Extracellular Localisation of the C-Terminus of DDX4 Confirmed by Immunocytochemistry and Fluorescence-Activated Cell Sorting
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunostaining of Human Tissue
2.1.1. DAB Staining
2.1.2. Cell Culture and Transfections, and Generation of the pFLAG-DDX4-myc Construct
2.1.3. Double Digests to Confirm Full-Length DDX4 Is Present within the Construct
2.1.4. Western Blot
2.1.5. pFLAG-CD2-myc
2.2. Immunostaining
2.2.1. Immunofluorescence
2.2.2. FACS
2.2.3. RNA Extraction and RT-PCR
3. Results
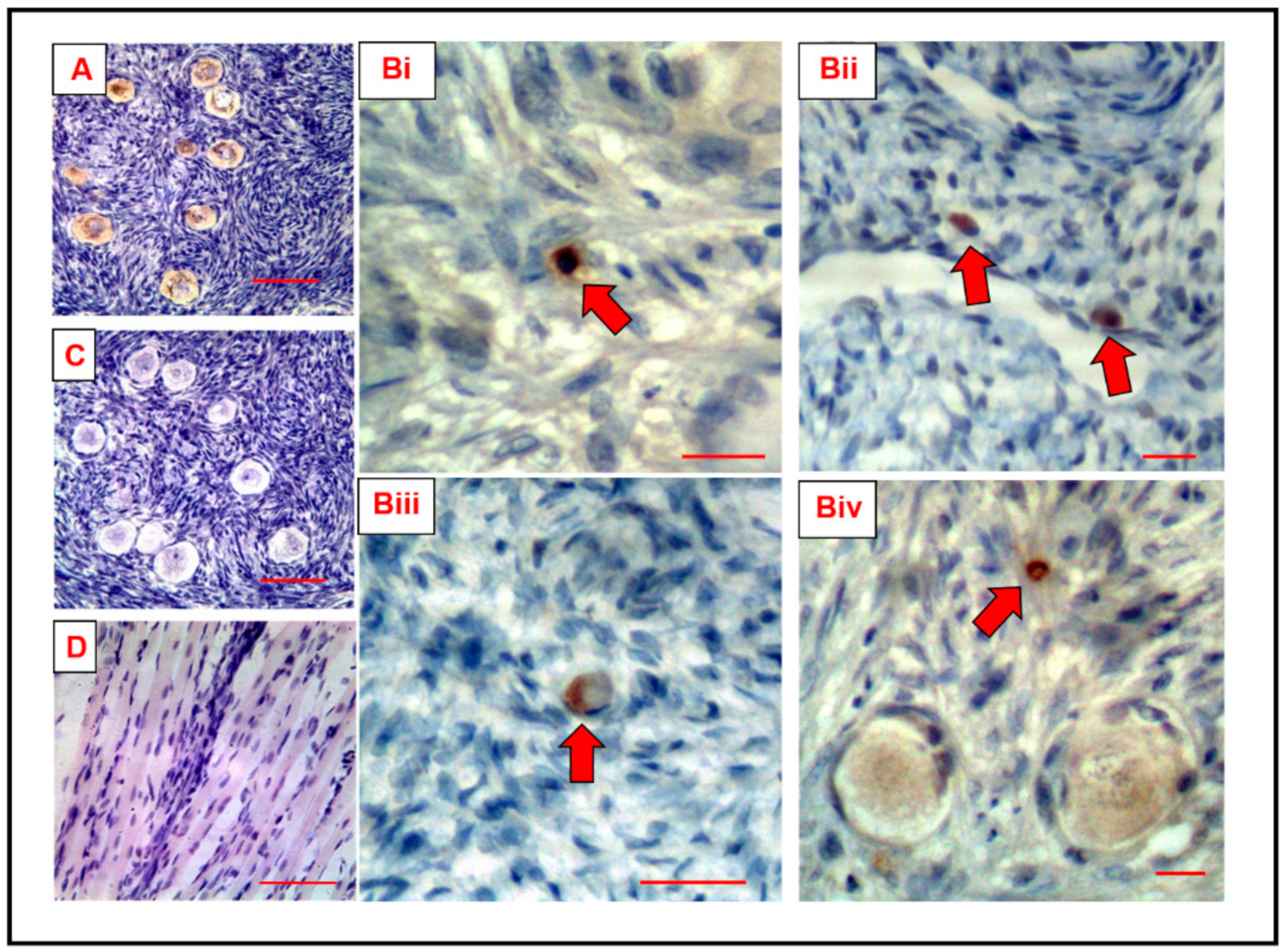
3.1. DDX4 Is Expressed in Small Ovarian Cells, in Addition to Human Oocytes
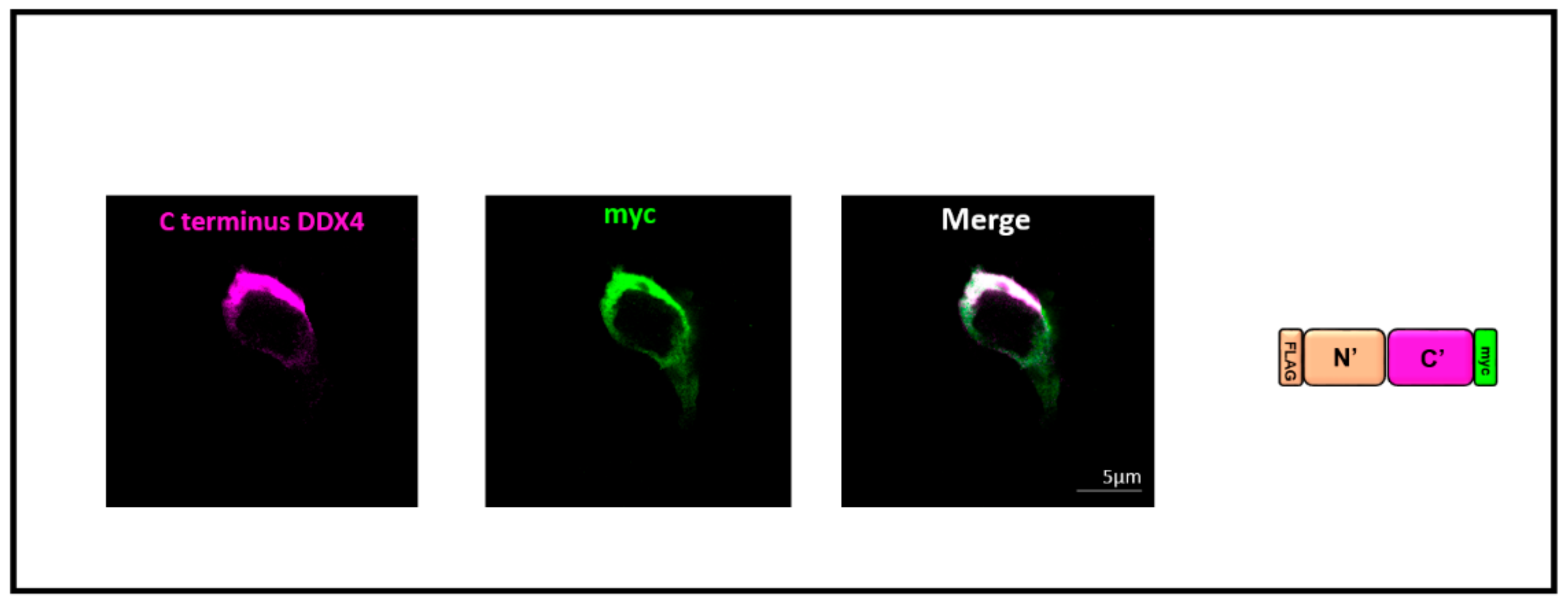
3.2. Double-Epitope-Tagged DDX4 Is Expressed in HEK 293T Cells
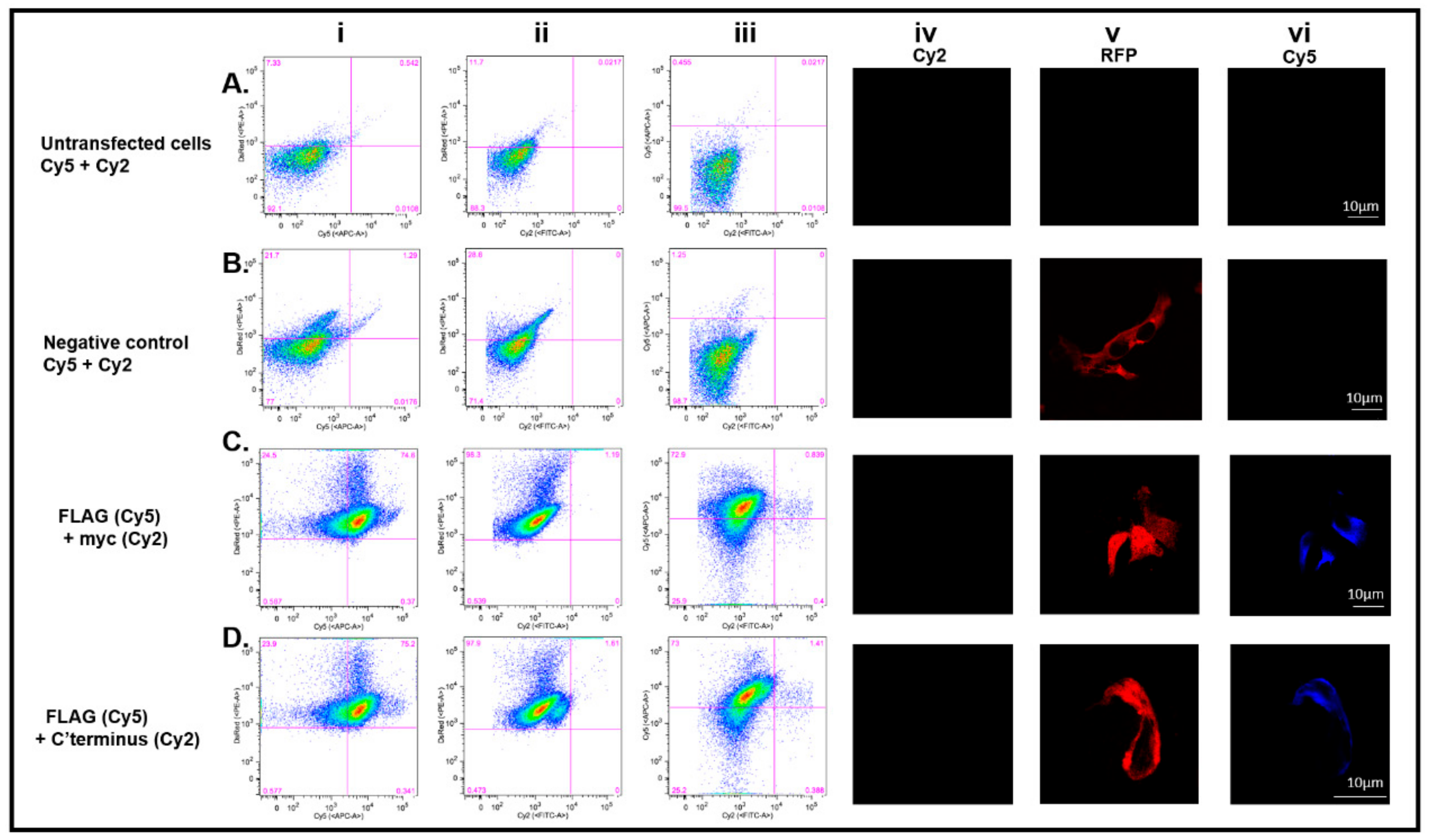
3.3. Immunocytochemistry Confirms That the C-Terminus of DDX4 Has an Extracellular Epitope
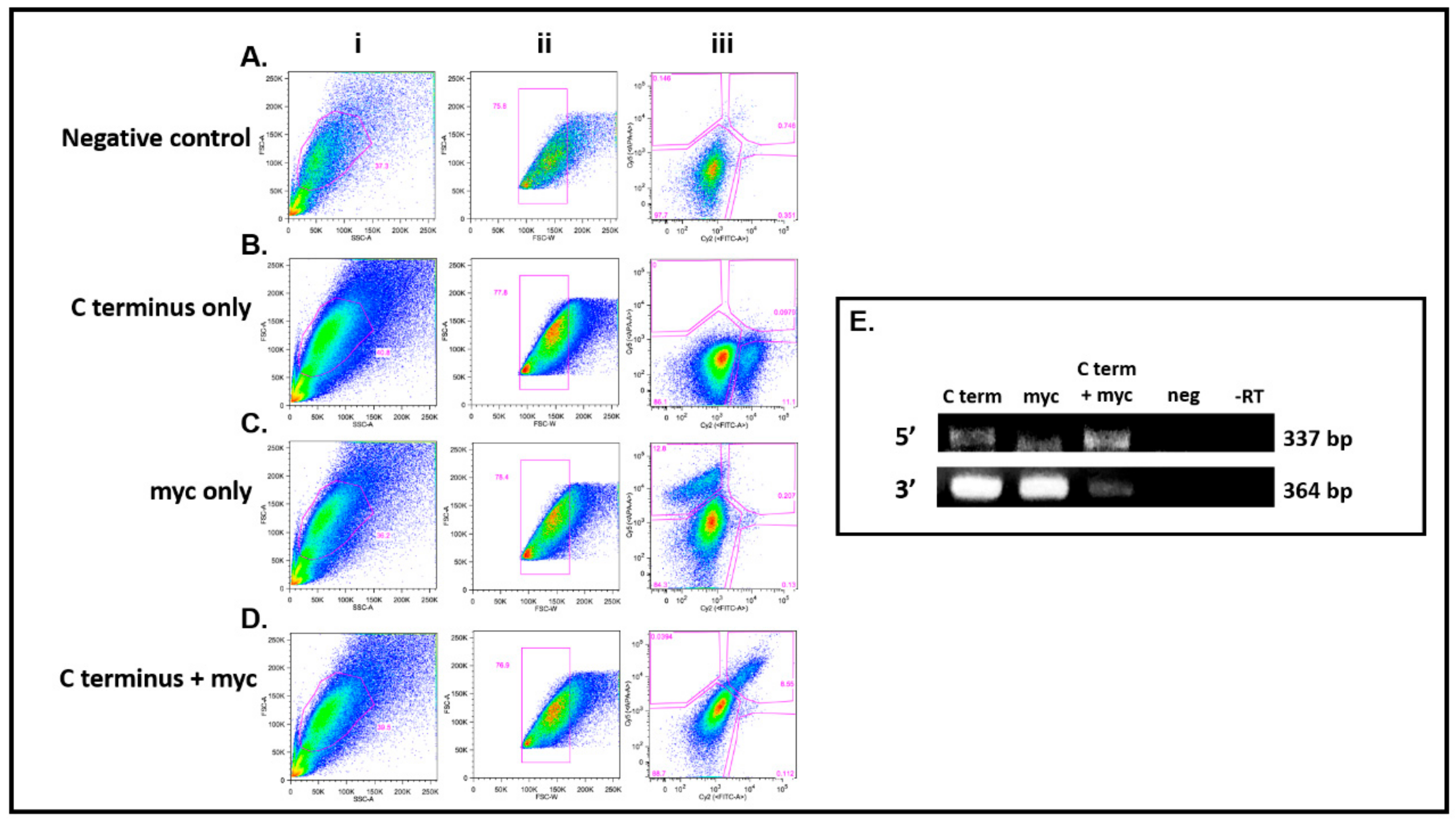
3.4. DDX4-Positive Cells Isolated by FACS
3.5. The C-Terminus DDX4 Antibody Is Specific to DDX4
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozakpinar, O.B.; Maurer, A.M.; Ozsavci, D. Ovarian stem cells: From basic to clinical applications. World. J. Stem. Cells. 2015, 7, 757–768. [Google Scholar] [CrossRef]
- Zuckerman, S. The number of oocytes in the mature ovary. Recent. Prog. Horm. Res. 1951, 6, 63–109. [Google Scholar]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef]
- Zou, K.; Yuan, Z.; Yang, Z.; Luo, H.; Sun, K.; Zhou, L.; Xiang, J.; Shi, L.; Yu, Q.; Zhang, Y.; et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009, 11, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, J.; Maki, C.; Ramos, T.; Marh, J.; Howerton, K.; Wong, J.; Pham, J.; Anorve, S.; Chow, Y.C.; Izadyar, F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation 2010, 79, 159–170. [Google Scholar] [CrossRef] [PubMed]
- White, Y.A.; Woods, D.C.; Takai, Y.; Ishihara, O.; Seki, H.; Tilly, J.L. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat. Med. 2012, 18, 413–421. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Yang, Y.; Wang, S.; Shi, L.; Xie, W.; Sun, K.; Zou, K.; Wang, L.; Xiong, J.; et al. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J. Mol. Cell. Biol. 2011, 3, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Hou, L.; Sun, K.; Xie, W.; Wu, J. Improved efficiency of female germline stem cell purification using fragilis-based magnetic bead sorting. Stem Cell. Dev. 2011, 20, 2197–2204. [Google Scholar] [CrossRef]
- Gong, S.P.; Lee, S.T.; Lee, E.J.; Kim, D.Y.; Lee, G.; Chi, S.G.; Ryu, B.K.; Lee, C.H.; Yum, K.E.; Lee, H.J.; et al. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil. Steril. 2010. [Google Scholar] [CrossRef]
- Kerr, J.B.; Duckett, R.; Myers, M.; Britt, K.L.; Mladenovska, T.; Findlay, J.K. Quantification of healthy follicles in the neonatal and adult mouse ovary: Evidence for maintenance of primordial follicle supply. Reproduction 2006, 132, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Niikura, Y.; Niikura, T.; Tilly, J.L. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging 2009, 1, 971–978. [Google Scholar] [CrossRef]
- Sriraman, K.; Bhartiya, D.; Anand, S.; Bhutda, S. Mouse ovarian very small embryonic-like stem cells resist chemotherapy and retain ability to initiate oocyte-specific differentiation. Rep. Sci. 2015, 22, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fouad, H.; Zoma, W.D.; Salama, S.A.; Wentz, M.J.; Al-Hendy, A. Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Rep. Sci. 2008, 15, 139–146. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Kang, J.X.; Xie, W.; Li, X.; Wu, C.; Xu, B.; Wu, J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol. Hum. Reprod. 2014, 20, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, C.E.; Bayne, R.A.; McLaughlin, M.; Telfer, E.E.; Anderson, R.A. Isolation, purification, and culture of oogonial stem cells from adult human and bovine ovarian cortex. Lancet 2014, 383. [Google Scholar] [CrossRef]
- Anderson, R.; McLaughlin, M.; Woods, D.; Tilly, J.; Telfer, E. Evaluation of oogonial stem cells and neo-oogenesis in ovaries of girls and women with turner syndrome: A pilot study. Hum. Rep. 2013, 28, i52–i55. [Google Scholar] [CrossRef]
- Parte, S.; Bhartiya, D.; Telang, J.; Daithankar, V.; Salvi, V.; Zaveri, K.; Hinduja, I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cell. Dev. 2011, 20, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Stimpfel, M.; Skutella, T.; Cvjeticanin, B.; Meznaric, M.; Dovc, P.; Novakovic, S.; Cerkovnik, P.; Vrtacnik-Bokal, E.; Virant-Klun, I. Isolation, characterization and differentiation of cells expressing pluripotent/multipotent markers from adult human ovaries. Cell Tissue Res. 2013, 354, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Skutella, T. Stem cells in aged mammalian ovaries. Aging 2010, 2, 3–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silvestris, E.; Cafforio, P.; D’Oronzo, S.; Felici, C.; Silvestris, F.; Loverro, G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: Single-cell isolation and molecular characterization. Hum. Rep. 2018, 33, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, Y.L.; McLaughlin, M.; Waterfall, M.; Dunlop, C.E.; Skehel, P.A.; Anderson, R.A.; Telfer, E.E. Initial characterisation of adult human ovarian cell populations isolated by ddx4 expression and aldehyde dehydrogenase activity. Sci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, G.; Xu, B.; Wu, C.; Hui, N.; Ni, X.; Wang, J.; Du, M.; Teng, X.; Wu, J. Human gv oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Kenda-Suster, N.; Smrkolj, S. Small putative nanog, sox2, and ssea-4-positive stem cells resembling very small embryonic-like stem cells in sections of ovarian tissue in patients with ovarian cancer. J. Ovarian. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Zech, N.; Rozman, P.; Vogler, A.; Cvjeticanin, B.; Klemenc, P.; Malicev, E.; Meden-Vrtovec, H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008, 76, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Wolff, E.F.; Libfraind, L.L.; Weitzel, R.P.; Woods, D.; Feng, Y.; Tilly, J.; DeCherney, A.H.; Tisdale, J.F. In Oogonial stem cells generate mature oocytes in an autologous rhesus macaque transplantation model. Fertil. Steril. 2013. [Google Scholar] [CrossRef]
- Hernandez, S.F.; Vahidi, N.A.; Park, S.; Weitzel, R.P.; Tisdale, J.; Rueda, B.R.; Wolff, E.F. Characterization of extracellular ddx4- or ddx4-positive ovarian cells. Nat. Med. 2015, 21, 1114–1116. [Google Scholar] [CrossRef]
- Imudia, A.N.; Wang, N.; Tanaka, Y.; White, Y.A.; Woods, D.C.; Tilly, J.L. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil. Steril. 2013, 100, 1451–1458. [Google Scholar] [CrossRef]
- Khosravi-Farsani, S.; Amidi, F.; Habibi Roudkenar, M.; Sobhani, A. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015, 16, 406–415. [Google Scholar]
- Park, E.S.; Tilly, J.L. Use of dead-box polypeptide-4 (ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol. Hum. Rep. 2015, 21, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Woods, D.C.; Tilly, J.L. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via smad1/5/8 signaling. Fertil. Steril. 2013, 100, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Thierry-Mieg, D.T.-M. Ncbi aceview. Available online: https://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/av.cgi?db=human&c=Gene&l=DDX4 (accessed on 9 May 2019).
- Fujiwara, Y.; Komiya, T.; Kawabata, H.; Sato, M.; Fujimoto, H.; Furusawa, M.; Noce, T. Isolation of a dead-family protein gene that encodes a murine homolog of drosophila vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. USA 1994, 91, 12258–12262. [Google Scholar] [CrossRef] [PubMed]
- Noce, T.; Okamoto-Ito, S.; Tsunekawa, N. Vasa homolog genes in mammalian germ cell development. Cell Struct. Funct. 2001, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Rocak, S.; Linder, P. Dead-box proteins: The driving forces behind rna metabolism. Nat. Rev. Mol. Cell Biol. 2004, 5, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Panula, S.; Petropoulos, S.; Edsgard, D.; Busayavalasa, K.; Liu, L.; Li, X.; Risal, S.; Shen, Y.; Shao, J.; et al. Adult human and mouse ovaries lack ddx4-expressing functional oogonial stem cells. Nat. Med. 2015, 21, 1116–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, W.; Shen, Y.; Adhikari, D.; Ueno, H.; Liu, K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc. Natl. Acad. Sci. USA 2012, 109, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.; Middleton, S.; Kremer, A.M.; Wardrope, C.; Hannah, M.; Gillingwater, T.H.; Skehel, P. Vapb interacts with and modulates the activity of atf6. Hum. Mol. Gen. 2008, 17, 1517–1526. [Google Scholar] [CrossRef]
- Bustos, S.P.; Reithmeier, R.A. Protein 4.2 interaction with hereditary spherocytosis mutants of the cytoplasmic domain of human anion exchanger 1. Biochem. J. 2011, 433, 313–322. [Google Scholar] [CrossRef]
- Terpe, K. Overview of tag protein fusions: From molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2003, 60, 523–533. [Google Scholar] [CrossRef]
- Gougeon, A.; Notarianni, E. There is no neo-oogenesis in the adult mammalian ovary. J. Turk. Ger. Gynecol. Assoc. 2011, 12, 270–273. [Google Scholar] [CrossRef]
- Lei, L.; Spradling, A.C. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc. Natl. Acad. Sci. USA 2013, 110, 8585–8590. [Google Scholar] [CrossRef]
- Oatley, J.; Hunt, P.A. Of mice and (wo)men: Purified oogonial stem cells from mouse and human ovaries. Biol. Reprod. 2012, 86. [Google Scholar] [CrossRef] [PubMed]
- Telfer, E.E.; Gosden, R.G.; Byskov, A.G.; Spears, N.; Albertini, D.; Andersen, C.Y.; Anderson, R.; Braw-Tal, R.; Clarke, H.; Gougeon, A.; et al. On regenerating the ovary and generating controversy. Cell 2005, 122, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, D.; Wang, L.; Liu, M.; Mao, J.; Yin, Y.; Ye, X.; Liu, N.; Han, J.; Gao, Y.; et al. No evidence for neo-oogenesis may link to ovarian senescence in adult monkey. Stem Cell. 2013, 31, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Zarate-Garcia, L.; Lane, S.I.; Merriman, J.A.; Jones, K.T. Facs-sorted putative oogonial stem cells from the ovary are neither ddx4-positive nor germ cells. Sci Rep. 2016, 6, 27991. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G. Germline stem cells in the postnatal ovary: Is the ovary more like a testis? Hum. Reprod. Update 2004, 10, 193–195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linder, P.; Jankowsky, E. From unwinding to clamping—The DEAD box RNA helicase family. Nat. Rev. Mol. Cell Biol. 2011, 12, 505–516. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarkson, Y.L.; Weatherall, E.; Waterfall, M.; McLaughlin, M.; Lu, H.; Skehel, P.A.; Anderson, R.A.; Telfer, E.E. Extracellular Localisation of the C-Terminus of DDX4 Confirmed by Immunocytochemistry and Fluorescence-Activated Cell Sorting. Cells 2019, 8, 578. https://doi.org/10.3390/cells8060578
Clarkson YL, Weatherall E, Waterfall M, McLaughlin M, Lu H, Skehel PA, Anderson RA, Telfer EE. Extracellular Localisation of the C-Terminus of DDX4 Confirmed by Immunocytochemistry and Fluorescence-Activated Cell Sorting. Cells. 2019; 8(6):578. https://doi.org/10.3390/cells8060578
Chicago/Turabian StyleClarkson, Yvonne L., Emma Weatherall, Martin Waterfall, Marie McLaughlin, Haojiang Lu, Paul A. Skehel, Richard A. Anderson, and Evelyn E. Telfer. 2019. "Extracellular Localisation of the C-Terminus of DDX4 Confirmed by Immunocytochemistry and Fluorescence-Activated Cell Sorting" Cells 8, no. 6: 578. https://doi.org/10.3390/cells8060578
APA StyleClarkson, Y. L., Weatherall, E., Waterfall, M., McLaughlin, M., Lu, H., Skehel, P. A., Anderson, R. A., & Telfer, E. E. (2019). Extracellular Localisation of the C-Terminus of DDX4 Confirmed by Immunocytochemistry and Fluorescence-Activated Cell Sorting. Cells, 8(6), 578. https://doi.org/10.3390/cells8060578




