Distinct Expression of Inflammatory Features in T Helper 17 Cells from Multiple Sclerosis Patients
Abstract
1. Introduction
2. Methods
2.1. MS Subjects
2.2. Purification of Naive CD4+ T Lymphocytes from Adult blood
2.3. Th Cell Differentiation Assay
2.4. Flow Cytometry Analysis
2.5. Cytokine Quantification
2.6. Statistical Analysis
3. Results
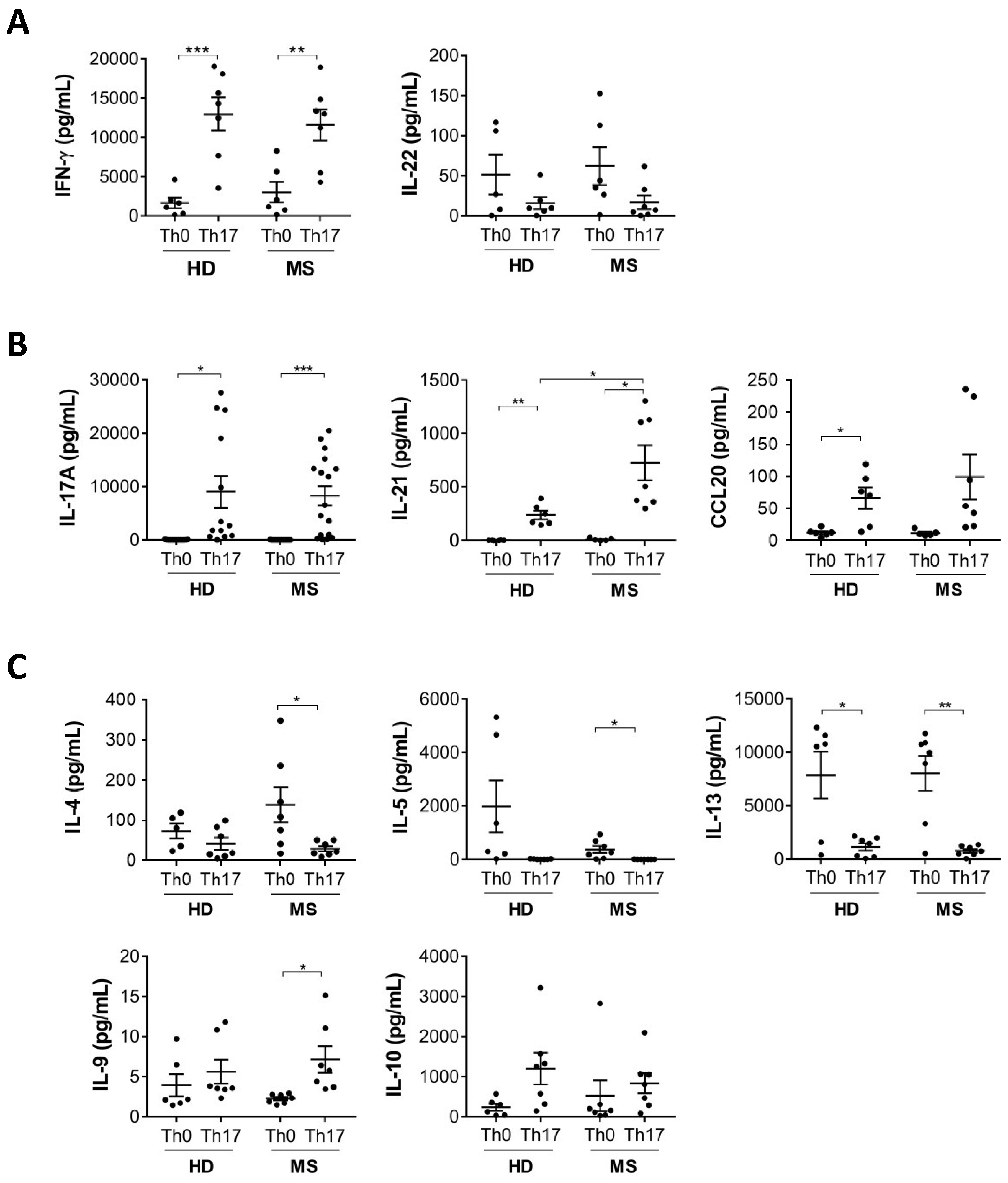
3.1. IL-21 Production by Th17 Cells Is Increased in MS Patients Compared to Healthy Donors
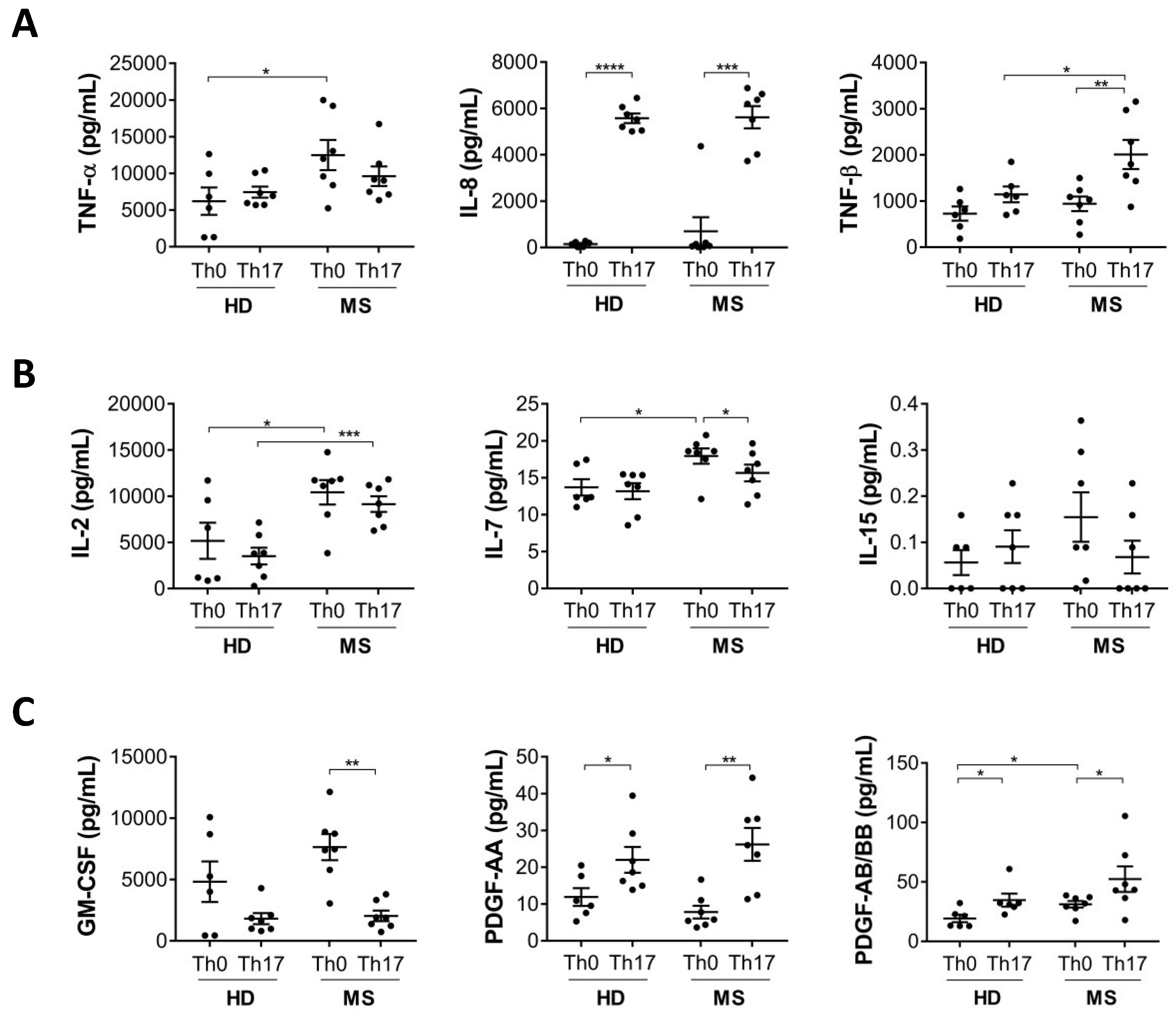
3.2. Production of Cytokines Involved in Inflammation and T Cell Activation Are Increased in Th17 Cells from MS Patients
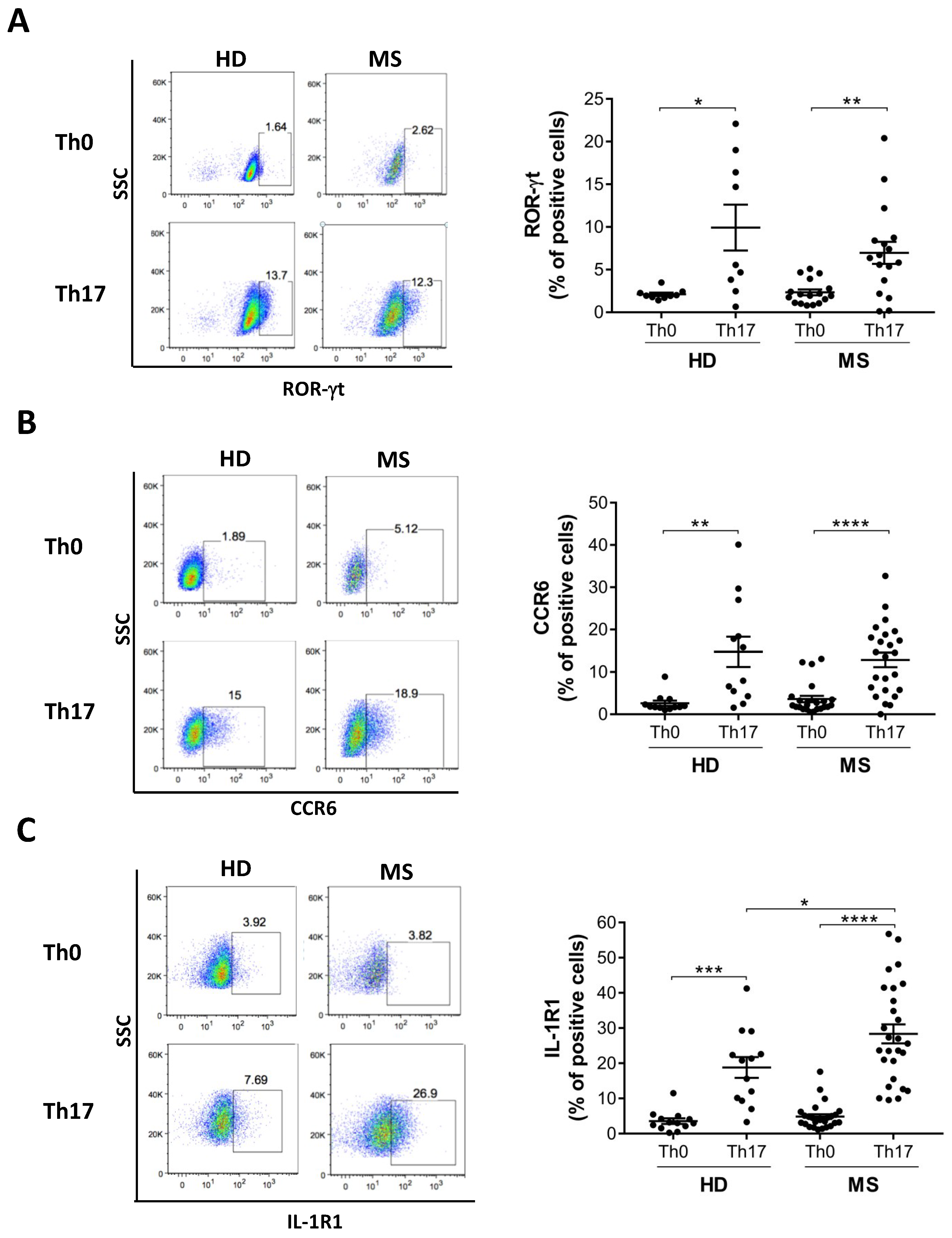
3.3. Th17 Cells Differentiated from MS Patients Express Higher IL-1R1 Than Those Differentiated from Healthy Donors
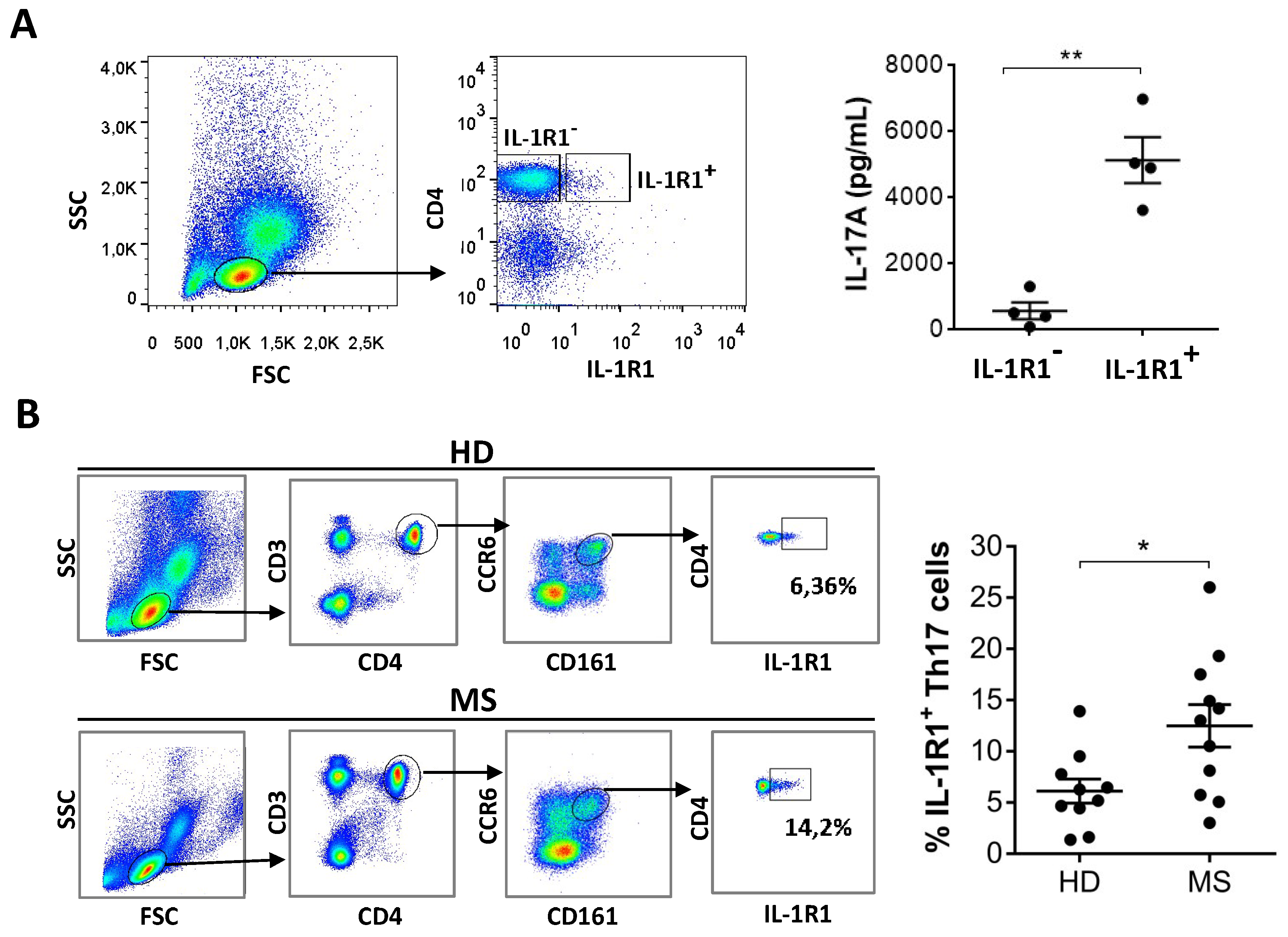
3.4. IL-1R1+ Cells in the Blood Are Th17 Cells and Are Increased in MS Patients Compared to Healthy Donors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Diego, R.S.; Weiner, H.L. Novel therapeutic strategies for multiple sclerosis—A multifaceted adversary. Nat. Rev. Drug Discov. 2008, 7, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Oksenberg, J.R. The Neurobiology of Multiple Sclerosis: Genes, Inflammation, and Neurodegeneration. Neuron 2006, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.M.; Ebers, G.C. Progress in deciphering the genetics of multiple sclerosis. Curr. Opin. Neurol. 2003, 16, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, B.; Archelos, J.J.; Hartung, H.-P. New concepts in the immunopathogenesis of multiple sclerosis. Nat. Rev. Neurosci. 2002, 3, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Brucklacher-Waldert, V.; Stuerner, K.; Kolster, M.; Wolthausen, J.; Tolosa, E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009, 132, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Battistini, L.; Borsellino, G. Advances in T Helper 17 Cell Biology: Pathogenic Role and Potential Therapy in Multiple Sclerosis. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C chemokine receptor 6–regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.; Touzot, M.; Servant, N.; Marloie-Provost, M.-A.; Hupé, P.; Barillot, E.; Soumelis, V. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood 2009, 114, 3610–3614. [Google Scholar] [CrossRef]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupe, P.; Barillot, E.; Soumelis, V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef]
- Zielinski, C.E.; Mele, F.; Aschenbrenner, D.; Jarrossay, D.; Ronchi, F.; Gattorno, M.; Monticelli, S.; Lanzavecchia, A.; Sallusto, F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature 2012, 484, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587. [Google Scholar] [CrossRef]
- Ruggiero, V. Involvement of IL-1R/TLR Signalling in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Mol. Med. 2012, 12, 218–236. [Google Scholar] [CrossRef]
- Loiarro, M.; Ruggiero, V.; Sette, C. Targeting TLR/IL-1R Signalling in Human Diseases. Mediat. Inflamm. 2010, 2010, 1–12. [Google Scholar] [CrossRef]
- Sutton, C.; Brereton, C.; Keogh, B.; Mills, K.H.; Lavelle, E.C. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1685–1691. [Google Scholar] [CrossRef]
- Sha, Y.; Markovic-Plese, S. Activated IL-1RI Signaling Pathway Induces Th17 Cell Differentiation via Interferon Regulatory Factor 4 Signaling in Patients with Relapsing-Remitting Multiple Sclerosis. Front. Immunol. 2016, 7, 942. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.K.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Ruocco, G.; Rossi, S.; Motta, C.; Macchiarulo, G.; Barbieri, F.; De Bardi, M.; Borsellino, G.; Finardi, A.; Grasso, M.G.; Ruggieri, S.; et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clin. Sci. 2015, 129, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Yang, X.O.; Chung, Y.; Fukunaga, A.; Nurieva, R.; Pappu, B.; Martin-Orozco, N.; Kang, H.S.; Ma, L.; Panopoulos, A.D.; et al. CCR6 Regulates the Migration of Inflammatory and Regulatory T Cells. J. Immunol. 2008, 181, 8391–8401. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, L.; De Palma, R.; Santarlasci, V.; Maggi, L.; Capone, M.; Frosali, F.; Rodolico, G.; Querci, V.; Abbate, G.; Angeli, R.; et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 2008, 205, 1903–1916. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Tzartos, J.S.; Craner, M.J.; Friese, M.A.; Jakobsen, K.B.; Newcombe, J.; Esiri, M.M.; Fugger, L. IL-21 and IL-21 Receptor Expression in Lymphocytes and Neurons in Multiple Sclerosis Brain. Am. J. Pathol. 2011, 178, 794–802. [Google Scholar] [CrossRef]
- Camperio, C.; Muscolini, M.; Volpe, E.; Di Mitri, D.; Mechelli, R.; Buscarinu, M.C.; Ruggieri, S.; Piccolella, E.; Salvetti, M.; Gasperini, C.; et al. CD28 ligation in the absence of TCR stimulation up-regulates IL-17A and pro-inflammatory cytokines in relapsing-remitting multiple sclerosis T lymphocytes. Immunol. Lett. 2014, 158, 134–142. [Google Scholar] [CrossRef]
- Wei, L.; Laurence, A.; Elias, K.M.; O’Shea, J.J. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT-3-dependent manner. J. Boil. Chem. 2007, 282, 34605–34610. [Google Scholar] [CrossRef]
- Nurieva, R.; Yang, X.O.; Martínez, G.; Zhang, Y.; Panopoulos, A.D.; Ma, L.; Schluns, K.; Tian, Q.; Watowich, S.S.; Jetten, A.M.; et al. 112 Essential Autocrine Regulation by IL-21 in the Generation of Inflammatory T Cells. Cytokine 2007, 39, 31. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: a double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Jelcic, I.; Al Nimer, F.; Wang, J.; Lentsch, V.; Planas, R.; Jelcic, I.; Madjowski, A.; Ruhrmann, S.; Faigle, W.; Frauenknecht, K.; et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell 2018, 175, 85–100. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.; Vora, A.; Serafini, B.; Nicholas, R.; Puopolo, M.; Reynolds, R.; Aloisi, F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007, 130, 1089–1104. [Google Scholar] [CrossRef]
- Lovato, L.; Willis, S.N.; Rodig, S.J.; Caron, T.; Almendinger, S.E.; Howell, O.W.; Reynolds, R.; O’Connor, K.C.; Hafler, D.A. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 2011, 134, 534–541. [Google Scholar] [CrossRef]
- Romme Christensen, J.; Bornsen, L.; Hesse, D.; Krakauer, M.; Sorensen, P.S.; Sondergaard, H.B.; Sellebjerg, F. Cellular sources of dysregulated cytokines in relapsing-remitting multiple sclerosis. J. Neuroinflammation 2012, 9, 215. [Google Scholar] [CrossRef]
- Bauer, J.; Namineni, S.; Reisinger, F.; Zoller, J.; Yuan, D.; Heikenwalder, M. Lymphotoxin, NF-kB, and cancer: the dark side of cytokines. Dig. Dis. 2012, 30, 453–468. [Google Scholar] [CrossRef]
- Gallo, P.; Piccinno, M.G.; Pagni, S.; Argentiero, V.; Giometto, B.; Bozza, F.; Tavolato, B. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J. Neurol. Sci. 1989, 92, 9–15. [Google Scholar] [CrossRef]
- Carbone, F.; De Rosa, V.; Carrieri, P.B.; Montella, S.; Bruzzese, D.; Porcellini, A.; Procaccini, C.; La Cava, A.; Matarese, G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat. Med. 2014, 20, 69–74. [Google Scholar] [CrossRef]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef]
- Hannink, M.; Donoghue, D.J. Structure and function of platelet-derived growth factor (PDGF) and related proteins. Biochim. Biophys. Acta (BBA)-Rev. Cancer 1989, 989, 1–10. [Google Scholar] [CrossRef]
- Distler, J.H.W.; Hirth, A.; Kurowska-Stolarska, M.; E Gay, R.; Gay, S.; Distler, O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q. J. Nucl. Med. 2003, 47, 149–161. [Google Scholar] [PubMed]
- Hu, D.; Notarbartolo, S.; Croonenborghs, T.; Patel, B.; Cialic, R.; Yang, T.-H.; Aschenbrenner, D.; Andersson, K.M.; Gattorno, M.; Pham, M.; et al. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 2017, 8, 1600. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Lee, J.U.; Kim, D.H.; Lim, S.; Kang, I.; Choi, J.M. Pathogenic function of bystander-activated memory-like CD4(+) T cells in autoimmune encephalomyelitis. Nat. Commun. 2019, 10, 709. [Google Scholar] [CrossRef] [PubMed]
| Number | 31 |
| Gender (male/female) | 5/26 |
| Age (years) | 43 ± 9.7 |
| EDSS | 2 ± 1.35 |
| MRI (gadolinium +/−) | 7/24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capone, A.; Bianco, M.; Ruocco, G.; De Bardi, M.; Battistini, L.; Ruggieri, S.; Gasperini, C.; Centonze, D.; Sette, C.; Volpe, E. Distinct Expression of Inflammatory Features in T Helper 17 Cells from Multiple Sclerosis Patients. Cells 2019, 8, 533. https://doi.org/10.3390/cells8060533
Capone A, Bianco M, Ruocco G, De Bardi M, Battistini L, Ruggieri S, Gasperini C, Centonze D, Sette C, Volpe E. Distinct Expression of Inflammatory Features in T Helper 17 Cells from Multiple Sclerosis Patients. Cells. 2019; 8(6):533. https://doi.org/10.3390/cells8060533
Chicago/Turabian StyleCapone, Alessia, Manuela Bianco, Gabriella Ruocco, Marco De Bardi, Luca Battistini, Serena Ruggieri, Claudio Gasperini, Diego Centonze, Claudio Sette, and Elisabetta Volpe. 2019. "Distinct Expression of Inflammatory Features in T Helper 17 Cells from Multiple Sclerosis Patients" Cells 8, no. 6: 533. https://doi.org/10.3390/cells8060533
APA StyleCapone, A., Bianco, M., Ruocco, G., De Bardi, M., Battistini, L., Ruggieri, S., Gasperini, C., Centonze, D., Sette, C., & Volpe, E. (2019). Distinct Expression of Inflammatory Features in T Helper 17 Cells from Multiple Sclerosis Patients. Cells, 8(6), 533. https://doi.org/10.3390/cells8060533






