A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy
Abstract
1. Introduction
2. Autophagy: A Brief Overview
2.1. Mechanisms of Autophagy
2.1.1. Macroautophagy
2.1.2. Microautophagy
2.1.3. Chaperone-Mediated Autophagy (CMA)
2.2. Molecular Mechanisms of Autophagy
2.3. Autosis: A Novel Form of Autophagy
2.4. Association between Autophagy and Other Cell Death Mechanisms
2.4.1. Links between Autophagy and Apoptosis
2.4.2. Autophagy and Necroptosis
2.4.3. Autophagy and Necrosis
3. Role of Autophagy
3.1. Role of Autophagy against Infectious Diseases
3.1.1. Anti-Bacterial Role of Autophagy
3.1.2. Anti-Viral Role of Autophagy
3.1.3. Proviral Role of Autophagy
3.2. Autophagy in Tumor Suppression
3.3. Autophagy in Tumor Progression
3.4. Autophagy in Brain Development
3.5. Autophagy in Neurodegeneration
3.6. Autophagy in the Immune System and Autoimmune Diseases
3.6.1. Autophagy in the Immune System
Pro-Inflammatory Signaling Regulated by Autophagy
Interplay between Cytokine Secretion and Autophagy
3.6.2. Autophagy and Autoimmunity
3.7. Autophagy in Cardiovascular Diseases
3.8. Autophagy in Iron Homeostasis
3.9. Autophagy in Obesity and Diabetes
3.10. Diseases Caused by Autophagy Gene Defects
3.10.1. Static Encephalopathy of Childhood with Neurodegeneration in Adulthood (SENDA)
3.10.2. Crohn’s Disease
3.10.3. Hereditary Spastic Paraparesis (HSP)
3.10.4. Danon Disease
3.10.5. X-Linked Myopathy with Excessive Autophagy (XMEA)
3.10.6. Sporadic Inclusion Body Myositis (sIBM)
4. Treatment of Autophagy-Associated Diseases
4.1. Strategies to Inhibit Autophagy
4.1.1. Vacuolar-Type H (+)-ATPase Inhibitors
4.1.2. Cycloheximide
4.1.3. Lysosome Alkalizers
4.1.4. Acidic Protease Inhibitors
4.1.5. Genetic Modifications
4.2. Autophagy Activators
4.2.1. Rapamycin
4.2.2. Small-Molecule Enhancers of Rapamycin (SMERs)
4.2.3. Trehalose
4.2.4. Inositol Monophosphatase (IMPase) Inhibitors
4.2.5. Epigenetic Changes
4.2.6. Other Molecules
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, K.F. Till death do us part: The marriage of autophagy and apoptosis. Oxidative Med. Cell Longev. 2018, 2018, 4701275. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy, process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy, core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S. Overview of the minireviews on autophagy. Mol. Cells 2018, 41, 1–2. [Google Scholar] [PubMed]
- Lee, Y.A.; Noon, L.A.; Akat, K.M.; Ybanez, M.D.; Lee, T.F.; Berres, M.L.; Fujiwara, N.; Goossens, N.; Chou, H.I.; Parvin-Nejad, F.P.; et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nat. Commun. 2018, 9, 4962. [Google Scholar] [CrossRef] [PubMed]
- Füllgrabe, J.; Ghislat, C.; Cho, D.H.; Rubinsztein, D.C. Transcriptional regulation of mammalian autophagy at a glance. J. Cell Sci. 2016, 129, 3059–3066. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Kunz, J.B.; Schwarz, H.; Mayer, A. Determination of four sequential stages during microautophagy in vitro. J. Biol. Chem. 2004, 279, 9987–9996. [Google Scholar] [CrossRef]
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy, lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Ueno, T.; Komatsu, M. Autophagy in the liver, functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R. Autophagy: A brief overview in perspective of dermatology. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Paolini, A.; Omairi, S.; Mitchell, R.; Vaughan, D.; Matsakas, A.; Vaiyapuri, S.; Ricketts, T.; Rubinsztein, D.C.; Patel, K. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci. Rep. 2018, 8, 9062. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Morris, H.; Schapira, A. Chaperone-mediated autophagy as a therapeutic target for Parkinson disease. Expert Opin. Ther. Targets 2018, 22, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Majeski, A.E.; Dice, J.F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, T.; Zhu, H.; Zhang, P.; Han, R.; Liu, Y.; Ni, P.; Shen, H.; Xu, W.; Xu, H. HMGB1 modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-Erk1/2 positive feedback during nutrient depletion. Immunobiology 2015, 220, 539–544. [Google Scholar] [CrossRef]
- Hsu, P.; Shi, Y. Regulation of autophagy by mitochondrial phospholipids in health and diseases. Biochim. Biophys. Acta 2017, 1862, 114–129. [Google Scholar] [CrossRef]
- Fujikake, N.; Shin, M.; Shimizu, S. Association between autophagy and neurodegenerative diseases. Front. Neurosci. 2018, 12, 255. [Google Scholar] [CrossRef]
- Huang, F.; Wang, B.R.; Wang, Y.G. Role of autophagy in tumorigenesis, metastasis, targeted therapy and drug resistance of hepatocellular carcinoma. World J. Gastroenterol. 2018, 24, 4643–4651. [Google Scholar] [CrossRef]
- Pleet, M.L.; Branscome, H.; DeMarino, C.; Pinto, D.O.; Zadeh, M.A.; Rodriguez, M.; Sariyer, I.K.; El-Hage, N.; Kashanchi, F. Autophagy, EVs, and infections: A perfect question for a perfect time. Front. Cell Infect. Microbiol. 2018, 8, 362. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, S.; Seranova, E.; Sarkar, S.; Kumar, D. Selective autophagy and xenophagy in infection and disease. Front. Cell Dev. Biol. 2018, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Majdoul, S.; Cosette, J.; Seye, A.K.; Bernard, E.; Frin, S.; Holic, N.; Chazal, N.; Briant, L.; Espert, L.; Galy, A.; et al. Peptides derived from evolutionarily conserved domains in Beclin-1 and Beclin-2 enhance the entry of lentiviral vectors into human cells. J. Biol. Chem. 2017, 292, 18672–18681. [Google Scholar] [CrossRef] [PubMed]
- Metaxakis, A.; Ploumi, C.; Tavernarakis, N. Autophagy in age-associated neurodegeneration. Cells 2018, 7, E37. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tzeng, Y.M.; Yeh, C.T.; Wu, T.H.A. Method for Inhibiting Growth of Ovarian Cancer Cells. U.S. Patent 20180050012, 22 February 2018. [Google Scholar]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Mowers, E.E.; Sharifi, M.N.; Macleod, K.F. Novel insights into how autophagy regulates tumor cell motility. Autophagy 2016, 12, 1679–1680. [Google Scholar] [CrossRef]
- Janji, B.; Chouaib, S. Role of autophagy in tumor progression and regression. In Targeting Autophagy in Cancer Therapy; Springer: Cham, Switzerland, 2016; pp. 117–131. [Google Scholar]
- Gozuacik, D.; Akkoc, Y.; Ozturk, D.G.; Kocak, M. Autophagy regulating microRNAs and cancer. Front. Oncol. 2017, 7, 65. [Google Scholar] [CrossRef]
- Deng, Z.; Purtell, K.; Lachance, V.; Wold, M.S.; Chen, S.; Yue, Z. Autophagy receptors and neurodegenerative diseases. Trends Cell Biol. 2017, 27, 491–504. [Google Scholar] [CrossRef]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Becker, A.C.; Gannagé, M.; Giese, S.; Hu, Z.; Abou-Eid, S.; Roubaty, C.; Paul, P.; Bühler, L.; Gretzmeier, C.; Dumit, V.I.; et al. Influenza A virus induces autophagosomal targeting of ribosomal proteins. Mol. Cell. Proteomics 2018, 17, 1909–1921. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, L.; Wei, F.; Fang, Q.; Bao, F.; Mi, S.; Li, N.; Wang, C.; Liu, Y.; Tu, C. mTORC1 negatively regulates the replication of classical swine fever virus through autophagy and IRES-dependent translation. iScience 2018, 3, 87–101. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.; Dhama, K.; Katthik, K.; Tiwari, R.; Malik, Y.P.S.; Singh, R.K.; Chaicumpa, W. Modulation of dengue/zika virus pathogenicity by antibody dependent enhancement and strategies to protect against enhancement in zika virus infection. Front. Immunol. 2018, 9, 597. [Google Scholar] [CrossRef]
- Peng, H.; Liu, B.; Yves, T.D.; He, Y.; Wang, S.; Tang, H.; Ren, H.; Zhao, P.; Qi, Z.; Qin, Z. Zika virus induces autophagy in human umbilical vein endothelial cells. Viruses 2018, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Nabar, N.R.; Shi, C.S.; Kamenyeva, O.; Xiao, X.; Hwang, I.Y.; Wang, M.; Kehrl, J.H. SARS-Coronavirus open reading frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Z.; Chu, J. The interplay of viral and host factors in Chikungunya virus infection, targets for antiviral strategies. Viruses 2018, 10, 294. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kuo, S.H.; Lin, C.Y.; Fu, P.J.; Lin, Y.S.; Yeh, T.M.; Liu, H.S. Dengue virus-induced ER stress is required for autophagy activation, viral replication, and pathogenesis both in vitro and in vivo. Sci. Rep. 2018, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.; Mostowy, S.; Sancho-Shimizu, V. Autophagy-virus interplay: From cell biology to human disease. Front. Cell Dev. Biol. 2018, 6, 155. [Google Scholar] [CrossRef]
- Barnwal, B.; Karlberg, H.; Mirazimi, A.; Tan, Y.J. The non-structural protein of Crimean-Congo hemorrhagic fever virus disrupts the mitochondrial membrane potential and induces apoptosis. J. Biol. Chem. 2016, 291, 582–592. [Google Scholar] [CrossRef]
- Mandhana, R.; Qian, L.K.; Horvath, C.M. Constitutively active MDA5 proteins are inhibited by paramyxovirus V proteins. J. Int. Cytokine Res. 2018, 38, 319–332. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Zhang, Q.; Meng, S.; Ding, C. Autophagy in negative-strand RNA virus infection. Front. Microbiol. 2018, 9, 206. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Blázquez, A.B.; Saiz, J.C. Reconciling West Nile virus with the autophagic pathway. Autophagy 2015, 11, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Patel, N.; Levy, M.; Storeygard, A.; Balk, D.; Gittleman, J.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Tiwari, R.; Chakraborty, S.; Kumar, A.; Karikalan, M.; Singh, R.; Rai, R.B. Global warming and emerging infectious diseases of animals and humans: Current scenario, challenges, solutions and future perspectives—A review. Int. J. Curr. Res. 2013, 5, 1942–1958. [Google Scholar]
- Singh, R.K.; Dhama, K.; Malik, Y.S.; Ramakrishnan, M.A.; Karthik, K.; Khandia, R.; Tiwari, R.; Munjal, A.; Saminathan, M.; Sachan, S.; et al. Ebola virus—Epidemiology, diagnosis, and control: Threat to humans, lessons learnt, and preparedness plans—An update on its 40 year’s journey. Vet. Q. 2017, 37, 98–135. [Google Scholar] [CrossRef]
- Han, B.A.; Kramer, A.M.; Drake, J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016, 32, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Dhama, K.; Malik, Y.S.; Ramakrishnan, M.A.; Karthik, K.; Tiwari, R.; Saurabh, S.; Sachan, S.; Joshi, S.K. Zika virus—Emergence, evolution, pathology, diagnosis, and control: Current global scenario and future perspectives—A comprehensive review. Vet. Q. 2016, 36, 150–175. [Google Scholar] [CrossRef]
- Fabri, M.; Realegeno, S.E.; Jo, E.K.; Modlin, R.L. Role of autophagy in the host response to microbial infection and potential for therapy. Curr. Opin. Immunol. 2011, 23, 65–70. [Google Scholar] [CrossRef]
- Mitchell, G.; Cheng, M.I.; Chen, C.; Nguyen, B.N.; Whiteley, A.T.; Kianian, S.; Cox, J.S.; Green, D.R.; McDonald, K.L.; Portnoy, D.A. Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E210–E217. [Google Scholar] [CrossRef]
- Singh, V.; Finke-Isami, J.; Hopper-Chidlaw, A.C.; Schwerk, P.; Thompson, A.; Tedin, K. Salmonella co-opts host cell chaperone-mediated autophagy for intracellular growth. J. Biol. Chem. 2016, 292, 1847–1864. [Google Scholar] [CrossRef]
- Roehrich, A.D.; Bordignon, E.; Mode, S.; Shen, D.K.; Liu, X.; Pain, M.; Murillo, I.; Martinez-Argudo, I.; Sessions, R.B.; Blocker, A.J. Steps for Shigella gatekeeper protein mxic function in hierarchical type III secretion regulation. J. Biol. Chem. 2017, 292, 1705–1723. [Google Scholar] [CrossRef]
- Nakajima, S.; Aikawa, C.; Nozawa, T.; Minowa-Nozawa, A.; Toh, H.; Nakagawa, I. Bcl-xL affects group A Streptococcus-induced autophagy directly by inhibiting fusion between autophagosomes and lysosomes, and indirectly by inhibiting bacterial internalization via interaction with Beclin 1-UVRAG. PLoS ONE 2017, 12, e0170138. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, A.I.; Brady, N.R.; Bartenschlager, R. Divergent roles of autophagy in virus infection. Cells 2013, 2, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Mahima Rahal, A.; Deb, R.; Latheef, S.K.; Samad, H.A.; Tiwari, R.; Verma, A.K.; Kumar, A.; Dhama, K. Immunomodulatory and therapeutic potentials of herbal, traditional/indigenous and ethnoveterinary medicines. Pak. J. Biol. Sci. 2012, 15, 754–774. [Google Scholar]
- Dhama, K.; Chakraborty, S.; Mahima Wani, M.Y.; Verma, A.K.; Deb, R.; Tiwari, R.; Kapoor, S. Novel and emerging therapies safeguarding health of humans and their companion animals: A review. Pak. J. Biol. Sci. 2013, 16, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Chakraborty, S.; Wani, M.Y.; Tiwari, R.; Barathidasan, R. Cytokine therapy for combating animal and human diseases—A review. Res. Opin. Anim. Vet. Sci. 2013, 3, 195–208. [Google Scholar]
- Dhama, K.; Chakraborty, S.; Tiwari, R.; Verma, A.K.; Saminathan, M.; Amarpal, A.; Malik, Y.S.; Nikousefat, Z.; Javdani, M.; Khan, R.U. A concept paper on novel technologies boosting production and safeguarding health of humans and animals. Res. Opin. Anim. Vet. Sci. 2014, 4, 353–370. [Google Scholar]
- Dhama, K.; Saminathan, M.; Jacob, S.S.; Singh, M.; Karthik, K.; Amarpal, A.; Tiwari, R.; Sunkara, L.T.; Malik, Y.S.; Singh, R.K. Effect of immunomodulation and immunomodulatory agents on health with some bioactive principles, modes of action and potent biomedical applications. Int. J. Pharmacol. 2015, 11, 253–290. [Google Scholar] [CrossRef]
- Dhama, K.; Latheef, S.K.; Munjal, A.K.; Khandia, R.; Samad, H.A.; Iqbal, H.M.N.; Joshi, S.K. Probiotics in curing allergic and inflammatory conditions—Research progress and futuristic vision. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Sharma, K.; Jeena, L.M.; Kumar, N.; Sircar, S.; Rajak, K.K.; Dhama, K. Toll-like receptors: The innate immune receptors with ingenious anti-viral paradigm. South Asian J. Exp. Biol. 2013, 3, 207–213. [Google Scholar]
- Iqbal, H.M.; Villalba, A.; Khandia, R.; Munjal, A.; Dhama, K. Recent trends in nanotechnology-based drugs and formulations for targeted therapeutic delivery. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Sachan, S.; Munjal, A.K.; Tiwari, R.; Dhama, K. Tumor Homing Peptides: Promising Futuristic Hope for Cancer Therapy; Patent eBook Series Topics in Anti-Cancer Research; Bentham Science Publishers: Sharjah, UAE, 2016; Volume 5, pp. 43–86. ISSN 2213-3585. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A.K.; Iqbal, H.M.; Dhama, K. Heat shock proteins: Therapeutic perspectives in inflammatory disorders. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 10, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D.; Kimmelman, A.C. Mechanisms of selective autophagy in normal physiology and cancer. J. Mol. Biol. 2016, 428 Pt A, 1659–1680. [Google Scholar]
- Khambu, B.; Yan, S.; Huda, N.; Liu, G.; Yin, X.M. Homeostatic role of autophagy in hepatocytes. Semin. Liver Dis. 2018, 38, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Driver, J.P.; Van Kaer, L. The role of autophagy in iNKT cell development. Front. Immunol. 2018, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Kuchitsu, Y.; Homma, Y.; Fujita, N.; Fukuda, M. Rab7 knockout unveils regulated autolysosome maturation induced by glutamine starvation. J. Cell Sci. 2018, 131, jcs215442. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Rezabakhsh, A.; Pezeshkian, M.; Rahbarghazi, R.; Nouri, M. Distinct role of autophagy on angiogenesis: Highlights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res. Ther. 2018, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Moras, M.; Lefevre, S.D.; Ostuni, M.A. From erythroblasts to mature red blood cells: Organelle clearance in mammals. Front. Physiol. 2017, 8, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Nguyen, H.Q.; Hwang, J.S.; Zada, S.; Lai, T.H.; Kang, S.S.; Kim, D.R. Systematic characterization of autophagy-related genes during the adipocyte differentiation using public-access data. Oncotarget 2018, 9, 15526–15541. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S. Biological roles of alternative autophagy. Mol. Cells 2018, 41, 50–54. [Google Scholar] [PubMed]
- Mortensen, M.; Ferguson, D.J.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA. 2010, 107, 832–837. [Google Scholar] [CrossRef]
- Goldman, S.J.; Zhang, Y.; Jin, S. Autophagic degradation of mitochondria in white adipose tissue differentiation. Antioxid. Redox Signal. 2011, 14, 1971–1978. [Google Scholar] [CrossRef]
- Datan, E.; Roy, S.G.; Germain, G.; Zali, N.; McLean, J.E.; Golshan, G. Dengue-induced autophagy, virus replication and protection from cell death require ER stress (PERK) pathway activation. Cell Death Dis. 2016, 7, e2127. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Sanchez, M.J.; Karabiyik, C.; et al. Autophagy and neurodegeneration, pathogenic mechanisms and therapeutic opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Papandreou, M.E.; Tavernarakis, N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2014, 22, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Dawson, P.W.; Richardson, C.D. Viral interactions with macroautophagy, a double-edged sword. Virology 2010, 402, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shoji-Kawata, S.; Levine, B. Autophagy, antiviral immunity, and viral countermeasures. Biochim. Biophys. Acta 2009, 1793, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, M.; Di Rienzo, M.; Piacentini, M.; Fimia, G.M. Emerging mechanisms in initiating and terminating autophagy. Trends Biomed. Sci. 2017, 42, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Goronzy, J.J.; Weyand, C.M. Autophagy in autoimmune disease. J. Mol. Med. (Berl.) 2015, 93, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Anding, A.L.; Baehrecke, E.H. Cleaning house, Selective autophagy of organelles. Dev. Cell. 2017, 41, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Sabir, N.; Mangi, M.H.; Yang, L. p62-Keap1-NRF2-ARE pathway: A contentious player for selective targeting of autophagy, oxidative stress and mitochondrial dysfunction in prion diseases. Front. Mol. Neurosci. 2018, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.; Jung, J.U. When autophagy meets viruses: A double-edged sword with functions in defense and offense. Semin. Immunopathol. 2010, 32, 323–341. [Google Scholar] [CrossRef]
- Zhu, H.; Tannous, P.; Johnstone, J.L.; Kong, Y.; Shelton, J.M.; Richardson, J.A.; Le, V.; Levine, B.; Rothermel, B.A.; Hill, J.A. Cardiac autophagy is a maladaptive response to hemodynamic stress. J. Clin. Investig. 2007, 117, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, D.; Yu, J.; Dong, H.; Zhang, J.; Yang, S. Targeting autophagy in cancer stem cells as an anticancer therapy. Cancer Lett. 2017, 393, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.R.I.; Bhattacharya, T.; Newton, I.L.G.; Hardy, R.W. Conflict in the intracellular lives of endosymbionts and viruses: A mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses 2018, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Gkikas, I.; Palikaras, K.; Tavernarakis, N. The role of mitophagy in innate immunity. Front. Immunol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- You, L.; Jin, S.; Zhu, L.; Qian, W. Autophagy, autophagy-associated adaptive immune responses and its role in hematologic malignancies. Oncotarget 2016, 8, 12374–12388. [Google Scholar] [CrossRef]
- Bussi, C.; Ramos, J.M.; Arroyo, D.S.; Gaviglio, E.A.; Gallea, J.I.; Wang, J.M.; Celej, M.S.; Iribarren, P. Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death. Sci. Rep. 2017, 7, 43153. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro. J. Neurochem. 2017, 142, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Kissová, I.; Salin, B.; Schaeffer, J.; Bhatia, S.; Manon, S.; Camougrand, N. Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy 2007, 3, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, G.; Martens, S. Mechanisms of selective autophagy. J. Mol. Biol. 2016, 428 Pt A, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.; Gaffke, L.; Cyske, Z.; Puchalski, M.; Rintz, E.; Bartkowski, M.; Osiadły, M.; Pierzynowski, M.; Mantej, J.; Piotrowska, E.; et al. Autophagy stimulation as a promising approach in treatment of neurodegenerative diseases. Metab. Brain Dis. 2018, 33, 989–1008. [Google Scholar] [CrossRef] [PubMed]
- Moulis, M.; Vindis, C. Autophagy in metabolic age-related human diseases. Cells 2018, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Yao, Y.; Liu, J.; Yu, Z.; Cheung, S.; Xie, A.; Liang, X.; Bi, X. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1−/− mouse brain. Am. J. Pathol. 2007, 171, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.Y.; Reid, P.C.; Sugii, S.; Ohgami, N.; Cruz, J.C.; Chang, C.C. Niemann-Pick type C disease and intracellular cholesterol trafficking. J. Biol. Chem. 2005, 280, 20917–20920. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006, 580, 5518–5524. [Google Scholar] [CrossRef] [PubMed]
- Battu, S.; Afroz, S.; Giddaluru, J.; Naz, S.; Huang, W.; Khumukcham, S.S.; Khan, R.A.; Bhat, S.Y.; Qureshi, I.A.; Manavathi, B.; et al. Amino acid starvation sensing dampens IL-1β production by activating riboclustering and autophagy. PLoS Biol. 2018, 16, e2005317. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E. Sex differences in autophagy contribute to female vulnerability in Alzheimer’s disease. Front. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef]
- Joy, S.; Thirunavukkarasu, L.; Agrawal, P.; Singh, A.; Sagar, B.; Manjithaya, R.; Surolia, N. Basal and starvation-induced autophagy mediates parasite survival during intraerythrocytic stages of Plasmodium falciparum. Cell Death Discov. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Arenas, F.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci. 2017, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Horbinski, C.; Guo, F.; Watkins, S.; Uchiyama, Y.; Chu, C.T. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 2007, 170, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, P.; Zhang, J. Crosstalk between autophagy and apoptosis, potential and emerging therapeutic targets for cardiac diseases. Int. J. Mol. Sci. 2016, 17, 332. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Sun, S.J.; Gao, J.; Sun, F.B. Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cancer cells. Oncol. Lett. 2016, 12, 5059–5067. [Google Scholar] [CrossRef]
- Hurley, J.H.; Young, L.N. Mechanisms of autophagy initiation. Ann. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Frankel, L.B.; Lubas, M.; Lund, A.H. Emerging connections between RNA and autophagy. Autophagy 2016, 13, 3–23. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Li, M.; Zhang, Q. Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J. Cell Physiol. 2017, 232, 2977–2984. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Wada, K.; Kabuta, T. Lysosomal degradation of intracellular nucleic acids-multiple autophagic pathways. J. Biochem. 2017, 161, 145–154. [Google Scholar] [CrossRef]
- Yin, Z.; Klionsky, D.J. HS1BP3 provides a novel mechanism of negative autophagy regulation through membrane lipids. Autophagy 2017, 13, 779–780. [Google Scholar] [CrossRef]
- Kumsta, C.; Hansen, M. Hormetic heat shock and HSF-1 overexpression improve C. elegans survival and proteostasis by inducing autophagy. Autophagy 2017, 13, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, H.; Kim, K.I.; Baek, S.H. Epigenetic and transcriptional regulation of autophagy. Autophagy 2016, 12, 2248–2249. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Mendoza, L.E.; Westerling, T.; Libby, E.; Marusyk, A.; Cato, L.; Cassani, R.; Cameron, L.A.; Ficarro, S.B.; Marto, J.A.; Klawitter, J.; et al. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2186–E2194. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.A.; Teis, D. Lysosomal signaling in control of degradation pathways. Curr. Opin. Cell Biol. 2016, 39, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and measuring autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kanki, T. Mechanisms and physiological roles of mitophagy in yeast. Mol. Cells 2018, 41, 35–44. [Google Scholar] [PubMed]
- Isakson, P.; Holland, P.; Simonsen, A. The role of Alfy in selective autophagy. Cell Death Differ. 2013, 20, 12–20. [Google Scholar] [CrossRef]
- Tallóczy, Z.; Jiang, W.; Virgin, H.W.; Leib, D.A.; Scheuner, D.; Kaufman, R.J.; Eskelinen, E.L.; Levine, B. Regulation of starvation-and virus-induced autophagy by the eif2α kinase signaling pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Okamoto, K.; Kondo-Okamoto, N.; Ohsumi, Y. Mitochondria-anchored receptor Atg32 mediates degradation of mitochondria via selective autophagy. Dev. Cell 2009, 17, 87–97. [Google Scholar] [CrossRef]
- Ding, W.X.; Yin, X.M. Mitophagy: Mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Kanki, T.; Wang, K.; Cao, Y.; Baba, M.; Klionsky, D.J. Atg32 is a mitochondrial protein that confers selectivity during mitophagy. Dev. Cell 2009, 17, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Kirkin, V.; McEwan, D.G.; Zhang, J.; Wild, P.; Rozenknop, A. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Dikic, I. Autophagy receptors in developmental clearance of mitochondria. Autophagy 2011, 7, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, M.U.; Veenhuis, M.; Klionsky, D.J. Peroxisome degradation in Saccharomyces cerevisiae is dependent on machinery of macroautophagy and the Cvt pathway. J. Cell Sci. 1999, 112, 4079–4087. [Google Scholar]
- Nazarko, T.Y.; Farré, J.C.; Subramani, S. Peroxisome size provides insights into the function of autophagy-related proteins. Mol. Biol. Cell 2009, 20, 3828–3939. [Google Scholar] [CrossRef]
- Yamasaki, A.; Noda, N.N. Structural biology of the Cvt pathway. J. Mol. Biol. 2017, 429, 531–542. [Google Scholar] [CrossRef]
- Molino, D.; Zemirli, N.; Codogno, P.; Morel, E. The journey of the autophagosome through mammalian cell organelles and membranes. J. Mol. Biol. 2016, 429, 497–514. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Yarana, C.; St Clair, D.K. Chemotherapy-induced tissue injury, an insight into the role of extracellular vesicles-mediated oxidative stress responses. Antioxidants 2017, 6, 75. [Google Scholar] [CrossRef]
- Dice, J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 1990, 15, 305–309. [Google Scholar] [CrossRef]
- Tasset, I.; Cuervo, A.M. Role of chaperone-mediated autophagy in metabolism. FEBS J. 2016, 283, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Dice, J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ye, Y. The roles of endo-lysosomes in unconventional protein secretion. Cells 2018, 7, E198. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 2009, 452, 297–324. [Google Scholar] [PubMed]
- Orvedahl, A.; Macpherson, S.; Sumpter, R.; Tallóczy, Z.; Zou, Z.; Levine, B. Autophagy protects against sindbis virus infection of the central nervous system. Cell Host Microbe 2010, 7, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Egger, D.; Wölk, B.; Gosert, R.; Bianchi, L.; Blum, H.E.; Moradpour, D.; Bienz, K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 2002, 76, 5974–5984. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lei, H.Y.; Liu, M.T.; Wang, J.R.; Chen, S.H.; Jiang-Shieh, Y.F.; Lin, Y.S.; Yeh, T.M.; Liu, C.C.; Liu, H.S. Autophagic machinery activated by dengue virus enhances virus replication. Virology 2008, 374, 240–248. [Google Scholar] [CrossRef]
- Jackson, W.T.; Giddings, T.H., Jr.; Taylor, M.P.; Mulinyawe, S.; Rabinovitch, M.; Kopito, R.R.; Kirkegaard, K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005, 3, e156. [Google Scholar] [CrossRef]
- Balvers, R.; Jiang, H.; Piya, S.; Gomez-Manzano, C.; Fueyo, J. Adenovirus, autophagy and lysis: Ecstasies and agonies. Future Virol. 2011, 6, 1161–1164. [Google Scholar] [CrossRef]
- Yang, Z.; Zhong, L.; Zhong, S.; Xian, R.; Yuan, B. Hypoxia induces microglia autophagy and neural inflammation injury in focal cerebral ischemia model. Exp. Mol. Pathol. 2015, 98, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jacobi, A.; Vater, C.; Zou, L.; Zou, X.; Stiehler, M. Icariin promotes angiogenic differentiation and prevents oxidative stress-induced autophagy in endothelial progenitor cells. Stem Cells 2015, 33, 1863–1877. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Shi, H.; Ren, Y.; Guo, F.; Ni, W.; Qiao, J.; Wang, P.; Zhang, H.; Chen, C. Bovine viral diarrhea virus infection induces autophagy in MDBK cells. J. Microbiol. 2014, 52, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Sung, M.S.; Lee, E.G.; Yoo, H.G.; Cheon, Y.H.; Chae, H.J.; Yoo, W.H. A pathogenic role for ER stress-induced autophagy and ER chaperone GRP78/BiP in T lymphocyte systemic lupus erythematosus. J. Leukoc. Biol. 2015, 97, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Yoshino, K.; Kondo, C.; Kawamata, T.; Oshiro, N.; Yonezawa, K.; Ohsumi, Y. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol. 2010, 30, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Mizushima, N. Role of ULK-FIP200 complex in mammalian autophagy: FIP200, a counterpart of yeast Atg17? Autophagy 2009, 5, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 2010, 22, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.G.; Hurley, J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell Biol. 2016, 39, 61–68. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy, renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Mercer, T.J.; Gubas, A.; Tooze, S.A. A molecular perspective of mammalian autophagosome biogenesis. J. Biol. Chem. 2018, 293, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; McPhee, C.K.; Zheng, L.; Mardones, G.A.; Rong, Y.; Peng, J.; Mi, N.; Zhao, Y.; Liu, Z.; Wan, F.; et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010, 465, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Gluschko, A.; Schramm, M. LC3-associated phagocytosis—The highway to hell for phagocytosed microbes. Semin. Cell Dev. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bader, C.A.; Shandala, T.; Ng, Y.S.; Johnson, I.R.; Brooks, D.A. Atg9 is required for intraluminal vesicles in amphisomes and autolysosomes. Biol. Open 2015, 4, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Griffith, J.; Rieter, E.; Krishnappa, L.; Klionsky, D.J.; Reggiori, F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J. Cell Biol. 2010, 190, 1005–1022. [Google Scholar] [CrossRef]
- Maron, B.J.; Roberts, W.C.; Arad, M.; Haas, T.S.; Spirito, P.; Wright, G.B.; Almquist, A.K.; Baffa, J.M.; Saul, J.P.; Ho, C.Y.; et al. Clinical outcome and phenotypic expression in LAMP2 cardiomyopathy. JAMA 2009, 301, 1253–1259. [Google Scholar] [CrossRef]
- Holland, P.; Knævelsrud, H.; Søreng, K.; Mathai, B.J.; Lystad, A.H.; Pankiv, S.; Bjørndal, G.T.; Schultz, S.W.; Lobert, V.H.; Chan, R.B.; et al. HS1BP3 negatively regulates autophagy by modulation of phosphatidic acid levels. Nat. Commun. 2016, 7, 13889. [Google Scholar] [CrossRef]
- Wen, H.; Zhan, L.; Chen, S.; Long, L.; Xu, E. Rab7 may be a novel therapeutic target for neurologic diseases as a key regulator in autophagy. J. Neurosci. Res. 2017, 95, 1993–2004. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, F.; Cui, L.; Zhou, B.; Guo, Y. Up-regulation of the active form of small GTPase Rab13 promotes macroautophagy in vascular endothelial cells. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 613–624. [Google Scholar] [CrossRef]
- Behrends, C.; Sowa, M.E.; Gygi, S.P.; Harper, J.W. Network organization of the human autophagy system. Nature 2010, 466, 68–76. [Google Scholar] [CrossRef] [PubMed]
- McKnight, N.C.; Jefferies, H.B.J.; Alemu, E.A.; Saunders, R.E.; Howell, M.; Johansen, T.; Tooze, S.A. Genome-wide siRNA screen reveals amino acid starvation-induced autophagy requires SCOC and WAC. EMBO J. 2012, 31, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, X.; Zhang, P.; Qi, L.; Liang, Q.; Zhang, X.; Huang, J.; Fang, B.; Hou, W.; Han, J.; et al. Genome-wide screen identifies signalling pathways that regulate autophagy during Caenorhabditis elegans development. EMBO Rep. 2014, 15, 705–713. [Google Scholar] [PubMed]
- Liu, Y.; Levine, B. Autosis and autophagic cell death: The dark side of autophagy. Cell Death Differ. 2015, 22, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M., Jr.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis is a Na+, K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef] [PubMed]
- Shoji-Kawata, S.; Sumpter, R.; Leveno, M.; Campbell, G.R.; Zou, Z.; Kinch, L.; Wilkins, A.D.; Sun, Q.; Pallauf, K.; MacDuff, D.; et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature 2013, 494, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pinedo, C.; Martin, S.J. Autosis, a new addition to the cell death Tower of Babel. Cell Death Dis. 2014, 5, e1319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puyal, J.; Ginet, V.; Clarke, P.G. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage, a challenge for neuroprotection. Prog. Neurobiol. 2013, 105, 24–48. [Google Scholar] [CrossRef]
- Kheloufi, M.; Boulanger, C.M.; Codogno, P.; Rautou, P.E. Autosis occurs in the liver of patients with severe anorexia nervosa. Hepatology 2015, 62, 657–658. [Google Scholar] [CrossRef]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef]
- Fitzwalter, B.E.; Thorburn, A. Recent insights into cell death and autophagy. FEBS J. 2015, 282, 4279–4288. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998, 72, 8586–8596. [Google Scholar] [PubMed]
- Kvansakul, M.; Caria, S.; Hinds, M.G. The Bcl-2 family in host-virus interactions. Viruses 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.B.; Dhamija, S. Beclin 1 phosphorylation—At the center of autophagy regulation. Front. Cell Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Mrakovcic, M.; Fröhlich, L.F. P53-mediated molecular control of autophagy in tumor cells. Biomolecules 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimorim, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.D.; Eisenstein, M.; Ber, Y.; Bialik, S.; Kimchi, A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell 2011, 44, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Gump, J.M.; Thorburn, A. Sorting cells for basal and induced autophagic flux by quantitative ratiometric flow cytometry. Autophagy 2014, 10, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, J.; Andrysik, Z.; Staskiewicz, L.; Gump, J.; Maycotte, P.; Oberst, A.; Green, D.R.; Espinosa, J.M.; Thorburn, A. Autophagy controls the kinetics and extent of mitochondrial apoptosis by regulating PUMA levels. Cell Rep. 2014, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nezis, I.P.; Shravage, B.V.; Sagona, A.P.; Johansen, T.; Baehrecke, E.H.; Stenmark, H. Autophagy as a trigger for cell death: Autophagic degradation of inhibitor of apoptosis dBruce controls DNA fragmentation during late oogenesis in Drosophila. Autophagy 2010, 6, 1214–1215. [Google Scholar] [CrossRef] [PubMed]
- Oami, T.; Watanabe, E.; Hatano, M.; Sunahara, S.; Fujimura, L.; Sakamoto, A.; Ito, C.; Toshimori, K.; Oda, S. Suppression of T cell autophagy results in decreased viability and function of T cells through accelerated apoptosis in a murine sepsis model. Crit. Care Med. 2017, 45, e77–e85. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, J.; Shi, S.; Wang, Q.; Saxton, B.; Xing, C.; Lin, Y. Combination of TRAIL and Chal-24 synergistically induces autophagy-mediated apoptosis in lung cancer cells. Clin. Cancer Res. 2017, 23 (Suppl. 1), A06. [Google Scholar]
- Bonapace, L.; Bornhauser, B.C.; Schmitz, M.; Cario, G.; Ziegler, U.; Niggli, F.K.; Schäfer, B.W.; Schrappe, M.; Stanulla, M.; Bourquin, J.P. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J. Clin. Investig. 2010, 120, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.D.; Leverrier, S.; Weist, B.M.; Newton, R.H.; Arechiga, A.F.; Luhrs, K.A.; Morrissette, N.S.; Walsh, C.M. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc. Natl. Acad. Sci. USA 2008, 105, 16677–16682. [Google Scholar] [CrossRef] [PubMed]
- Farkas, T.; Daugaard, M.; Jäättelä, M. Identification of small molecule inhibitors of phosphatidylinositol 3-kinase and autophagy. J. Biol. Chem. 2011, 286, 38904–38912. [Google Scholar] [CrossRef] [PubMed]
- Osborn, S.L.; Diehl, G.; Han, S.J.; Xue, L.; Kurd, N.; Hsieh, K.; Cado, D.; Robey, E.A.; Winoto, A. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 13034–13039. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; McGill, M.R.; Chao, X.; Woolbright, B.L.; Jaeschke, H.; Ding, W.X. Caspase inhibition prevents tumor necrosis factor-α-induced apoptosis and promotes necrotic cell death in mouse hepatocytes in vivo and in vitro. Am. J. Pathol. 2016, 186, 2623–2636. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, B.; Su, X.; Chen, G.; Li, Y.; Yu, L.; Li, L.; Wei, W. An ursolic acid derived small molecule triggers cancer cell death through hyperstimulation of macropinocytosis. J. Med. Chem. 2017, 60, 6638–6648. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Tan, H.L.; Huang, Q.; Sun, X.J.; Zhu, X.; Shen, H.M. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ. 2011, 18, 26–37. [Google Scholar] [CrossRef]
- Milne, J.C.; Denu, J.M. The sirtuin family, therapeutic targets to treat diseases of aging. Curr. Opin. Chem. Biol. 2008, 12, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Yang, J.; Zhang, P.; Ma, K.; Zhou, J.; Liao, W.; Zhu, W.G. Antineoplastic activity of the cytosolic FoxO1 results from autophagic cell death. Autophagy 2010, 6, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Chan, F.K. RIP3, a molecular switch for necrosis and inflammation. Genes Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Dai, Z.; Cai, C.; Zhang, X.; Li, A.; Zhang, H.; Yan, Y.; Lin, W.; Wu, Y.; Li, H.; et al. Knockout of Atg5 inhibits proliferation and promotes apoptosis of DF-1 cells. In Vitro Cell Dev. Biol. Anim. 2019, 55, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Goodall, M.L.; Fitzwalter, B.E.; Zahedi, S.; Wu, M.; Rodriguez, D.; Mulcahy-Levy, J.M.; Green, D.R.; Morgan, M.; Cramer, S.D.; Thorburn, A. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell 2016, 37, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, P.; Chioureas, D.; Baltatzis, G.; Fonseca, P.; Rodriguez, P.; Gogvadze, V.; Lennartsson, L.; Björklund, A.C.; Zhivotovsky, B.; Grandér, D.; et al. Sorafenib-induced defective autophagy promotes cell death by necroptosis. Oncotarget 2015, 6, 37066–37082. [Google Scholar] [CrossRef] [PubMed]
- Pajuelo, D.; Gonzalez-Juarbe, N.; Tak, U.; Sun, J.; Orihuela, C.J.; Niederweis, M. NAD+ depletion triggers macrophage necroptosis, a cell death pathway exploited by Mycobacterium tuberculosis. Cell Rep. 2018, 24, 429–440. [Google Scholar] [CrossRef]
- Caruso, L.B.; Martin, K.A.; Lauretti, E.; Hulse, M.; Siciliano, M.; Lupey-Green, L.N.; Abraham, A.; Skorski, T.; Tempera, I. Poly(ADP-ribose) polymerase 1, PARP1, modifies EZH2 and inhibits EZH2 histone methyltransferase activity after DNA damage. Oncotarget 2018, 9, 10585–10605. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. AMP-activated protein kinase, an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Cai, S.L.; Kim, J.; Nanez, A.; Sahin, M.; MacLean, K.H.; Inoki, K.; Guan, K.L.; Shen, J.; Person, M.D.; et al. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc. Natl. Acad. Sci. USA 2010, 107, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Brumell, J.H. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy 2006, 2, 156–158. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ogawa, M.; Hain, T.; Chakraborty, T.; Sasakawa, C. Listeria monocytogenes ActA is a key player in evading autophagic recognition. Autophagy 2009, 5, 1220–1221. [Google Scholar] [CrossRef] [PubMed]
- Krokowski, S.; Mostowy, S. Interactions between Shigella flexneri and the autophagy machinery. Front. Cell Infect. Microbiol. 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Amano, A.; Mizushima, N.; Yamamoto, A.; Yamaguchi, H.; Kamimoto, T.; Nara, A.; Funao, J.; Nakata, M.; Tsuda, K.; et al. Autophagy defends cells against invading group A Streptococcus. Science 2004, 306, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Ellison, L.K.; Ramjeet, M.; Travassos, L.H.; Jones, N.L.; Girardin, S.E.; Philpott, D.J. The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity 2013, 39, 858–873. [Google Scholar] [CrossRef]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Jia, K.; Thomas, C.; Akbar, M.; Sun, Q.; Adams-Huet, B.; Gilpin, C.; Levine, B. Autophagy genes protect against Salmonella typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc. Natl. Acad. Sci. USA 2009, 106, 14564–14569. [Google Scholar] [CrossRef]
- Chen, H.D.; Kao, C.Y.; Liu, B.Y.; Huang, S.W.; Kuo, C.J.; Ruan, J.W.; Lin, Y.H.; Huang, C.R.; Chen, Y.H.; Wang, H.D.; et al. HLH-30/TFEB-mediated autophagy functions in a cell-autonomous manner for epithelium intrinsic cellular defense against bacterial pore-forming toxin in C. elegans. Autophagy 2017, 13, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, H.M.; Park, K.S.; Shin, D.M.; Kim, T.S.; Kim, Y.S.; Suh, H.W.; Kim, S.Y.; Kim, I.S.; Kim, J.M.; et al. MIR144* inhibits antimicrobial responses against Mycobacterium tuberculosis in human monocytes and macrophages by targeting the autophagy protein DRAM2. Autophagy 2017, 13, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zou, H.; Wang, B.; Sun, Q.; Fu, A.; Wang, Y.; Wang, Y.; Xu, X.; Li, W. Probiotic Bacillus amyloliquefaciens SC06 induces autophagy to protect against pathogens in macrophages. Front. Microbiol. 2017, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Kulp, S.K.; Soni, S.; Wang, D.; Gunn, J.S.; Schlesinger, L.S.; Chen, C.S. Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob. Agents Chemother. 2009, 53, 5236–5244. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Soni, S.; Kulp, S.K.; Curry, H.; Wang, D.; Gunn, J.S.; Schlesinger, L.S.; Chen, C.S. Eradication of intracellular Francisella tularensis in THP-1 human macrophages with a novel autophagy inducing agent. J. Biomed. Sci. 2009, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.R.; Spector, S.A. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012, 8, e1002689. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.; Brunton, J.; Kawula, T. The role of autophagy in intracellular pathogen nutrient acquisition. Front. Cell. Infect. Microbiol. 2015, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Xiong, Q.; Yamamoto, A.; Hayashi-Nishino, M.; Rikihisa, Y. Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc. Natl. Acad. Sci. USA 2012, 109, 20800–20807. [Google Scholar] [CrossRef]
- Niu, H.; Yamaguchi, M.; Rikihisa, Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell. Microbiol. 2008, 10, 593–605. [Google Scholar] [CrossRef]
- Moreau, K.; Lacas-Gervais, S.; Fujita, N.; Sebbane, F.; Yoshimori, T.; Simonet, M.; Lafont, F. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell. Microbiol. 2010, 12, 1108–1123. [Google Scholar] [CrossRef]
- Alem, F.; Yao, K.; Lane, D.; Calvert, V.; Petricoin, E.F.; Kramer, L.; Hale, M.L.; Bavari, S.; Panchal, R.G.; Hakami, R.M. Host response during Yersinia pestis infection of human bronchial epithelial cells involves negative regulation of autophagy and suggests a modulation of survival-related and cellular growth pathways. Front. Microbiol. 2015, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.L.; Colombo, M.I. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ. 2010, 17, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; López-Otin, C.; Virgin, H.W.; Celli, J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Dorn, B.R.; Dunn, W.A., Jr.; Progulske-Fox, A. Bacterial interactions with the autophagic pathway. Cell. Microbiol. 2002, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; López-Otín, C. Autophagy: Molecular mechanisms, physiological functions and relevance in human pathology. Cell. Mol. Life Sci. 2004, 61, 1439–1454. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Powers, T.R.; Knodler, L.A. Measurement of Salmonella enterica internalization and vacuole lysis in epithelial cells. Methods Mol. Biol. 2017, 1519, 285–296. [Google Scholar] [PubMed]
- Ganesan, R.; Hos, N.J.; Gutierrez, S.; Fischer, J.; Stepek, J.M.; Daglidu, E.; Krönke, M.; Robinson, N. Salmonella typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog. 2017, 13, e1006227. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Silva, B.J.; de Mattos Barbosa, M.G.; Andrade, P.R.; Ferreira, H.; da Costa Nery, J.A.; Coˆrte-Real, S.; da Silva, G.M.S.; Rosa, P.S.; Fabri, M.; Sarno, E.N.; et al. Autophagy is an innate mechanism associated with leprosy polarization. PLoS Pathog. 2017, 13, e1006103. [Google Scholar] [CrossRef]
- Deretic, V. Multiple regulatory and effector roles of autophagy in immunity. Curr. Opin. Immunol. 2009, 21, 53–62. [Google Scholar] [CrossRef]
- Kudchodkar, S.B.; Levine, B. Viruses and autophagy. Rev. Med. Virol. 2009, 19, 359–378. [Google Scholar] [CrossRef]
- Paul, P.; Münz, C. Autophagy and mammalian viruses: Roles in immune response, viral replication, and beyond. Adv. Virus Res. 2016, 95, 149–195. [Google Scholar] [CrossRef]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippé, R. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Milosevic, S.; Behrends, U.; Jaffee, E.M.; Pardoll, D.M.; Bornkamm, G.W.; Mautner, J. Major Histocompatibility complex class ii-restricted presentation of a cytosolic antigen by autophagy. Eur. J. Immunol. 2003, 33, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Pypaert, M.; Münz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 2007, 26, 79–92. [Google Scholar] [CrossRef]
- Shelly, S.; Lukinova, N.; Bambina, S.; Berman, A.; Cherry, S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 2009, 30, 588–598. [Google Scholar] [CrossRef]
- Nakamoto, M.; Moy, R.H.; Xu, J.; Bambina, S.; Yasunaga, A.; Shelly, S.S.; Gold, B.; Cherry, S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 2012, 36, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.H.; Gold, B.; Molleston, J.M.; Schad, V.; Yanger, K.; Salzano, M.V.; Yagi, Y.; Fitzgerald, K.A.; Stanger, B.Z.; Soldan, S.S. Antiviral autophagy restricts rift valley fever virus infection and is conserved from flies to mammals. Immunity 2014, 40, 51–65. [Google Scholar] [CrossRef]
- Owczarczyk, A.B.; Schaller, M.A.; Reed, M.; Rasky, A.J.; Lombard, D.B.; Lukacs, N.W. Sirtuin 1 regulates dendritic cell activation and autophagy during respiratory syncytial virus–induced immune responses. J Immunol. 2015, 195, 1637–1646. [Google Scholar] [CrossRef]
- Needs, S.; Kaas, S.; Horne, T.; Alonzi, D.; Allman, S. Stimulation of autophagy by salicylamide derivatives-implications for viral infection. In Proceedings of the Frankfurt Conference on Ubiquitin and Autophagy “Quality Control in life Process”, Frankfurt am Main, Germany, 4–7 July 2016. [Google Scholar]
- Fan, X.; Han, S.; Yan, D.; Gao, Y.; Wei, Y.; Liu, X.; Liao, Y.; Guo, H.; Sun, S. Foot-and-mouth disease virus infection suppresses autophagy and NF-κB antiviral responses via degradation of ATG5-ATG12 by 3Cpro. Cell Death Dis. 2017, 8, e2561. [Google Scholar] [CrossRef]
- Shintani, T.; Klionsky, D.J. Autophagy in health and disease: A double-edged sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Li, C.; Fu, X.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Li, N. Autophagy promoted infectious kidney and spleen necrosis virus replication and decreased infectious virus yields in CPB cell line. Fish Shellfish Immunol. 2017, 60, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Monastyrska, I.; Verheije, M.H.; Calì, T.; Ulasli, M.; Bianchi, S.; Bernasconi, R.; De Haan, C.A.; Molinari, M. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe 2010, 7, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Zhang, J.; Si, X.; Gao, G.; Mao, I.; Mcmanus, B.M.; Luo, H. Autophagosome supports coxsackievirus B3 replication in host cells. J. Virol. 2008, 82, 9143–9153. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.P.; Kirkegaard, K. Modification of cellular autophagy protein LC3 by poliovirus. J. Virol. 2007, 81, 12543–12553. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Choi, J.; Wakita, T.; Yen, T.; Ou, J.H.J. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 2008, 48, 1054–1061. [Google Scholar]
- Ke, P.Y.; Chen, S.S.L. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Investig. 2011, 121, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Raychoudhuri, A.; Steele, R.; Ray, R.; Ray, R.B. Knockdown of autophagy enhances the innate immune response in hepatitis C virus–infected hepatocytes. Hepatology 2011, 53, 406–414. [Google Scholar] [CrossRef]
- Gannagé, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Rämer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G.; et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef]
- Rossman, J.S.; Lamb, R.A. Autophagy, apoptosis, and the influenza virus M2 protein. Cell Host Microbe 2009, 6, 299–300. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, X.; Liu, D.; Fan, Z.; Hu, X.; Yan, J.; Wang, M.; Gao, G.F. Autophagy is involved in influenza a virus replication. Autophagy 2009, 5, 321–328. [Google Scholar] [CrossRef]
- Pissawong, T.; Maneewatch, S.; Thueng-in, K.; Srimanote, P.; Dong-din-on, F.; Thanongsaksrikul, J.; Songserm, T.; Tongtawe, P.; Bangphoomi, K.; Chaicumpa, W. Human monoclonal ScFv that bind to different functional domains of M2 and inhibit H5N1 influenza virus replication. Virol. J. 2013, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.D.; Johnsen, I.B.; Stiberg, K.A.; Sherstova, T.; Wakita, T.; Richard, G.M.; Kandasamy, R.K.; Meurs, E.F.; Anthonsen, M.W. Hepatitis C virus triggers Golgi fragmentation and autophagy through the immunity-related GTPase M. Proc. Natl. Acad. Sci. USA 2017, 114, E3462–E3471. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhou, Z.; Jiang, K.; Yu, S.; Jia, L.; Wu, Y.; Liu, Y.; Meng, S.; Ding, C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch. Virol. 2012, 157, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, S.; Ding, N.; Meng, C.; Meng, S.; Zhang, S.; Zhan, Y.; Qiu, X.; Tan, L.; Chen, H. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J. Virol. 2014, 88, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Spector, S.A. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS 2008, 22, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kyei, G.B.; Dinkins, C.; Davis, A.S.; Roberts, E.; Singh, S.B.; Dong, C.; Wu, L.; Kominami, E.; Ueno, T.; Yamamoto, A. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009, 186, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Saribas, A.S.; Khalili, K.; Sariyer, I.K. Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle 2015, 14, 2899–2904. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zheng, Y.H.; He, Y.; Chen, Z.; He, B. The role of autophagy in THP-1 macrophages resistance to HIV-vpr-induced apoptosis. Exp. Cell Res. 2017, 351, 68–73. [Google Scholar] [CrossRef]
- Taylor, G.S.; Mautner, J.; Münz, C. Autophagy in herpesvirus immune control and immune escape. Herpesviridae 2011, 2, 2. [Google Scholar] [CrossRef]
- Liang, Q.; Chang, B.; Brulois, K.F.; Castro, K.; Min, C.K.; Rodgers, M.A.; Shi, M.; Ge, J.; Feng, P.; Oh, B.H.; et al. Kaposi’s sarcoma-associated herpesvirus K7 modulates Rubicon-mediated inhibition of autophagosome maturation. J. Virol. 2013, 87, 12499–12503. [Google Scholar] [CrossRef]
- Su, M.; Mei, Y.; Sanishvili, R.; Levine, B.; Colbert, C.L.; Sinha, S. Targeting γ-herpesvirus 68 Bcl-2-ediated down-regulation of autophagy. J. Biol. Chem. 2014, 289, 8029–8040. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.L.; Jackson, W.T. Intracellular vesicle acidification promotes maturation of infectious poliovirus particles. PLoS Pathog. 2012, 8, e1003046. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, Z.; Zhang, L.; Zhao, C.; Zhao, X.; Gu, X.; Chen, H. Autophagy knocked down by high-risk HPV infection and uterine cervical carcinogenesis. Int. J. Clin. Exp. Med. 2015, 8, 10304. [Google Scholar] [PubMed]
- Mclean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 2011, 286, 22147–22159. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.J.; Britton, P. Involvement of autophagy in coronavirus replication. Viruses 2012, 4, 3440–3451. [Google Scholar] [CrossRef] [PubMed]
- Cottam, E.M.; Maier, H.J.; Manifava, M.; Vaux, L.C.; Chandra-Schoenfelder, P.; Gerner, W.; Britton, P.; Ktistakis, N.T.; Wileman, T. Coronavirus Nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy 2011, 7, 1335–1347. [Google Scholar] [CrossRef]
- O’donnell, V.; Pacheco, J.M.; Larocco, M.; Burrage, T.; Jackson, W.; Rodriguez, L.L.; Borca, M.V.; Baxt, B. Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication. Virology 2011, 410, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Devhare, P.; Sujijantarat, N.; Steele, R.; Kwon, Y.C.; Ray, R.; Ray, R.B. Knockdown of autophagy inhibits infectious hepatitis C virus release by the exosomal pathway. J. Virol. 2016, 90, 1387–1396. [Google Scholar] [CrossRef]
- Dash, S.; Chava, S.; Aydin, Y.; Chandra, P.K.; Ferraris, P.; Chen, W.; Balart, L.A.; Wu, T.; Garry, R.F. Hepatitis C virus infection induces autophagy as a prosurvival mechanism to alleviate hepatic ER-stress response. Viruses 2016, 8, 150. [Google Scholar] [CrossRef]
- Huang, X.; Yue, Y.; Li, D.; Zhao, Y.; Qiu, L.; Chen, J.; Pan, Y.; Xi, J.; Wang, X.; Sun, Q. Antibody-dependent enhancement of dengue virus infection inhibits rlr-mediated type-1 IFN-independent signalling through upregulation of cellular autophagy. Sci. Rep. 2016, 6, 22303. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Han, C.; Yao, S.; Qi, X.; Gao, Y.; Maier, H.J.; Britton, P.; Chen, L.; Zhang, L.; et al. Infectious bursal disease virus subverts autophagic vacuoles to promote viral maturation and release. J. Virol. 2017, 91, e01883-16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; White, E.J.; Gomez-Manzano, C.; Fueyo, J. Adenovirus’s last trick: You say lysis, we say autophagy. Autophagy 2008, 4, 118–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, B.; Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Da, L.; Mao, Y.; Li, Y.; Li, D.; Xu, Z.; Li, F.; Wang, Y.; Tiollais, P.; Li, T. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 2009, 49, 60–71. [Google Scholar] [CrossRef]
- Park, S.; Buck, M.D.; Desai, C.; Zhang, X.; Loginicheva, E.; Martinez, J.; Freeman, M.L.; Saitoh, T.; Akira, S.; Guan, J.L. Autophagy genes enhance murine gamma herpesvirus 68 reactivation from latency by preventing virus-induced systemic inflammation. Cell Host Microbe 2016, 19, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Orvedahl, A.; Alexander, D.; Talloczy, Z.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, C.; Shu, Y.; Ju, X.; Zou, Z.; Wang, H.; Rao, S.; Guo, F.; Liu, H.; Nan, W.; et al. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci. Signal. 2012, 5, Ra16. [Google Scholar] [CrossRef]
- Surviladze, Z.; Sterk, R.T.; Deharo, S.A.; Ozbun, M.A. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/akt/mtor pathway and inhibition of autophagy. J Virol. 2013, 87, 2508–2517. [Google Scholar] [CrossRef]
- Prentice, E.; Jerome, W.G.; Yoshimori, T.; Mizushima, N.; Denison, M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004, 279, 10136–10141. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Marinković, M.; Šprung, M.; Buljubašić, M.; Novak, I. Autophagy modulation in cancer: Current knowledge on action and therapy. Oxidative Med. Cell Longev. 2018, 2018, 8023821. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J., 3rd; et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Komatsu, M.; Hara, T.; Sakamoto, A.; Kishi, C.; Waguri, S.; Eishi, Y.; Hino, O.; Tanaka, K.; Mizushima, N. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011, 25, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Levine, B. Cell biology, autophagy and cancer. Nature 2007, 446, 745–747. [Google Scholar] [CrossRef]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, T.; Li, G.; Xu, C.; Xu, Z.; Zhang, J.; He, K.; Zheng, L.; Guan, Z.; Su, X.; et al. Lower Beclin 1 downregulates HER2 expression to enhance tamoxifen sensitivity and predicts a favorable outcome for ER positive breast cancer. Oncotarget 2016, 8, 52156–52177. [Google Scholar] [CrossRef]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.H.; Jung, J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006, 8, 688–699. [Google Scholar] [CrossRef]
- Jin, S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2006, 2, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Kongara, S.; Beaudoin, B.; Karp, C.M.; Bray, K.; Degenhardt, K.; Chen, G.; Jin, S.; White, E. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007, 21, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Tcherkezian, J.; Roux, P.P. The expanding role of mTOR in cancer cell growth and proliferation. Mutagenesis 2015, 30, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Scaravilli, F.; Easton, D.F.; Duden, R.; O’Kane, C.J.; et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, H.; Levine, A.J.; Jin, S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. USA 2005, 102, 8204–8209. [Google Scholar] [CrossRef] [PubMed]
- Tsuchihara, K.; Fujii, S.; Esumi, H. Autophagy and cancer, dynamism of the metabolism of tumor cells and tissues. Cancer Lett. 2009, 278, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.X.; Yang, Y.T.; Yu, S.; Li, Y.Z.; Wang, W.W.; Huang, J.; Xie, X.F.; Xiong, L.; Lei, S.; Peng, C. Pogostone induces autophagy and apoptosis involving PI3K/Akt/mTOR axis in human colorectal carcinoma HCT116 cells. J. Ethnopharmacol. 2017, 202, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Rocchi, S. New anti-cancer molecules targeting HSPA5/BIP to induce endoplasmic reticulum stress, autophagy and apoptosis. Autophagy 2017, 13, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Qi, H.; Chen, F.; He, J. Trichosanthin inhibits human ovarian cancer cells growth due to apoptosis and autophagy. Int. J. Clin. Exp. Med. 2017, 10, 5497–5503. [Google Scholar]
- Sato, K.; Tsuchihara, K.; Fujii, S.; Sugiyama, M.; Goya, T.; Atomi, Y.; Ueno, T.; Ochiai, A.; Esumi, H. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007, 67, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Ogier-Denis, E.; Houri, J.J.; Bauvy, C.; Codogno, P. Guanine nucleotide exchange on heterotrimeric Gi3 protein controls autophagic sequestration in HT-29 cells. J. Biol. Chem. 1996, 271, 28593–28600. [Google Scholar] [CrossRef] [PubMed]
- Schonewolf, C.A.; Mehta, M.; Schiff, D.; Wu, H.; Haffty, B.G.; Karantza, V.; Jabbour, S.K. Autophagy inhibition by chloroquine sensitizes HT-29 colorectal cancer cells to concurrent chemoradiation. World J. Gastrointest Oncol. 2014, 6, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, E.G.; Yoo, N.J.; Lee, S.H. Mutational and expressional analysis of BNIP3, a pro-apoptotic Bcl-2 member, in gastric carcinomas. APMIS 2007, 115, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Tylichová, Z.; Straková, N.; Vondráček, J.; Vaculová, A.H.; Kozubík, A.; Hofmanová, J. Activation of autophagy and PPARγ protect colon cancer cells against apoptosis induced by interactive effects of butyrate and DHA in a cell type-dependent manner: The role of cell differentiation. J. Nutr. Biochem. 2017, 39, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.S.; Ebbinghaus, M.; Muley, V.Y.; Zhou, Z.; Al-Saadi, K.R.; Pacyna-Gengelbach, M.; O’Sullivan, G.A.; Betz, H.; König, R.; Wang, Z.Q.; et al. Tumor suppression in mice lacking GABARAP, an Atg8/LC3 family member implicated in autophagy, is associated with alterations in cytokine secretion and cell death. Cell Death Dis. 2016, 7, e2205. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Chen, H.Y.; Mathew, R.; Fan, J.; Strohecker, A.M.; Karsli-Uzunbas, G. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011, 25, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Investig. 2008, 118, 3917–3929. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Karp, C.; Strohecker, A.M.; Guo, Y.; Mathew, R. Role of autophagy in suppression of inflammation and cancer. Curr. Opin. Cell Biol. 2010, 22, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy 2009, 5, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Narita, M.; Ferreira, M.; Kirschner, K.; Sadaie, M.; Darot, J.F.; Tavaré, S.; Arakawa, S.; Shimizu, S.; Watt, F.M.; et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009, 23, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Fu, J.; Xu, A.; Su, B.; Ren, Y.; Li, N.; Zhu, J.; Zhao, X.; Dai, R.; Cao, J.; et al. PSMD10/Gankyrin induces autophagy to promote tumor progression through cytoplasmic interaction with ATG7 and nuclear transactivation of ATG7 expression. Autophagy 2016, 12, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 2008, 4, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Viscomi, M.T.; D’Amelio, M.; Nobili, A.; Cavallucci, V.; Latini, L.; Bisicchia, E.; Sasso, V.; Molinari, M. Autophagy mechanisms for brain recovery. Keep it clean, keep it alive. In Neurobiological and Psychological Aspects of Brain Recovery; Springer: Cham, Switzerland, 2017; pp. 35–53. [Google Scholar]
- Girault, V.; Gilard, V.; Marguet, F.; Lesueur, C.; Hauchecorne, M.; Ramdani, Y.; Laquerrière, A.; Marret, S.; Jégou, S.; Gonzalez, B.J.; et al. Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis. 2017, 8, e2610. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fleming, A.; Ricketts, T.; Pavel, M.; Virgin, H.; Menzies, F.M.; Rubinsztein, D.C. Autophagy regulates Notch degradation and modulates stem cell development and neurogenesis. Nat Commun. 2016, 7, 10533. [Google Scholar] [CrossRef]
- Nixon, R.A.; Yang, D.S.; Lee, J.H. Neurodegenerative lysosomal disorders: A continuum from development to late age. Autophagy 2008, 4, 590–599. [Google Scholar] [CrossRef]
- Tooze, S.A.; Schiavo, G. Liaisons dangereuses, autophagy, neuronal survival and neurodegeneration. Curr. Opin. Neurobiol. 2008, 18, 504–515. [Google Scholar] [CrossRef]
- Wong, E.; Cuervo, A.M. Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 2010, 13, 805–811. [Google Scholar] [CrossRef]
- Sahni, S.; Merlot, A.M.; Krishan, S.; Jansson, P.J.; Richardson, D.R. Gene of the month: BECN1. J. Clin. Pathol. 2014, 67, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Cho, H.; Kim, E.K. Brain metabolism as a modulator of autophagy in neurodegeneration. Brain Res. 2016, 1649, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A.; Cataldo, A.M. Lysosomal system pathways, genes to neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [PubMed]
- Vogiatzi, T.; Xilouri, M.; Vekrellis, K.; Stefanis, L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 2008, 283, 23542–23556. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; She, H.; Gearing, M.; Colla, E.; Lee, M.; Shacka, J.J.; Mao, Z. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 2009, 323, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Davies, J.E.; Huang, Z.; Tunnacliffe, A.; Rubinsztein, D.C. Trehalose, a novel mTOR-independent autophagy enhancer accelerates the clearance of mutant huntingtin and alphasynuclein. J. Biol. Chem. 2007, 282, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Fimia, G.M.; Stoykova, A.; Romagnoli, A.; Giunta, L.; Di Bartolomeo, S.; Nardacci, R.; Corazzari, M.; Fuoco, C.; Ucar, A.; Schwartz, P.; et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007, 447, 1121–1125. [Google Scholar] [CrossRef]
- Webb, J.L.; Ravikumar, B.; Atkins, J.; Skepper, J.N.; Rubinsztein, D.C. Alpha synuclein is degraded by both autophagy and the proteasome. J. Biol. Chem. 2003, 278, 25009–25013. [Google Scholar] [CrossRef]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss- Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [PubMed]
- Dahmene, M.; Bérard, M.; Oueslati, A. Dissecting the molecular pathway involved in PLK2 kinase-mediated α-synuclein-selective autophagic degradation. J. Biol. Chem. 2017, 292, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, A.J.; Pallanck, L.J. PINK1/Parkin mitophagy and neurodegeneration—What do we really know in vivo? Curr. Opin. Genet. Dev. 2017, 44, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cesari, R.; Martin, E.S.; Calin, G.A.; Pentimalli, F.; Bichi, R.; McAdams, H.; Trapasso, F.; Drusco, A.; Shimizu, M.; Masciullo, V.; et al. Parkin, a gene implicated in autosomal recessive juvenile parkinsonism, is a candidate tumor suppressor gene on chromosome 6q25-q27. Proc. Natl. Acad. Sci. USA 2003, 100, 5956–5961. [Google Scholar] [CrossRef] [PubMed]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; de Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2010, 107, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Hart, A.C.; Levine, B. Autophagy genes protect against disease caused by polyglutamine expansion proteins in Caenorhabditis elegans. Autophagy 2007, 3, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Billes, V.; Komlós, M.; Hotzi, B.; Manzéger, A.; Tarnóci, A.; Papp, D.; Szikszai, F.; Szinyákovics, J.; Rácz, A.; et al. The small molecule AUTEN-99 (autophagy enhancer-99) prevents the progression of neurodegenerative symptoms. Sci. Rep. 2017, 7, 42014. [Google Scholar] [CrossRef]
- Bi, X.; Liao, G. Autophagic-lysosomal dysfunction and neurodegeneration in Niemann-Pick type C mice: Lipid starvation or indigestion? Autophagy 2007, 3, 646–648. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.J.; Zhang, H. Autophagy in immunity, implications in etiology of autoimmunity/autoinflammatory diseases. Autophagy 2012, 8, 1286–1299. [Google Scholar] [CrossRef]
- Wu, D.J.; Adamopoulos, I.E. Autophagy and autoimmunity. Clin. Immunol. 2017, 176, 55–62. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, J.H.; Byun, S. Modulation of autophagy for controlling immunity. Cells 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Murera, D.; Arbogast, F.; Fauny, J.D.; Muller, S.; Gros, F. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 2016, 23, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ 2019, 26, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ 2019, 26, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.J.; Simon, A.K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 2019, 19, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, H.; Kodali, S.; Wang, J. Regulation of B cell fate, survival, and function by mitochondria and autophagy. Mitochondrion 2018, 41, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Pengo, N.; Scolari, M.; Oliva, L.; Milan, E.; Mainoldi, F.; Raimondi, A.; Fagioli, C.; Merlini, A.; Mariani, E.; Pasqualetto, E.; et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013, 14, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Kashyap, A.K.; Jia, W.; He, Y.W.; Schaefer, B.C. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-κB. Immunity 2012, 36, 947–958. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Harris, J.; Hartman, M.; Roche, C.; Zeng, S.G.; O’Shea, A.; Sharp, F.A.; Lambe, E.M.; Creagh, E.M.; Golenbock, D.T.; Tschopp, J.; et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. J. Biol. Chem. 2011, 286, 9587–9597. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.J.; Kim, S.H.; Han, J.; Bae, J.; Kim, S.J.; Park, C.G.; Chun, T. IL-10 inhibits the starvation induced autophagy in macrophages via class I phosphatidylinositol 3-kinase (PI3K) pathway. Mol. Immunol. 2011, 48, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wu, H.; Chen, Y.; Zhang, J.; Zheng, M.; Chen, G.; Li, L.; Lu, Q. The therapeutic and pathogenic role of autophagy in autoimmune diseases. Front. Immunol. 2018, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. Systemic lupus erythematosus: Defective noncanonical autophagy in SLE-like disease. Nat. Rev. Rheumatol. 2016, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Twelves, S.; Farkas, K.; Setta-Kaffetzi, N.; Burden, A.D.; Gach, J.E.; Irvine, A.D.; Képíró, L.; Mockenhaupt, M.; Oon, H.H.; et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and up-regulating IL-36 production. J. Investig. Dermatol. 2016, 136, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lund, J.M.; Ramanathan, B.; Mizushima, N.; Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007, 315, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tu, Q.; Gong, J.; Zhao, L.; Liang, S.; He, Q. Autophagy activity and expression pattern of autophagy-related markers in the podocytes of patients with lupus nephritis: Association with pathological classification. Ren. Fail. 2019, 41, 294–302. [Google Scholar] [CrossRef]
- Wang, F.; Muller, S. Manipulating autophagic processes in autoimmune diseases: A special focus on modulating chaperone-mediated autophagy, an emerging therapeutic target. Front. Immunol. 2015, 6, 252. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.D.; Yan, F.; Jing, Y.Y.; Han, Z.P.; Sun, K.; Liang, L.; Hou, J.; Wei, L.X. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci. 2015, 5, 14. [Google Scholar] [CrossRef]
- Jiang, G.M.; Tan, Y.; Wang, H.; Peng, L.; Chen, H.T.; Meng, X.J.; Li, L.L.; Liu, Y.; Li, W.F.; Shan, H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 2019, 18, 17. [Google Scholar] [CrossRef]
- Rothermel, B.A.; Hill, J.A. Autophagy in load-induced heart disease. Circ. Res. 2008, 103, 1363–1369. [Google Scholar] [CrossRef]
- Gustafsson, A.B.; Gottlieb, R.A. Autophagy in ischemic heart disease. Circ. Res. 2009, 104, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Brunk, U.T. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc. Res. 2005, 68, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Pattison, J.S.; Robbins, J. Autophagy and proteotoxicity in cardiomyocytes. Autophagy 2011, 7, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Fu, J.; Tanji, K.; Yamada, T.; Shimojo, S.; Koori, T.; Mora, M.; Riggs, J.E.; Oh, S.J.; Koga, Y.; et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000, 406, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Guhde, G.; Suter, A.; Eskelinen, E.L.; Hartmann, D.; Lüllmann-Rauch, R.; Janssen, P.M.; Blanz, J.; von Figura, K.; Saftig, P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000, 406, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef]
- Huang, C.; Andres, A.M.; Ratliff, E.P.; Hernandez, G.; Lee, P.; Gottlieb, R.A. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS ONE 2011, 6, e20975. [Google Scholar] [CrossRef]
- Takemura, G.; Miyata, S.; Kawase, Y.; Okada, H.; Maruyama, R.; Fujiwara, H. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy 2006, 2, 212–214. [Google Scholar] [CrossRef]
- Akazawa, H.; Komazaki, S.; Shimomura, H.; Terasaki, F.; Zou, Y.; Takano, H.; Nagai, T.; Komuro, I. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J. Biol. Chem. 2004, 279, 41095–41103. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Hill, J.A. Therapeutic targeting of autophagy in cardiovascular disease. J. Mol. Cell Cardiol. 2015, 95, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, W.; Zhang, Y.; Zhang, F.; Huang, K. PARP1 promote autophagy in cardiomyocytes via modulating FoxO3a transcription. Cell Death Dis. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Hamaï, A.; Mehrpour, M. Autophagy and iron homeostasis. Med. Sci. 2017, 33, 260–267. [Google Scholar] [CrossRef][Green Version]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch. Neurosci. 2015, 2, e20078. [Google Scholar]
- Donovan, A.; Roy, C.N.; Andrews, N.C. The ins and outs of iron homeostasis. Physiology 2006, 21, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Gustafsson, B.; Brunk, U.T. Cell sensitivity to oxidative stress is influenced by ferritin autophagy. Free Radic. Biol. Med. 2011, 50, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Frennesson, C.; Gustafsson, T.; Brunk, U.T.; Nilsson, S.E.; Kurz, T. Autophagy of iron-binding proteins may contribute to the oxidative stress resistance of ARPE-19 cells. Exp. Eye Res. 2013, 116, 359–365. [Google Scholar] [CrossRef]
- Krishan, S.; Jansson, P.J.; Gutierrez, E.; Lane, D.J.; Richardson, D.; Sahni, S. Iron metabolism and autophagy: A poorly explored relationship that has important consequences for health and disease. Nagoya J. Med. Sci. 2015, 77, 1–6. [Google Scholar]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G.; et al. Selective vps34 inhibitor blocks autophagy and uncovers a role for ncoa4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef]
- Berndt, J.D. Ironing out the details with autophagy. Sci. Signal. 2014, 7, ec99. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies ncoa4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Wang, Z.P.; Yuan, Y.; Zhang, N.; Jin, Y.G.; Wan, C.X.; Tang, Q.Z. Role of autophagy in a model of obesity: A long-term high fat diet induces cardiac dysfunction. Mol. Med. Rep. 2018, 18, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Mukhopadhyay, M.; Bhattacharyya, M.; Karmakar, P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018, 17, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Fu, S.; Calay, E.S.; Hotamisligil, G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010, 11, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Ebato, C.; Uchida, T.; Arakawa, M.; Komatsu, M.; Ueno, T.; Komiya, K.; Azuma, K.; Hirose, T.; Tanaka, K.; Kominami, E.; et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chung, K.W.; Won Kim, J.; Kim, J.; Komatsu, M.; Tanaka, K.; Nguyen, Y.H.; Kang, T.M.; Yoon, K.H.; Kim, J.W.; et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008, 8, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Arsham, A.M.; Neufeld, T.P. A genetic screen in Drosophila reveals novel cytoprotective functions of the autophagy-lysosome pathway. PLoS ONE 2009, 4, e6068. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M.; Forrester, M.T.; Stamler, J.S. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. “NO” to autophagy: Fat does the trick for diabetes. Diabetes 2018, 67, 180–181. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, Z.; Orwig, A.; Chen, S.; Ding, W.X.; Xu, Y.; Kunz, R.C.; Lind, N.R.L.; Stamler, J.S.; Yang, L. S-Nitrosoglutathione reductase dysfunction contributes to obesity-associated hepatic insulin resistance via regulating autophagy. Diabetes 2018, 67, 193–207. [Google Scholar] [CrossRef]
- Subudhi, B.B.; Chattopadhyay, S.; Mishra, P.; Kumar, A. Current strategies for inhibition of Chikungunya infection. Viruses 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.H.; Nair, V.R.; Scharn, C.R.; Xavier, R.J.; Torrealba, J.R.; Shiloh, M.U.; Levine, B. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe. 2017, 21, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Stige, K.E.; Gjerde, I.O.; Houge, G.; Knappskog, P.M.; Tzoulis, C. Beta-propeller protein-associated neurodegeneration: A case report and review of the literature. Clin. Case Rep. 2018, 6, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pott, J.; Maloy, K.J. Epithelial autophagy controls chronic colitis by reducing TNF-induced apoptosis. Autophagy 2018, 14, 1460–1461. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Onodera, K.; Nakase, H. Role of autophagy in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 1944–1953. [Google Scholar] [CrossRef]
- Gorski, S.M.; Qadir, M.A. Inhibition of Autophagy Genes in Cancer Chemotherapy. U.S. Patent 8076308, 13 December 2011. [Google Scholar]
- Amaravadi, R.K.; Winkler, J. Dimeric Quinacrine Derivatives as Autophagy Inhibitors for Cancer Therapy. Patent WO 2016168721, 20 October 2016. [Google Scholar]
- Xu, H.; Zhang, H.; Lao, Y.; Wang, X.; Chen, K.; Yang, D.; Chen, S.; Lin, C.; Bian, Z.; Lu, A.; et al. Anti-Cervical Cancer Compound and Method of Use Thereof. U.S. Patent 9339488, 17 May 2016. [Google Scholar]
- Carew, J.; Phillips, J.G. Substituted Thioxanthenone Autophagy Inhibitors. U.S. Patent 9926326, 27 March 2018. [Google Scholar]
- Law, Y.K.; Wu, A.G.; Wong, K.W.; Liu, L. Autophagy enhancer for treatment of neurodegenerative diseases. U.S. Patent 9005677, 14 April 2015. [Google Scholar]
- D’amelio, M.; Molinari, M.; Viscomi, M.T.; Cecconi, F. Autophagy Enhancing Compounds, Peptides and Peptidomimetic Compounds for Use in the Treatment of Neuronal Diseases. Patent WO 2012076555, 14 June 2012. [Google Scholar]
- Jung, J.U.; Lee, J.S. Highly Potent Peptides to Control Cancer and Neurodegenerative Diseases. Patent WO 2010011952, 24 June 2010. [Google Scholar]
- Middleton, R.P.; Zanghi, B.M. Methods for Reducing Neurodegeneration. Patent EP2717695, 2 November 2016. [Google Scholar]
- Bradner, J.E.; Shen, J.P.; Perlstein, E.O.; Rubinsztein, D.; Sarkar, S.; Wood, S.L.S. Regulating Autophagy. Patent WO2008122038A1, 9 August 2008. [Google Scholar]
- Manjithaya, R.; Mishra, P.; Santhi Natesan, S.; Bats, S.; Ammanathan, V.; Chavalmane, A. Method for Modulating Autophagy and Applications Thereof. Patent WO2017098467A1, 15 June 2017. [Google Scholar]
- Li, M.; Song, J.; Zeng, Y.; Liu, L. MTOR-Independent Activator of TFEB for Autophagy Enhancement and Uses Thereof. U.S. Patent 9351946, 31 May 2016. [Google Scholar]
- Zaupa, C.; Hortelano, J.; Silvestre, N.; Spindler, A. Combination Product with Autophagy Modulator. Patent WO2016131945, 25 August 2016. [Google Scholar]
- Halfon, P. Treatment of Hepatitis C Virus Related Diseases Using Hydroxychloroquine or a Combination of Hydroxychloroquine and an Anti-Viral Agent. U.S. Patent 8987302, 24 March 2015. [Google Scholar]
- Dent, P.; Zukiwski, A.; Proniuk, S. Enhancing the Anti-Tumor, Anti-Viral, and Anti-Protozoan Effects of 2-amino-n-[4-[5-phenanthren-2-yl-3-(trifluoromethyl) pyrazol-1-yl] phenyl]acetamide (osu-03012) and Other Pharmaceutical Drugs. Patent WO2016069854, 6 May 2016. [Google Scholar]
- Levine, B.C.; Shoji-Kawata, S.; Lichtarge, O.; Wilkins, A.D. Autophagy-inducing peptide. Patent CA 2864145C, 14 February 2017. [Google Scholar]
- Liu, L.; Ward, D.; Leung, E.L.H.; Yao, X.J.; Wong, V.K.W.; Luo, L.X. Combination Treatment of RAS-Positive Diseases with PDE-Delta Inhibitor and Direct Autophagy Inhibitor. U.S. Patent 9861623B1, 9 January 2018. [Google Scholar]
- Kimmelman, A.C.; Mancias, J.D.; Harper, J.W. Compositions and Methods for Modulating ncoa4-Mediated Autophagic Targeting of Ferritin. International and National Patent Collections. Patent WO 2015149006, 26 November 2015. [Google Scholar]
- Hidenao, S.; Jun, U.; Koichi, W. MicroRNA that Regulate Autophagy. Patent JPWO 2015037656, 2 March 2017. [Google Scholar]
- Nicolas, G.; Vaulont, S.; Kahn, A. Use of Hepcidin as a Regulator of Iron Homeostasis. U.S. Patent 7169758B2, 30 January 2007. [Google Scholar]
- Ganz, T.; Nemeth, E.; Kautz, L. Erythroferrone and Erfe Polypeptides and Methods of Regulating Iron Metabolism. Patent CA 2890040A1, 8 May 2014. [Google Scholar]
- Schneider, S.A.; Bhatia, K.P. Syndromes of neurodegeneration with brain iron accumulation. Semin. Pediatr. Neurol. 2012, 19, 57–66. [Google Scholar] [CrossRef]
- Haack, T.B.; Hogarth, P.; Kruer, M.C.; Gregory, A.; Wieland, T.; Schwarzmayr, T.; Graf, E.; Sanford, L.; Meyer, E.; Kara, E.; et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am. J. Hum. Genet. 2012, 91, 1144–1149. [Google Scholar] [CrossRef]
- Saitsu, H.; Nishimura, T.; Muramatsu, K.; Kodera, H.; Kumada, S.; Sugai, K.; Kasai-Yoshida, E.; Sawaura, N.; Hoshino, A.; Ryujin, F.; et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet. 2013, 45, 445–449. [Google Scholar] [CrossRef]
- Schneider, S.; Zorzi, G.; Nardocci, N. Pathophysiology and treatment of neurodegeneration with brain iron accumulation in the pediatric population. Curr. Treat Opt. Neurol. 2013, 15, 652–667. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, P.; Huang, X.; Hu, W.; Guo, B.; Wu, F. The WD40 repeat PtdIns(3)P-binding protein EPG-6 regulates progression of omegasomes to autophagosomes. Dev. Cell 2011, 21, 343–357. [Google Scholar] [CrossRef]
- Polson, H.E.; de Lartigue, J.; Rigden, D.J.; Reedijk, M.; Urbé, S.; Clague, M.J.; Tooze, S.A. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy 2010, 6, 506–522. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Saitoh, T.; Fujita, N.; Jang, M.H.; Uematsu, S.; Yang, B.G.; Satoh, T. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef]
- Fujita, N.; Saitoh, T.; Kageyama, S.; Akira, S.; Noda, T.; Yoshimori, T. Differential involvement of Atg16L1 in Crohn disease and canonical autophagy: Analysis of the organization of the Atg16L1 complex in fibroblasts. J. Biol. Chem. 2009, 284, 32602–32609. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.S.; Rioux, J.D.; Mizoguchi, A.; Saitoh, T.; Huett, A.; Darfeuille-Michaud, A.; Wileman, T.; Mizushima, N.; Carding, S.; et al. Crohn disease: A current perspective on genetics, autophagy and immunity. Autophagy 2011, 7, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Oz-Levi, D.; Ben-Zeev, B.; Ruzzo, E.K.; Hitomi, Y.; Gelman, A.; Pelak, K.; Anikster, Y.; Reznik-Wolf, H.; Bar-Joseph, I.; Olender, T.; et al. Mutation in TECPR2 reveals a role for autophagy in hereditary spastic paraparesis. Am. J. Hum. Genet. 2012, 91, 1065–1072. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Crimella, C.; Airoldi, G.; Polishchuk, R.; Bonato, S.; Brighina, E.; Scarlato, M.; Musumeci, O.; Toscano, A.; Martinuzzi, A.; et al. Defective autophagy in spastizin mutated patients with hereditary spastic paraparesis type 15. Brain 2013, 136, 3119–3139. [Google Scholar] [CrossRef]
- Vantaggiato, C.; Panzeri, E.; Castelli, M.; Citterio, A.; Arnoldi, A.; Santorelli, F.M.; Liguori, R.; Scarlato, M.; Musumeci, O.; Toscano, A.; et al. ZFYVE26/SPASTIZIN and SPG11/SPATACSIN mutations in hereditary spastic paraplegia types AR-SPG15 and AR-SPG11 have different effects on autophagy and endocytosis. Autophagy 2019, 15, 34–57. [Google Scholar] [CrossRef]
- Rothaug, M.; Stroobants, S.; Schweizer, M.; Peters, J.; Zunke, F.; Allerding, M.; D’Hooge, R.; Saftig, P.; Blanz, J. LAMP-2 deficiency leads to hippocampal dysfunction but normal clearance of neuronal substrates of chaperone-mediated autophagy in a mouse model for Danon disease. Acta Neuropathol. Commun. 2015, 3, 6. [Google Scholar] [CrossRef]
- Ng, K.M.; Mok, P.Y.; Butler, A.W.; Ho, J.C.; Choi, S.W.; Lee, Y.K.; Lai, W.H.; Au, K.W.; Lau, Y.M.; Wong, L.Y.; et al. Amelioration of X-linked related autophagy failure in Danon disease with DNA methylation inhibitor. Circulation 2016, 134, 1373–1389. [Google Scholar] [CrossRef]
- Rowland, T.J.; Sweet, M.E.; Mestroni, L.; Taylor, M.R. Danon disease—Dysregulation of autophagy in a multisystem disorder with cardiomyopathy. J. Cell Sci. 2016, 129, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.J.; Moore, S.A.; Kalimo, H.; Minassian, B.A. X-linked myopathy with excessive autophagy: A failure of self-eating. Acta Neuropathol. 2015, 129, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Ramachandran, N.; Wang, P.; Haan, E.; Kneebone, C.; Manavis, J.; Morandi, L.; Moroni, I.; Blumbergs, P.; Mora, M.; et al. Non-coding VMA21 deletions cause X-linked myopathy with excessive autophagy. Neuromuscular Disord. 2015, 25, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Herrington, D.M. The function of cathepsins B, D, and X in atherosclerosis. Am. J. Cardiovasc. Dis. 2016, 6, 163–170. [Google Scholar]
- Nogalska, A.; D’Agostino, C.; Terracciano, C.; Engel, W.K.; Askanas, V. Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers. Am. J. Pathol. 2010, 177, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, F.; Pen, Q.; Lu, X.; Tian, X.; Wang, Y.; Wang, G. Potential role of autophagy in T-cell survival in polymyositis and dermatomyositis. Mol. Med. Rep. 2017, 16, 1180–1188. [Google Scholar] [CrossRef]
- Mastaglia, F.L. Sporadic inclusion body myositis, variability in prevalence and phenotype and influence of the MHC. Acta Myol. 2009, 28, 66–71. [Google Scholar]
- Ozawa, T.; Koide, R.; Nakata, Y.; Saitsu, H.; Matsumoto, N.; Takahashi, K.; Nakano, I.; Orimo, S. A novel WDR45 mutation in a patient with static encephalopathy of childhood with neurodegeneration in adulthood (SENDA). Am. J. Med. Genet. A 2014, 164, 2388–2390. [Google Scholar] [CrossRef]
- D’souza, R.S.; Levandowski, C.; Slavov, D.; Graw, S.L.; Allen, L.A.; Adler, E.; Mestroni, L.; Taylor, M.R. Danon disease: Clinical features, evaluation, and management. Circ. Heart Fail. 2014, 7, 843–849. [Google Scholar] [CrossRef]
- Cullup, T.; Kho, A.L.; Dionisi-Vici, C.; Brandmeier, B.; Smith, F.; Urry, Z.; Simpson, M.A.; Yau, S.; Bertini, E.; McClelland, V.; et al. Recessive mutations in EPG5 cause Vici syndrome, a multisystem disorder with defective autophagy. Nat Genet. 2013, 45, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Zhao, H.; Sun, H.; Zhang, H. Role of Epg5 in selective neurodegeneration and Vici syndrome. Autophagy 2013, 9, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Carroll, B.; Buganim, Y.; Maetzel, D.; Ng, A.H.; Cassady, J.P.; Cohen, M.A.; Chakraborty, S.; Wang, H.; Spooner, E.; et al. Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell Rep. 2013, 5, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.G.; McElroy, J.A.; Grange, R.W.; Fuller, D.D.; Walter, G.A.; Byrne, B.J.; Falk, D.J. Correcting neuromuscular deficits with gene therapy in Pompe disease. Ann. Neurol. 2015, 78, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Deng, W.; Zhang, S.; Cai, N.; Jiao, S.; Song, J.; Wei, L. Paradoxical roles of autophagy in different stages of tumorigenesis, protector for normal or cancer cells. Cell Biosci. 2013, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.S.; Mortensen, M.; Simon, A.K. Autophagy in the pathogenesis of myelodysplastic syndrome and acute myeloid leukemia. Cell Cycle 2011, 10, 1719–1725. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.J.; Zhang, H. Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front. Immunol. 2018, 9, 2334. [Google Scholar] [CrossRef]
- Yao, Q.M.; Zhu, Y.F.; Wang, W.; Song, Z.Y.; Shao, X.Q.; Li, L.; Song, R.H.; An, X.F.; Qin, Q.; Li, Q.; et al. Polymorphisms in autophagy-related gene IRGM are associated with susceptibility to autoimmune thyroid diseases. Biomed. Res. Int. 2018, 2018, 7959707. [Google Scholar] [CrossRef]
- Usategui-Martín, R.; García-Aparicio, J.; Corral-Gudino, L.; Calero-Paniagua, I.; Del Pino-Montes, J.; González Sarmiento, R. Polymorphisms in autophagy genes are associated with Paget disease of bone. PLoS ONE 2015, 10, e0128984. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Meazza, C.; Pagani, S.; Martinelli, C.; Pasqua, N.; Pelizzo, G.; Biggiogera, M.; Bozzola, M. Identification of autophagy-related genes and their regulatory mirnas associated with celiac disease in children. Int. J. Mol. Sci. 2017, 18, 391. [Google Scholar] [CrossRef]
- Barboza, L.A.; Ghisi, N.C. Evaluating the current state of the art of Huntington disease research: A scientometric analysis. Braz. J. Med. Biol. Res. 2018, 51, e6299. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and autophagy in the heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, M.Y.; Li, P.F.; Cao, J.M. MicroRNAs in cardiac autophagy: Small molecules and big role. Cells 2018, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, B.L.; Yang, X.; Zhang, X.; Liu, J. The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br. J. Pharmacol. 2013, 168, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Sinicrope, F.A. Celecoxib-induced apoptosis is enhanced by ABT-737 and by inhibition of autophagy in human colorectal cancer cells. Autophagy 2010, 6, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Oot, R.A.; Couoh-Cardel, S.; Sharma, S.; Stam, N.J.; Wilkens, S. Breaking up and making up: The secret life of the vacuolar H+-ATPase. Protein Sci. 2017, 26, 896–909. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, W.K.; Li, Y.; Yu, L.; Li, Z.J.; Wong, C.C.; Li, H.T.; Sung, J.J.; Cho, C.H. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem. Biophys. Res. Commun. 2009, 382, 451–456. [Google Scholar] [CrossRef]
- Yuan, N.; Song, L.; Zhang, S.; Lin, W.; Cao, Y.; Xu, F.; Fang, Y.; Wang, Z.; Zhang, H.; Li, X.; et al. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica 2015, 100, 345–356. [Google Scholar] [CrossRef]
- Kissing, S.; Rudnik, S.; Damme, M.; Lüllmann-Rauch, R.; Ichihara, A.; Kornak, U.; Eskelinen, E.L.; Jabs, S.; Heeren, J.; et al. Disruption of the vacuolar-type H+-ATPase complex in liver causes MTORC1-independent accumulation of autophagic vacuoles and lysosomes. Autophagy 2017, 13, 670–685. [Google Scholar] [CrossRef]
- Yano, K.; Yanagisawa, T.; Mukae, K.; Niwa, Y.; Inoue, Y.; Moriyasu, Y. Dissection of autophagy in tobacco BY-2 cells under sucrose starvation conditions using the vacuolar H(+)-ATPase inhibitor concanamycin A and the autophagy-related protein Atg8. Plant Signal Behav. 2015, 10, e1082699. [Google Scholar] [CrossRef]
- Rez, G.; Kovacs, J. Prevention by cycloheximide of cellular autophagy induced by hyperosmotic sucrose or cadmium chloride in mouse pancreatic acinar cells. Acta Biol. Acad. Sci. Hung. 1973, 24, 201–205. [Google Scholar] [PubMed]
- Zhu, Z.C.; Liu, J.W.; Li, K.; Zheng, J.; Xiong, Z.Q. KPNB1 inhibition disrupts proteostasis and triggers unfolded protein response-mediated apoptosis in glioblastoma cells. Oncogene 2018, 37, 2936–2952. [Google Scholar] [CrossRef] [PubMed]
- Machiya, Y.; Hara, S.; Arawaka, S.; Fukushima, S.; Sato, H.; Sakamoto, M.; Koyama, S.; Kato, T. Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J. Biol. Chem. 2010, 285, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Parke, A.L.; Clifford-Rashotte, M.; Kean, W.F. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015, 23, 231–269. [Google Scholar] [CrossRef] [PubMed]
- Harhaji-Trajkovic, L.; Arsikin, K.; Kravic-Stevovic, T.; Petricevic, S.; Tovilovic, G.; Pantovic, A.; Zogovic, N.; Ristic, B.; Janjetovic, K.; Bumbasirevic, V.; et al. Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm. Res. 2012, 29, 2249–2263. [Google Scholar] [CrossRef] [PubMed]
- McAfee, Q.; Zhang, Z.; Samanta, A.; Levi, S.M.; Ma, X.H.; Piao, S.; Lynch, J.P.; Uehara, T.; Sepulveda, A.R.; Davis, L.E.; et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc. Natl. Acad. Sci. USA 2012, 109, 8253–8258. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, Y.; Inoue, Y. Use of protease inhibitors for detecting autophagy in plants. Methods Enzymol. 2008, 451, 557–580. [Google Scholar]
- Jung, M.; Lee, J.; Seo, H.Y.; Lim, J.S.; Kim, E.K. Cathepsin inhibition-induced lysosomal dysfunction enhances pancreatic beta-cell apoptosis in high glucose. PLoS ONE 2015, 10, e0116972. [Google Scholar] [CrossRef]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef]
- Redmann, M.; Benavides, G.A.; Berryhill, T.F.; Wani, W.Y.; Ouyang, X.; Johnson, M.S.; Ravi, S.; Barnes, S.; Darley-Usmar, V.M.; Zhang, J. Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol. 2017, 11, 73–81. [Google Scholar] [CrossRef]
- Tanida, I. Autophagy basics. Microbiol. Immunol. 2011, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liersch, R.; Detmar, M. The miR-290-295 cluster suppresses autophagic cell death of melanoma cells. Sci. Rep. 2012, 2, 808. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chuang, A.Y.; Ratovitski, E.A. Phospho-ΔNp63α/miR-885-3p axis in tumor cell life and cell death upon cisplatin exposure. Cell Cycle 2011, 10, 3938–3947. [Google Scholar] [CrossRef] [PubMed]
- Menghini, R.; Casagrande, V.; Marino, A.; Marchetti, V.; Cardellini, M.; Stoehr, R. MiR-216a: A link between endothelial dysfunction and autophagy. Cell Death Dis. 2014, 5, e1029. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Chattopadhyay, D.; Chakrabarti, G. miR-17-5p downregulation contributes to paclitaxel resistance of lung cancer cells through altering beclin 1 expression. PLoS ONE 2014, 9, e95716. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Han, W.; Sui, X.; Xie, J.; Pan, H. Interaction of autophagy with microRNAs and their potential therapeutic implications in human cancers. Cancer Lett. 2015, 356 Pt B, 332–338. [Google Scholar] [CrossRef]
- Lassen, K.G.; Xavier, R.J. Genetic control of autophagy underlies pathogenesis of inflammatory bowel disease. Mucosal Immunol. 2017, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Boada-Romero, E.; Serramito-Gomez, I.; Sacristan, M.P.; Boone, D.L.; Xavier, R.J.; Pimentel-Muinos, F.X. The T300A Crohn’s disease risk polymorphism impairs function of the WD40 domain of ATG16L1. Nat. Commun. 2016, 7, 11821. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Gentilella, A.; Kozma, S.C.; Thomas, G. A liaison between mTOR signalling, ribosome biogenesis and cancer. Biochim. Biophys. Acta 2015, 1849, 812–820. [Google Scholar] [CrossRef]
- Frederick, C.; Ando, K.; Leroy, K.; Héraud, C.; Suain, V.; Buée, L.; Brion, J.P. Rapamycin ester analog CCI-779/Temsirolimus alleviates tau pathology and improves motor deficit in mutant tau transgenic mice. J. Alzheimers Dis. 2015, 44, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Vignot, S.; Faivre, S.; Aguirre, D.; Raymond, E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann. Oncol. 2005, 16, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Lin, Y.C.; Yang, S.C.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Hwang, T.I. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des. Dev. Ther. 2016, 10, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, S.; Ma, A.; Pan, X.; Wang, H.; Li, N.; Liu, S.; Wu, M. Expression of miRNA-155 in carotid atherosclerotic plaques of apolipoprotein E knockout (ApoE−/−) mice and the interventional effect of rapamycin. Int. Immunopharmacol. 2017, 46, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Chen, G.; He, L.; Tang, C.; Wang, C.; Yang, D.; Li, H.; Dong, Z.; Liu, H. Rapamycin enhances repressed autophagy and attenuates aggressive progression in a rat model of IgA nephropathy. Am. J. Nephrol. 2017, 45, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Floto, R.A.; Sarkar, S.; Perlstein, E.O.; Kampmann, B.; Schreiber, S.L.; Rubinsztein, D. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy 2007, 3, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Siswanto, S.; Fredrich, M.; Bouzrou, M.; Brunt, E.R.; van Leeuwen, F.W.; Kampinga, H.H.; Korf, H.W.; et al. Polyglutamine aggregation in Huntington’s disease and spinocerebellar ataxia type 3: Similar mechanisms in aggregate formation. Neuropathol. Appl. Neurobiol. 2016, 42, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Mardones, P.; Rubinsztein, D.C.; Hetz, C. Mystery solved: Trehalose kick starts autophagy by blocking glucose transport. Sci. Signal. 2016, 9, fs2. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.L.; Grose, C. Focus: Drug development: Variable effects of autophagy induction by trehalose on Herpes viruses depending on conditions of infection. Yale J. Biol. Med. 2017, 90, 25. [Google Scholar] [PubMed]
- Honma, Y.; Sato-Morita, M.; Katsuki, Y.; Mihara, H.; Baba, R.; Harada, M. Trehalose activates autophagy and decreases proteasome inhibitor-induced endoplasmic reticulum stress and oxidative stress-mediated cytotoxicity in hepatocytes. Hepatol. Res. 2018, 48, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, M.; Li, L.; Xu, S.; Huang, D.; Ju, M.; Huang, J.; Chen, K.; Gu, H. Trehalose, sucrose and raffinose are novel activators of autophagy in human keratinocytes through an mTOR-independent pathway. Sci. Rep. 2016, 6, 28423. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Koprich, J.B.; Wang, Y.; Yu, W.B.; Xiao, B.G.; Brotchie, J.M.; Wan, J. Treatment with trehalose prevents behavioral and neurochemical deficits produced in an AAV α-Synuclein rat model of Parkinson’s disease. Mol. Neurobiol. 2016, 53, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Kruger, U.; Wang, Y.; Kumar, S.; Mandelkow, E.M. Autophagic degradation of tau in primary neurons and its enhancement by trehalose. Neurobiol. Aging 2012, 33, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Aguib, Y.; Heiseke, A.; Gilch, S.; Riemer, C.; Baier, M.; Schatzl, H.M.; Ertmer, A. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 2009, 5, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kubota, Y.; Sawa, R.; Umekita, M.; Hatano, M.; Ohba, S.I.; Hayashi, C.; Igarashi, M.; Nomoto, A. Novel autophagy inducers lentztrehaloses A., B and C. J. Antibiot. 2015, 68, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Motoi, Y.; Shimada, K.; Ishiguro, K.; Hattori, N. Lithium and autophagy. ACS Chem. Neurosci. 2014, 5, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Rane, S.; Lussiernbb, S.; Andersen, J.K. Lithium protects against oxidative stress-mediated cell death in alpha-synuclein over-expressing in vitro and in vivo models of Parkinson’s disease. J. Neurosci. Res. 2011, 89, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef]
- Xia, Q.; Zheng, Y.; Jiang, W.; Huang, Z.; Wang, M.; Rodriguez, R.; Jin, X. Valproic acid induces autophagy by suppressing the Akt/mTOR pathway in human prostate cancer cells. Oncol. Lett. 2016, 12, 1826–1832. [Google Scholar] [CrossRef]
- Ji, M.M.; Wang, L.; Zhan, Q.; Xue, W.; Zhao, Y.; Zhao, X.; Xu, P.P.; Shen, Y.; Liu, H.; Janin, A.; et al. Induction of autophagy by valproic acid enhanced lymphoma cell chemosensitivity through HDAC-independent and IP3-mediated PRKAA activation. Autophagy 2015, 11, 2160–2171. [Google Scholar] [CrossRef]
- Pietrocola, F.; Lachkar, S.; Enot, D.P.; Niso-Santano, M.; Bravo-San Pedro, J.M.; Sica, V.; Izzo, V.; Maiuri, M.C.; Madeo, F.; Mariño, G.; et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015, 22, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Finkel, T. Regulation of autophagy by the p300 acetyltransferase. J. Biol. Chem. 2009, 284, 6322–6328. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Malik, S.A.; Mariño, G.; Vacchelli, E.; Senovilla, L.; Chaba, K.; Niso-Santano, M.; Maiuri, M.C.; Madeo, F.; Kroemer, G. Coffee induces autophagy in vivo. Cell Cycle 2014, 13, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Zimmermann, A.; Maiuri, M.C.; Kroemer, G. Essential role for autophagy in life span extension. J. Clin. Investig. 2015, 125, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.A.; Barczak, A.K.; Silvis, M.R.; Luo, S.S.; Sogi, K.; Vokes, M.; Carpenter, A.E.; Moore, C.B.; Siddiqi, N.; Rubin, E.J.; et al. Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 2014, 10, e1003946. [Google Scholar] [CrossRef]
- Schiebler, M.; Brown, K.; Hegyi, K.; Newton, S.M.; Renna, M.; Hepburn, L.; Klapholz, C.; Coulter, S.; Obregón-Henao, A.; Henao-Tamayo, M.; et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol. Med. 2014, 7, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Ravikumar, B.; Floto, R.A.; Rubinsztein, D.C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009, 16, 46–56. [Google Scholar] [CrossRef]
- Renna, M.; Jimenez-Sanchez, M.; Sarkar, S.; Rubinsztein, D.C. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J. Biol. Chem. 2010, 285, 11061–11067. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lai, H.Y.; Chen, Y.C.; Li, C.F.; Huang, H.S.; Liu, H.S.; Tsai, Y.S.; Wang, J.M. Metformin promotes apoptosis in hepatocellular carcinoma through the CEBPD-induced autophagy pathway. Oncotarget 2017, 8, 13832–13845. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Li, L.; Zhang, S.Y.; Wu, Q.; Mei, W.J.; Liu, H.M.; Wang, X.C. Phenanthroimidazole derivatives act as potentinducer of autophagy by activating DNA damage pathway. Bioorg. Chem. 2019, 19, 102940. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wu, H.M.; Li, S.; Wang, M.Z.; Fang, L.; Liu, R.Y. Melatonin enhances autophagy and decreases apoptosis induced by nanosilica in RAW264.7 cells. IUBMB Life 2019. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.P.; Xu, T.Q.; Liu, B.L.; Lei, X.P.; Hambrook, J.R.; Zhang, D.M.; Zhou, G.X. Sasanquasaponin ΙΙΙ from Schima crenata Korth induces autophagy through Akt/mTOR/p70S6K pathway and promotes apoptosis in human melanoma A375 cells. Phytomedicine 2018, 20, 152769. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.; Zhu, M.; Du, J.; Xu, J.; Qin, X.; Xu, X.; Song, E. Epigallocatechin-3-gallate stimulate autophagy and reduces apoptosis levels in retinal Müller cells under high-glucose conditions. Exp. Cell Res. 2019, 380, 149–158. [Google Scholar] [CrossRef] [PubMed]
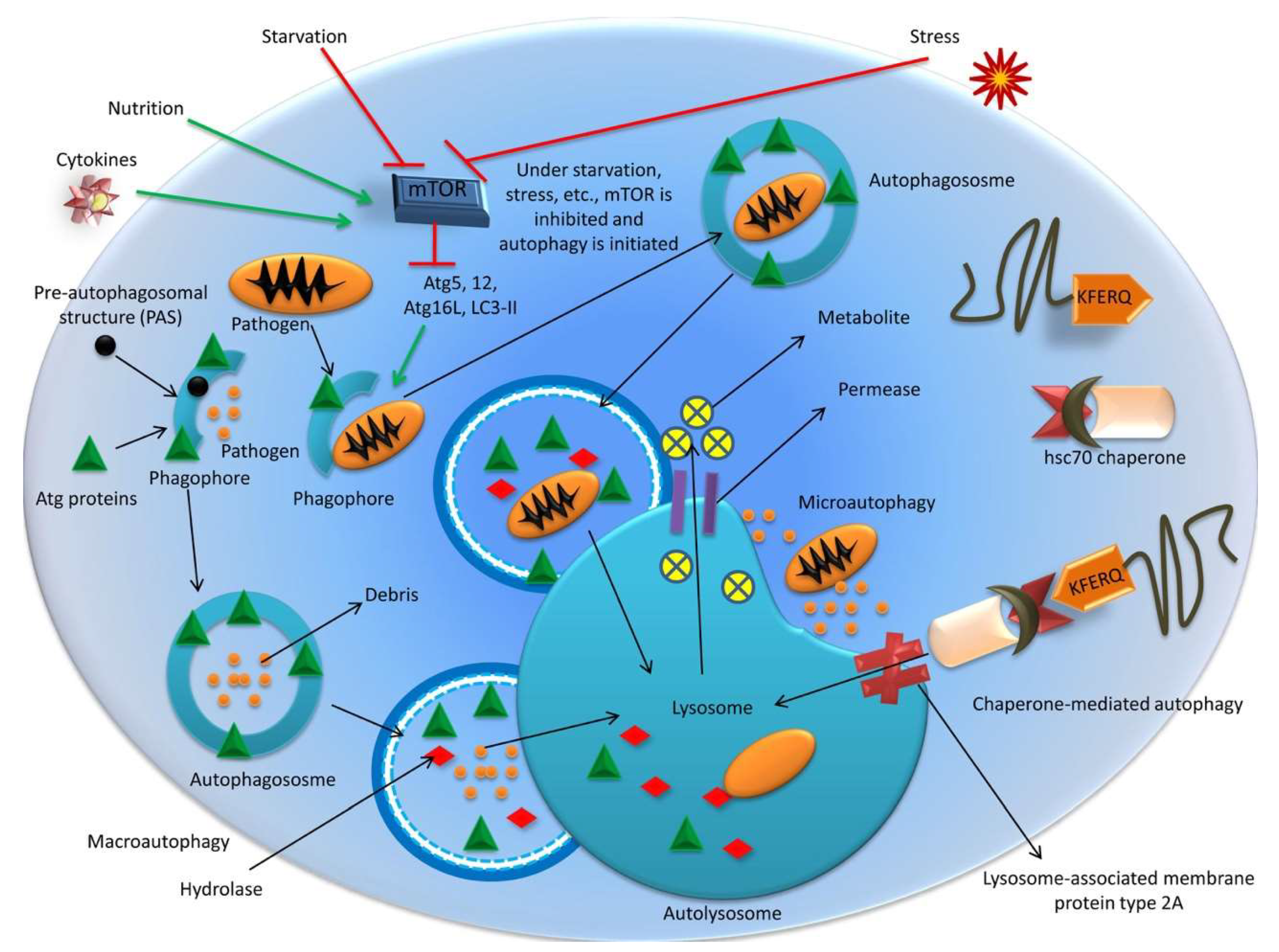
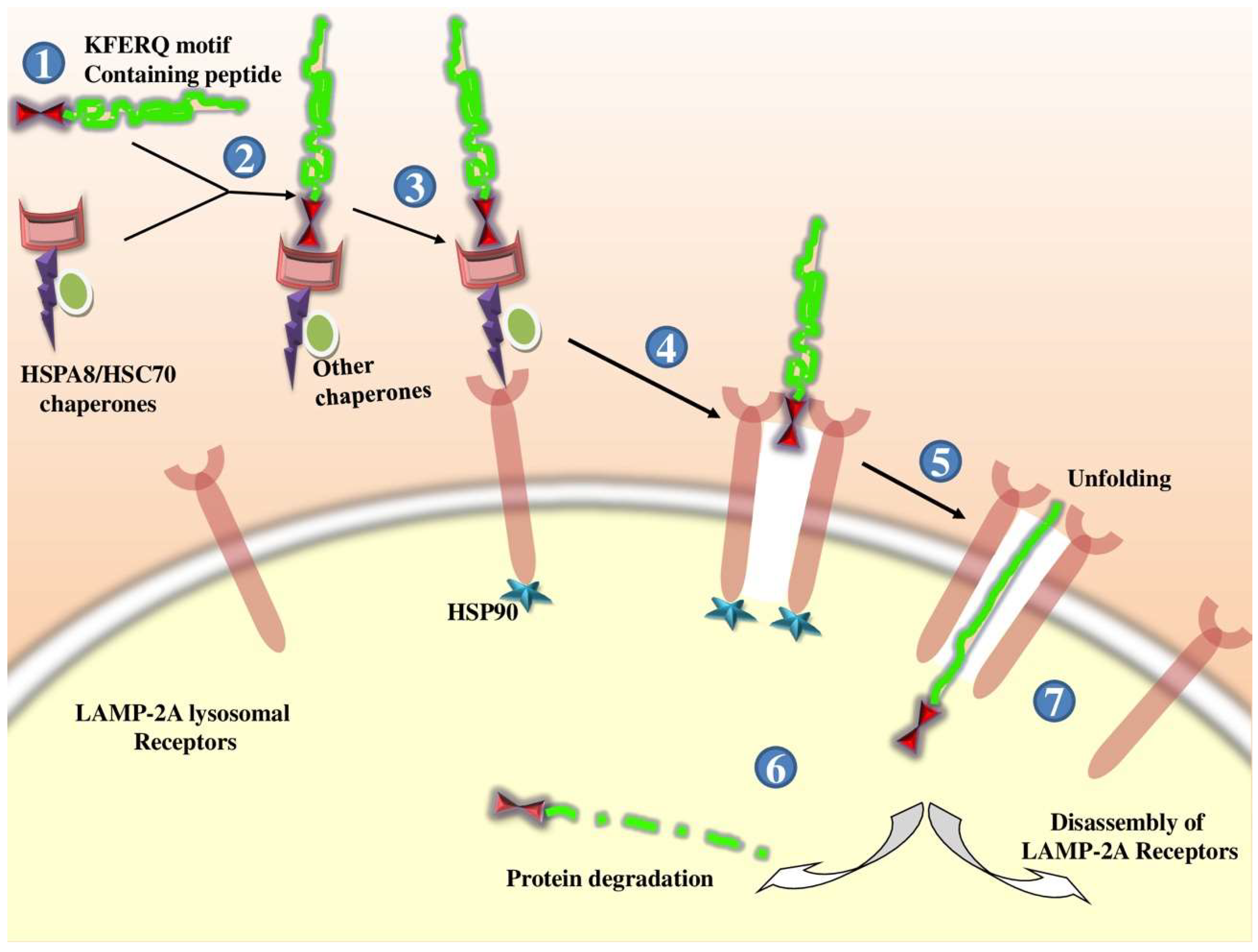

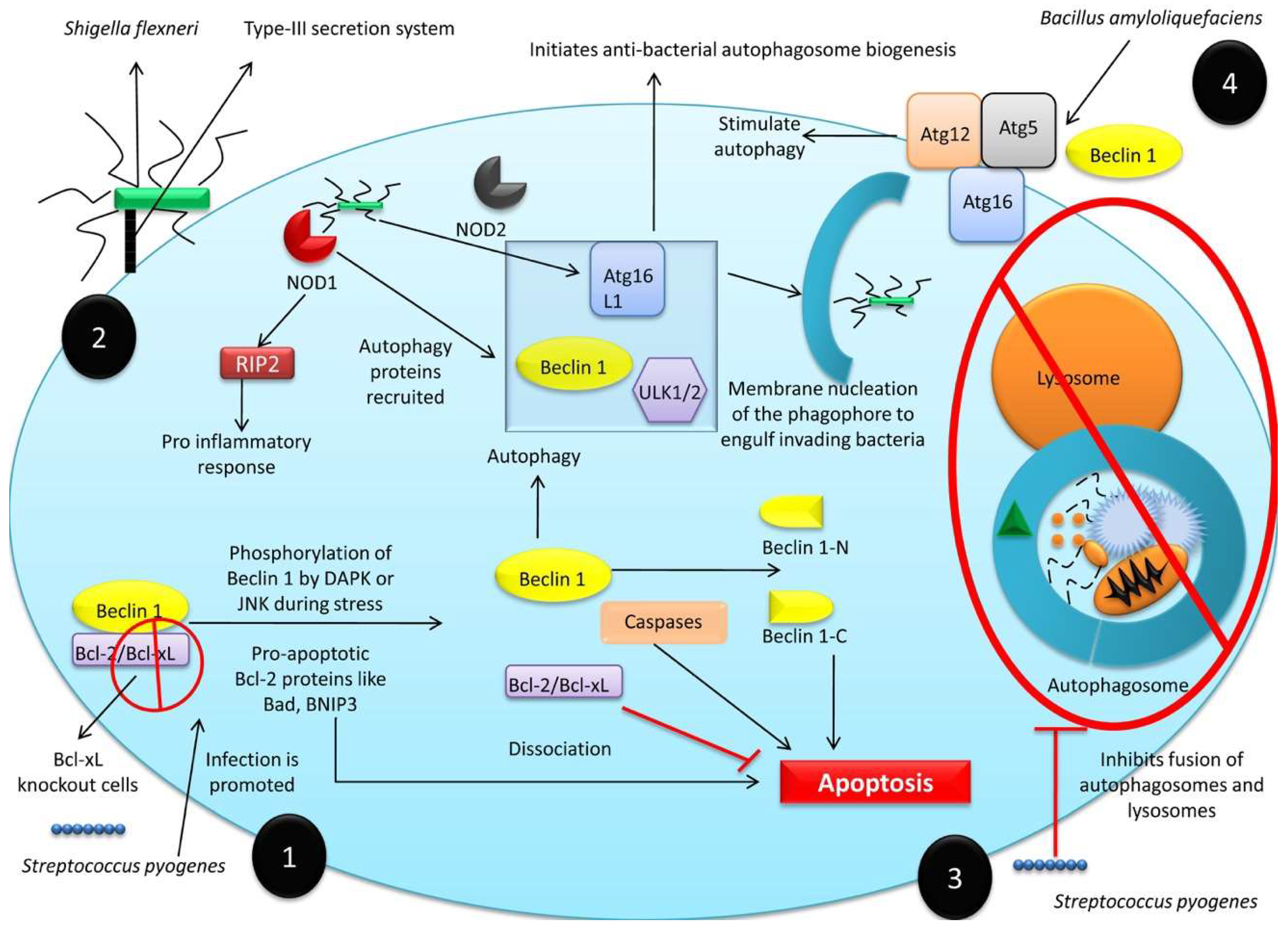
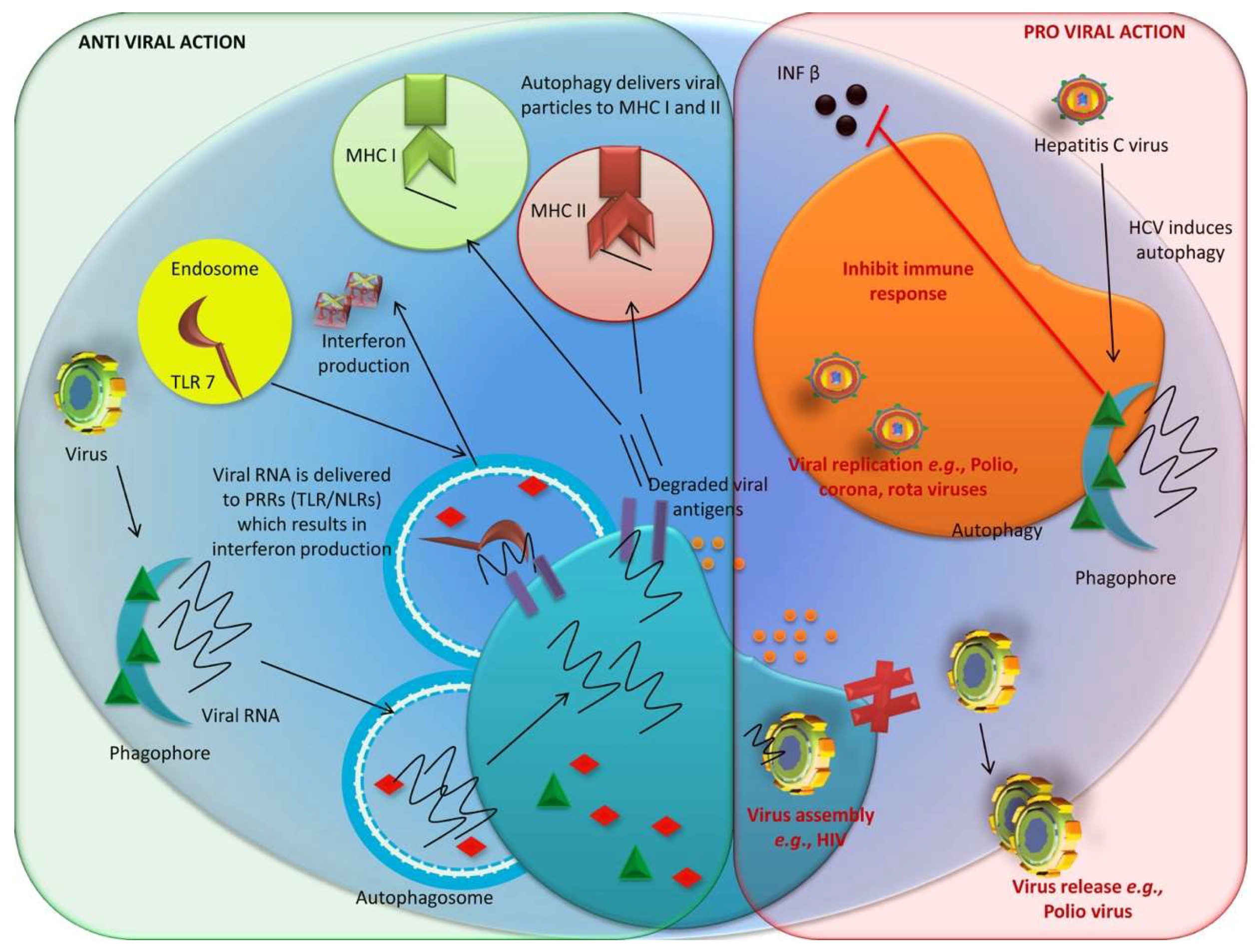

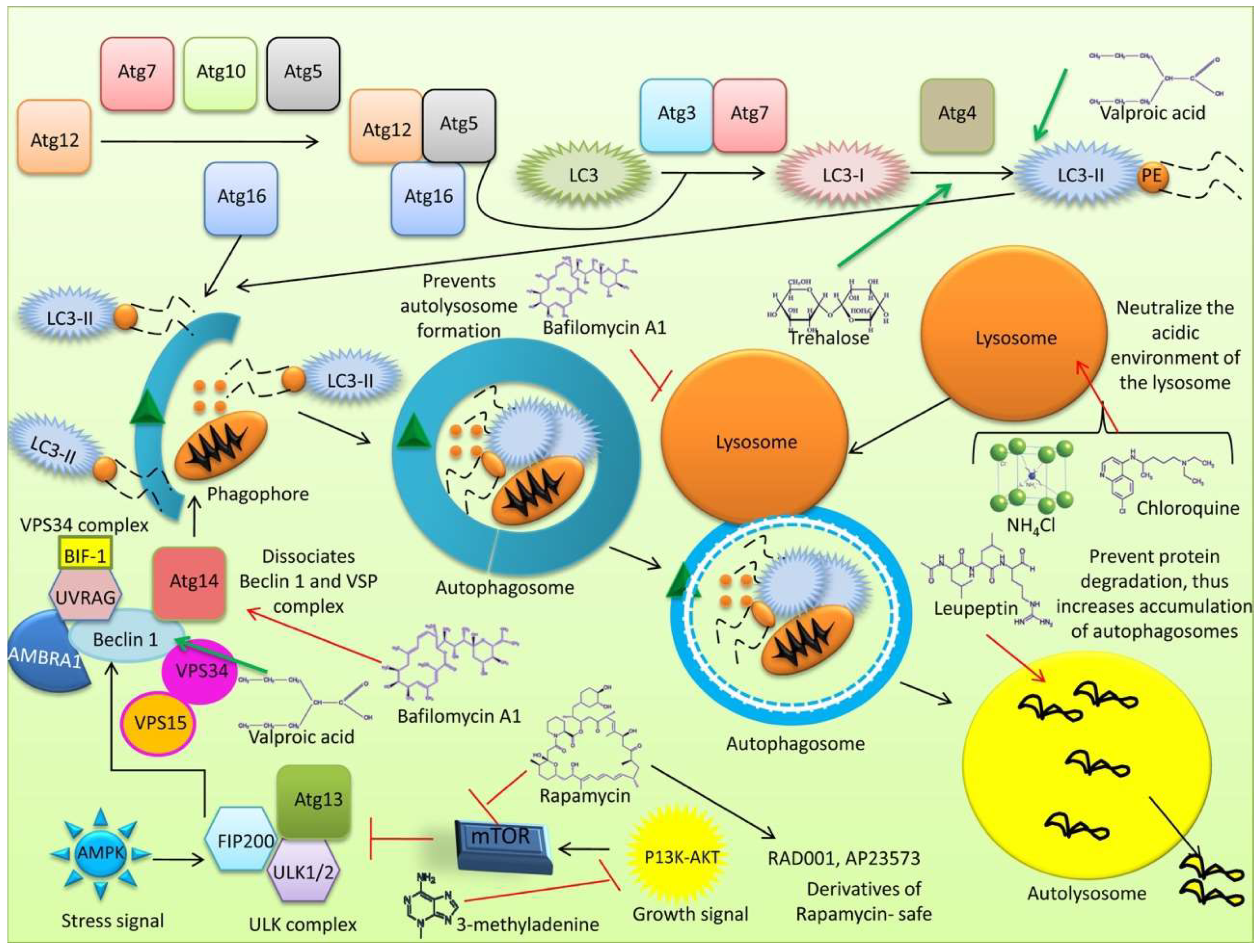
| S. No. | Activity Associated with Autophagy | Effect of Autophagy | Modus Operandi of Related Activity and Example/Proof of Concept | Reference(s) |
|---|---|---|---|---|
| 1 | Viral infection | Anti-viral activity | Endogenous viral antigen presentation on MHC class-1 in Herpes simplex virus type 1 (HSV-1) infection | English et al., 2009 [236] |
| Delivery of viral antigens to Toll-like receptors (TLRs)- in Vesicular stomatitis virus (VSV) infection; Pattern recognition receptor Toll-7 mediated PI3K-Akt-signaling | Shelly et al., 2009 [239], Nakamoto et al., 2012 [240] | |||
| Sirtuin 1, a NAD(+)-dependent deacetylase mediated dendritic cell and autophagy induction - Respiratory syncytial virus (RSV) | Owczarczyk et al., 2015 [242] | |||
| Autophagy by salicylamide derivates- anti-viral activity against - Cytopathic bovine viral diarrhea virus (cp-BVDV) flavivirus | Needs et al., 2016 [243] | |||
| Inhibition of Sindbis virus replication by overexpression of Beclin 1 | Liang et al., 1998 [176] | |||
| Enhanced autophagy by 1α,25-dihydroxycholecalciferol reduces HIV replication | Campbell and Spector, 2012 [220] | |||
| During foot and mouth disease virus infection, Atg5-Atg12 enhances NF-κB and IRF3 pathways | Fan et al., 2017 [244] | |||
| Targeting glycoproteins E1 and E2 and non-structural proteins of Chikungunya virus (CHKV) | Subudhi et al., 2018 [397] | |||
| Pro-viral activity | Rapamycin, chloroquine and small interfering RNAs target Atg5 and Beclin 1- virus production is hampered in New Castle disease virus (NCDV) | Sun et al., 2014 [259] | ||
| Induction of early stages of autophagy and inhibition of later destructive stages – to conquer suppression of new virion production- HIV | Kyei et al., 2009 [261] | |||
| Nef-mediated inhibition of maturation of autophagosome- HIV | ||||
| NS4A-induced autophagy in epithelial cells induces virus replication – Flavivirus | McLean et al., 2011 [269] | |||
| Limitation of autophagosomal by 3-methyladenine or small-interfering RNAs- diminished replication of virus- FMDV | O’Donnell et al., 2011 [272] | |||
| Virus-induced autophagy-mediated impairment of innate immune response- Hepatitis C virus (HCV) | Shrivastava et al., 2011 [252] | |||
| Diminished viral clearance by IFN-α /RBV-based antiviral therapy-HCV | Dash et al., 2016 [274] | |||
| Inhibition of RLR-mediated type-I IFN-independent signaling resulting in antibody-dependent enhancement (ADE) of Dengue virus (DENV) | Huang et al., 2016 [275] | |||
| Adenoviral infection may be privileged by autophagy via an increase in ATP; Atg12-Atg5 complex is significantly upregulated. | Jiang et al., 2008 [277] | |||
| Activation of the phosphatidylinositol 3 kinase/Akt/mTOR pathway and inhibition of autophagy- induce cellular entry of –Human Papilloma virus (HPV) type 16. | Surviladze et al., 2013 [283] | |||
| Replication of Infectious Spleen and Kidney Necrosis virus (ISKNV) is increased when autophagy is induced | Li et al., 2017 [246] | |||
| Human nuclear ribonucleoprotein K (hnRNP-K) and ubiquilin 4 (UBQLN4) help in viral replication. NDP52 human autophagy receptor interacts with CHIKV nsP2 and acts as proviral factor | Wong and Chu, 2018 [37] | |||
| Classical swine fever virus replication is negatively regulated through mTORC1 | Luo et al., 2018 [33] | |||
| Autophagosomal targeting of ribosomal proteins by influenza A virus (IAV) | Becker et al., 2018 [32] | |||
| Autophagy of endothelial cells of umbilical vein by Zika virus (ZIKV) helps in replication | Peng et al., 2018 [35] | |||
| Necrosis of cells through severe acute respiratory syndrome-coronavirus (SARS-CoV) open reading frame-3a for multiplication | Yue et al., 2018 [36] | |||
| ER stress by DENV infection helps in autophagy and replication, both in vitro and in vivo | Lee et al., 2018 [38] | |||
| Non-structural protein of virus affects mitochondrial membrane in Crimean-Congo Hemorrhagic fever causing apoptosis | Barnwal et al., 2016 [40] | |||
| MDA5 protein inhibition by paramyxovirus V proteins | Mandhana et al., 2018 [41] | |||
| Altering nonstructural proteins of West Nile virus (WNV) affects LC3 modification and aggregation | Martín-Acebes et al., 2015 [43] | |||
| 2 | Bacterial infection | Anti-bacterial activity | In Bcl-xL knockout cells, Streptococcus pyogenes infection is promoted | Nakajima et al., 2017 [53] |
| NOD proteins interaction with Atg16L1 and initiation of anti-bacterial autophagosome biogenesis | Sorbara et al., 2013 [212] | |||
| Protection from Caenorhabditis elegans infection by transcription factor HLH-30/TFEB-mediated autophagy | Chen et al., 2017 [215] | |||
| Inhibition of Mycobacterium tuberculosis in human macrophages by SMAD specific E3 ubiquitin protein ligase 1 (SMURF1) | Franco et al., 2017 [398] | |||
| Pro-bacterial activity | Effector Ats-1 is used to enhance autophagosomes formation containing LC3, Beclin 1, Atg8 and Atg6, without lysosomal marker by Anaplasma phagocytophilum | Niu et al., 2012 [222] | ||
| Yersinia-containing vacuoles (YCVs) contains autophagy markers but not acidified | Moreau et al., 2010 [224] | |||
| Coxiella-replicative vacuoles contains LC3, Beclin 1, and Rab24 | Vázquez and Colombo, 2010 [226] | |||
| Inside BCVs, replication of Brucella requires ULK1, Beclin 1, and Atg14L | Starr et al., 2012 [227] | |||
| Secreted phospholipases C (PLCs; PlcA and PlcB) and a surface protein (ActA) help Listeria monocytogenes multiplication | Mitchell et al., 2018 [50] | |||
| Shigella gatekeeper protein MxiC regulate type III secretion | Roehrich et al., 2017 [52] | |||
| 3 | Tumor | Tumor suppression | Monoallelic loss of Atg6/Beclin 1 gene – correlated with human prostate, breast, and ovarian cancers | Choi et al., 2013 [285] |
| Beclin 1 overexpression inhibits tumor progression | Liang et al., 1999 [286] | |||
| Inhibition of necrosis and chronic inflammation through inhibiting- high mobility group box 1 protein (HMGB1) | Tang et al., 2010 [288] | |||
| Autophagy deficiency- leads to benign hepatoma cell death | Takamura et al., 2011 [291] | |||
| Autophagy induced by PTEN and TSC, the tumor suppressor protein | Feng et al., 2005 [301]; Tsuchihara et al., 2009 [302] | |||
| In mice tumor model, inactivation of Beclin 1 and Atg5 affects autophagy | Levine, 2007 [292] | |||
| Heterozygous disturbance of Beclin 1 lead to development of cancer | Qu et al., 2003 [289]; Yue et al., 2003 [293] | |||
| UV radiation resistance associated gene (UVRAG) can suppress tumorigenicity and proliferation of colon cancer | Liang et al., 2006 [295] | |||
| Pogostone stimulate autophagy and apoptosis through PI3K/Akt/mTOR axis and have anti-colorectal tumor activities | Cao et al., 2017 [303] | |||
| Tumor induction | Autophagy alleviates stressed condition – in hypoxic conditions, metabolic stress, shortage of energy, damaged mitochondria and other organelles | Sato et al., 2007 [306] | ||
| Increased autophagy-associated protein LC3 and BNIP3- linked to colorectal and gastric cancers; Elevated expression of NIP3 (a pro-apoptotic member of the Bcl-2 family of cell death factor) in gastric carcinomas | Lee et al., 2007a [309] | |||
| Autophagy inhibition leads to cell death in tumors acting like an RAS-activated tumor | Guo et al., 2011 [312] | |||
| In the absence of autophagy -accumulation of ubiquitinylated protein aggregates and higher p62 level- responsible for liver tumor | Takamura et al., 2011 [291] | |||
| Activation of autophagy and peroxisome proliferator-activated receptor gamma (PPARγ) protect colon cancer cells against apoptosis | Tylichová et al., 2017 [310] | |||
| In RAS-activated tumors, inhibition of autophagy leads to increased cancer cell death | Guo et al., 2011 [312] | |||
| Post-chemotherapy, increased autophagy may cause cancer cells to go into dormancy and proliferate later | White et al., 2010 [315] | |||
| Proteasome 26S subunit, non-ATPase 10 (PSMD10) or gankyrin induced autophagy in hepatocellular carcinoma causes tumor progression | Luo et al., 2016 [318] | |||
| 4 | Neuronal health | Brain development | Clear protein aggregates / old organelles in old neurons | Hara et al., 2006 [319] |
| Atg5 mutation confined to neural tissue leads to impaired growth, progressive motor and behavioral deficits, prominent neurodegeneration and axonal swelling | ||||
| Absence of Atg59 and Atg710- leads to neuronal degeneration | Liao et al., 2007 [100] | |||
| Upon ethanol exposure, autophagy dysregulation in cortical microvessels affects cortical vascular development | Girault et al., 2017 [322] | |||
| Neurodegeneration | Dysregulated autophagy results in accumulation of damaged and toxic molecules- leads to Alzheimer’s, Parkinson’s and Huntington’s diseases | Sahni et al., 2014 [327] | ||
| Anomalies in endosomal-lysosomal pathway and accumulation of autophagosomes- lead to Alzheimer’s, Parkinson’s and Huntington’s diseases | Pickford et al., 2008 [330] | |||
| Beclin 1 deficiency- leads to deposition of β-amyloid protein and neurodegeneration | ||||
| Atg7 mutation in mice causes accumulate ubiquitin and results in neurodegeneration and death | Komatsu et al., 2006 [334]; Nixon, 2013 [31] | |||
| Embryos of Ambra1-deficient mice possess defects in the neuronal tube | Fimia et al., 2007 [335] | |||
| Mutations in the phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) and Parkin genes result in defective mitophagy which leads to Parkinson’s disease | Whitworth and Pallanck, 2017 [339] | |||
| Beta-propeller protein causes neurodegeneration | Stige et al., 2018 [399] | |||
| 5 | Iron availability in body | Homeostasis | Iron in the form of ferritin complex- redox-active iron is sequestered in lysosome | Kurz et al., 2011 [382]; Krishan et al., 2015 [384] |
| Knockdown of nuclear receptor co-activator 4 (NCOA4), which is responsible for directing ferritin to autophagosome, increases iron-responsive element-binding protein 2 (IRP2)- prevent cell death by exogenous reactive oxygen species | Berndt, 2014 [386] | |||
| Ferritinophagy | Iron storage protein called ferritin is degraded in the lysosome; thus, resulting in a form of selective macroautophagy | Hamaï and Mehrpour, 2017 [379] | ||
| 6 | Chronic inflammatory bowel disease | Anti-effect | Reduction in TNF-α induced apoptosis in gut epithelium | Pott and Maloy, 2018 [400] |
| Pro-effect | Goblet cell function, cytokine production or NOD2, ATG16L1, and IRGM gene regulation affect pathogenesis of inflammatory bowel disease | Iida et al., 2017 [401] | ||
| 7 | Lifestyle diseases | Obesity | Causes biochemical disturbance, ER stress, mitochondrial dysfunction induces obesity-cardiac disorders | Che et al., 2018 [388] |
| Diabetes mellitus | Affects beta-cells of pancreas, insulin target tissues, glucose metabolism | Bhattacharya et al., 2018 [389] | ||
| Cardiovascular disease | Perturbations in autophagic machinery in cardiomyocytes and other cardiovascular cell types | Schiattarella and Hill, 2015 [377] | ||
| Autophagy through PARP1 modulation of FoxO3a transcription in cardiomyocytes | Wang et al., 2018a [378] |
| S. No. | Targeted Ailment | Title of Patent | Patent Number | Modus Operandi | Inventers | Date of Publication | Status | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| 1. | Tumor treatment | Inhibition of autophagy genes in cancer chemotherapy | US 8076308 | Compositions comprise a siRNA directed against an Atg gene to inhibit its expression | Gorski SM, Qadir MA | 13.12.11 | Granted | Gorski and Qadir 2011 [402] |
| Dimeric quinacrine derivatives as autophagy inhibitors for cancer therapy | WO 2016168721 | Chloroquine compounds and derivatives mediated inhibition of lysosome | Amaravadi RK, Winkler J. | 20.10.16 | Application | Amaravadi and Winkler, 2016 [403] | ||
| Anti-cervical cancer compound and method of use thereof | US 9339488 | Griffipavixanthone, a novel cytotoxic Bixanthone from Garcinia griffithii and G. pavifolia selectively kill cervical cancer cells via inducing autophagy | XU H, Zhang H, Lao Y, Wang X, Chen K, Yang D, Chen S, Lin C, Bian Z, Lu A, Chan ASC, | 17.05.16 | Grant | Xu et al., 2016 [404] | ||
| Substituted thioxanthenone autophagy inhibitors | US 9926326 | Inhibition of autophagy through autophagy inhibitors developed from substitution of chemical groups, can help in treatment of cancers | Carew J, Phillips JG | 27.03.18 | Grant | Carew and Phillips, 2018 [405] | ||
| Method for inhibiting growth of ovarian cancer cells | US20180050012 | Method of inhibition of ovarian cancer cells by 4-acetyl-antroquinonol B or its salt | Huang CC, Tzeng YM, Yeh CT, Wu THA | 22.02.18 | Application | Huang et al., 2018 [25] | ||
| 2. | Neuroprotection | Autophagy enhancer for treatment of neurodegenerative diseases | US 9005677 | Onjisaponin B derived and isolated from Radix polygalae enhances autophagy | Law YK, Wu AG, Wong KW, Liu L | 14.04.15 | Grant | Law et al., 2015 [406] |
| Autophagy enhancing compounds, peptides and peptidomimetic compounds for use in the treatment of neuronal diseases | WO 2012076555 | Pharmaceutical compositions enhancing autophagy in acute focal brain lesions | D’amelio M, Molinari M, Viscomi MT, Cecconi F | 14.06.12 | Application | D’amelio et al., 2012 [407] | ||
| Highly potent peptides to control cancer and neurodegenerative diseases | WO 2010011952 | Inhibition of autophagy by administering a FLIP protein interfering with the formation of the LC3-Atg4-Atg7-Atg3 conjugation complex | Jung JU, Lee JS | 24.06.10 | Application | Jung and Lee, 2010 [408] | ||
| Methods for reducing neurodegeneration | EP 2717695 | Inhibiting the expression of mTOR in canine | Middleton RP, Zanghi BM | 02.11.16 | Grant | Middleton and Zanghi, 2016 [409] | ||
| Regulating autophagy | WO2008122038A1 | Regulating autophagy helps in prevention and treatment of neurodeneration or other diseases | Bradner JE , Shen JP, Perlstein EO, Rubinsztein D, Sarkar S, Wood SLS | 09.10.08 | Application | Bradner et al., 2008 [410] | ||
| Method for modulating autophagy and applications thereof | WO2017098467A1 | Autophagy modulators (pyridines; hydrogenated derivatives) regulate all types of autophagy by increasing or decreasing autophagic flux | Manjithaya R, Mishra P, Santhi Natesan S, Bats S, Ammanathan V, Chavalmane A | 15.06.17 | Application | Manjithaya et al., 2017 [411] | ||
| mTOR-independent activator of TFEB for autophagy enhancement and uses thereof | US 9351946 | Small molecules enhance autophagy and lysosome biogenesis by activating the gene TFEB | Li M, Song J, Zeng Y, Liu L | 31.05.16 | grant | Li et al., 2016 [412] | ||
| Combination product with autophagy modulator | WO 2016131945 | Autophagy modulator directly or indirectly acting on a complex involved in autophagy such as ULKl/2-Atgl3- FIP200 complex, Atg9 complex, STING complex, class III PI3K complex, ubiquitin-like conjugation systems Atg5-Atgl2, LC3, fusion complex, SNARE protein and transcription factor EB | Zaupa C, Hortelano J, Silvestre N, Spindler A | 25.08.16 | Application | Zaupa et al., 2016 [413] | ||
| 3. | Viral inhibitor | Treatment of hepatitis C virus-related diseases using hydroxychloroquine or a combination of hydroxychloroquine and an anti-viral agent | US 8987302 | Chloroquine cause pH-dependent inhibition of degradation of cargo delivered to the lysosome | Halfon P | 24.03.15 | Grant | Halfon, 2015 [414] |
| Enhancing the anti-tumor, anti-viral, and anti-protozoan effects of 2-amino-n-[4-[5-phenanthren-2-yl-3-(trifluoromethyl)pyrazol-1-yl] phenyl]acetamide (osu-03012) and other pharmaceutical drugs | WO 2016069854 | Drug OSU-03012 (AR-12) in combination with multikinase inhibitor | Dent P, Zukiwski A, Proniuk S | 06.05.16 | Application | Dent et al., 2016 [415] | ||
| Autophagy-inducing peptide | CA2864145C | Autophagy-inducing peptide derived from beclin-1 (residues 269-283) has antiviral role against West Nile Virus, chikungunya virus, HIV and Ebola viru | Levine BC, Sanae Shoji-Kawata S, Lichtarge O, Wilkins AD | 14.02.17 | Grant | Levine et al., 2017 [416] | ||
| Combination treatment of RAS-positive diseases with PDE-delta inhibitor and direct autophagy inhibitor | US 9861623B1 | Potentiating the apoptotic activity of deltarasin, a PDE-delta inhibitor by 3-methyladenine, a direct autophagy inhibitor for treatment of RAS positive cases | Liu L, Ward D, Leung ELH, Yao XJ, Wong VKW, Luo LX | 09.01.18 | Grant | Liu et al., 2018 [417] | ||
| 4. | Iron homeostasis | Compositions and methods for modulating nuclear receptor coactivator 4 (NCOA4) -mediated autophagic targeting of ferritin | WO 2015149006 | Modulation of the level and activity of nuclear receptor coactivator 4 (NCOA4) | Kimmelman AC, Mancias JD, Harper JW | 26.11.15 | Application | Kimmelman et al., 2015 [418] |
| MicroRNA that regulate autophagy | JPWO 2015037656A1 | miRNA-mediated targeting the WDR45 gene and ATP13A2 gene | Hidenao S, Jun U, Koichi W | 02.03.15 | Application | Hidenao et al., 2015 [419] | ||
| Use of hepcidin as a regulator of iron homeostasis | US7169758B2 | Hepcidin, a key regulator of the entry of iron into the circulation can be used for disorders of iron overload | Nicolas G, Vaulont S, Kahn A | 30.01.07 | Grant | Nicolas et al., 2007 [420] | ||
| Erythroferrone and erfe polypeptides and methods of regulating iron metabolism | CA2890040A1 | Hepcidin concentration can be regulated by herein polypeptides called as erythroferrone and erfe polypeptides | Ganz T, Nemeth E, Kautz L | 08.05.14 | Application | Ganz et al., 2014 [421] |
| S. No. | Name of disorder | Type of disorder | Mutant gene | Outcome of pathological condition | Symptoms | Reference(s) |
|---|---|---|---|---|---|---|
| 1 | Static encephalopathy of childhood with neurodegeneration in adulthood (SENDA) | Neurodegenerative disorder | WIPI4 located at Xp11.23 | WIPI4, homologous to yeast Atg18, is recruited to autophagosome formation site, severely reduced in affected individuals. Iron accumulation in brain | In childhood- early-onset of spastic paraplegia and mental retardation | Ozawa et al. 2014 [445]; Haack et al., 2012 [423]; Saitsu et al., 2013 [424] |
| In adult age- symptoms of parkinsonism and dystonia | ||||||
| 2 | Crohn’s disease | Inflammatory bowel disease | Atg16L1 | Inhibited LC3 conjugations to phosphatidylethanolamine (PE) | Abdominal pain, diarrhea, vomiting, and weight loss | Fujita et al., 2009 [430] |
| 3 | Hereditary spastic paraparesis (HSP) | Increased muscle spasticity | A recessive mutation in TECPR2 | Autophagosome accumulation due to impaired fusion with lysosome | Lower extremity weakness and spasticity | Vantaggiato et al., 2013 [433]; Oz-Levi et al., 2012 [432] |
| 4 | Danon disease | Cardiomyopathy and intellectual dysfunction | Lysosome-associated membrane protein 2 (LAMP-2B isoform) | Accumulation of autophagic vacuoles in liver, kidney, pancreas, and cardiac and skeletal muscles | Weakening of skeletal muscles | D’souza et al., 2014 [446]; Rothaug et al., 2015 [435]; Ng et al., 2016 [436] |
| 5 | X-linked myopathy with excessive autophagy (XMEA) | Skeletal myopathy | VMA21 | Elevated levels of CPK | Weakness in proximal muscles of the legs | Ruggieri et al., 2015 [440]; Dowling et al., 2015 [439] |
| Interrupted sarcolemma membrane homeostasis Impaired autophagy and lysosomal function Vps15 knockouts exhibit muscle pathology similar to the XMEA, indicative of role of aberrant autophagy in XMEA disease. | ||||||
| 6 | Sporadic inclusion body myositis (sIBM) | Progressive muscle disorder | MYH2 | Muscle tissues exhibits both inflammatory and degenerative changes. Impaired autophagy with inhibited lysosomal protein degradation. | Progressive quadriceps femoris and deep finger flexors weakness and atrophy | Mastaglia, 2009 [444]; Nogalska et al., 2010 [442] |
| 7 | Vici syndrome | Callosal agenesis, cataracts, hypopigmentation, cardiomyopathy, psychomotor retardation, and immunodeficiency with cleft lip and palate | Recessive mutations in EPG5 | Deficiency blocks the maturation of autophagosomes into degradative autolysosomes resulting in accumulation of non-degradative autolysosomes. | Psychomotor abnormalities | Cullup et al., 2013 [447] |
| Defect in the endocytic pathway Epg5 deficiency blocks the maturation of autophagosomes into degradative autolysosomes | Facial dysmorphism and cataracts | Zhao et al., 2013 [448] | ||||
| 8 | Lysosomal storage disorder Niemann-Pick type C (NPC) | Neurodegeneration and liver dysfunction | NPC1 or NPC2 | Compromised autophagy with accumulation of autophagosomes as evidenced by elevated LC3-II levels and LC3 positive vesicles in the NPC1 mutant cells Cholesterol accumulation | Increased death of brain and liver cells | Sarkar et al., 2013 [449] |
| 9 | Pompe disease | Pathology of the neuromuscular junction | Lysosomal acid α-glucosidase | Accumulation of glycogen in the nervous system | Muscle atrophy, weakness, loss of muscle function and cardio-respiratory failure | Todd et al., 2015 [450] |
| 10 | Cancer | High percentage of human breast cancers and ovarian cancer | Monoallelic deletion of BECN1 | Activation of inflammasome leading to the maturation of inflammatory cytokines like IL-1β and IL-18 | Cell necrosis, chronic inflammation and ultimate tumorigenesis | Sun et al., 2013 [451] |
| 11 | Myelodysplastic syndromes / acute myeloid leukemia (AML) | Accumulation of damaged mitochondria | Atg7 | Increased levels of ROS | Bone marrow cells are characterized by mitochondrial abnormalities and increased cell death | Watson et al., 2011 [452] |
| 12 | Autoimmune/Autoinflammatory diseases | Immune dysfunction | Atg5 | Disturbance in autophagic vesicle formation, immune cell development and function, mitochondrial ROS, antimicrobial immunity [(retinoic acid receptor responder 3 (RARRES3) and mitochondrial anti-viral signalling protein (MAVS)] | Autoimmune diseases, susceptibility to infections, e.g., HIV, bacteria | Ye et al., 2018 [453] |
| 13 | Autoimmune thyroid diseases | Autoimmune thyroid diseases and Graves’ disease | IRGM | Lymphocytic infiltration in thyroid, antibodies to antigens including thyroid-stimulating hormone receptor (TSHR), thyroid antigens including thyroglobulin (Tg), and thyroid peroxidase (TPO), anti-TSHR autoantibodies | Graves’ disease, hyperthyroidism | Yao et al., 2018 [454] |
| 14 | Paget disease of bone (PDB) | Osteoclast size, number and activity increase causing bone cleavage, cytoplasmic inclusions with protein aggregates responsible for autophagy | Atg2B, Atg5 | Focal bone disorder affecting the skeleton segmentally, bone defects, softening, breakage | Bone defects, softening, breakage, pain | Usategui-Martín et al., 2015 [455] |
| 15 | Celiac disease in children | Chronic systemic autoimmune disease of the small intestine, gluten induced damage resulting in celiac sprue and gluten-sensitive enteropathy | Atg7 and Beclin 1 | Chronic systemic autoimmune disorder, enteropathy | Diarrhea, dehydration, indigestion, decreased appetite, stomach-ache and bloating, poor growth, and weight loss | Comincini et al., 2017 [456] |
| 16 | Huntington disease | Neurodegenerative disorder, CAG trinucleotide repeats in the 5′ coding region of the IT15 (Interesting Transcript 15) gene of 4th chromosome | IT15 | Neurodegeneration, deformity in autosomal-lysosome degradation, neurodegenerative proteinopathy, accumulation of toxic materials | Depression, apathy, irritability, suicidal behaviours, involuntary movements/chorea, progressive dementia, severe weight loss | Barboza and Ghisi, 2018 [457] |
| 17 | Cardiac disease | Cardiac autophagy disorder | Beclin 1, microRNAs (miRNAs) | Over or under expression of genes regulating cardiac autophagy (Beclin 1), miRNAs regulate cardiac autophagy by suppressing the expression of autophagy-related genes in a targeted manner | Signs of cardiovascular disease- like heart attack, pain, fainting, dysrhythmia | Shirakabe et al., 2016 [458]; Sun et al., 2018 [459] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khandia, R.; Dadar, M.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Iqbal, H.M.N.; Singh, K.P.; Joshi, S.K.; et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells 2019, 8, 674. https://doi.org/10.3390/cells8070674
Khandia R, Dadar M, Munjal A, Dhama K, Karthik K, Tiwari R, Yatoo MI, Iqbal HMN, Singh KP, Joshi SK, et al. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells. 2019; 8(7):674. https://doi.org/10.3390/cells8070674
Chicago/Turabian StyleKhandia, Rekha, Maryam Dadar, Ashok Munjal, Kuldeep Dhama, Kumaragurubaran Karthik, Ruchi Tiwari, Mohd. Iqbal Yatoo, Hafiz M.N. Iqbal, Karam Pal Singh, Sunil K. Joshi, and et al. 2019. "A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy" Cells 8, no. 7: 674. https://doi.org/10.3390/cells8070674
APA StyleKhandia, R., Dadar, M., Munjal, A., Dhama, K., Karthik, K., Tiwari, R., Yatoo, M. I., Iqbal, H. M. N., Singh, K. P., Joshi, S. K., & Chaicumpa, W. (2019). A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells, 8(7), 674. https://doi.org/10.3390/cells8070674









