CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. MS Patients and Healthy Subjects
2.2. Cells Abs and Reagents
2.3. Cell Stimulation and Western Blotting
2.4. Chromatin Immunoprecipitation (ChIP)
2.5. Multicolour Analysis of Surface Activation Markers
2.6. Real-Time PCR
2.7. Real Time Analysis of Cell Metabolism
2.8. Statistical Analysis
3. Results
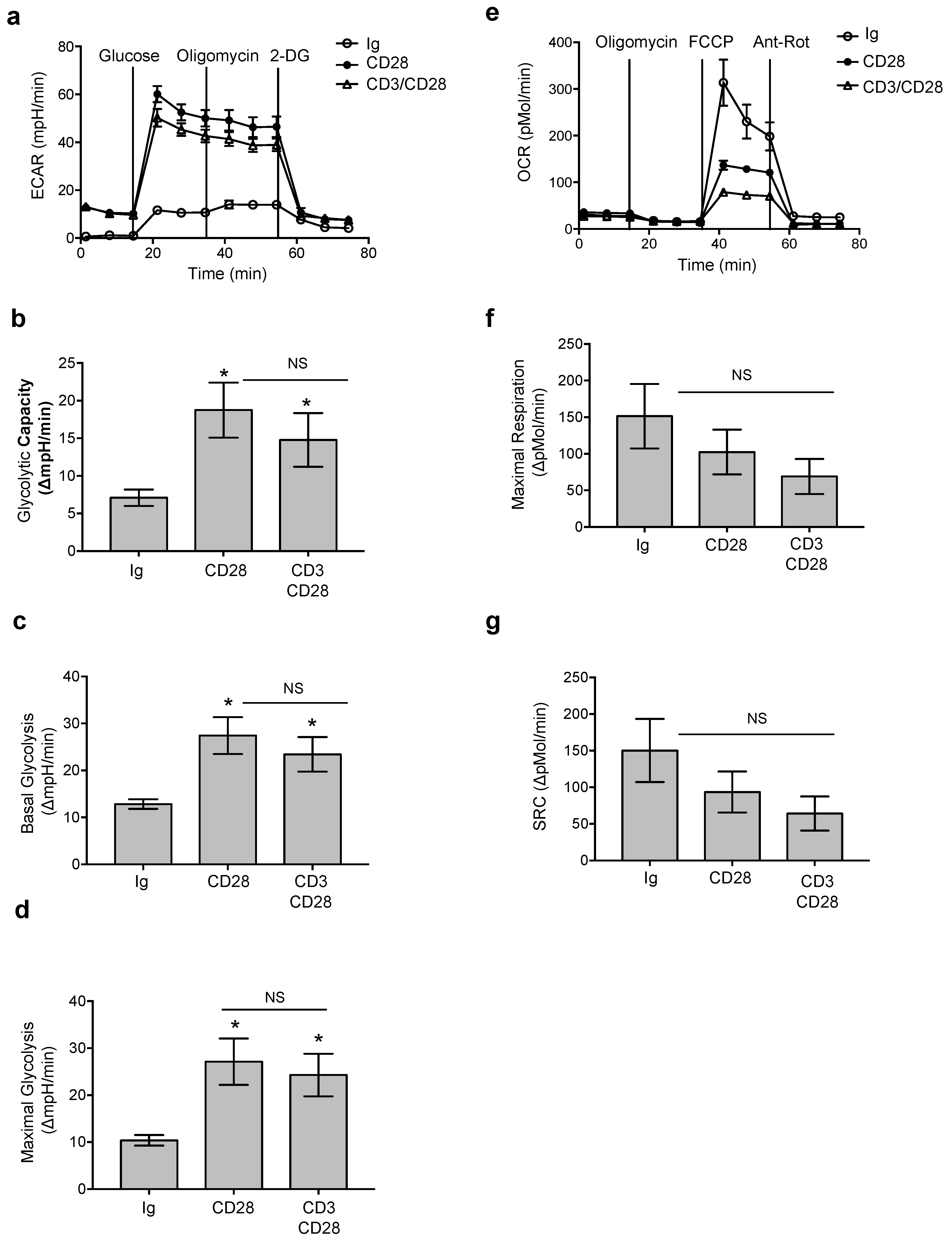
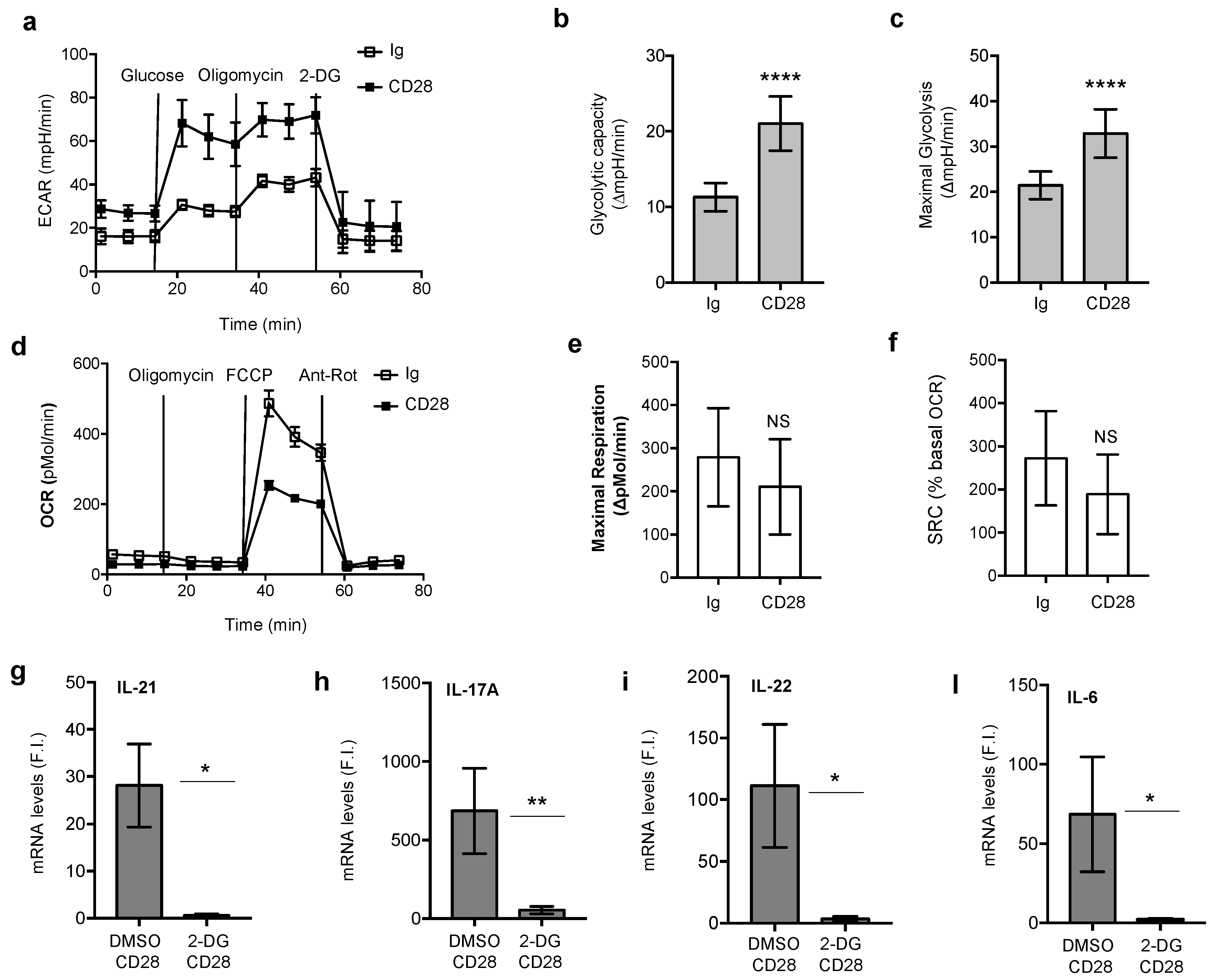
3.1. CD28 Pro-Inflammatory Functions Are Associated With a Glycolytic Metabolic Reprogramming
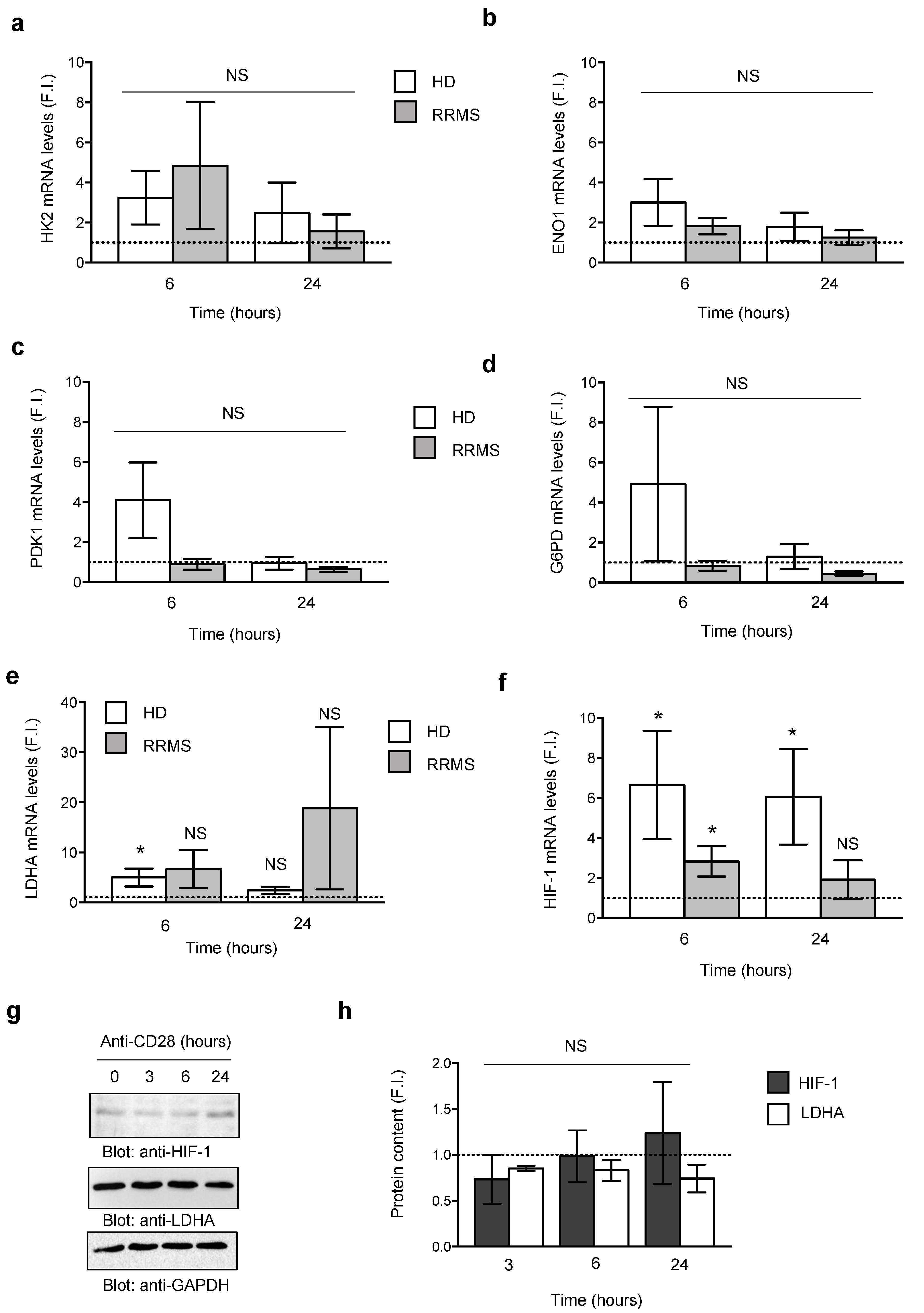
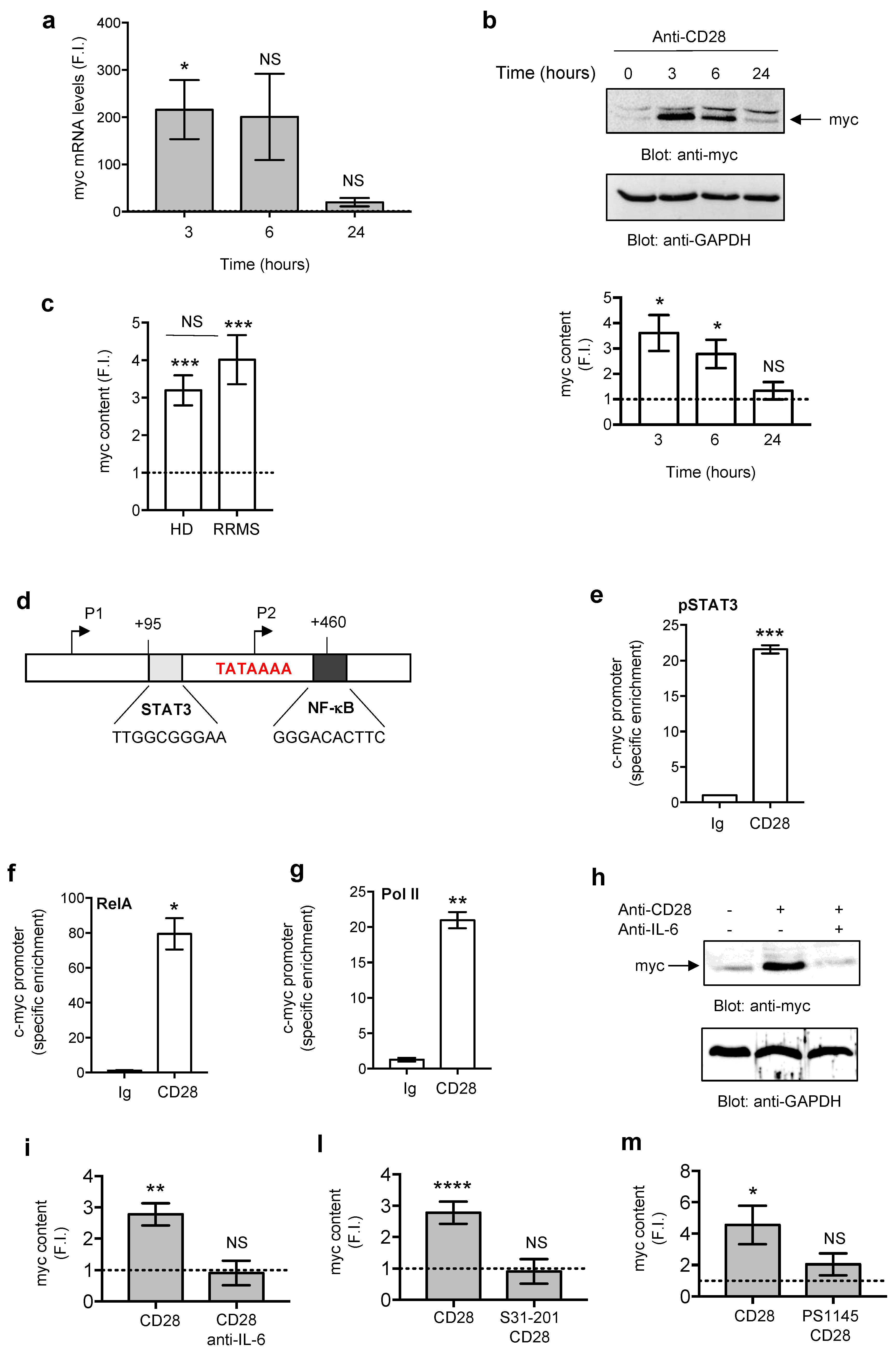
3.2. CD28-Induced Metabolic Reprogramming is Related to the Increase of C-Myc
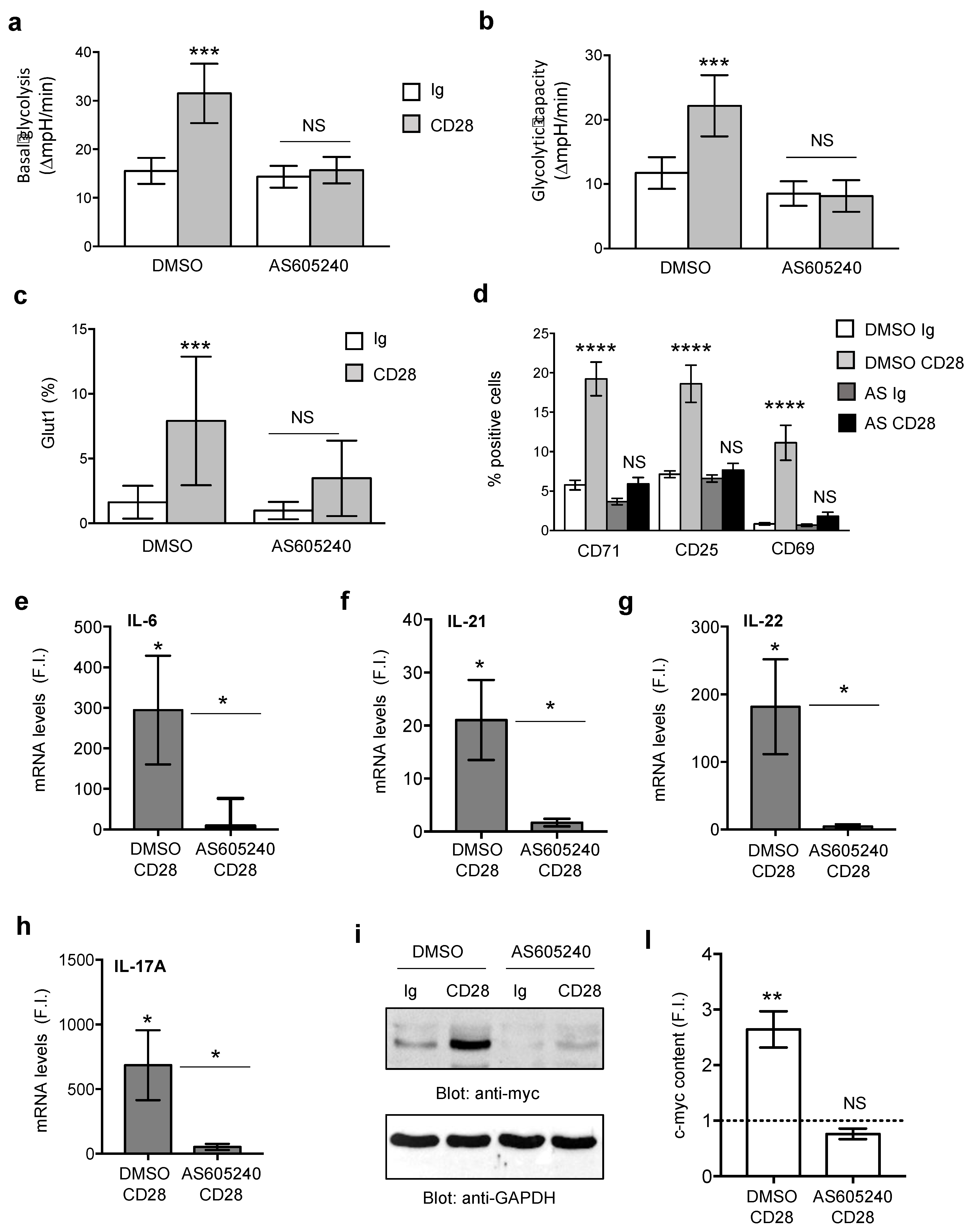
3.3. Class 1A PI3K Is Required for CD28-Induced Metabolic Reprogramming and Inflammatory Functions of RRMS T Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Batoulis, H.; Addicks, K.; Kuerten, S. Emerging concepts in autoimmune encephalomyelitis beyond the CD4/T(h)1 paradigm. Ann. Anat. 2010, 192, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Dos Passos, G.R.; Sato, D.K.; Becker, J.; Fujihara, K. Th17 cells pathways in Multiple Sclerosis and Neuromyelitis Optica spectrum disorders: Pathophysiological and therapeutic implications. Mediators Inflamm. 2016, 2016, 5314541. [Google Scholar] [CrossRef] [PubMed]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in Multiple Sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef]
- Matusevicius, D.; Kivisakk, P.; He, B.; Kostulas, N.; Ozenci, V.; Fredrikson, S.; Link, H. Interleukin-17 mrna expression in blood and csf mononuclear cells is augmented in Multiple Sclerosis. Mult. Scler. 1999, 5, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Durelli, L.; Conti, L.; Clerico, M.; Boselli, D.; Contessa, G.; Ripellino, P.; Ferrero, B.; Eid, P.; Novelli, F. T-helper 17 cells expand in Multiple Sclerosis and are inhibited by Interferon-beta. Ann. Neurol. 2009, 65, 499–509. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Friese, M.A.; Craner, M.J.; Palace, J.; Newcombe, J.; Esiri, M.M.; Fugger, L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in Multiple Sclerosis. Am. J. Pathol. 2008, 172, 146–155. [Google Scholar] [CrossRef]
- Hedegaard, C.J.; Krakauer, M.; Bendtzen, K.; Lund, H.; Sellebjerg, F.; Nielsen, C.H. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in Multiple Sclerosis. Immunology 2008, 125, 161–169. [Google Scholar] [CrossRef]
- Shabgah, A.G.; Navashenaq, J.G.; Shabgah, O.G.; Mohammadi, H.; Sahebkar, A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun. Rev. 2017, 16, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Namaka, M.P.; Dou, C.; Zhang, Y. Exploring the role of Interleukin-22 in neurological and autoimmune disorders. Int. Immunopharmacol. 2015, 28, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, R.; Dai, Y.; Wu, A.; Wang, H.; Cheng, C.; Qiu, W.; Lu, Z.; Zhong, X.; Shu, Y.; et al. IL-22 secreting CD4+ T cells in the patients with Neuromyelitis Optica and Multiple Sclerosis. J. Neuroimmunol. 2013, 261, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Perriard, G.; Mathias, A.; Enz, L.; Canales, M.; Schluep, M.; Gentner, M.; Schaeren-Wiemers, N.; Du Pasquier, R.A. Interleukin-22 is increased in Multiple Sclerosis patients and targets astrocytes. J. Neuroinflamm. 2015, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Muls, N.; Nasr, Z.; Dang, H.A.; Sindic, C.; van Pesch, V. IL-22, GM-CSF and IL-17 in peripheral CD4+ T cell subpopulations during Multiple Sclerosis relapses and remission. Impact of corticosteroid therapy. PLoS ONE 2017, 12, e0173780. [Google Scholar] [CrossRef] [PubMed]
- Wing, A.C.; Hygino, J.; Ferreira, T.B.; Kasahara, T.M.; Barros, P.O.; Sacramento, P.M.; Andrade, R.M.; Camargo, S.; Rueda, F.; Alves-Leon, S.V.; et al. Interleukin-17- and Interleukin-22-secreting myelin-specific CD4(+) T cells resistant to corticoids are related with active brain lesions in Multiple Sclerosis patients. Immunology 2016, 147, 212–220. [Google Scholar] [CrossRef]
- Rolla, S.; Bardina, V.; De Mercanti, S.; Quaglino, P.; De Palma, R.; Gned, D.; Brusa, D.; Durelli, L.; Novelli, F.; Clerico, M. Th22 cells are expanded in Multiple Sclerosis and are resistant to IFN-beta. J. Leukoc. Biol. 2014, 96, 1155–1164. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Joseph, A.M.; Monticelli, L.A.; Sonnenberg, G.F. Metabolic regulation of innate and adaptive lymphocyte effector responses. Immunol. Rev. 2018, 286, 137–147. [Google Scholar] [CrossRef]
- Palmer, C.S.; Ostrowski, M.; Balderson, B.; Christian, N.; Crowe, S.M. Glucose metabolism regulates T cell activation, differentiation, and functions. Front. Immunol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Amersfoort, J.; Kuiper, J. T cell metabolism in metabolic disease-associated autoimmunity. Immunobiology 2017, 222, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Galgani, M.; De Rosa, V.; Matarese, G. T cell metabolism and susceptibility to autoimmune diseases. Mol. Immunol. 2015, 68, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Z.; Wang, R.; Huang, G.; Vogel, P.; Neale, G.; Green, D.R.; Chi, H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of Th17 and Treg cells. J. Exp. Med. 2011, 208, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Berod, L.; Friedrich, C.; Nandan, A.; Freitag, J.; Hagemann, S.; Harmrolfs, K.; Sandouk, A.; Hesse, C.; Castro, C.N.; Bahre, H.; et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 2014, 20, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Michalek, R.D.; Gerriets, V.A.; Jacobs, S.R.; Macintyre, A.N.; MacIver, N.J.; Mason, E.F.; Sullivan, S.A.; Nichols, A.G.; Rathmell, J.C. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 2011, 186, 3299–3303. [Google Scholar] [CrossRef]
- De Rosa, V.; Galgani, M.; Porcellini, A.; Colamatteo, A.; Santopaolo, M.; Zuchegna, C.; Romano, A.; De Simone, S.; Procaccini, C.; La Rocca, C.; et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 2015, 16, 1174–1184. [Google Scholar] [CrossRef]
- Carbone, F.; De Rosa, V.; Carrieri, P.B.; Montella, S.; Bruzzese, D.; Porcellini, A.; Procaccini, C.; La Cava, A.; Matarese, G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014, 20, 69–74. [Google Scholar] [CrossRef]
- Porciello, N.; Tuosto, L. CD28 costimulatory signals in T lymphocyte activation: Emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor Rev. 2016, 28, 11–19. [Google Scholar] [CrossRef]
- Marinari, B.; Costanzo, A.; Marzano, V.; Piccolella, E.; Tuosto, L. CD28 delivers a unique signal leading to the selective recruitment of rela and p52 NF-kappaB subunits on IL-8 and BCL-xL gene promoters. Proc. Natl. Acad. Sci. USA 2004, 101, 6098–6103. [Google Scholar] [CrossRef]
- Porciello, N.; Grazioli, P.; Campese, A.F.; Kunkl, M.; Caristi, S.; Mastrogiovanni, M.; Muscolini, M.; Spadaro, F.; Favre, C.; Nunes, J.A.; et al. A non-conserved amino acid variant regulates differential signalling between human and mouse CD28. Nat. Commun. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Porciello, N.; Kunkl, M.; Tuosto, L. CD28 between tolerance and autoimmunity: The side effects of animal models. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Mastrogiovanni, M.; Porciello, N.; Caristi, S.; Monteleone, E.; Arcieri, S.; Tuosto, L. CD28 individual signaling up-regulates human IL-17A expression by promoting the recruitment of RelA/NF-κB and STAT3 transcription factors on the proximal promoter. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kunkl, M.; Porciello, N.; Mastrogiovanni, M.; Capuano, C.; Lucantoni, F.; Moretti, C.; Persson, J.L.; Galandrini, R.; Buzzetti, R.; Tuosto, L. ISA-2011B, a phosphatidylinositol 4-phosphate 5-kinase alpha inhibitor, impairs CD28-dependent costimulatory and pro-inflammatory signals in human T lymphocytes. Front. Immunol. 2017, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Camperio, C.; Muscolini, M.; Volpe, E.; Di Mitri, D.; Mechelli, R.; Buscarinu, M.C.; Ruggieri, S.; Piccolella, E.; Salvetti, M.; Gasperini, C.; et al. CD28 ligation in the absence of TCR stimulation up-regulates IL-17A and pro-inflammatory cytokines in relapsing-remitting Multiple Sclerosis T lymphocytes. Immunol. Lett. 2014, 158, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Frauwirth, K.A.; Riley, J.L.; Harris, M.H.; Parry, R.V.; Rathmell, J.C.; Plas, D.R.; Elstrom, R.L.; June, C.H.; Thompson, C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002, 16, 769–777. [Google Scholar] [CrossRef]
- Sanchez-Lockhart, M.; Marin, E.; Graf, B.; Abe, R.; Harada, Y.; Sedwick, C.E.; Miller, J. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J. Immunol. 2004, 173, 7120–7124. [Google Scholar] [CrossRef] [PubMed]
- Muscolini, M.; Camperio, C.; Capuano, C.; Caristi, S.; Piccolella, E.; Galandrini, R.; Tuosto, L. Phosphatidylinositol 4-phosphate 5-kinase alpha activation critically contributes to CD28-dependent signaling responses. J. Immunol. 2013, 190, 5279–5286. [Google Scholar] [CrossRef]
- Muscolini, M.; Camperio, C.; Porciello, N.; Caristi, S.; Capuano, C.; Viola, A.; Galandrini, R.; Tuosto, L. Phosphatidylinositol 4-phosphate 5-kinase alpha and Vav1 mutual cooperation in CD28-mediated actin remodeling and signaling functions. J. Immunol. 2015, 194, 1323–1333. [Google Scholar] [CrossRef]
- Tuosto, L.; Capuano, C.; Muscolini, M.; Santoni, A.; Galandrini, R. The multifaceted role of PIP2 in leukocyte biology. Cell Mol. Life Sci. 2015, 72, 4461–4474. [Google Scholar] [CrossRef]
- Porciello, N.; Kunkl, M.; Viola, A.; Tuosto, L. Phosphatidylinositol 4-phosphate 5-kinases in the regulation of T cell activation. Front. Immunol. 2016, 7, 186. [Google Scholar] [CrossRef]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mtor regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011, 12, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.Y.; Chen, G.; Munk, R.; Eitan, E.; Martindale, J.; Longo, D.L.; Ghosh, P. P((7)(0)S(6)K(1)) in the TORC1 pathway is essential for the differentiation of Th17 cells, but not Th1, Th2, or Treg cells in mice. Eur. J. Immunol. 2016, 46, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Abanni, L.; Cali, G.; Porcellini, A.; Carbone, F.; Fontana, S.; Horvath, T.L.; La Cava, A.; et al. An oscillatory switch in mtor kinase activity sets regulatory T cell responsiveness. Immunity 2010, 33, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 revisions of the mcdonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sorensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of Multiple Sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Cianfrocca, R.; Muscolini, M.; Marzano, V.; Annibaldi, A.; Marinari, B.; Levrero, M.; Costanzo, A.; Tuosto, L. Rela/NF-kappaB recruitment on the bax gene promoter antagonizes p73-dependent apoptosis in costimulated T cells. Cell Death Differ. 2008, 15, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Barre, B.; Avril, S.; Coqueret, O. Opposite regulation of myc and p21waf1 transcription by STAT3 proteins. J. Biol. Chem. 2003, 278, 2990–2996. [Google Scholar] [CrossRef]
- Kiuchi, N.; Nakajima, K.; Ichiba, M.; Fukada, T.; Narimatsu, M.; Mizuno, K.; Hibi, M.; Hirano, T. Stat3 is required for the gp130-mediated full activation of the c-myc gene. J. Exp. Med. 1999, 189, 63–73. [Google Scholar] [CrossRef]
- Ji, L.; Arcinas, M.; Boxer, L.M. Nf-kappa b sites function as positive regulators of expression of the translocated c-myc allele in Burkitt’s lymphoma. Mol. Cell. Biol. 1994, 14, 7967–7974. [Google Scholar] [CrossRef]
- Kim, D.W.; Gazourian, L.; Quadri, S.A.; Romieu-Mourez, R.; Sherr, D.H.; Sonenshein, G.E. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AHR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 2000, 19, 5498–5506. [Google Scholar] [CrossRef]
- Northrop, J.K.; Thomas, R.M.; Wells, A.D.; Shen, H. Epigenetic remodeling of the IL-2 and IFN-gamma loci in memory CD8 T cells is influenced by CD4 T cells. J. Immunol. 2006, 177, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, J.N.R.; Sherman, J.W.; Wang, R. Myc and HIF in shaping immune response and immune metabolism. Cytokine Growth Factor Rev. 2017, 35, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Fitzgerald, P.; Chi, H.; Munger, J.; et al. The transcription factor myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Wierstra, I.; Alves, J. The c-myc promoter: Still mystery and challenge. Adv. Cancer Res. 2008, 99, 113–333. [Google Scholar] [PubMed]
- Kallikourdis, M.; Trovato, A.E.; Roselli, G.; Muscolini, M.; Porciello, N.; Tuosto, L.; Viola, A. Phosphatidylinositol 4-phosphate 5-kinase β controls recruitment of lipid rafts into the immunological synapse. J. Immunol. 2016, 196, 1955–1963. [Google Scholar] [CrossRef]
- Shen, H.; Shi, L.Z. Metabolic regulation of Th17 cells. Mol. Immunol. 2019, 109, 81–87. [Google Scholar] [CrossRef]
- Patel, C.H.; Powell, J.D. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr. Opin. Immunol. 2017, 46, 82–88. [Google Scholar] [CrossRef]
- Pardo, G.; Jones, D.E. The sequence of disease-modifying therapies in relapsing Multiple Sclerosis: Safety and immunologic considerations. J. Neurol. 2017, 264, 2351–2374. [Google Scholar] [CrossRef]
- Gasperini, C.; Ruggieri, S.; Mancinelli, C.R.; Pozzilli, C. Advances in the treatment of relapsing-remitting Multiple Sclerosis–critical appraisal of fingolimod. Ther. Clin. Risk Manag. 2013, 9, 73–85. [Google Scholar]
- Jacobs, S.R.; Herman, C.E.; Maciver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose uptake is limiting in Tcell activation and requires CD28-mediated AKT-dependent and independent pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef] [PubMed]
- Klein Geltink, R.I.; O’Sullivan, D.; Corrado, M.; Bremser, A.; Buck, M.D.; Buescher, J.M.; Firat, E.; Zhu, X.; Niedermann, G.; Caputa, G.; et al. Mitochondrial priming by CD28. Cell 2017, 171, 385–397 e311. [Google Scholar] [CrossRef]
- Haas, R.; Smith, J.; Rocher-Ros, V.; Nadkarni, S.; Montero-Melendez, T.; D’Acquisto, F.; Bland, E.J.; Bombardieri, M.; Pitzalis, C.; Perretti, M.; et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015, 13, e1002202. [Google Scholar] [CrossRef] [PubMed]
- Menk, A.V.; Scharping, N.E.; Moreci, R.S.; Zeng, X.; Guy, C.; Salvatore, S.; Bae, H.; Xie, J.; Young, H.A.; Wendell, S.G.; et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 2018, 22, 1509–1521. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, C.; Carbone, F.; De Rosa, V.; Colamatteo, A.; Galgani, M.; Perna, F.; Lanzillo, R.; Brescia Morra, V.; Orefice, G.; Cerillo, I.; et al. Immunometabolic profiling of T cells from patients with relapsing-remitting Multiple Sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism 2017, 77, 39–46. [Google Scholar] [CrossRef] [PubMed]
- De Riccardis, L.; Rizzello, A.; Ferramosca, A.; Urso, E.; De Robertis, F.; Danieli, A.; Giudetti, A.M.; Trianni, G.; Zara, V.; Maffia, M. Bioenergetics profile of CD4(+) T cells in relapsing remitting Multiple Sclerosis subjects. J. Biotechnol. 2015, 202, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, A.N.; Gerriets, V.A.; Nichols, A.G.; Michalek, R.D.; Rudolph, M.C.; Deoliveira, D.; Anderson, S.M.; Abel, E.D.; Chen, B.J.; Hale, L.P.; et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 2014, 20, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Wieman, H.L.; Wofford, J.A.; Rathmell, J.C. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol. Biol. Cell 2007, 18, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lockhart, M.; Rojas, A.V.; Fettis, M.M.; Bauserman, R.; Higa, T.R.; Miao, H.; Waugh, R.E.; Miller, J. T cell receptor signaling can directly enhance the avidity of CD28 ligand binding. PLoS ONE 2014, 9, e89263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Procaccini, C.; Carbone, F.; Di Silvestre, D.; Brambilla, F.; De Rosa, V.; Galgani, M.; Faicchia, D.; Marone, G.; Tramontano, D.; Corona, M.; et al. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity 2016, 44, 712. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Vincent, E.E.; Cronin, J.G.; Panetti, S.; Chambers, M.; Holm, S.R.; Owens, S.E.; Francis, N.J.; Finlay, D.K.; Thornton, C.A. Akt and STAT5 mediate naïve human CD4+ T-cell early metabolic response to TCR stimulation. Nat. Commun. 2019, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- Pollizzi, K.N.; Powell, J.D. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 2014, 14, 435–446. [Google Scholar] [CrossRef]
- Angelin, A.; Gil-de-Gomez, L.; Dahiya, S.; Jiao, J.; Guo, L.; Levine, M.H.; Wang, Z.; Quinn, W.J., 3rd; Kopinski, P.K.; Wang, L.; et al. FOXP3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017, 25, 1282–1293 e1287. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.J.; Grosso, J.F.; Yen, H.R.; Xin, H.; Kortylewski, M.; Albesiano, E.; Hipkiss, E.L.; Getnet, D.; Goldberg, M.V.; Maris, C.H.; et al. Cutting edge: An in vivo requirement for STAT3 signaling in Th17 development and Th17-dependent autoimmunity. J. Immunol. 2007, 179, 4313–4317. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.K.; Lin, X.; Gaffen, S.L. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J. Biol. Chem. 2004, 279, 52762–52771. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium; Sawcer, S.; Hellenthal, G.; Pirinen, M.; Spencer, C.C.A.; Patsopoulos, N.A.; Moutsianas, L.; Dilthey, A.; Su, Z.; et al. Genetic risk and a primary role for cell-mediated immune mechanisms in Multiple Sclerosis. Nature 2011, 476, 214. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunkl, M.; Sambucci, M.; Ruggieri, S.; Amormino, C.; Tortorella, C.; Gasperini, C.; Battistini, L.; Tuosto, L. CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis. Cells 2019, 8, 575. https://doi.org/10.3390/cells8060575
Kunkl M, Sambucci M, Ruggieri S, Amormino C, Tortorella C, Gasperini C, Battistini L, Tuosto L. CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis. Cells. 2019; 8(6):575. https://doi.org/10.3390/cells8060575
Chicago/Turabian StyleKunkl, Martina, Manolo Sambucci, Serena Ruggieri, Carola Amormino, Carla Tortorella, Claudio Gasperini, Luca Battistini, and Loretta Tuosto. 2019. "CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis" Cells 8, no. 6: 575. https://doi.org/10.3390/cells8060575
APA StyleKunkl, M., Sambucci, M., Ruggieri, S., Amormino, C., Tortorella, C., Gasperini, C., Battistini, L., & Tuosto, L. (2019). CD28 Autonomous Signaling Up-Regulates C-Myc Expression and Promotes Glycolysis Enabling Inflammatory T Cell Responses in Multiple Sclerosis. Cells, 8(6), 575. https://doi.org/10.3390/cells8060575




