Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Experiments and Ethics Statements
2.3. Immunization Protocols
2.4. Induction of Trinitrobenzene Sulfonic Acid (TNBS) Colitis and Inflammation Scoring
2.5. Patients and Human Fecal Samples
2.6. Microbiota Analysis
2.7. Antibiotic Treatment and Fecal Microbiota Transplant
2.8. Statistical Analysis
3. Results
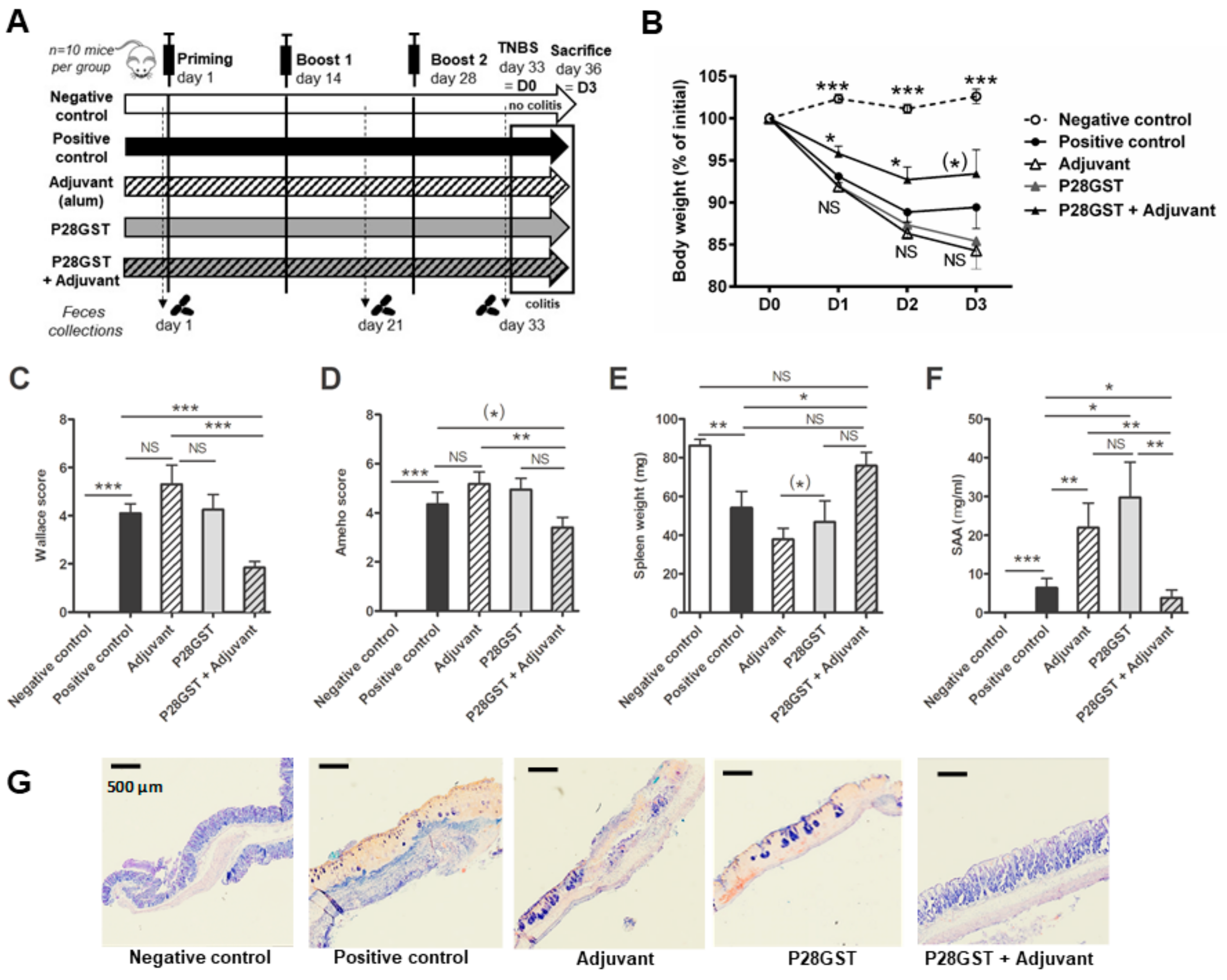
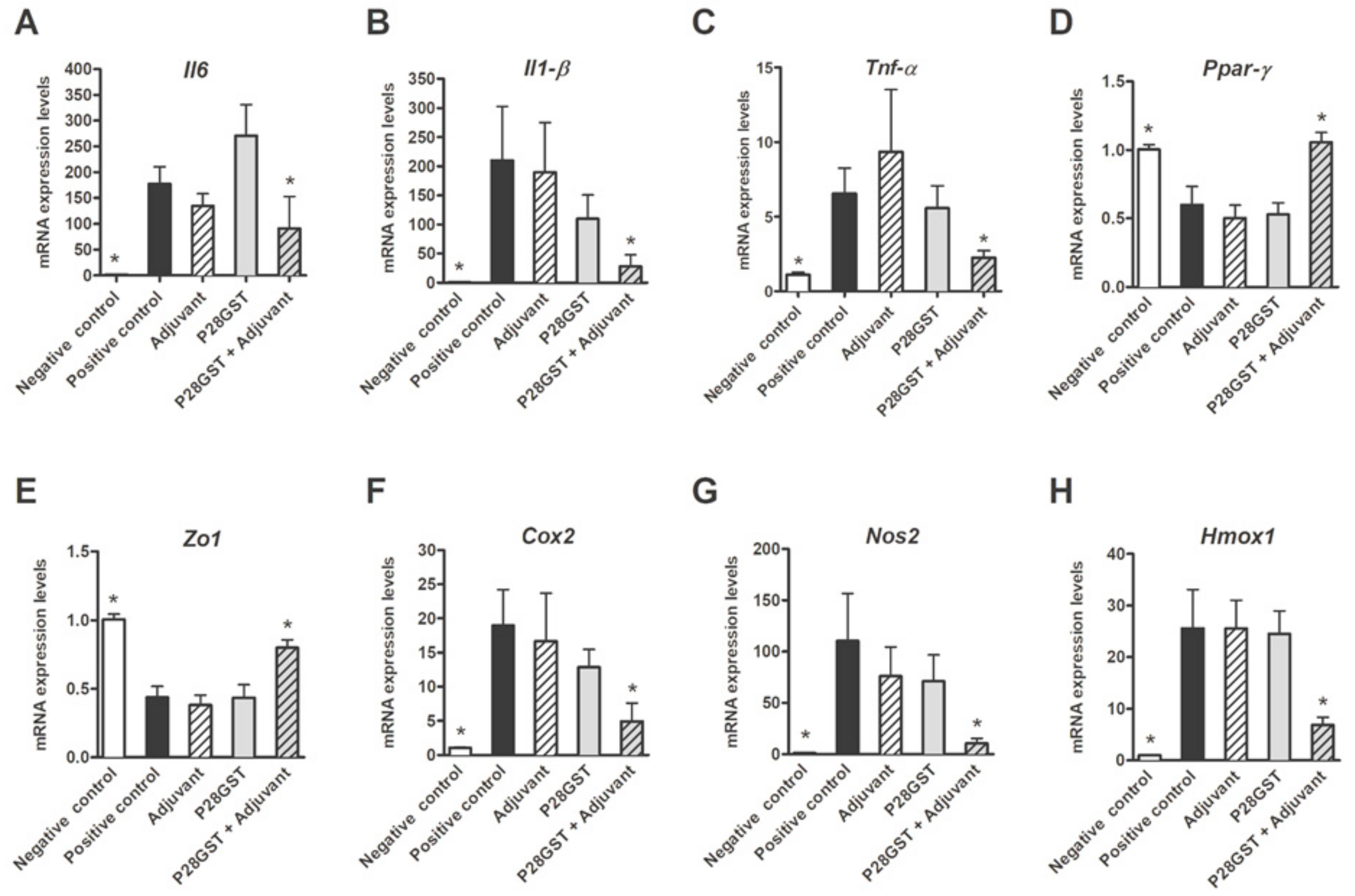
3.1. P28GST Immunization Prevents Experimental Colitis in Mice
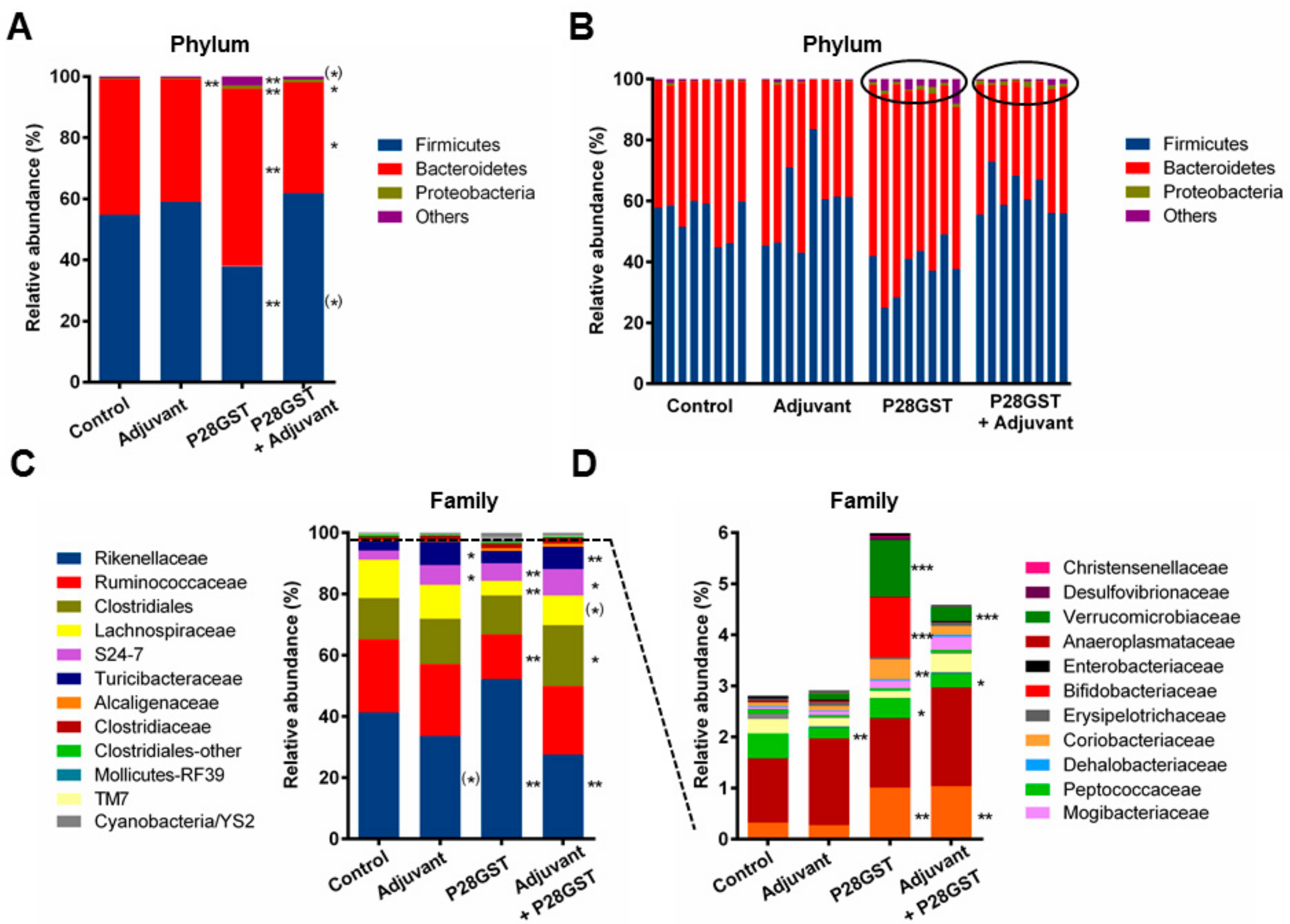
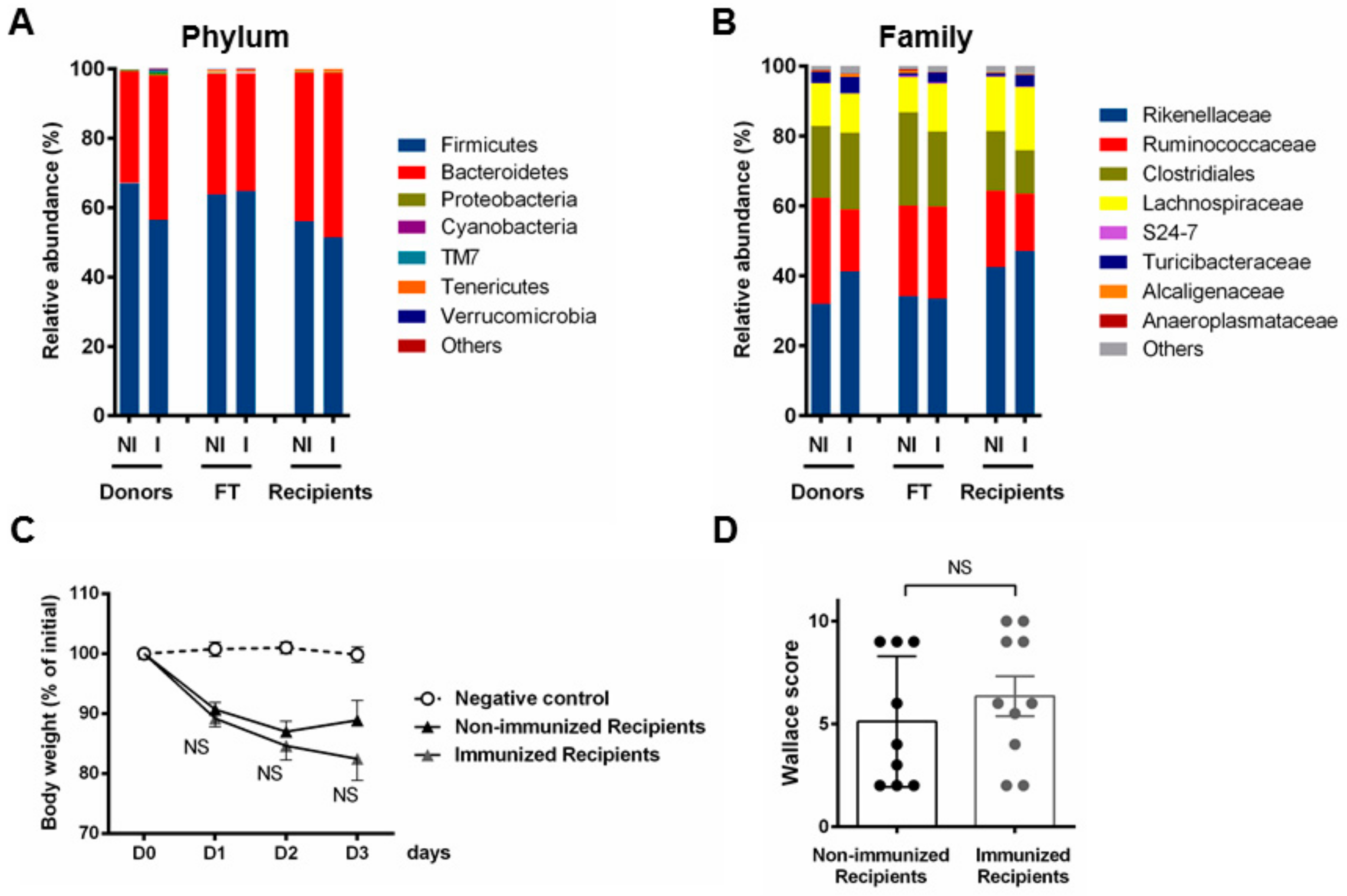
3.2. P28GST Immunomodulation Slightly Modifies the Mouse Gut Microbiota Composition
3.3. Fecal Transplantation Conditions Are Crucial to Address Microbiota Functionality
3.4. Fecal Transplantation in Naive Mice Is Not Sufficient to Restore the Phenotypic P28GST Induced Anti-Inflammatory Effects
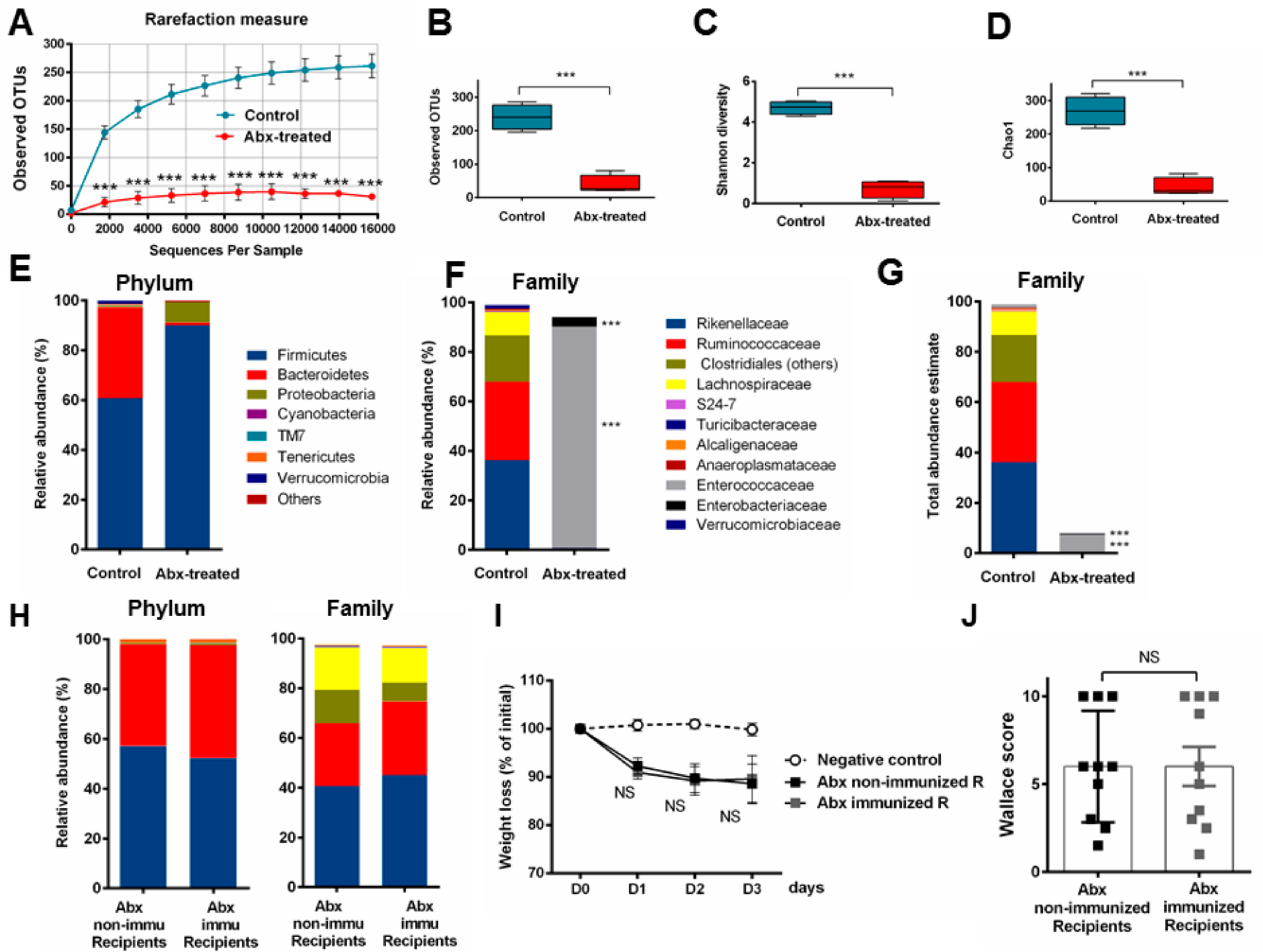
3.5. Fecal Transplantation in Mice Treated with Antibiotics is Not Sufficient to Restore the P28GST Induced Beneficial Anti-Inflammatory Effects
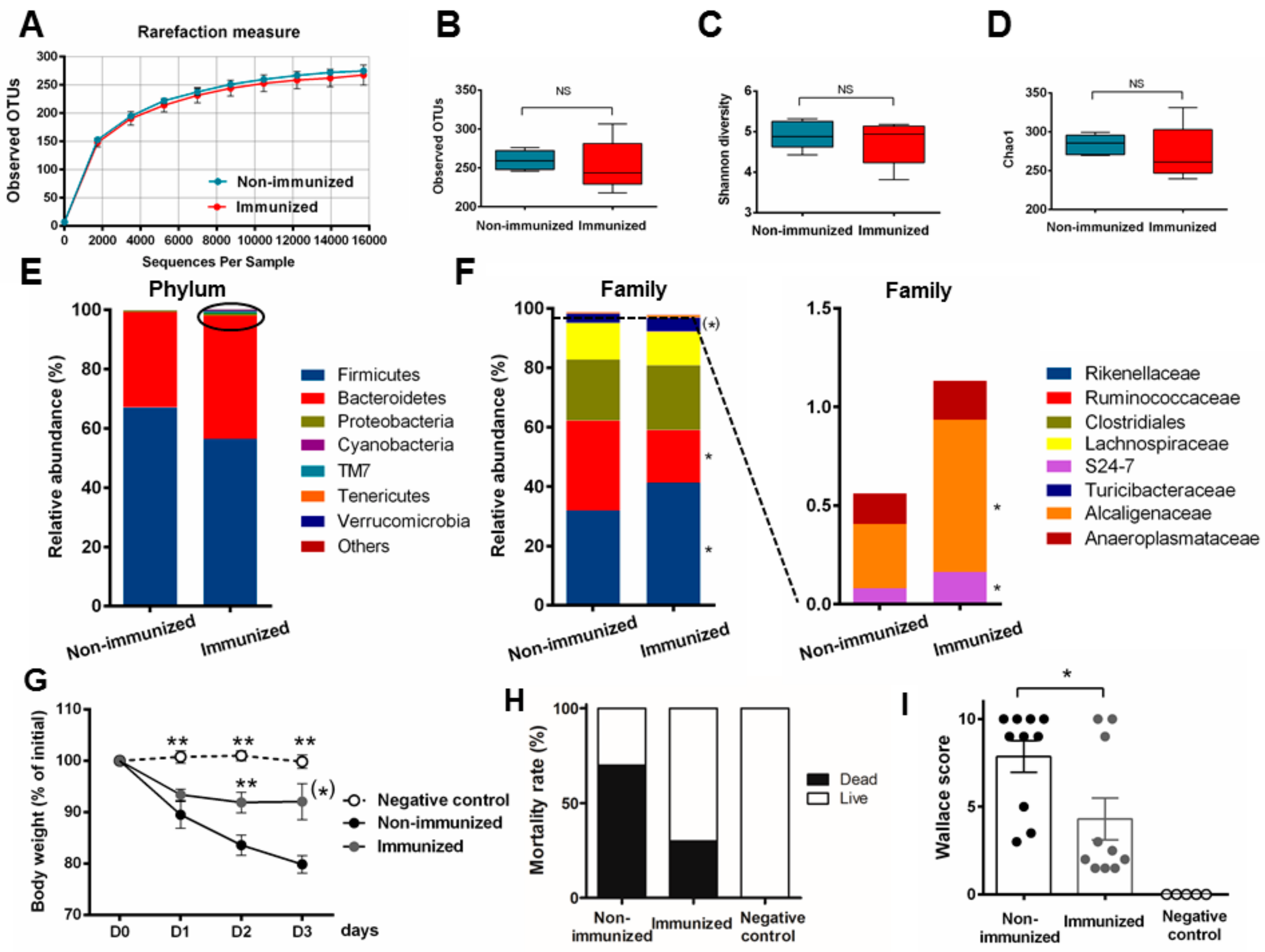
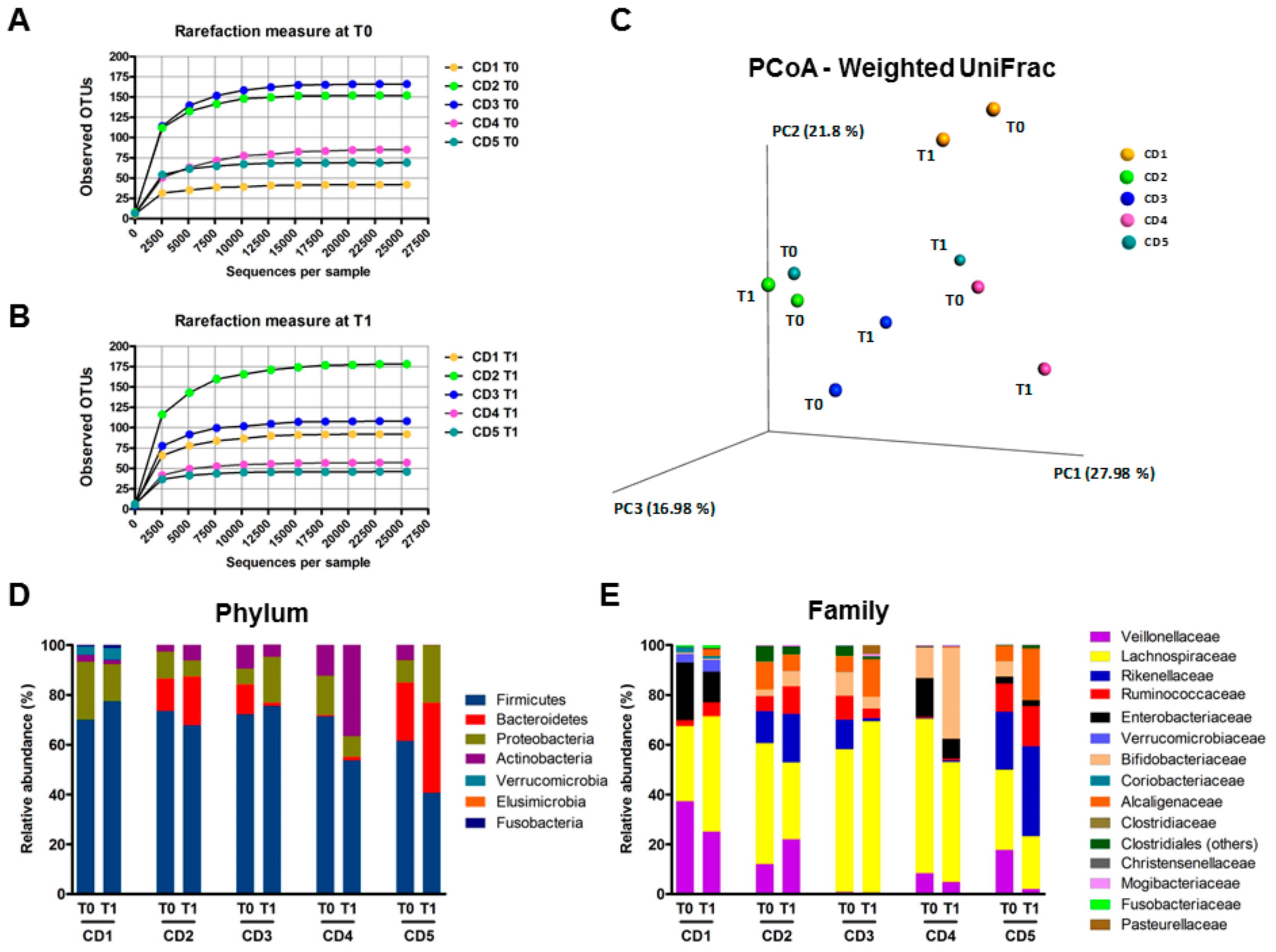
3.6. Human Fecal Microbiota Composition Following P28GST Immunomodulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smallwood, T.B.; Giacomin, P.R.; Loukas, A.; Mulvenna, J.P.; Clark, R.J.; Miles, J.J. Helminth immunomodulation in autoimmune disease. Front. Immunol. 2017, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Stanford, J.L. Give us this day our daily germs. Immunol. Today 1998, 19, 113–116. [Google Scholar] [CrossRef]
- Heylen, M.; Ruyssers, N.E.; De Man, J.G.; Timmermans, J.-P.; Pelckmans, P.A.; Moreels, T.G.; De Winter, B.Y. Worm proteins of Schistosoma mansoni reduce the severity of experimental chronic colitis in mice by suppressing colonic proinflammatory immune responses. PLoS ONE 2014, 9, e110002. [Google Scholar] [CrossRef] [PubMed]
- Heylen, M.; Ruyssers, N.E.; Nullens, S.; Schramm, G.; Pelckmans, P.A.; Moreels, T.G.; De Man, J.G.; De Winter, B.Y. Treatment with egg antigens of Schistosoma mansoni ameliorates experimental colitis in mice through a colonic T-cell-dependent mechanism. Inflamm. Bowel Dis. 2015, 21, 48–59. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Tang, Y.; Sun, X. Parasite-derived proteins for the treatment of allergies and autoimmune diseases. Front. Microbiol. 2017, 8, 2164. [Google Scholar] [CrossRef]
- Driss, V.; El Nady, M.; Delbeke, M.; Rousseaux, C.; Dubuquoy, C.; Sarazin, A.; Gatault, S.; Dendooven, A.; Riveau, G.; Colombel, J.F.; et al. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol. 2016, 9, 322–335. [Google Scholar] [CrossRef]
- Sarazin, A.; Dendooven, A.; Delbeke, M.; Gatault, S.; Pagny, A.; Standaert, A.; Rousseaux, C.; Desreumaux, P.; Dubuquoy, L.; Capron, M. Treatment with P28GST, a schistosome-derived enzyme, after acute colitis induction in mice: Decrease of intestinal inflammation associated with a down regulation of Th1/Th17 responses. PLoS ONE 2018, 13, e0209681. [Google Scholar] [CrossRef]
- Riveau, G.; Deplanque, D.; Remoué, F.; Schacht, A.-M.; Vodougnon, H.; Capron, M.; Thiry, M.; Martial, J.; Libersa, C.; Capron, A. Safety and immunogenicity of rSh28GST antigen in humans: Phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl. Trop. Dis. 2012, 6, e1704. [Google Scholar] [CrossRef]
- Hervé, M.; Angeli, V.; Pinzar, E.; Wintjens, R.; Faveeuw, C.; Narumiya, S.; Capron, A.; Urade, Y.; Capron, M.; Riveau, G.; et al. Pivotal roles of the parasite PGD2 synthase and of the host D prostanoid receptor 1 in schistosome immune evasion. Eur. J. Immunol. 2003, 33, 2764–2772. [Google Scholar] [CrossRef]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Walk, S.T.; Blum, A.M.; Ewing, S.A.-S.; Weinstock, J.V.; Young, V.B. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm. Bowel Dis. 2010, 16, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.A.; Smith, K.A.; Filbey, K.J.; Harcus, Y.; Hewitson, J.P.; Redpath, S.A.; Valdez, Y.; Yebra, M.J.; Finlay, B.B.; Maizels, R.M. Commensal-pathogen interactions in the intestinal tract: Lactobacilli promote infection with, and are promoted by, helminth parasites. Gut Microbes 2014, 5, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Sorobetea, D.; Kiilerich, P.; Ramayo-Caldas, Y.; Estellé, J.; Ma, T.; Madsen, L.; Kristiansen, K.; Svensson-Frej, M. Chronic Trichuris muris infection decreases diversity of the Iitestinal microbiota and concomitantly increases the abundance of Lactobacilli. PLoS ONE 2015, 10, e0125495. [Google Scholar] [CrossRef] [PubMed]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: Effects reversed by pathogen clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, D.; Bowcutt, R.; Lee, S.C.; Tang, M.S.; Kurtz, Z.D.; Ding, Y.; Honda, K.; Gause, W.C.; Blaser, M.J.; Bonneau, R.A.; et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 2016, 352, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Su, L.; Li, Y.; Long, S.R.; Chang, J.; Zhang, W.; Walker, W.A.; Xavier, R.J.; Cherayil, B.J.; Shi, H.N. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol. 2018, 11, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Wegener Parfrey, L.; Jirků, M.; Šíma, R.; Jalovecká, M.; Sak, B.; Grigore, K.; Jirků Pomajbíková, K. A benign helminth alters the host immune system and the gut microbiota in a rat model system. PLoS ONE 2017, 12, e0182205. [Google Scholar] [CrossRef]
- Broadhurst, M.J.; Ardeshir, A.; Kanwar, B.; Mirpuri, J.; Gundra, U.M.; Leung, J.M.; Wiens, K.E.; Vujkovic-Cvijin, I.; Kim, C.C.; Yarovinsky, F.; et al. Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog. 2012, 8, e1003000. [Google Scholar] [CrossRef]
- Cooper, P.; Walker, A.W.; Reyes, J.; Chico, M.; Salter, S.J.; Vaca, M.; Parkhill, J. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS ONE 2013, 8, e76573. [Google Scholar] [CrossRef]
- Lee, S.C.; Tang, M.S.; Lim, Y.A.L.; Choy, S.H.; Kurtz, Z.D.; Cox, L.M.; Gundra, U.M.; Cho, I.; Bonneau, R.; Blaser, M.J.; et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 2014, 8, e2880. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Zakrzewski, M.; Jenkins, T.P.; Su, X.; Al-Hallaf, R.; Croese, J.; de Vries, S.; Grant, A.; Mitreva, M.; Loukas, A.; et al. Changes in duodenal tissue-associated microbiota following hookworm infection and consecutive gluten challenges in humans with coeliac disease. Sci. Rep. 2016, 6, 36797. [Google Scholar] [CrossRef] [PubMed]
- Brosschot, T.P.; Reynolds, L.A. The impact of a helminth-modified microbiome on host immunity. Mucosal Immunol. 2018, 11, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, P.; Croese, J.; Krause, L.; Loukas, A.; Cantacessi, C. Suppression of inflammation by helminths: A role for the gut microbiota? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140296. [Google Scholar] [CrossRef] [PubMed]
- Doonan, J.; Tarafdar, A.; Pineda, M.A.; Lumb, F.E.; Crowe, J.; Khan, A.M.; Hoskisson, P.A.; Harnett, M.M.; Harnett, W. The parasitic worm product ES-62 normalises the gut microbiota bone marrow axis in inflammatory arthritis. Nat. Commun. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.J.; Pulendran, B. The potential of the microbiota to influence vaccine responses. J. Leukoc. Biol. 2018, 103, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Foligne, B.; Dessein, R.; Marceau, M.; Poiret, S.; Chamaillard, M.; Pot, B.; Simonet, M.; Daniel, C. Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein. Gastroenterology 2007, 133, 862–874. [Google Scholar] [CrossRef]
- Breton, J.; Daniel, C.; Vignal, C.; Body-Malapel, M.; Garat, A.; Plé, C.; Foligné, B. Does oral exposure to cadmium and lead mediate susceptibility to colitis? The dark-and-bright sides of heavy metals in gut ecology. Sci. Rep. 2016, 6, 19200. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Bahl, M.I.; Licht, T.R.; Toft, M.F.; Hansen, A.K. Microbiota composition of simultaneously colonized mice housed under either a gnotobiotic isolator or individually ventilated cage regime. Sci. Rep. 2017, 7, 42245. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L.; Wilson, M.S.; Wynn, T.A. Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol. 2018, 18, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, Y.; Yang, X.; Wang, X.; Yan, K.; Zhong, Z.; Wang, X.; Xu, Y.; Zhang, Y.; Liu, F.; et al. Therapeutic potential of recombinant cystatin from Schistosoma japonicum in TNBS-induced experimental colitis of mice. Parasit. Vectors 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Matisz, C.E.; Leung, G.; Reyes, J.L.; Wang, A.; Sharkey, K.A.; McKay, D.M. Adoptive transfer of helminth antigen-pulsed dendritic cells protects against the development of experimental colitis in mice. Eur. J. Immunol. 2015, 45, 3126–3139. [Google Scholar] [CrossRef]
- Sorobetea, D.; Svensson-Frej, M.; Grencis, R. Immunity to gastrointestinal nematode infections. Mucosal Immunol. 2018, 11, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Mangan, N.E.; Walsh, C.M.; Fallon, R.E.; McKenzie, A.N.J.; van Rooijen, N.; Fallon, P.G. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol. 2007, 178, 4557–4566. [Google Scholar] [CrossRef]
- Noor, Z.; Watanabe, K.; Abhyankar, M.M.; Burgess, S.L.; Buonomo, E.L.; Cowardin, C.A.; Petri, W.A. Role of eosinophils and tumor necrosis factor alpha in interleukin-25-mediated protection from amebic colitis. MBio 2017, 8. [Google Scholar] [CrossRef]
- Huang, F.-J.; Ma, Y.-L.; Tang, R.-Y.; Gong, W.-C.; Li, J.; Chen, C.-X.; Yin, L.; Chen, X.-P. Interleukin-4- and NACHT, LRR and PYD domains-containing protein 3-independent mechanisms of alum enhanced T helper type 2 responses on basophils. Immunology 2016, 149, 238–251. [Google Scholar] [CrossRef]
- Czarnewski, P.; Araújo, E.C.B.; Oliveira, M.C.; Mineo, T.W.P.; Silva, N.M. Recombinant TgHSP70 immunization protects against Toxoplasma gondii brain cyst formation by enhancing inducible nitric oxide expression. Front. Cell Infect. Microbiol. 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Matziouridou, C.; Rocha, S.D.C.; Haabeth, O.A.; Rudi, K.; Carlsen, H.; Kielland, A. iNOS- and NOX1-dependent ROS production maintains bacterial homeostasis in the ileum of mice. Mucosal Immunol. 2018, 11, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.M.; Rapin, A.; Lebon, L.; Dubey, L.K.; Mosconi, I.; Sarter, K.; Piersigilli, A.; Menin, L.; Walker, A.W.; Rougemont, J.; et al. The intestinal microbiota contributes to the ability of helminths to modulate allergic inflammation. Immunity 2015, 43, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, M.; Vilchez-Vargas, R.; Bussche, J.V.; Truchado, P.; Jauregui, R.; El Hage, R.A.; Pieper, D.H.; Vanhaecke, L.; Van de Wiele, T. High-fiber and high-protein diets shape different gut microbial communities, which ecologically behave similarly under stress conditions, as shown in a gastrointestinal simulator. Mol. Nutr. Food Res. 2017, 61, 1600150. [Google Scholar] [CrossRef]
- Valdez, Y.; Brown, E.M.; Finlay, B.B. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014, 35, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Lex, J.R.; Azizi, A. Microbiota, a forgotten relic of vaccination. Expert Rev. Vaccines 2017, 16, 1171–1173. [Google Scholar] [CrossRef][Green Version]
- Ang, L.; Arboleya, S.; Lihua, G.; Chuihui, Y.; Nan, Q.; Suarez, M.; Solís, G.; de los Reyes-Gavilán, C.G.; Gueimonde, M. The establishment of the infant intestinal microbiome is not affected by rotavirus vaccination. Sci. Rep. 2014, 4, 7417. [Google Scholar] [CrossRef]
- Hays, M.P.; Ericsson, A.C.; Yang, Y.; Hardwidge, P.R. Vaccinating with conserved Escherichia coli antigens does not alter the mouse intestinal microbiome. BMC Res. Notes 2016, 9, 401. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Myers-Morales, T.; Bussell, K.M.; D’Orazio, S.E. Fecal transplantation does not transfer either susceptibility or resistance to food borne listeriosis in C57BL/6 and BALB/c/By mice. F1000Res 2013, 2, 177. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Vidyarthi, A.; Nadeem, S.; Negi, S.; Nair, G.; Agrewala, J.N. Alteration in the gut microbiota provokes susceptibility to tuberculosis. Front. Immunol. 2016, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Schuijt, T.J.; Lankelma, J.M.; Scicluna, B.P.; de Sousa e Melo, F.; Roelofs, J.J.T.H.; de Boer, J.D.; Hoogendijk, A.J.; de Beer, R.; de Vos, A.; Belzer, C.; et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016, 65, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ferrere, G.; Wrzosek, L.; Cailleux, F.; Turpin, W.; Puchois, V.; Spatz, M.; Ciocan, D.; Rainteau, D.; Humbert, L.; Hugot, C.; et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017, 66, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foligné, B.; Plé, C.; Titécat, M.; Dendooven, A.; Pagny, A.; Daniel, C.; Singer, E.; Pottier, M.; Bertin, B.; Neut, C.; et al. Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights. Cells 2019, 8, 577. https://doi.org/10.3390/cells8060577
Foligné B, Plé C, Titécat M, Dendooven A, Pagny A, Daniel C, Singer E, Pottier M, Bertin B, Neut C, et al. Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights. Cells. 2019; 8(6):577. https://doi.org/10.3390/cells8060577
Chicago/Turabian StyleFoligné, Benoît, Coline Plé, Marie Titécat, Arnaud Dendooven, Aurélien Pagny, Catherine Daniel, Elisabeth Singer, Muriel Pottier, Benjamin Bertin, Christel Neut, and et al. 2019. "Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights" Cells 8, no. 6: 577. https://doi.org/10.3390/cells8060577
APA StyleFoligné, B., Plé, C., Titécat, M., Dendooven, A., Pagny, A., Daniel, C., Singer, E., Pottier, M., Bertin, B., Neut, C., Deplanque, D., Dubuquoy, L., Desreumaux, P., Capron, M., & Standaert, A. (2019). Contribution of the Gut Microbiota in P28GST-Mediated Anti-Inflammatory Effects: Experimental and Clinical Insights. Cells, 8(6), 577. https://doi.org/10.3390/cells8060577




