Epilepsy in Tubulinopathy: Personal Series and Literature Review
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nabbout, R.; Scheffer, I.E. Genetics of idiopathic epilepsies. Handb. Clin. Neurol. 2013, 111, 567–578. [Google Scholar]
- Barkovich, A.J.; Guerrini, R.; Kuzniecky, R.I.; Jackson, G.D.; Dobyns, W.B. A developmental and genetic classification for malformations of cortical development: Update. Brain 2012, 135, 1348–1369. [Google Scholar] [CrossRef]
- Andrade, D.M. Genetic basis in epilepsies caused by malformations of cortical development and in those with structurally normal brain. Hum. Genet. 2009, 126, 1731–1793. [Google Scholar] [CrossRef]
- Guerrini, R.; Dobyns, W.B. Malformations of cortical development: Clinical features and genetic causes. Lancet Neurol. 2014, 13, 710–726. [Google Scholar] [CrossRef]
- Guerrini, R.; Barba, C. Malformations of cortical development and aberrant cortical networks: Epileptogenesis and functional organization. J. Clin. Neurophysiol. 2010, 27, 372–379. [Google Scholar] [CrossRef]
- Kuzniecky, R. Epilepsy and malformations of cortical development: New developments. Curr. Opin. Neurol. 2015, 28, 151–157. [Google Scholar] [CrossRef]
- Parrini, E.; Conti, V.; Dobyns, W.B.; Guerrini, R. Genetic basis of brain malformations. Mol. Syndr. 2016, 7, 220–233. [Google Scholar] [CrossRef]
- Kato, M. Genotype-phenotype correlation in neuronal migration disorders and cortical dysplasias. Front Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Fang, A.; Li, R.; Bai, Y.; Kriegstein, A.R.; Wang, X. The dynamics of neuronal migration. Adv. Exp. Med. Biol 2014, 800, 25–36. [Google Scholar]
- Romaniello, R.; Arrigoni, F.; Bassi, M.T.; Borgatti, R. Mutations in α- and β-tubulin encoding genes: Implications in brain malformations. Brain Dev. 2015, 37, 273–280. [Google Scholar] [CrossRef]
- Jaglin, X.H.; Chelly, J. Tubulin-related cortical dysgeneses: Microtubule dysfunction underlying neuronal migration defects. Trends Genet. 2009, 25, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Keays, D.A.; Tian, G.; Poirier, K.; Huang, G.J.; Siebold, C.; Cleak, J.; Piñon, M.C. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 2007, 128, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Mutch, C.A.; Poduri, A.; Sahin, M.; Barry, B.; Walsh, C.A.; Barkovich, A.J. Disorders of microtubule function in neurons: Imaging correlates. Ajnr. Am. J. Neuroradiol. 2016, 37, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Tischfield, M.A.; Engle, E.C. Distinct alpha- and beta-tubulin isotypes are required for the positioning, differentiation and survival of neurons: New support for the ‘multi-tubulin’ hypothesis. Biosci. Rep. 2010, 30, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Pilz, D.T.; Babatz, T.D.; Cushion, T.D.; Harvey, K.; Topf, M.; Rees, M.I. TUBA1A mutations cause wide spectrum lissencephaly (smooth brain) and suggest that multiple neuronal migration pathways converge on alpha tubulins. Hum. Mol. Genet. 2010, 19, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Tsai, L.H. Micro TUB (B3) ules and brain development. Cell 2009, 140, 30–32. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Cederquist, G.Y.; Gupta, M.L., Jr.; Engle, E.C. Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr. Opin. Genet. Dev. 2011, 21, 286–294. [Google Scholar] [CrossRef]
- Cushion, T.D.; Dobyns, W.B.; Mullins, J.G.; Stoodley, N.; Chung, S.K.; Fry, A.E.; Uyanik, G. Overlapping cortical malformations and mutations in TUBB2B and TUBA1A. Brain 2013, 136, 536–548. [Google Scholar] [CrossRef]
- Poirier, K.; Saillour, Y.; Bahi-Buisson, N.; Jaglin, X.H.; Fallet-Bianco, C.; Nabbout, R.; Genevieve, D. Mutations in the neuronal β-tubulin subunit TUBB3 result in malformation of cortical development and neuronal migration defects. Hum. Mol. Genet. 2010, 19, 4462–4473. [Google Scholar] [CrossRef]
- Morris-Rosendahl, D.J.; Najm, J.; Lachmeijer, A.M.A.; Sztriha, L.; Martins, M.; Kuechler, A.; Vasconcelos, C. Refining the phenotype of alpha-1a Tubulin (TUBA1A) mutation in patients with classical lissencephaly. Clin. Genet. 2008, 74, 425–433. [Google Scholar] [CrossRef]
- Tian, G.; Jaglin, X.H.; Keays, D.A.; Francis, F.; Chelly, J.; Cowan, N.J. Disease-associated mutations in TUBA1A result in a spectrum of defects in the tubulin folding and heterodimer assembly pathway. Hum. Mol. Genet. 2010, 19, 3599–3613. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.C.; Oostra, A.; Desprechins, B.; De Vlaeminck, Y.; Verhelst, H.; Regal, L.; Verloo, P.; Bockaert, N.; Keymolen, K.; Seneca, S.; et al. TUBA1A mutations: From isolated lissencephaly to familial polymicrogyria. Neurology 2011, 76, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Romaniello, R.; Tonelli, A.; Arrigoni, F.; Baschirotto, C.; Triulzi, F.; Bresolin, N.; Borgatti, R. A novel mutation in the β-tubulin gene TUBB2B associated with complex malformation of cortical development and deficits in axonal guidance. Dev. Med. Child. Neurol. 2012, 54, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Sohal, A.P.; Montgomery, T.; Mitra, D.; Ramesh, V. TUBA1A mutation-associated lissencephaly: Case report and review of the literature. Pediatr. Neurol. 2012, 46, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Okumura, A.; Hayashi, M.; Tsurui, H.; Yamakawa, Y.; Abe, S.; Kudo, T.; Yamamoto, T. Lissencephaly with marked ventricular dilation, agenesis of corpus callosum, and cerebellar hypoplasia caused by TUBA1A mutation. Brain Dev. 2013, 35, 274–279. [Google Scholar] [CrossRef]
- Poirier, K.; Saillour, Y.; Fourniol, F.; Francis, F.; Souville, I.; Valence, S.; Beldjord, C. Expanding the spectrum of TUBA1A-related cortical dysgenesis to Polymicrogyria. Eur. J. Hum. Genet. 2013, 21, 381–385. [Google Scholar] [CrossRef]
- Zanni, G.; Colafati, G.S.; Barresi, S.; Randisi, F.; Talamanca, L.F.; Genovese, E.; Bertini, E. Description of a novel TUBA1A mutation in Arg-390 associated with asymmetrical polymicrogyria and mid-hindbrain dysgenesis. Eur. J. Paediatr. Neurol. 2013, 17, 361–365. [Google Scholar] [CrossRef]
- Amrom, D.; Tanyalcin, I.; Verhelst, H.; Deconinck, N.; Brouhard, G.J.; Décarie, J.C.; Michaud, J.L. Polymicrogyria with dysmorphic basal ganglia? Think tubulin! Clin. Genet. 2014, 85, 178–183. [Google Scholar] [CrossRef]
- Blumkin, L.; Halevy, A.; Ben-Ami-Raichman, D.; Dahari, D.; Haviv, A. Expansion of the spectrum of TUBB4A-related disorders: A new phenotype associated with a novel mutation in the TUBB4A gene. Neurogenetics 2014, 15, 107–113. [Google Scholar]
- Hikita, N.; Hattori, H.; Kato, M.; Sakuma, S.; Morotomi, Y.; Ishida, H.; Tokuhara, D. A case of TUBA1A mutation presenting with lissencephaly and Hirschsprung disease. Brain Dev. 2014, 36, 159–162. [Google Scholar] [CrossRef]
- Shimojima, K.; Narita, A.; Maegaki, Y.; Saito, A.; Furukawa, T.; Yamamoto, T. Whole-exome sequencing identifies a de novo TUBA1A mutation in a patient with sporadic malformations of cortical development: A case report. BMC Res. Notes 2014, 7, 465. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.A.; Bello-Espinosa, L.E.; Kherani, A.; Wei, X.C.; Innes, A.M. TUBA1A Mutation associated with eye abnormalities in addition to brain malformation. Pediatr. Neurol. 2015, 53, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, S.; Ishihara, N.; Miya, F.; Tsutsumi, M.; Yanagihara, I.; Fujita, N.; Yamasaki, M. TUBA1A mutation can cause a hydranencephaly-like severe form of cortical dysgenesis. Sci. Rep. 2015, 5, 15165. [Google Scholar] [CrossRef] [PubMed]
- Bahi-Buisson, N.; Cavallin, M. Tubulinopathies Overview. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- Laquerriere, A.; Gonzales, M.; Saillour, Y.; Cavallin, M.; Joyē, N.; Quēlin, C.; Chelly, J. De novo TUBB2B mutation causes fetal akinesia deformation sequence with microlissencephaly: An unusual presentation of tubulinopathy. Eur. J. Med. Genet. 2016, 59, 249–256. [Google Scholar] [CrossRef]
- Gardner, J.; Cushion, T.; Niotakis, G.; Olson, H.; Grant, P.; Scott, R.; Bonnières, M. Clinical and functional characterization of the recurrent TUBA1A p. (Arg2His) mutation. Brain Sci. 2018, 8, 145. [Google Scholar] [CrossRef]
- Romaniello, R.; Arrigoni, F.; Fry, A.E.; Bassi, M.T.; Rees, M.I.; Borgatti, R.; Cushion, T.D. Tubulin genes and malformations of cortical development. Eur. J. Med. Genet. 2018, 61, 744–754. [Google Scholar] [CrossRef]
- Sato, T.; Kato, M.; Moriyama, K.; Haraguchi, K.; Saitsu, H.; Matsumoto, N.; Moriuchi, H. A case of tubulinopathy presenting with porencephaly caused by a novel missense mutation in the TUBA1A gene. Brain Dev. 2018, 40, 819–823. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Li, S.; Jiang, N.; Guo, J.; Zhang, W.; Xie, J. De novo mutated TUBB2B associated pachygyria diagnosed by medical exome sequencing and long-range PCR. Fetal Pediatr. Pathol. 2018, 1–9. [Google Scholar] [CrossRef]
- Oegema, R.; Cushion, T.D.; Phelps, I.G.; Chung, S.K.; Dempsey, J.C.; Collins, S.; Dobyns, W.B. Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum. Mol. Genet. 2015, 24, 5313–5325. [Google Scholar] [CrossRef]
- Romaniello, R.; Arrigoni, F.; Panzeri, E.; Poretti, A.; Micalizzi, A.; Citterio, A.; Ferraris, A. Tubulin-related cerebellar dysplasia: Definition of a distinct pattern of cerebellar malformation. Eur. Radiol. 2017, 27, 5080–5092. [Google Scholar] [CrossRef]
- Arrigoni, F.; Romaniello, R.; Peruzzo, D.; Poretti, A.; Bassi, M.T.; Pierpaoli, C.; Triulzi, F. The spectrum of brainstem malformations associated to mutations of the tubulin genes family: MRI and DTI analysis. Eur. Radiol. 2019, 29, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Leventer, R.J.; Guerrini, R.; Dobyns, W.B. Malformations of cortical development and epilepsy. Dialog. Clin. Neurosci. 2008, 10, 47–62. [Google Scholar]
- Romaniello, R.; Arrigoni, F.; Cavallini, A.; Tenderini, E.; Baschirotto, C.; Triulzi, F.; Borgatti, R. Brain malformations and mutations in α- and β-tubulin genes: A review of the literature and description of two new cases. Dev. Med. Child. Neurol. 2014, 56, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Prontera, P.; Stangoni, G.; Mencaroni, E.; Principi, N.; Esposito, S. Epileptogenic brain malformations and mutations in tubulin genes: A case report and review of the literature. Int. J. Mol. Sci. 2017, 18, 2273. [Google Scholar] [CrossRef] [PubMed]
- Poirier, K.; Keays, D.A.; Francis, F.; Saillour, Y.; Bahi, N.; Manouvrier, S.; Bienvenu, T. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A). Hum. Mutat. 2007, 28, 1055–1064. [Google Scholar] [CrossRef]
- Bahi-Buisson, N.; Poirier, K.; Boddaert, N.; Saillour, Y.; Castelnau, L.; Philip, N.; Bourgeois, M. Refinement of cortical dysgeneses spectrum associated with TUBA1A mutations. J. Med. Genet. 2008, 45, 647–653. [Google Scholar] [CrossRef]
- Bahi-Buisson, N.; Poirier, K.; Fourniol, F.; Saillour, Y.; Valence, S.; Lebrun, N.; Lascelles, K. The wide spectrum of tubulinopathies: What are the key features for the diagnosis? Brain 2014, 137, 1676–1700. [Google Scholar] [CrossRef]
- Rektor, I.; Kuba, R.; Bràzdil, M.; Chrastina, J. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav. 2012, 25, 56–59. [Google Scholar] [CrossRef]
- Recio, M.V.; Gallagher, M.J.; McLean, M.J.; Abou-Khalil, B. Clinical features of epilepsy in patients with cerebellar structural abnormalities in a referral center. Epilepsy Res. 2007, 76, 1–5. [Google Scholar] [CrossRef]
- Ivanova, E.L.; Gilet, J.G.; Sulimenko, V.; Duchon, A.; Rudolf, G.; Runge, K.; Tilly, P. TUBG1 missense variants underlying cortical malformations disrupt neuronal locomotion and microtubule dynamics but not neurogenesis. Nat. Commun. 2019, 10, 2129. [Google Scholar] [CrossRef]
- Barkovich, J. Complication begets clarification in classification. Brain 2013, 136, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Breuss, M.; Keays, D.A. Microtubules and neurodevelopmental disease: The movers and the makers. Adv. Exp. Med. Biol. 2014, 800, 75–96. [Google Scholar] [PubMed]
- Uribe, V. The beta-tubulin gene TUBB2B is involved in a large spectrum of neuronal migration disorders. Clin. Genet. 2010, 77, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Breuss, M.; Morandell, J.; Nimpf, S.; Gstrein, T.; Lauwers, M.; Hochstoeger, T.; Suplata, M. The expression of tubb2b undergoes a developmental transition in murine cortical neurons. J. Comp. Neurol. 2015, 523, 2161–2186. [Google Scholar] [CrossRef] [PubMed]
- Jaglin, X.H.; Poirier, K.; Saillour, Y.; Buhler, E.; Tian, G.; Bahi-Buisson, N.; Castelnau-Ptakhine, L. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. 2009, 41, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Cederquist, G.Y.; Luchniak, A.; Tischfield, M.A.; Peeva, M.; Song, Y.; Menezes, M.P.; Gomes, L. An inherited TUBB2B mutation alters a kinesinbinding site and causes polymicrogyria, CFEOM, and axon dysinnervation. Hum. Mol. Genet. 2012, 21, 5484–5499. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, R.; Mei, D.; Cordelli, D.M.; Pucatti, D.; Franzoni, E.; Parrini, E. Symmetric polymicrogyria and pachygyria associated with TUBB2B gene mutations. Eur. J. Hum. Genet. 2012, 20, 995–998. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Baris, H.N.; Wu, C.; Rudolph, G.; Van Maldergem, L.; He, W.; Mackey, D.A. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 2010, 140, 74–87. [Google Scholar] [CrossRef]
- Saillour, Y.; Broix, L.; Bruel-Jungerman, E.; Lebrun, N.; Muraca, G.; Rucci, J.; Chelly, J. Beta tubulin isoforms are not interchangeable for rescuing impaired radial migration due to Tubb3 knockdown. Hum. Mol. Genet. 2013, 23, 1516–1526. [Google Scholar] [CrossRef]
- Xu, X.; Shangguan, Y.; Lu, S.; Wang, W.; Du, C.; Xiao, F.; Yang, Y. Tubulin β-III modulates seizure activity in epilepsy. J. Pathol. 2017, 242, 297–308. [Google Scholar] [CrossRef]
- Guerrini, R. Epilepsy in children. Lancet 2006, 367, 499–524. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R.; Fields, R.D. EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol. 2015, 126, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Tanaka, N.; Diaz, J.; Edlow, B.L.; Wu, O.; Hämäläinen, M.; Kramer, M.A. EEG functional connectivity is partially predicted by underlying white matter connectivity. Neuroimage 2015, 108, 23–33. [Google Scholar] [CrossRef] [PubMed]
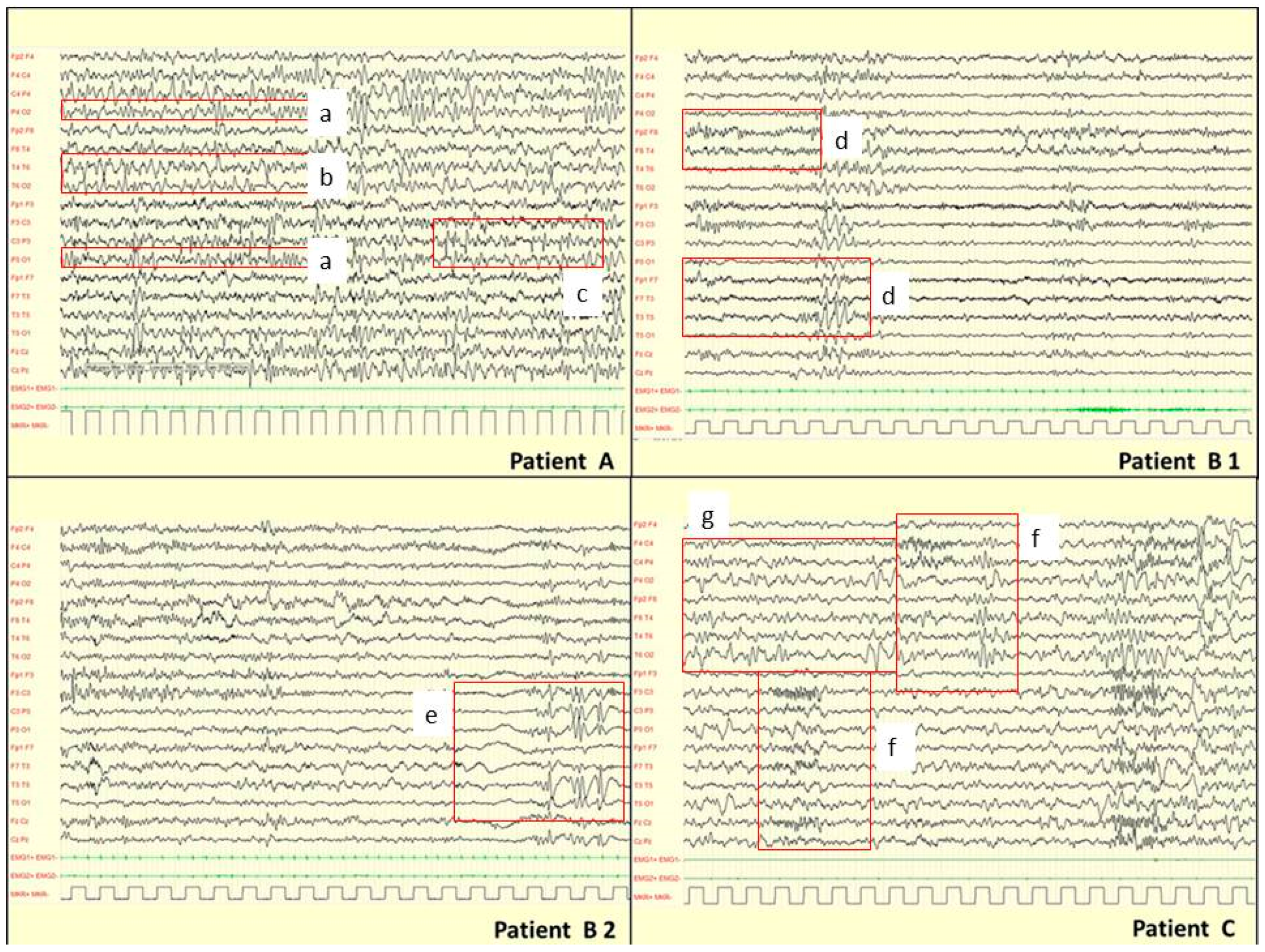
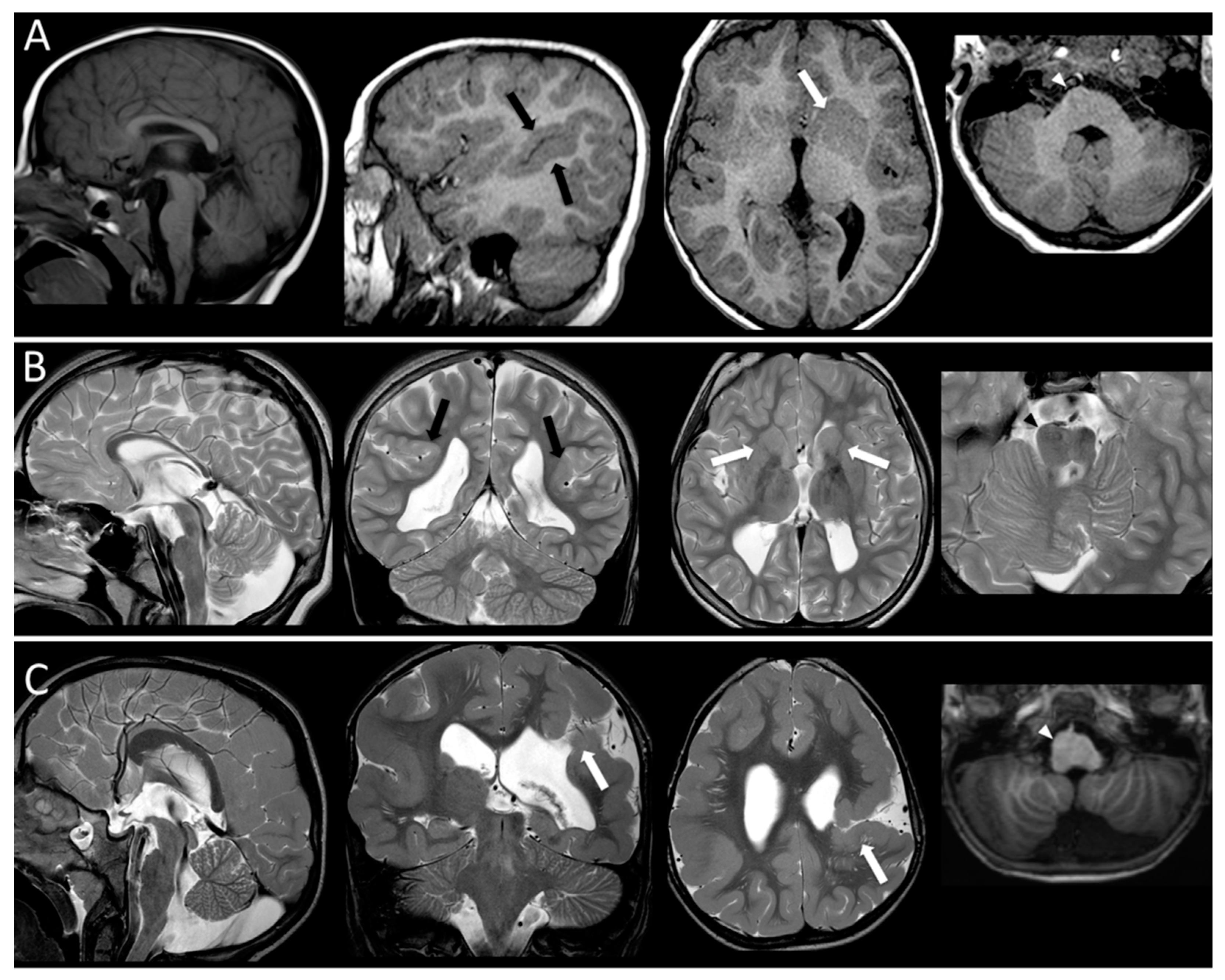

| Gender, Age | Epileptic Syndrome | Seizure Type | Age at Onset | Awake EEG BA | Awake EEG EA | Sleep EEG BA | Sleep EEG EA | Response to AEDs | MCDs on MRI | Genetic Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EPILEPTIC PATIENTS | |||||||||||
| TUBA1A | |||||||||||
| P45617 | F, 13 yrs 3 mo | Focal symptomatic epilepsy | Spasms, FS | 18 mo, 3 yrs | Irregular Low voltage | 0 | Irregular BFA Asynchronous | SA EA Right TO | Controlled (ACTH; VPA) | Perisylvian dysgyria | c.466C > G (p.R156G) |
| P89815 § | F, 3 yrs 3 mo | Focal symptomatic epilepsy | FS | 21 days | Irregular Asym Slowing BFA | SA paroxysmal Rght CO | Irregular Asym Asynchronous | SA EA Right CO Left CT asynchronous | Controlled (LEV) | PMG-multi | c.4C > A (p.R2S) |
| 17656 § | M, 13 yrs 4 mo | Focal symptomatic epilepsy | Myoclonic, focal SE, SF | 3 mo | Irregular Slowing | 0 | Irregular Asym | SA paroxysmal -EA Left C PO | Controlled (VPA, ETS) | No MCD | c.1169G > A (p.R390H) |
| TUBB2B | |||||||||||
| P76712 § | M, 10 yrs 9 mo | Focal symptomatic epilepsy | Spasms, FS | 18 mo | Irregular Asym BFA | SA Left CT | Irregular Asym BFA | SA EA Left CT and diffuse | Partially controlled (VPA) | Generalized PMG + SCH | c.1060T > C (p.C354R) de novo |
| P38408 § | F, 16 yrs 8 mo | Focal symptomatic epilepsy | Spasm, FS | 5 mo | Irregular Asym Slowing BFA | 0 | Irregular Asym BFA NEM | SA EA Right C | Controlled (VPA, LTG) | Generalized PMG + SCH | c.419G > C (p.G140A) de novo |
| P78511 § | F, 39 yrs | Focal symptomatic epilepsy | Spasms, FS | 7 mo | Irregular Slow voltage BFA | SA paroxysmal bilateral diffuse positive ILS | NA | NA | Controlled (ACTH, PB, CBZ) | Symp_Gyr, periv heterotopia, subcortical linear heterotopia, small temporal lobes | c.1080_1084delCCTGAinsACATCTTC [p.L361_K362delinsHLQ) de novo |
| NON EPILEPTIC PATIENTS | |||||||||||
| TUBA1A | |||||||||||
| P78411 § | M, 7 yrs 6 mo | no | no | / | Irregular Asym Slowing BFA | no | Irregular Asym BFA Asynchronous | SA C bilateral Left predominance | / | No MCD | c.175G > A (p.G59S) de novo |
| P113708 § I-3 | M, 56 yrs 4 mo | no | no | / | NA | NA | NA | / | Perisylvian-PMG | c.161G > A (p.S54N) | |
| P113708 § II-1 | F, 25 yrs 4 mo | no | no | / | Irregular Slowing Low voltage | 0 | NA | NA | / | Perisylvian-PMG | c.161G > A (p.S54N) |
| P113708 § II-2 | F, 25 yrs 4 mo | no | no | / | Irregular Slowing Low voltage BFA | 0 | NA | NA | / | Perisylvian-PMG | c.161G > A (p.S54N) |
| P76111 § | F, 12 yrs 3 mo | no | no | / | Irregular Asym. Slowing BFA Low voltage | 0 | Irregular Asym Asynchronous | SA bilateral diffuse | / | No MCD | c.1160C > T (p.A387V) de novo |
| TUBB3 | |||||||||||
| P17816 § | F, 4 yrs | no | no | / | NO | NA | NA | NA | / | no MCD | c.1228G > A (p.E410K) de novo |
| 105814 § | M, 4 yrs 9 mo | no | no | / | Irregular Slowing | 0 | Irregular Asym Asynchronous | SA Right PO and diffuse | / | no MCD | c.862G > A (p.E288K) de novo |
| P120818 | M, 34 yrs | no | no | / | NA | NA | NA | NA | / | No MCD | c.689C > T (p.S230L) |
| P109418 | M, 12 yrs | no | no | / | Irregular Slowing | SA PO bilateral | Irregular Asynchronous | SA paroxysmal PO bilateral | / | Perisylvian PMG | c.728C > T (p.P243L) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romaniello, R.; Zucca, C.; Arrigoni, F.; Bonanni, P.; Panzeri, E.; Bassi, M.T.; Borgatti, R. Epilepsy in Tubulinopathy: Personal Series and Literature Review. Cells 2019, 8, 669. https://doi.org/10.3390/cells8070669
Romaniello R, Zucca C, Arrigoni F, Bonanni P, Panzeri E, Bassi MT, Borgatti R. Epilepsy in Tubulinopathy: Personal Series and Literature Review. Cells. 2019; 8(7):669. https://doi.org/10.3390/cells8070669
Chicago/Turabian StyleRomaniello, Romina, Claudio Zucca, Filippo Arrigoni, Paolo Bonanni, Elena Panzeri, Maria T. Bassi, and Renato Borgatti. 2019. "Epilepsy in Tubulinopathy: Personal Series and Literature Review" Cells 8, no. 7: 669. https://doi.org/10.3390/cells8070669
APA StyleRomaniello, R., Zucca, C., Arrigoni, F., Bonanni, P., Panzeri, E., Bassi, M. T., & Borgatti, R. (2019). Epilepsy in Tubulinopathy: Personal Series and Literature Review. Cells, 8(7), 669. https://doi.org/10.3390/cells8070669






