3. Results
3.1. Statistical Comparison of CSP vs. Non-CSP Groups (GA Administered)
The group of patients treated with GA consisted of 72 patients (12 male and 60 female patients). Group A (CSP) consisted of 32 patients (6 male and 26 female patients), group B (non-CSP) consisted of 40 patients (6 male and 34 female patients). Therefore, there were 4.33 times more women than men in group A and 5.67 times more women than men in group B. The difference between the groups and the proportion of women was not statistically significant (p value 0.916) at a 5% significance level. The groups were not significantly different in age. The mean age (standard deviation) of patients in groups differed on average by 2.2 years (A 36.2 (8.09), and B 34.0 (9.9)). This is not a statistically significant difference (p value = 0.309). Groups with a statistically significant difference (p < 0.438) did not differ in basal EDSS (median A 2.0 vs. B 1.5).
Of the lymphocyte characteristics, the differences between A and B were statistically significant (at the 5% level of significance) for baseline CD4+/CD45RO+ (mean A 25.1 vs. B 19.9; p value < 0.001; effect size = 0.9), relative lymphocyte counts (26.3 vs. 32.2; 0.005; 0.69) and CD3+/CD69+ab (0.07 vs. 0.05; 0.043; 0.32).
In the fifth year, the groups differed significantly in absolute lymphocyte counts (2.0 vs. 2.11; 0.016; 0.59), CD3ab (1.5 vs. 1.8; 0.01; 0.63), CD8ab (0.48 vs. 0.53; 0.003; 0.72), CD4+/CD45RO+ (26.5 vs. 21.7; 0,001; 0,84), CD38ab (0.9 vs. 1.2; 0.005; 0.68), CD69ab (0.06 vs. 0.07; 0.029; 0.42), CD4+/CD45RA+ (0.41 vs. 0.54; 0.044; 0.49) and CD8+/CD38+ab (0.16 vs. 0.23; 0.003; 0.63).
Out of the changes in the course of five years, the change of CD3 was statistically significant at the 5% level of significance (absolute and relative). For groups of CSPs (A), CD3 decreased over five years; on the contrary, in the non-CSP groups, CD3 increased. For the CSP group, the mean absolute decrease was three units, and in the non CSP group, there was an increase of 2.2 (p value = 0.004; effect size = 0.73). Another significant change was for CD8. In the CSP group, there was a decrease (4.3 units, (11.7%)), and in the group non-CSP, there was an increase (one unit (4.8%).) The effect size was the highest of all parameters (0.88 for absolute change and 0.79 for relative change.) Other statistically significant differences were found in the changes in the characteristics: CD8ab, CD19, absolute total leukocyte count, CD3+/CD69+ab, CD5, CD40, CD69, CD69ab and CD8+/CD38+ab.
Changes and values obtained in the fifth year can be interpreted as significantly correlated with the CSP.
According to the effect size, the most significant correlation of the EDSS progression appears to be the change in CD8 (0.88), change in CD3 (0.73) and baseline value relative lymphocyte count (0.69), which can be thought of as the CSP predictor. In addition, the CD4+/CD45RO+ and CD3+/CD69+ab parameters are interesting: the groups differ both in the measured values and in their changes (
Table 1). The descriptive statistics for all 37 parameters are summarized in
Supplementary Table S2.
3.2. Validation of CSP Dependence on Significant Parameters after Correction of Influential Factors
The values of the lymphocyte characteristics at the baseline can be considered as potential biomarkers for MS if they can predict CSP with high reliability (accuracy). Using the logistic regression, we have estimated the predictive potential for each parameter. EDSS, age at baseline, and gender, based on our patient population, do not appear to be appropriate predictors (do not predict whether patient belongs has CSP; CSP = yes/no, pseudo R2 = 0; 0.02; 0).
The statistically significant parameters (baseline values of lymphocyte characteristics) and, therefore, potential biomarkers according to the pseudo R2 are: CD4+/CD45RO+ (0.31), CD3+/CD69+ab (0.27), CD69 (0.23), CD3+/CD69+ (0.19), CD19 (0.19), CD4+/CD45RA+ (0.18), CD4+/CD45RO+ab (0.18), relative lymphocyte count (0.15), and CD8 (0.07).
Except for CD19, the presented values of the lymphocyte characteristics relative to the baseline are statistically significant, even after adjusting for the influential factors EDSS, age, and sex.
Table 2 and
Supplementary Table S3 provide estimates of the odds ratio (OR) and pseudo R2 for all described parameters above (not only baseline) with ORs that are statistically significant without and/or with adjustment for influential factors.
3.3. Identification of Combinations of Explanatory Variables
3.3.1. Penalized Logistic Regression (Identification of All 37 Explanatory Variables)
The lasso logistic regression was used to identify a significant combination of explanatory variables from all 37 explanatory variables. A combination of four parameters was selected at the baseline: relative lymphocyte counts, CD3+/CD69+, CD4+/CD45RO+, and CD4+/CD45RA+ab.
The selected four parameters were entered as explanatory variables into a multiple model. The value of R2 is 0.38. This model, which contains four lymphocyte parameters to baseline, fits the data by 23% (0.38/0.31) better than the best univariate parameter, CD4+/CD45RO+, at baseline. The statistically significant difference at the 5% level of significance explaining the parameter is CD4+/CD45RO+ (even after the influential factor adjustment). This finding confirms the results of the univariate analysis.
The estimated ORs and their statistical significance are provided in
Table 3.
3.3.2. Combination of Statistically Significant Variables from One-dimensional Models
The statistically significant baseline parameters in the one-dimensional regression models are: CD4+/CD45RO+, CD3+/CD69+ab, CD69, CD3+/CD69, CD19, CD4+/CD45RA+, CD4+/CD45RO+ab, relative lymphocyte count, and CD8.
Two parameters are strongly correlated: CD3+/CD69+ and CD3+/CD69+ab (Spearman’s correlation coefficient 0.92). In the multiple regression model, the CD3+/CD69+ab parameter was selected from this pair based on the higher R2 value of the one-dimensional models.
As an explanatory variable for the multiple regression model, a combination of the following eight parameters was selected: CD4+/CD45RO+, CD3+/CD69+ab, CD69, CD19, CD4+/CD45RA+, CD4+/CD45RO+ab, relative lymphocyte count, and CD8.
The R2 value of the multiple model R2 is 0.52. This model, which contains eight lymphocyte parameters to the baseline, fits the data 68% (0.52/0.31) better than the best univariate parameter at baseline CD4+/CD45RO+. The results of this model (regression parameter estimates) are not statistically significant due to the small number of patients in the groups and the complexity of the model, in which nine model parameters are estimated (intercept + eight for each lymphocyte parameter).
This combination of lymphocytic characteristics also fits the data better (R2 = 0.46 versus 0.38) than the combination of four variables described in the previous section.
The estimated ORs and their statistical significance are provided in
Table 4.
The predicted probability of the CSP for the values of significant parameters, i.e., CD3+/CD69+ (at baseline), CD3+/CD69+ab (at baseline), CD4+/CD45RA+ (at baseline), CD4+/CD45R0+ (at baseline), CD4+/CD45RO+ab (at baseline), CD8 (at baseline), CD69 (at baseline), ad relative lymphocyte count (at baseline) is presented in
Supplementary Figures S1–S8.
3.3.3. ROC Analysis
Table 5 below presents the results of the ROC analysis for the four most appropriate parameters (lymphocyte characteristics).
The best discriminatory ability to distinguish the CSP patients from the non-CSP patients in our groups (sample) is illustrated by CD4+/CD45RO+ (AUC 76%). The higher the value of this parameter is, the more likely it is that the patient reaches CSP within five years. The cut-off for this parameter is 22.55 (accuracy 72.1%, sensitivity 70%, specificity 74%). The remaining parameters have a lower prediction potential (AUC 62–65%). Their cut-offs are provided in
Table 5.
The results (the CSP prediction ability of identified cut-offs) were further validated in an independent cohort of patients treated with interferon β-1b (IFN). In
Table 6, the original cut-offs (identified in the new, current data, i.e., patients treated with IFN) were verified.
Table 7 summarizes the search for cut-off values for eight parameters in the independent set of patients treated with IFN.
4. Discussion
The objective of this study was to find a parameter or a combination of parameters that can predict the progression of disability in MS patients treated with GA (evaluated over a five-year period). In our study, using the ROC analysis, we determined cut-off values to the baseline that can determine the CSP and the non-CSP patients. The parameter, which helps best to identify the patients with an increased risk of disease progression within five years, is CD4+/CD45RO+.
For the CD4+/CD45RO+ characteristics, the cut-off value is set to baseline 22.55. If the patient has a value greater than 22.55, 70% of the patients can reliably be identified in the CSP group (sensitivity); in other words, 70% of the patients with a value >22.55 actually have CSP, and with a 74% probability, this cut-off can correctly identify the non-CSP patients (specificity).
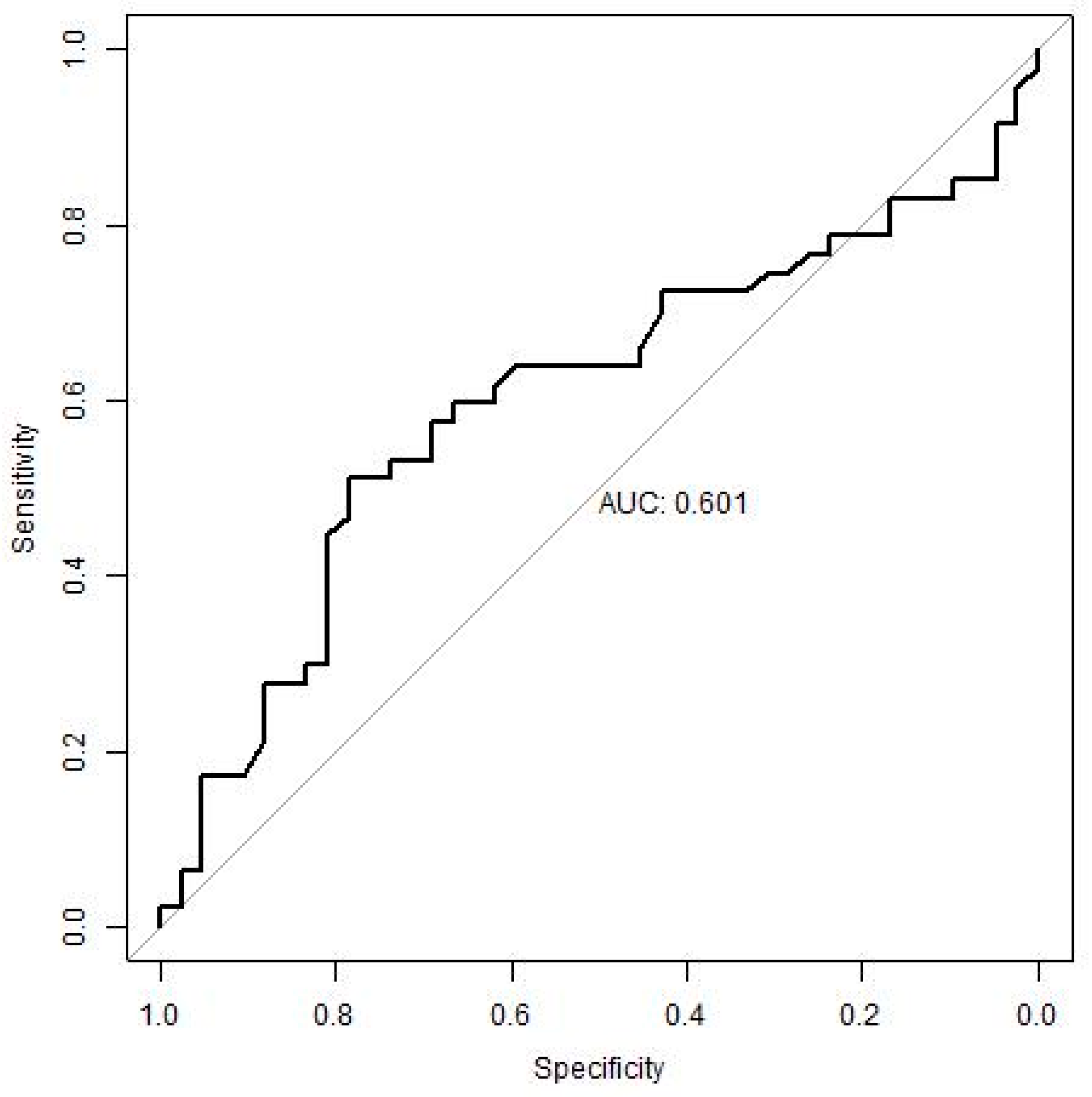
We validated the ability of identified potential biomarkers and their cut-offs to predict CSP on an independent group of patients treated with IFN, i.e., with a drug with a different mechanism of action against GA, in order to assess the robustness of these cut-offs (general utilization for patients with MS independent of their treatment). When comparing the results, the CD4+/CD45RO+ > 22.55 cut-off retained a high sensitivity (72%) on the test file, i.e., it was able to identify correctly with this CSP validator group but failed to correctly identify the non-CSP patients (low specificity) (
Figure 1). Other parameters did not reach satisfactory results.
The leucocyte molecule CD45 deserves special attention in the pathophysiology of MS. CD45 is a transmembrane molecule with tyrosine phosphatase activity, which is expressed at different density on all cells of haematopoietic origin, and exist in many different isoforms. Most naïve human T cells express a form of CD45R that is called CD45RA, and memory T cells express a different isoform called CD45RO. Several subtypes of memory T cells could be identified by mapping the expression of selected membrane molecules. Central memory T cells (TCM) express CD45RO, C-C chemokine receptor type 7 (CCR7), and L-selectin (CD62L) and are predominantly localized in lymphoid tissue. Effector memory T cells (TEM) that express CD45RO but lack expression of CCR7 and L-selectin are found both in the blood and tissues. Tissue resident memory T cells (TRM) occupy tissues without circulating in the blood.
The role of CD4+/CD45RO+ cells in the pathogenesis of MS is not yet fully understood. CD4+/CD45RO+ are memory helper T-lymphocytes, i.e., lymphocytes that have already been activated by antigens. This population changes with age, reaching a peak later in adulthood. In the experimental autoimmune encephalomyelitis, encephalitogenic T cells differ from the non-encephalitogenic ones by their expression of CD49d. The CD49d molecule is a β chain of the VLA4 integrin heterodimer adhesion molecule. The ligand for VLA4 adhesion molecule is VCAM1 (CD106) molecule expressed on endothelial cells of the brain-blood barrier. The interaction between VLA4 and VCAM1 molecules is a prerequisite for T cell entry from blood into the brain. The disease-inducing CD49d+(high) cells but not the CD49d+(low) cells enter the brain parenchyma. In this context, Barrau et al. characterized CD4+/CD45RO+/CD49d+(high) cells in RR MS patients. These cells, showing characteristics of activated cells able to produce proinflammatory cytokines, were found to be increased in the peripheral blood during relapses and present in high numbers in the cerebrospinal fluid. These results suggested that the CD4+/CD45RO+/CD49d+(high) subpopulation in RR MS patients includes autoreactive cells. CD4+/CD45RO+/CD49d+(high) cells show characteristics of activated T cells and are able to produce major TH1 cytokines such as IFN-γ [
14].
CD4+/CD45RO+ memory T-cells from MS patients also showed a reduced ability to suppress NLRP3 inflammasome activation. NLRP3 inflammasome, which is assembled via both damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) is activating caspase 1. Caspase 1 in cooperation with several other molecules is able to cleave latent forms of proIL-1β and IL-18 cytokines. Fully active pluripotent proinflammatory cytokines IL-1β and IL-18 are produced in this way. NLRP3 inflammasomes exert key roles in the initiation and propagation of the inflammation [
15].
As other potentially suitable biomarkers, we separately identified the relative lymphocyte count, CD3+/CD69+ and CD4+/CD45RA+ab or combined with CD4+/CD45RO+.
Blanco et al. and Pavelek et al. observed an increase in the CD4+/CD45RA+ count in GA-treatment responders. This observation is probably the result of the switch from CD4+/CD45RO+ memory T cells to naïve CD4+/CD45RA+ T cells, which is likely the result of GA action. The upregulation of CD4+/CD45RA+ appears to be one of the mechanisms by which GA inhibits MS activity [
16,
17]. CD3+/CD69+ cells represent early-activated T lymphocytes. A decrease in the CD3+/CD69+ count was also seen during the GA treatment [
17].
According to the ROC analysis, the cut-off values for relative lymphocyte count are 26.2, for CD3+/CD69+ are 2.25 and for CD4+/CD45RA+ab are 0.435. The cut-off value of 26.2 relative lymphocyte count has a specificity of 71%. The sensitivity of this cut-off is low at 50%. The specificity of cut-off 2.25 for CD3+/CD69 + again has a low sensitivity of 53% and a high specificity of 76%. Conversely, the cut-off of 0.435 for CD4+/CD45RA+ab has a high sensitivity of 70% and a low specificity of 51%.
Although much is left to be clarified about pathogenic mechanisms of MS, understanding the mechanisms of the immunity-mediated damage to CNS components with MS enables the introduction of new medicines that positively modulate the damaging inflammation. With the development of the MS treatment, the early escalation of treatment has gained ground, which has given hope to patients in terms of curbing irreversible disease progression and maintaining a satisfactory quality of life. To achieve this goal, the monitoring of the clinical and subclinical disease activity is crucial.
Currently, there are several potential biomarkers, which are known as responders to the GA therapy. Despite this, none of these biomarkers have been introduced in common clinical practice. A high IL-18 level at baseline and a reduction in TNF-alpha over time are associated with the response to GA [
18]. Regarding the association with clinical responders to the GA treatment, Mindur et al. [
19] reported an increase in serum IL-27 production. Another study found increased expression of the Response Gene to Complement 32 (RGC-32) (
p < 0.0001) and FasL (Fas ligand, CD178) (
p < 0.0001) and decreased expression of IL-21 (
p = 0.02) [
20] as potential biomarkers. RGC-32 was also detected by Tatomir et al. as a potential biomarker of relapse and response to the GA therapy, as the RGC-32 mRNA expression is significantly decreased during relapse and increased in responders to the GA treatment. The predictive accuracy of RGC-32 as a potential biomarker (by ROC analysis) is 90% for detecting relapses and 85% for detecting a response to the GA treatment [
21].
Other biomarkers for responders to the GA treatment may be SIRT1 mRNA (a NAD-dependent histone and protein deacetylase) and H3K9me2 (H3K9 dimethylation). GA responders had significantly higher SIRT1 mRNA (
p = 0.01) and H3K9me2 levels than non-responders (
p = 0.018) [
22]. The results were further evaluated by Cieriello et al. Statistically significant lower levels of p-SIRT1 protein (
p = 0.02) and H3K9me3 (
p = 0.004) were found in GA non-responders compared to the responders. Non-responders to the GA treatment were defined as patients who exhibited at least two relapses following initiation of the GA treatment. Using the receiver operating characteristic analysis, the area under the curve (AUC) for the prediction of relapse was 77% (
p = 0.007) for p-SIRT1 and was 81% (
p = 0.002) for H3K9me3. For predicting responsiveness to the GA treatment, the AUC was 75% (
p = 0.01) for H3K9me3. H3K9me3 could serve as potential biomarker to predict response to the GA treatment [
23].
Furthermore, patterns of TH1/TH2 cytokines can predict clinical response in MS patients treated with GA. The quotient (IL-2 + IFN-γ)/(IL-4 + IL-10) was elevated in patients with relapses compared to relapse-free patients after 12 (
p = 0.04), 24 (
p = 0.02) and 36 months (
p = 0.04) [
24].
This present study has some limitations. Pregnancy and compliance rate were not observed, and the results can be influenced by these factors. Although a worsening of the neurological findings measured by the EDSS scale was found in a number of patients over the five-year period, these patients did not meet the criteria for escalation of treatment or SPMS [
25]. As they were patients in routine clinical practice, escalation was tied to valid local Czech insurance reimbursement criteria for treatment.
The disadvantage of peripheral blood biomarkers is that they are released more extracerebrally than directly from the brain [
26]. However, the collection of cerebrospinal fluid to evaluate biomarkers is a relatively invasive method with more frequent adverse effects. In contrast, peripheral blood collection and subsequent analysis is a quick and simple method. We are aware that absolute lymphocyte counts are highly variable and may even change considerably during a day. Therefore, peripheral blood collection was performed in the morning at approximately the same time. We believe that analysing the CD4+/CD45RO+ parameter and finding baseline values before starting MS treatment can help doctors consider the use of GA in common clinical practice.