Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Brain Tissue Collection
2.3. Hippocampus Corticosterone Assay
2.4. Immunohistochemistry
2.5. Cell culture and Reagents
2.6. CORT Treatment
2.7. Cell Counting kit-8 (CCK-8) Assay
2.8. Annexin V-FITC/Propidium Iodide (PI) Staining
2.9. Reactive Oxygen Species (ROS) Assay
2.10. ATP/ADP/AMP Analyses
2.11. Measurement of the Mitochondrial Membrane Potential
2.12. Determination of Malondialdehye (MDA) Activity
2.13. Hippocampus Tissue and Cell Protein Extraction
2.14. Nuclear Protein Extraction
2.15. Western Blot Analysis
2.16. Cell Immunofluorescence
2.17. Binding Assay to the Antioxidant Response Element (ARE) Promoter
2.18. Co-Immunoprecipitation (CO-IP) Analysis
2.19. Transmission Electron Microscopy
2.20. Statistical Analysis
3. Results
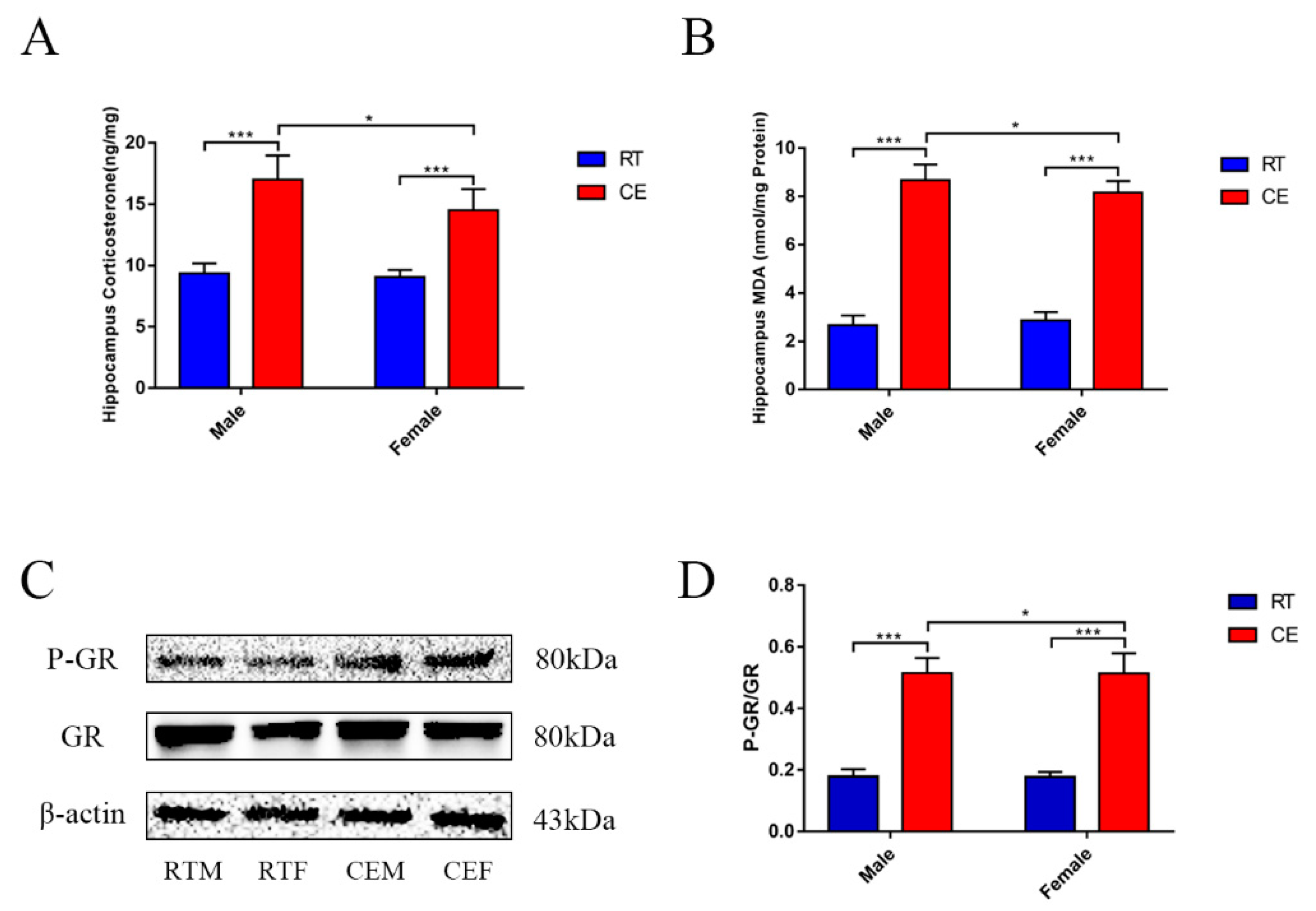
3.1. CORT Levels and Activated GR Expression in the Mouse Hippocampus Following Cold Exposure
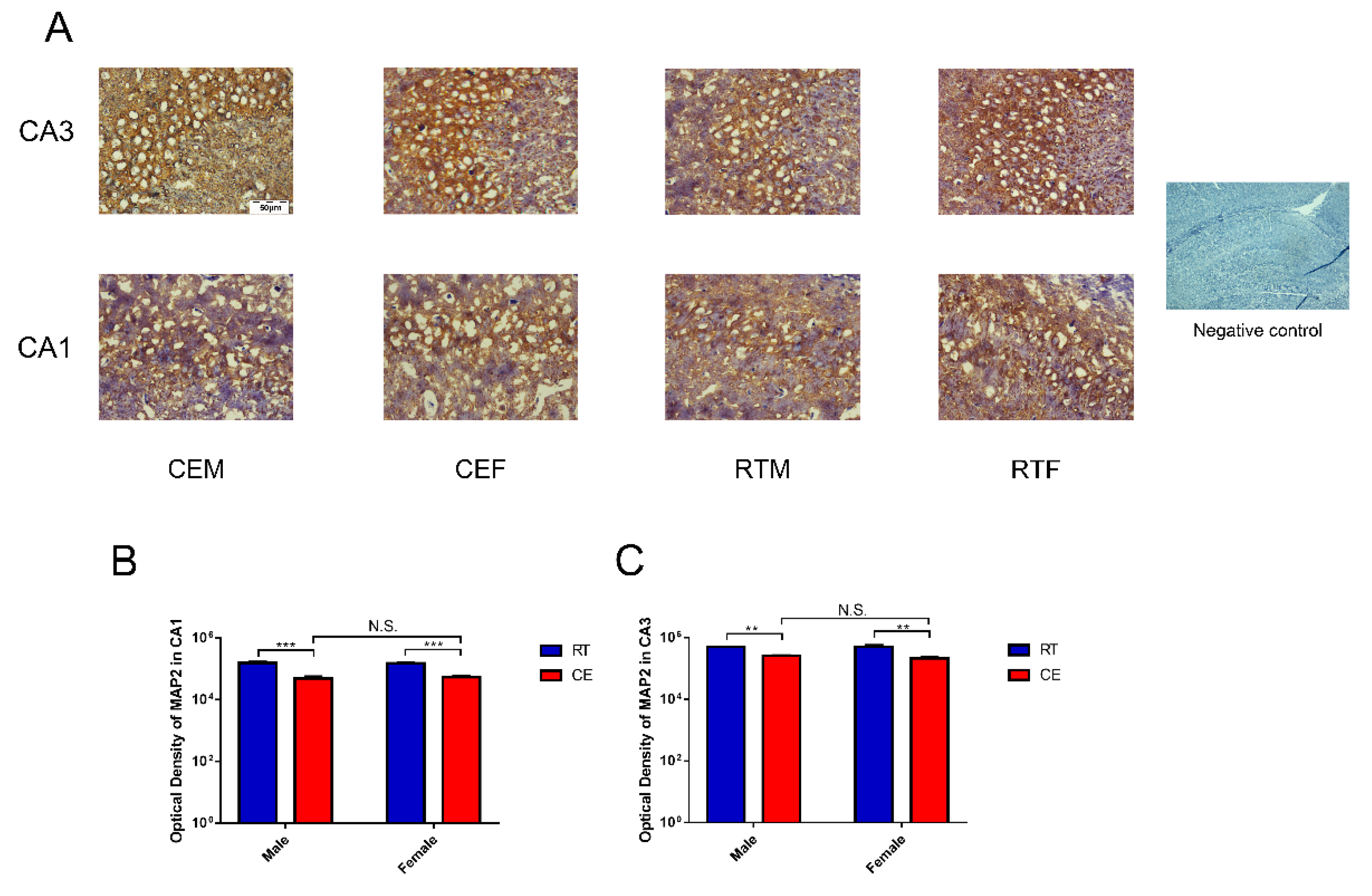
3.2. MAP2 Expression Levels and the Oxidative Stress Relevant Signaling Pathway in Hippocampus Tissue following Cold Exposure
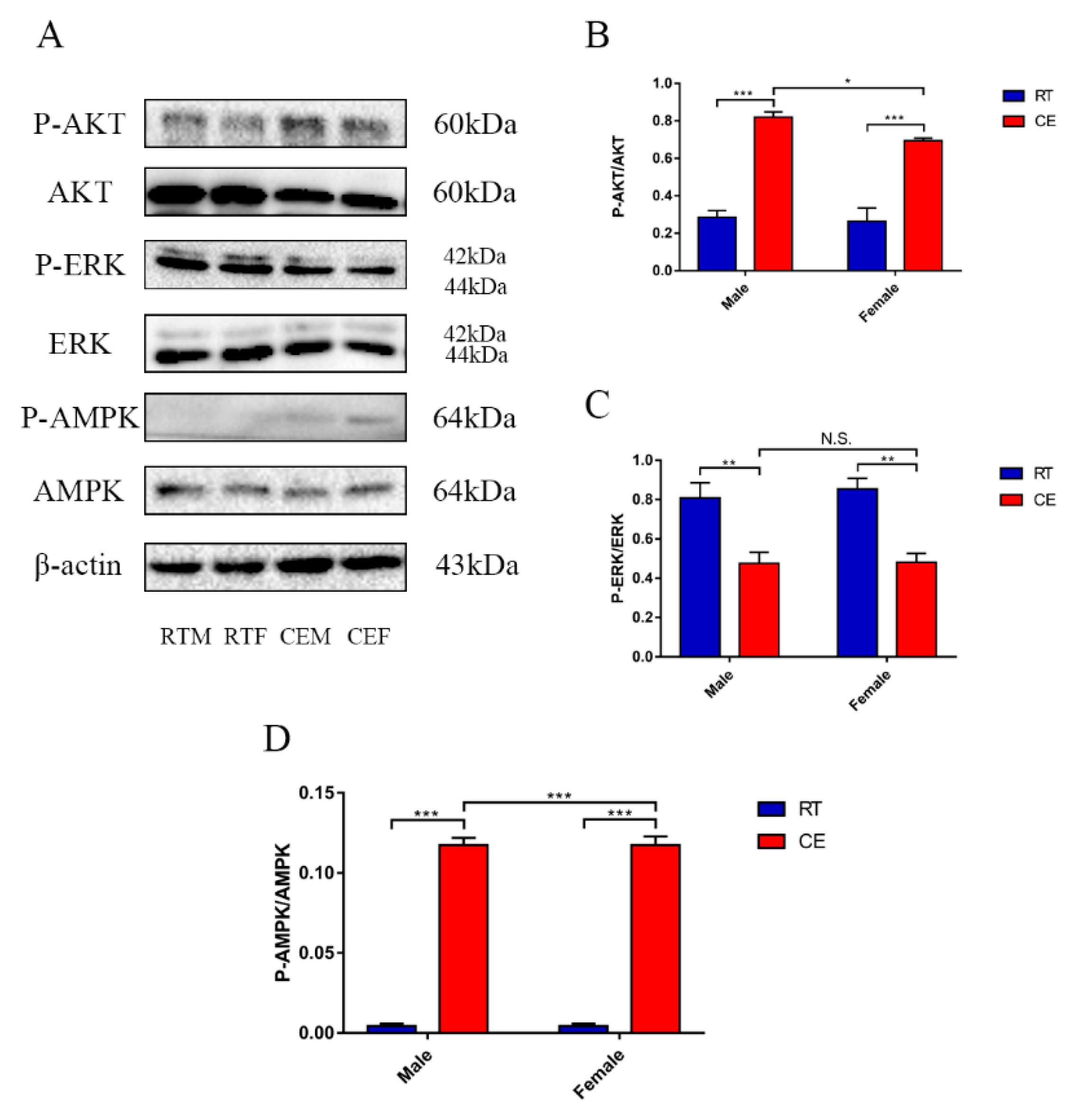
3.3. The Relevant Signaling Pathway Responses to Cold Stress of the Hippocampus
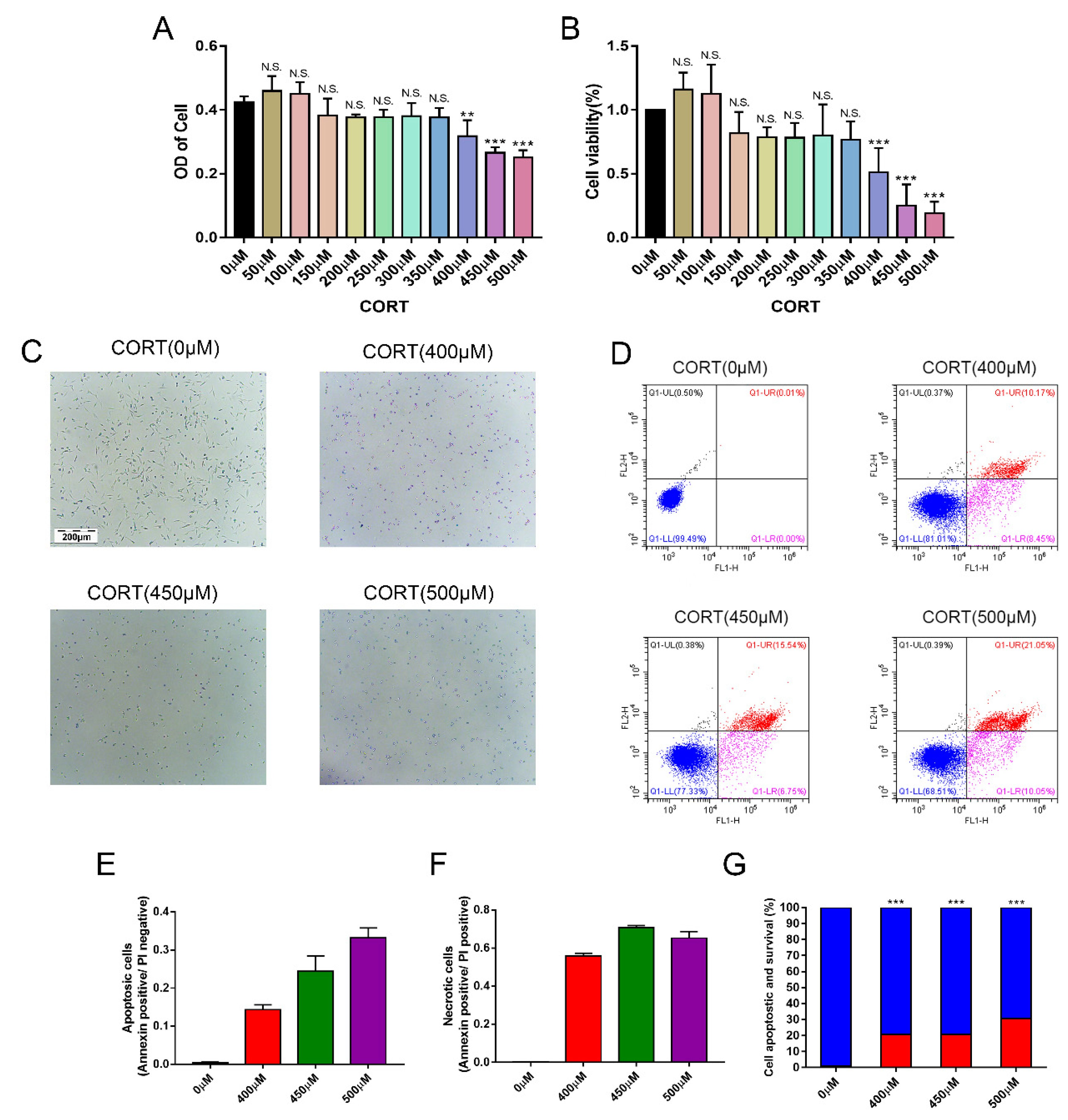
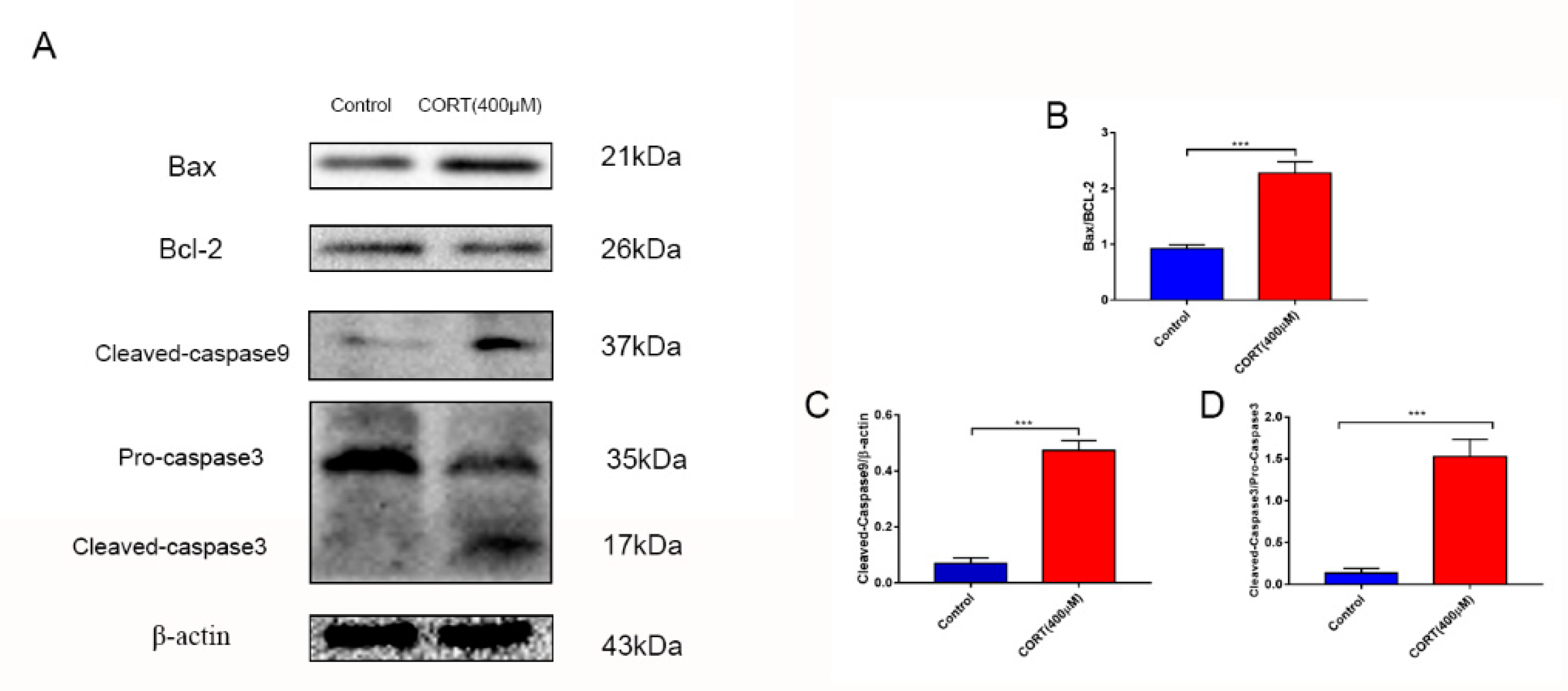
3.4. Apoptosis Induced by Excess CORT in HT22 Cells
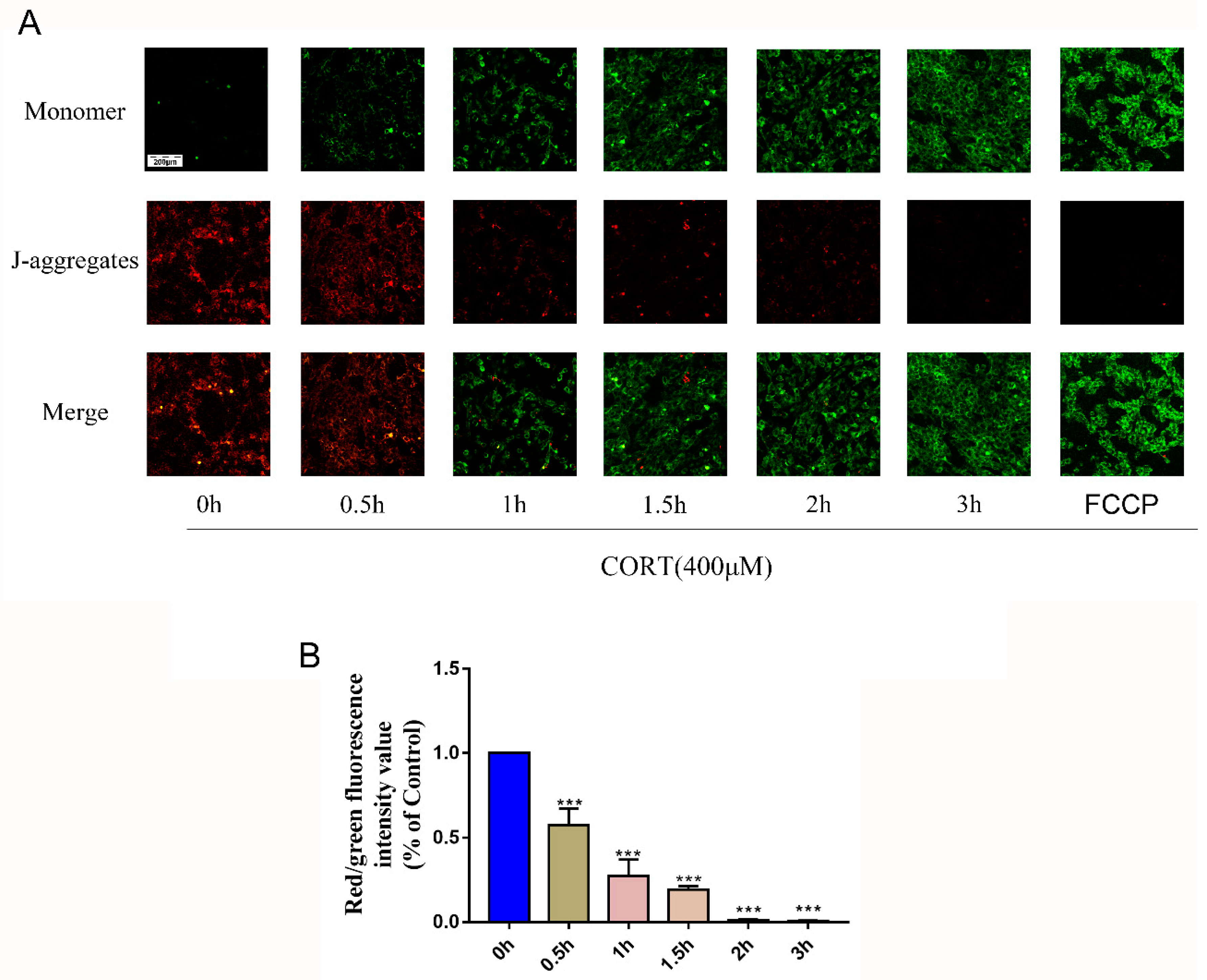
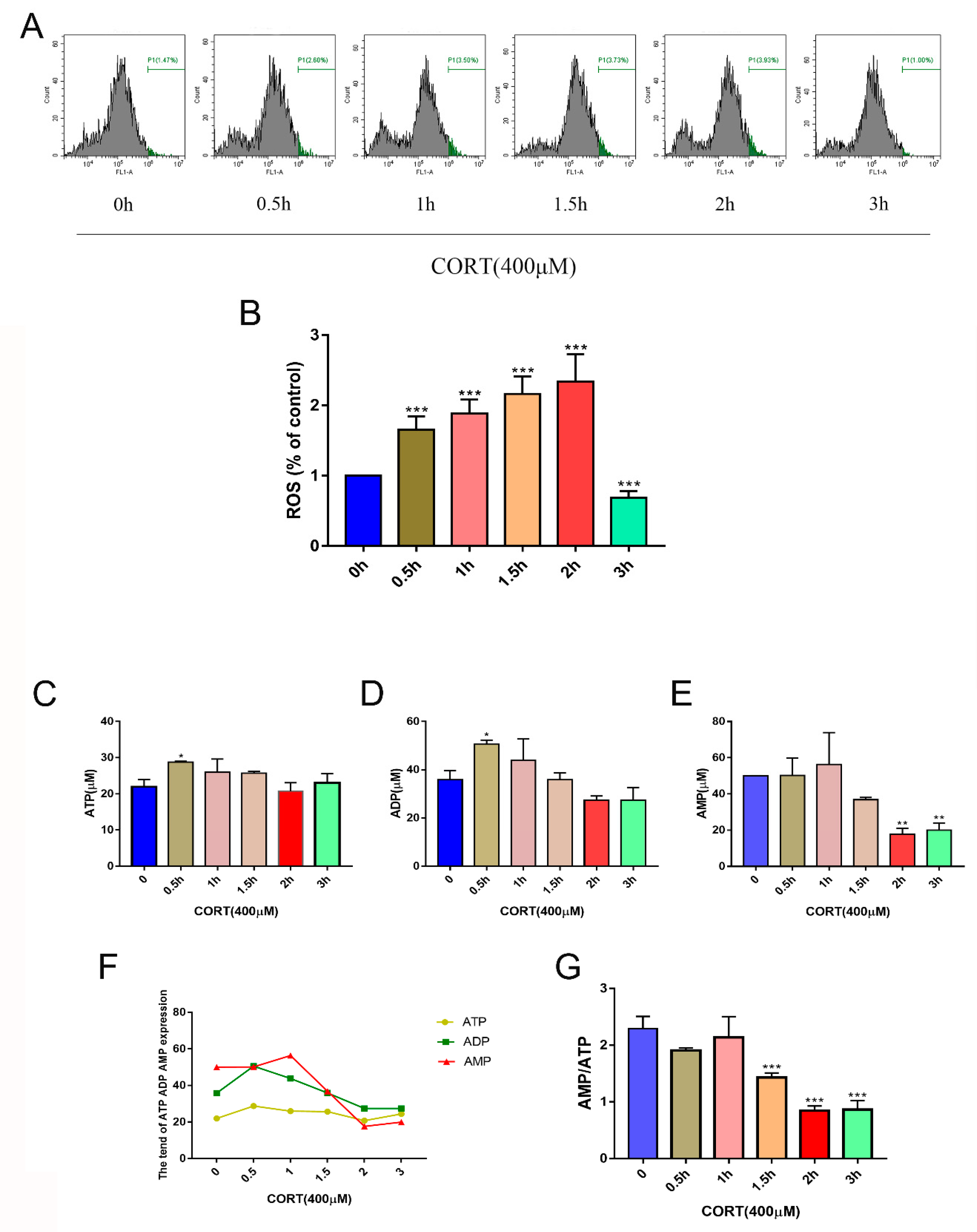
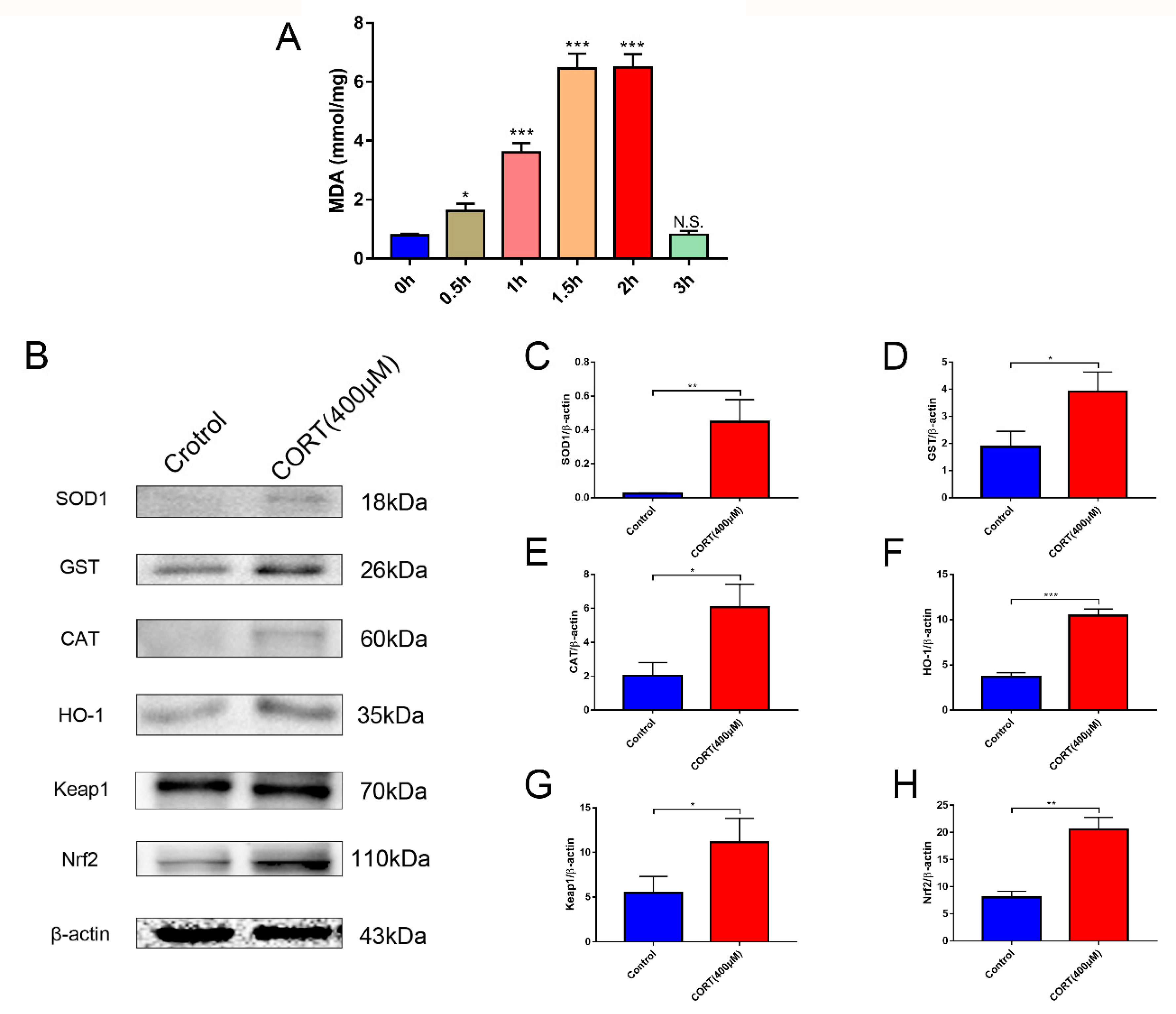
3.5. Oxidation Induced by Excess CORT in HT22 Cells
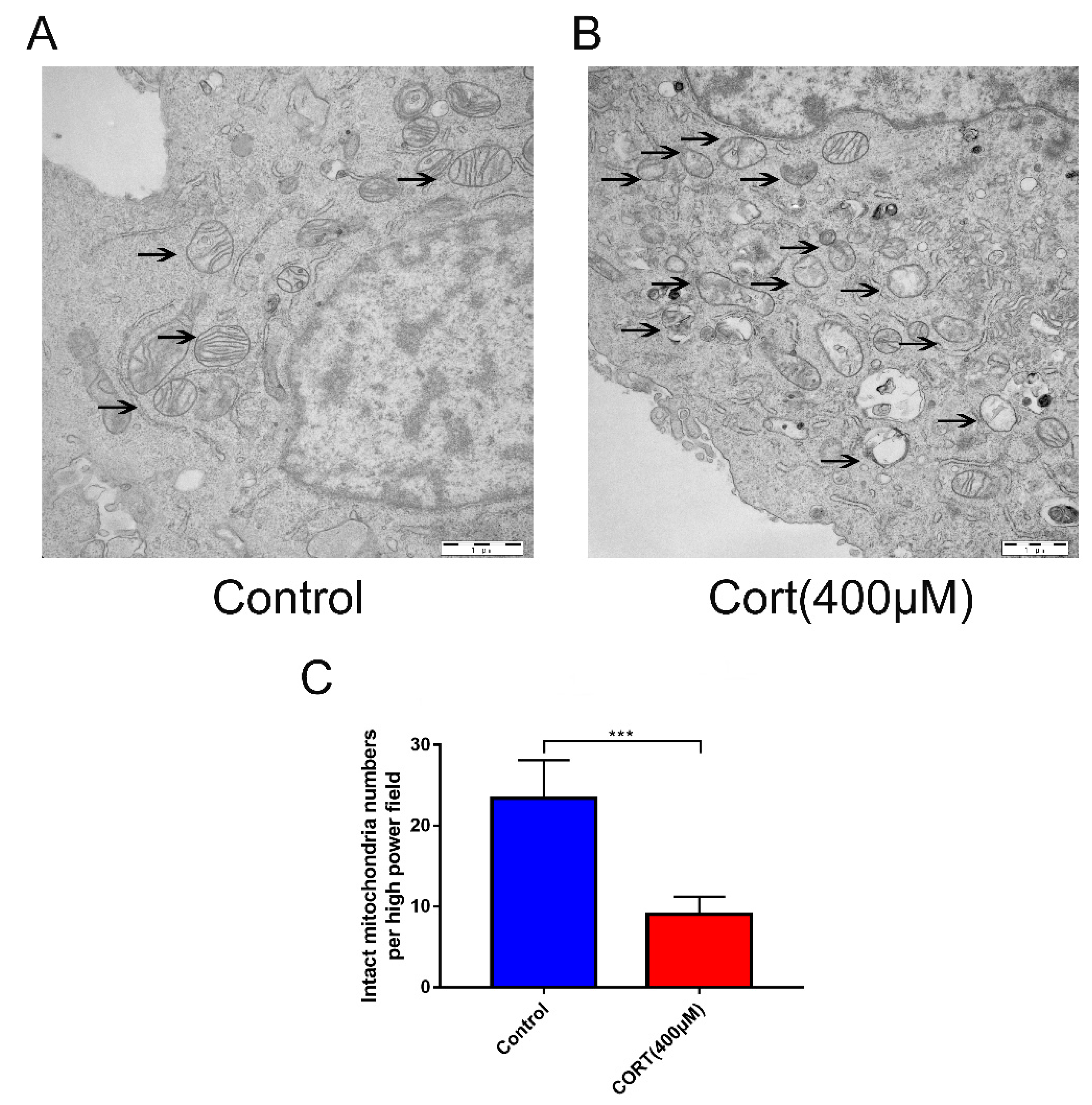
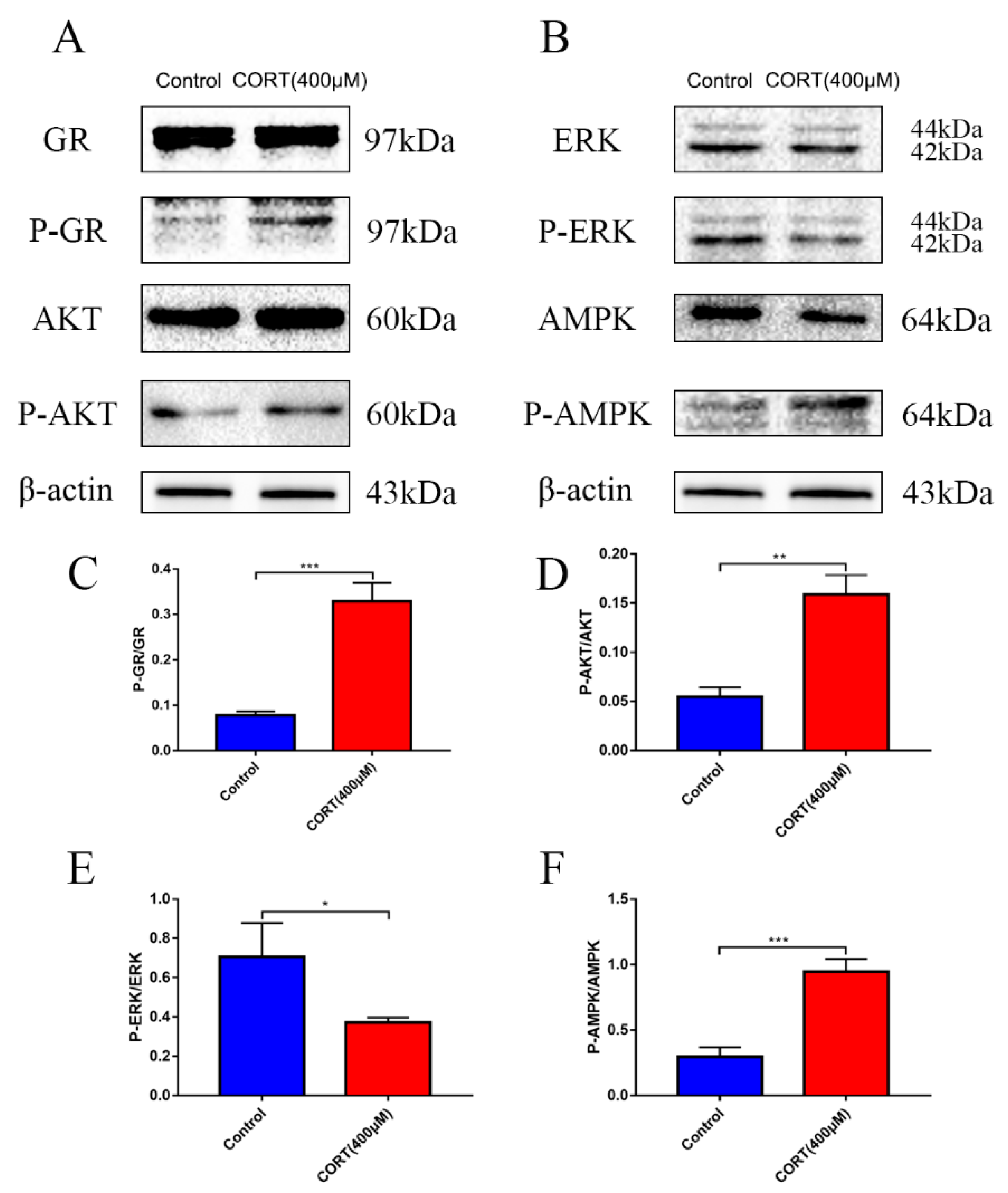
3.6. The Relevant Mechanism of Oxidative Stress Induced by Excess CORT Treatment of HT22 Cells
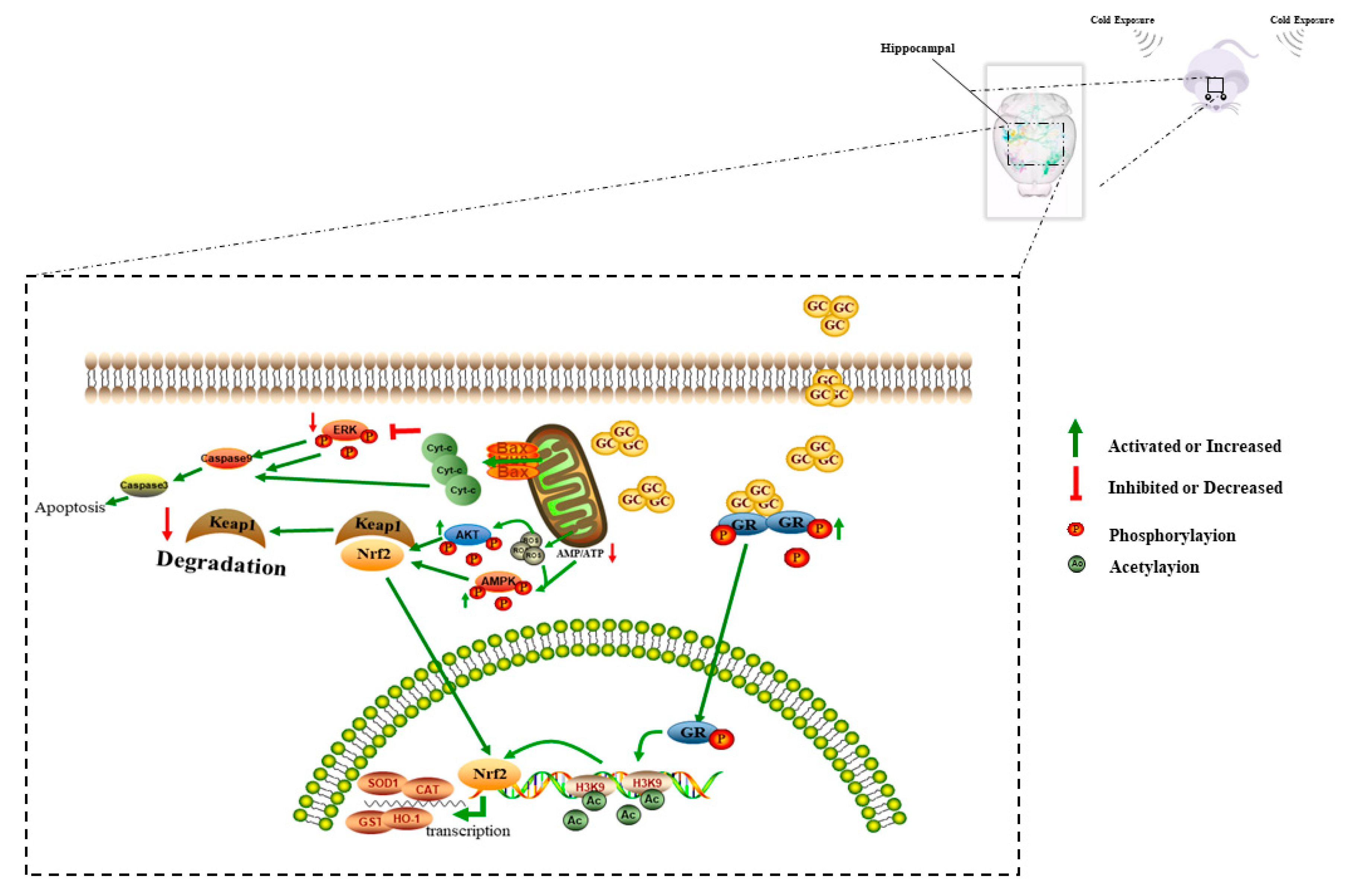
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, D.L.; Burke, R.J. Gender, Work Stress, and Health; American Psychological Association: Washington, WA, USA, 2002. [Google Scholar]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the art review: Depression, stress, anxiety, and cardiovascular disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Contractor, A.A.; Elhai, J.D.; Fine, T.H.; Tamburrino, M.B.; Cohen, G.; Shirley, E.; Chan, P.K.; Liberzon, I.; Galea, S.; Calabrese, J.R. Latent profile analyses of posttraumatic stress disorder, depression and generalized anxiety disorder symptoms in trauma-exposed soldiers. J. Psychiatr. Res. 2015, 68, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Zouikr, I.; Patience, M.; Clarkson, A.N.; Isgaard, J.; Johnson, S.J.; Spratt, N.; Nilsson, M.; Walker, F.R. Chronic stress exacerbates neuronal loss associated with secondary neurodegeneration and suppresses microglial-like cells following focal motor cortex ischemia in the mouse. Brain Behav. Immun. 2015, 48, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Martony, M.; Hernandez, J.A.; de Wit, M.; St Leger, J.; Erlacher-Reid, C.; Vandenberg, J.; Stacy, N.I. Clinicopathological prognostic indicators of survival and pathological findings in cold-stressed Florida manatees Trichechus manatus latirostris. Dis. Aquat Organ. 2019, 132, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Byun, G.; Choi, Y.; Song, H.; Lee, J.T. Difference in temporal variation of temperature-related mortality risk in seven major South Korean cities spanning 1998–2013. Sci. Total Environ. 2019, 656, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Cichon, M.; Chadzinska, M.; Ksiazek, A.; Konarzewski, M. Delayed effects of cold stress on immune response in laboratory mice. Proc. Biol. Sci. 2002, 269, 1493–1497. [Google Scholar] [CrossRef]
- Wu, M.; ong, L.; Zheng, X.; Zhang, L.; Liu, B.; Wang, L.; Li, H.; Xiong, C.; Cao, Z.; Wang, Y.; et al. Prenatal exposure of diurnal temperature range and preterm birth: Findings from a birth cohort study in China. Sci. Total Environ. 2019, 656, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zang, S.-C.; Li, S.-Z.; Guo, J.-R.; Wang, J.-F.; Wang, D.; Zhang, L.-P.; Yang, H.-M.; Lian, S. HMGB1-mediated differential response on hippocampal neurotransmitter disorder and neuroinflammation in adolescent male and female mice following cold exposure. Brain Behav. Immun. 2019, 76, 223–235. [Google Scholar]
- Dong, H.W.; Swanson, L.W.; Chen, L.; Fanselow, M.S.; Toga, A.W. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc. Natl. Acad. Sci. USA 2009, 106, 11794–11799. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.L.; Pathak, S.D.; Jeromin, A.; Ng, L.L.; MacPherson, C.R.; Mortrud, M.T.; Cusick, A.; Riley, Z.L.; Sunkin, S.M.; Bernard, A.; et al. Genomic anatomy of the hippocampus. Neuron 2008, 60, 1010–1021. [Google Scholar] [CrossRef]
- Stone, S.S.; Teixeira, C.M.; Zaslavsky, K.; Wheeler, A.L.; Martinez-Canabal, A.; Wang, A.H.; Sakaguchi, M.; Lozano, A.M.; Frankland, P.W. Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 2011, 21, 1348–1362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Aguilera, G. Vasopressin protects hippocampal neurones in culture against nutrient deprivation or glutamate-induced apoptosis. J. Neuroendocr. 2010, 22, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, U. Alzheimer’s disease. Der Radiol. 2015, 55, 386–388. [Google Scholar]
- McEwen, B.S.; Gray, J.D.; Nasca, C. 60 years of neuroendocrinology: Redefining neuroendocrinology: Stress, sex and cognitive and emotional regulation. J. Endocrinol. 2015, 226, T67–T83. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res. 2016, 1645, 50–54. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vasc. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, J.; Tao, L.; Shen, Z.; Li, H.; Mo, F.; Wang, X.; Wang, S.; Shen, H. Corticosterone increases intracellular Zn(2+) release in hippocampal HT-22 cells. Neurosci. Lett. 2015, 588, 172–177. [Google Scholar] [CrossRef]
- Zhang, C.S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Xu, B.; Lian, S.; Li, S.-Z.; Guo, J.-R.; Wang, J.-F.; Wang, D.; Zhang, L.-P.; Yang, H.-M. GABAB receptor mediate hippocampal neuroinflammation in adolescent male and female mice after cold expose. Brain Res. Bull. 2018, 142, 163–175. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016, 41, 3. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.-J.C.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Frank, M.G.; Gaudet, A.D.; D’Angelo, H.M.; Daut, R.A.; Hampson, E.C.; Ayala, M.T.; Watkins, L.R.; Maier, S.F. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav. Immun. 2018, 70, 257–267. [Google Scholar] [CrossRef]
- Romeo, R.D.; Patel, R.; Pham, L.; So, V.M. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci. Biobehav. Rev. 2016, 70, 206–216. [Google Scholar] [CrossRef]
- Yeste, M.; Estrada, E.; Rocha, L.; Marín, H.; Rodríguez-Gil, J.; Miró, J. Cryotolerance of stallion spermatozoa is related to ROS production and mitochondrial membrane potential rather than to the integrity of sperm nucleus. Andrology 2015, 3, 395–407. [Google Scholar] [CrossRef]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, D.; Liu, D.; Cui, Q.; Cai, H.; Li, Q.; Chen, H.; Kong, M.G. Production of simplex RNS and ROS by nanosecond pulse N2/O2 plasma jets with homogeneous shielding gas for inducing myeloma cell apoptosis. J. Phys. D Appl. Phys. 2017, 50, 195204. [Google Scholar] [CrossRef]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Suchy, M.-T.; Trettin, A.; Modun, D.; Stuke, N.; Maassen, N.; Frölich, J.C. Development, validation and biomedical applications of stable-isotope dilution GC–MS and GC–MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15 (S)-8-iso-prostaglandin F2α and nitric oxide (NO). J. Chromatogr. B 2016, 1019, 95–111. [Google Scholar]
- Lu, M.C.; Ji, J.A.; Jiang, Z.Y.; You, Q.D. The Keap1–Nrf2–ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.K.; Deepthi, N.; Sultana, R.; Banerjee, S.K. Resveratrol ameliorates cardiac oxidative stress in diabetes through deacetylation of NFkB-p65 and histone 3. J. Nutr. Biochem. 2015, 26, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Schaffer, B.E.; Brunet, A. AMPK: An energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016, 26, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Guan, L.; Zhang, F.; Zhang, W.; Ding, W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J. Appl. Toxicol. 2016, 36, 48–59. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Lang, L.-m.; Li, S.-Z.; Guo, J.-R.; Wang, J.-F.; Wang, D.; Zhang, L.-P.; Yang, H.-M.; Lian, S. Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure. Cells 2019, 8, 612. https://doi.org/10.3390/cells8060612
Xu B, Lang L-m, Li S-Z, Guo J-R, Wang J-F, Wang D, Zhang L-P, Yang H-M, Lian S. Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure. Cells. 2019; 8(6):612. https://doi.org/10.3390/cells8060612
Chicago/Turabian StyleXu, Bin, Li-min Lang, Shi-Ze Li, Jing-Ru Guo, Jian-Fa Wang, Di Wang, Li-Ping Zhang, Huan-Min Yang, and Shuai Lian. 2019. "Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure" Cells 8, no. 6: 612. https://doi.org/10.3390/cells8060612
APA StyleXu, B., Lang, L.-m., Li, S.-Z., Guo, J.-R., Wang, J.-F., Wang, D., Zhang, L.-P., Yang, H.-M., & Lian, S. (2019). Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure. Cells, 8(6), 612. https://doi.org/10.3390/cells8060612



