalou, C.; Watts, G.F.; Sandoval, J.L.; Ramalho, J.S.; Hsu, V.W.; Brenner, M.B. Arl13b regulates endocytic recycling traffic. Proc. Natl. Acad. Sci. USA 2012, 109, 21354–21359. [Google Scholar] [CrossRef] [Green Version]
- Cevik, S.; Hori, Y.; Kaplan, O.I.; Kida, K.; Toivenon, T.; Foley-Fisher, C.; Cottell, D.; Katada, T.; Kontani, K.; Blacque, O.E. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 2010, 188, 953–969. [Google Scholar] [CrossRef] [Green Version]
- Derivery, E.; Sousa, C.; Gautier, J.J.; Lombard, B.; Loew, D.; Gautreau, A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell 2009, 17, 712–723. [Google Scholar] [CrossRef]
- Gomez, T.S.; Billadeau, D.D. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev. Cell 2009, 17, 699–711. [Google Scholar] [CrossRef]
- Anton, O.M.; Andres-Delgado, L.; Reglero-Real, N.; Batista, A.; Alonso, M.A. MAL protein controls protein sorting at the supramolecular activation cluster of human T lymphocytes. J. Immunol. 2011, 186, 6345–6356. [Google Scholar] [CrossRef]
- Calabia-Linares, C.; Robles-Valero, J.; de la Fuente, H.; Perez-Martinez, M.; Martin-Cofreces, N.; Alfonso-Perez, M.; Gutierrez-Vazquez, C.; Mittelbrunn, M.; Ibiza, S.; Urbano-Olmos, F.R.; et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J. Cell Sci. 2011, 124, 820–830. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Arenas, E.; Calleja, E.; Martinez-Martin, N.; Gharbi, S.I.; Navajas, R.; Garcia-Medel, N.; Penela, P.; Alcami, A.; Mayor, F., Jr.; Albar, J.P.; et al. beta-Arrestin-1 mediates the TCR-triggered re-routing of distal receptors to the immunological synapse by a PKC-mediated mechanism. EMBO J. 2014, 33, 559–577. [Google Scholar] [CrossRef]
- Kaplan, O.I.; Molla-Herman, A.; Cevik, S.; Ghossoub, R.; Kida, K.; Kimura, Y.; Jenkins, P.; Martens, J.R.; Setou, M.; Benmerah, A.; et al. The AP-1 clathrin adaptor facilitates cilium formation and functions with RAB-8 in C. elegans ciliary membrane transport. J. Cell Sci. 2010, 123, 3966–3977. [Google Scholar] [CrossRef]
- Takiar, V.; Mistry, K.; Carmosino, M.; Schaeren-Wiemers, N.; Caplan, M.J. VIP17/MAL expression modulates epithelial cyst formation and ciliogenesis. Am. J. Physiol. Cell Physiol. 2012, 303, C862–C871. [Google Scholar] [CrossRef] [Green Version]
- Schroder, J.M.; Schneider, L.; Christensen, S.T.; Pedersen, L.B. EB1 is required for primary cilia assembly in fibroblasts. Curr. Biol. 2007, 17, 1134–1139. [Google Scholar] [CrossRef]
- Osborne, D.G.; Piotrowski, J.T.; Dick, C.J.; Zhang, J.S.; Billadeau, D.D. SNX17 affects T cell activation by regulating TCR and integrin recycling. J. Immunol. 2015, 194, 4555–4566. [Google Scholar] [CrossRef]
- Larghi, P.; Williamson, D.J.; Carpier, J.M.; Dogniaux, S.; Chemin, K.; Bohineust, A.; Danglot, L.; Gaus, K.; Galli, T.; Hivroz, C. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat. Immunol. 2013, 14, 723–731. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, B.; Xu, L.; Li, H.; Xia, J.; Yin, W.; Li, Z.; Shi, D.; Li, S.; Lin, S.; et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 2012, 22, 333–345. [Google Scholar] [CrossRef]
- Szalinski, C.M.; Labilloy, A.; Bruns, J.R.; Weisz, O.A. VAMP7 modulates ciliary biogenesis in kidney cells. PLoS ONE 2014, 9, e86425. [Google Scholar] [CrossRef]
- Hao, L.; Scholey, J.M. Intraflagellar transport at a glance. J. Cell Sci. 2009, 122, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Nachury, M.V. The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell Biol. 2018, 51, 124–131. [Google Scholar] [CrossRef]
- van Dam, T.J.; Townsend, M.J.; Turk, M.; Schlessinger, A.; Sali, A.; Field, M.C.; Huynen, M.A. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. USA 2013, 110, 6943–6948. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Wang, L.; Kim, S.; Li, J.; Dynlacht, B.D. Role for the IFT-A Complex in Selective Transport to the Primary Cilium. Cell Rep. 2016, 17, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Wen, X.; Ratti, N.; Loktev, A.; Rangell, L.; Scales, S.J.; Jackson, P.K. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 2013, 152, 210–223. [Google Scholar] [CrossRef]
- Berbari, N.F.; Lewis, J.S.; Bishop, G.A.; Askwith, C.C.; Mykytyn, K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 2008, 105, 4242–4246. [Google Scholar] [CrossRef]
- Seo, S.; Zhang, Q.; Bugge, K.; Breslow, D.K.; Searby, C.C.; Nachury, M.V.; Sheffield, V.C. A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet. 2011, 7, e1002358. [Google Scholar] [CrossRef]
- Su, X.; Driscoll, K.; Yao, G.; Raed, A.; Wu, M.; Beales, P.L.; Zhou, J. Bardet-Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum. Mol. Genet. 2014, 23, 5441–5451. [Google Scholar] [CrossRef]
- Ye, F.; Nager, A.R.; Nachury, M.V. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 2018, 217, 1847–1868. [Google Scholar] [CrossRef] [Green Version]
- Robichaux, M.A.; Potter, V.L.; Zhang, Z.; He, F.; Schmid, M.F.; Wensel, T.G. Defining the Layers of a Sensory Cilium with STORM and Cryo-Electron Nanoscopies. bioRxiv 2017, 198655. [Google Scholar] [CrossRef]
- Jekely, G.; Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 2006, 28, 191–198. [Google Scholar] [CrossRef]
- Pampliega, O.; Orhon, I.; Patel, B.; Sridhar, S.; Diaz-Carretero, A.; Beau, I.; Codogno, P.; Satir, B.H.; Satir, P.; Cuervo, A.M. Functional interaction between autophagy and ciliogenesis. Nature 2013, 502, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Vivar, O.I.; Masi, G.; Carpier, J.M.; Magalhaes, J.G.; Galgano, D.; Pazour, G.J.; Amigorena, S.; Hivroz, C.; Baldari, C.T. IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 386–391. [Google Scholar] [CrossRef]
- Sonnichsen, B.; De Renzis, S.; Nielsen, E.; Rietdorf, J.; Zerial, M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000, 149, 901–914. [Google Scholar] [CrossRef]
- Ullrich, O.; Reinsch, S.; Urbe, S.; Zerial, M.; Parton, R.G. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996, 135, 913–924. [Google Scholar] [CrossRef]
- van der Sluijs, P.; Hull, M.; Webster, P.; Male, P.; Goud, B.; Mellman, I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992, 70, 729–740. [Google Scholar] [CrossRef]
- Carpier, J.M.; Zucchetti, A.E.; Bataille, L.; Dogniaux, S.; Shafaq-Zadah, M.; Bardin, S.; Lucchino, M.; Maurin, M.; Joannas, L.D.; Magalhaes, J.G.; et al. Rab6-dependent retrograde traffic of LAT controls immune synapse formation and T cell activation. J. Exp. Med. 2018, 215, 1245–1265. [Google Scholar] [CrossRef]
- Eguether, T.; San Agustin, J.T.; Keady, B.T.; Jonassen, J.A.; Liang, Y.; Francis, R.; Tobita, K.; Johnson, C.A.; Abdelhamed, Z.A.; Lo, C.W.; et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev. Cell 2014, 31, 279–290. [Google Scholar] [CrossRef]
- Keady, B.T.; Samtani, R.; Tobita, K.; Tsuchya, M.; San Agustin, J.T.; Follit, J.A.; Jonassen, J.A.; Subramanian, R.; Lo, C.W.; Pazour, G.J. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev. Cell 2012, 22, 940–951. [Google Scholar] [CrossRef]
- Yang, N.; Li, L.; Eguether, T.; Sundberg, J.P.; Pazour, G.J.; Chen, J. Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis. Development 2015, 142, 2194–2202. [Google Scholar] [CrossRef] [Green Version]
- Chavez, M.; Ena, S.; Van Sande, J.; de Kerchove d’Exaerde, A.; Schurmans, S.; Schiffmann, S.N. Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev. Cell 2015, 34, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gonzalo, F.R.; Phua, S.C.; Roberson, E.C.; Garcia, G., 3rd; Abedin, M.; Schurmans, S.; Inoue, T.; Reiter, J.F. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev. Cell 2015, 34, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Vieira, O.V.; Gaus, K.; Verkade, P.; Fullekrug, J.; Vaz, W.L.; Simons, K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA 2006, 103, 18556–18561. [Google Scholar] [CrossRef]
- Han, S.; Miyoshi, K.; Shikada, S.; Amano, G.; Wang, Y.; Yoshimura, T.; Katayama, T. TULP3 is required for localization of membrane-associated proteins ARL13B and INPP5E to primary cilia. Biochem. Biophys. Res. Commun. 2019, 509, 227–234. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Wen, X.; Chih, B.; Nelson, C.D.; Lane, W.S.; Scales, S.J.; Jackson, P.K. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010, 24, 2180–2193. [Google Scholar] [CrossRef] [Green Version]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Schurman, S.J.; Scheinman, S.J. Inherited cerebrorenal syndromes. Nat. Rev. Nephrol. 2009, 5, 529–538. [Google Scholar] [CrossRef]
- Prosseda, P.P.; Luo, N.; Wang, B.; Alvarado, J.A.; Hu, Y.; Sun, Y. Loss of OCRL increases ciliary PI(4,5)P2 in Lowe oculocerebrorenal syndrome. J. Cell Sci. 2017, 130, 3447–3454. [Google Scholar] [CrossRef]
- Chouaki-Benmansour, N.; Ruminski, K.; Sartre, A.M.; Phelipot, M.C.; Salles, A.; Bergot, E.; Wu, A.; Chicanne, G.; Fallet, M.; Brustlein, S.; et al. Phosphoinositides regulate the TCR/CD3 complex membrane dynamics and activation. Sci. Rep. 2018, 8, 4966. [Google Scholar] [CrossRef] [Green Version]
- Fairn, G.D.; Ogata, K.; Botelho, R.J.; Stahl, P.D.; Anderson, R.A.; De Camilli, P.; Meyer, T.; Wodak, S.; Grinstein, S. An electrostatic switch displaces phosphatidylinositol phosphate kinases from the membrane during phagocytosis. J. Cell Biol. 2009, 187, 701–714. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Sui, D.; Wu, D.; Hu, J. The activation loop of PIP5K functions as a membrane sensor essential for lipid substrate processing. Sci. Adv. 2016, 2, e1600925. [Google Scholar] [CrossRef]
- Kunkl, M.; Porciello, N.; Mastrogiovanni, M.; Capuano, C.; Lucantoni, F.; Moretti, C.; Persson, J.L.; Galandrini, R.; Buzzetti, R.; Tuosto, L. ISA-2011B, a Phosphatidylinositol 4-Phosphate 5-Kinase alpha Inhibitor, Impairs CD28-Dependent Costimulatory and Pro-inflammatory Signals in Human T Lymphocytes. Front. Immunol. 2017, 8, 502. [Google Scholar] [CrossRef]
- Kallikourdis, M.; Trovato, A.E.; Roselli, G.; Muscolini, M.; Porciello, N.; Tuosto, L.; Viola, A. Phosphatidylinositol 4-Phosphate 5-Kinase beta Controls Recruitment of Lipid Rafts into the Immunological Synapse. J. Immunol. 2016, 196, 1955–1963. [Google Scholar] [CrossRef]
- Barkalow, K.; Witke, W.; Kwiatkowski, D.J.; Hartwig, J.H. Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J. Cell Biol. 1996, 134, 389–399. [Google Scholar] [CrossRef]
- Rozelle, A.L.; Machesky, L.M.; Yamamoto, M.; Driessens, M.H.; Insall, R.H.; Roth, M.G.; Luby-Phelps, K.; Marriott, G.; Hall, A.; Yin, H.L. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 2000, 10, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Szatmari, D.; Xue, B.; Kannan, B.; Burtnick, L.D.; Bugyi, B.; Nyitrai, M.; Robinson, R.C. ATP competes with PIP2 for binding to gelsolin. PLoS ONE 2018, 13, e0201826. [Google Scholar] [CrossRef]
- Zhao, H.; Hakala, M.; Lappalainen, P. ADF/cofilin binds phosphoinositides in a multivalent manner to act as a PIP(2)-density sensor. Biophys. J. 2010, 98, 2327–2336. [Google Scholar] [CrossRef]
- Gawden-Bone, C.M.; Griffiths, G.M. Phospholipids: Pulling Back the Actin Curtain for Granule Delivery to the Immune Synapse. Front. Immunol. 2019, 10, 700. [Google Scholar] [CrossRef]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 307–314. [Google Scholar] [CrossRef]
- Chauveau, A.; Le Floc’h, A.; Bantilan, N.S.; Koretzky, G.A.; Huse, M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 2014, 7, ra82. [Google Scholar] [CrossRef]
- Zilber, Y.; Babayeva, S.; Seo, J.H.; Liu, J.J.; Mootin, S.; Torban, E. The PCP effector Fuzzy controls cilial assembly and signaling by recruiting Rab8 and Dishevelled to the primary cilium. Mol. Biol. Cell 2013, 24, 555–565. [Google Scholar] [CrossRef]
- Fan, S.; Hurd, T.W.; Liu, C.J.; Straight, S.W.; Weimbs, T.; Hurd, E.A.; Domino, S.E.; Margolis, B. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr. Biol. 2004, 14, 1451–1461. [Google Scholar] [CrossRef]
- Real, E.; Faure, S.; Donnadieu, E.; Delon, J. Cutting edge: Atypical PKCs regulate T lymphocyte polarity and scanning behavior. J. Immunol. 2007, 179, 5649–5652. [Google Scholar] [CrossRef]
- Ludford-Menting, M.J.; Oliaro, J.; Sacirbegovic, F.; Cheah, E.T.; Pedersen, N.; Thomas, S.J.; Pasam, A.; Iazzolino, R.; Dow, L.E.; Waterhouse, N.J.; et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity 2005, 22, 737–748. [Google Scholar] [CrossRef]
- Oliaro, J.; Van Ham, V.; Sacirbegovic, F.; Pasam, A.; Bomzon, Z.; Pham, K.; Ludford-Menting, M.J.; Waterhouse, N.J.; Bots, M.; Hawkins, E.D.; et al. Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J. Immunol. 2010, 185, 367–375. [Google Scholar] [CrossRef]
- Arsenio, J.; Kakaradov, B.; Metz, P.J.; Kim, S.H.; Yeo, G.W.; Chang, J.T. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat. Immunol. 2014, 15, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.T.; Ciocca, M.L.; Kinjyo, I.; Palanivel, V.R.; McClurkin, C.E.; Dejong, C.S.; Mooney, E.C.; Kim, J.S.; Steinel, N.C.; Oliaro, J.; et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity 2011, 34, 492–504. [Google Scholar] [CrossRef]
- Chang, J.T.; Palanivel, V.R.; Kinjyo, I.; Schambach, F.; Intlekofer, A.M.; Banerjee, A.; Longworth, S.A.; Vinup, K.E.; Mrass, P.; Oliaro, J.; et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 2007, 315, 1687–1691. [Google Scholar] [CrossRef]
- Metz, P.J.; Arsenio, J.; Kakaradov, B.; Kim, S.H.; Remedios, K.A.; Oakley, K.; Akimoto, K.; Ohno, S.; Yeo, G.W.; Chang, J.T. Regulation of asymmetric division and CD8+ T lymphocyte fate specification by protein kinase Czeta and protein kinase Clambda/iota. J. Immunol. 2015, 194, 2249–2259. [Google Scholar] [CrossRef]
- Martin, P.; Villares, R.; Rodriguez-Mascarenhas, S.; Zaballos, A.; Leitges, M.; Kovac, J.; Sizing, I.; Rennert, P.; Marquez, G.; Martinez, A.C.; et al. Control of T helper 2 cell function and allergic airway inflammation by PKCzeta. Proc. Natl. Acad. Sci. USA 2005, 102, 9866–9871. [Google Scholar] [CrossRef]
- Yang, J.Q.; Leitges, M.; Duran, A.; Diaz-Meco, M.T.; Moscat, J. Loss of PKC lambda/iota impairs Th2 establishment and allergic airway inflammation in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1099–1104. [Google Scholar] [CrossRef]
- Aguera-Gonzalez, S.; Burton, O.T.; Vazquez-Chavez, E.; Cuche, C.; Herit, F.; Bouchet, J.; Lasserre, R.; Del Rio-Iniguez, I.; Di Bartolo, V.; Alcover, A. Adenomatous Polyposis Coli Defines Treg Differentiation and Anti-inflammatory Function through Microtubule-Mediated NFAT Localization. Cell Rep. 2017, 21, 181–194. [Google Scholar] [CrossRef] [Green Version]
- de la Roche, M.; Asano, Y.; Griffiths, G.M. Origins of the cytolytic synapse. Nat. Rev. Immunol. 2016, 16, 421–432. [Google Scholar] [CrossRef]
- Noda, K.; Kitami, M.; Kitami, K.; Kaku, M.; Komatsu, Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proc. Natl. Acad. Sci. USA 2016, 113, E2589–E2597. [Google Scholar] [CrossRef]
- Finetti, F.; Cassioli, C.; Cianfanelli, V.; Onnis, A.; Paccagnini, E.; Kabanova, A.; Baldari, C.T. The intraflagellar transport protein IFT20 controls lysosome biogenesis by regulating the post-Golgi transport of acid hydrolases. Cell Death Differ. 2019. [Google Scholar] [CrossRef]
- Choksi, S.P.; Lauter, G.; Swoboda, P.; Roy, S. Switching on cilia: Transcriptional networks regulating ciliogenesis. Development 2014, 141, 1427–1441. [Google Scholar] [CrossRef]
- Viau, A.; Bienaime, F.; Lukas, K.; Todkar, A.P.; Knoll, M.; Yakulov, T.A.; Hofherr, A.; Kretz, O.; Helmstadter, M.; Reichardt, W.; et al. Cilia-localized LKB1 regulates chemokine signaling, macrophage recruitment, and tissue homeostasis in the kidney. EMBO J. 2018, 37. [Google Scholar] [CrossRef]
- Gorska, M.M.; Alam, R. A mutation in the human Uncoordinated 119 gene impairs TCR signaling and is associated with CD4 lymphopenia. Blood 2012, 119, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
| Function | Immunological Synapse | Primary Cilium |
|---|---|---|
| GTPases | Rab3 [132]; Rab4 [33]; Rab5 [33]; Rab8 [132,133]; Rab11 [33,132]-FIP3 [134,135]; Rab27 [132]; Rab29 [136]; Rab35 [137]; Rab37 [132]; ARL3 [138] | Rab5 [139]; Rab6 [121,140]; Rab8 [123]; Rab10 [141]; Rab11 [124,142]-FIP3 [143]; Rab17 [125]; Rab23 [144]; Rab29 [136]; ARF4 [120]; ARL3 [131,145]; ARL6 [146] |
| GEFs and GAPs | EPI64C [137]; ARL13B [138] | RP2 [145,147]; Rabaptin5 [148]; ASAP-1 [121]; Rabin8 [123]; TBC1D7 [125]; EVI5like [125]; ARL13 [149,150] |
| Adaptors | WASH [151,152]; MAL [132,153]; clathrin [154]; β-arrestin1 [155]; EB-1 [48]; Unc119 [138]; ARPC3 [22] | TRAPPII [124]; AP-1 [156]; AP-2 [139]; MAL [157]; EB-1 [67,158]; Unc119 [131] |
| SNAREs and tethers | SNAP-23 [32]; Syntaxin-4 and -17 [32,159]; VAMP-2, -3 and -7 [32,132,133,160] | Exocyst subunits (Sec10, Exo70) [126,129], Syntaxin-10 [161]; VAMP-7 [162] |
| Others | IFT20; IFT52; IFT54; IFT57; IFT88 [21,33]; ERGIC-53 [22] | IFT proteins [163]; BLOC-1 [129]; BBSome complex [164] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassioli, C.; Baldari, C.T. A Ciliary View of the Immunological Synapse. Cells 2019, 8, 789. https://doi.org/10.3390/cells8080789
Cassioli C, Baldari CT. A Ciliary View of the Immunological Synapse. Cells. 2019; 8(8):789. https://doi.org/10.3390/cells8080789
Chicago/Turabian StyleCassioli, Chiara, and Cosima T. Baldari. 2019. "A Ciliary View of the Immunological Synapse" Cells 8, no. 8: 789. https://doi.org/10.3390/cells8080789
APA StyleCassioli, C., & Baldari, C. T. (2019). A Ciliary View of the Immunological Synapse. Cells, 8(8), 789. https://doi.org/10.3390/cells8080789





