Omics Studies Revealed the Factors Involved in the Formation of Colony Boundary in Myxococcus xanthus
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Fluorescence Microscopy Observation of Colony Boundaries
2.3. Scanning Electron Microscopy (SEM)
2.4. Sample Preparation for LC-MS/MS
2.5. Proteomics Analyses and Data Processing
2.6. T6SS-Knockout Sample Preparation
2.7. Extraction of Compounds from Boundary
2.8. Identification of Compounds Using High-Resolution HPLC-MS/MS System
2.9. Data Analysis
2.10. Proteomic and Metabolomics Data Accession Numbers
3. Results
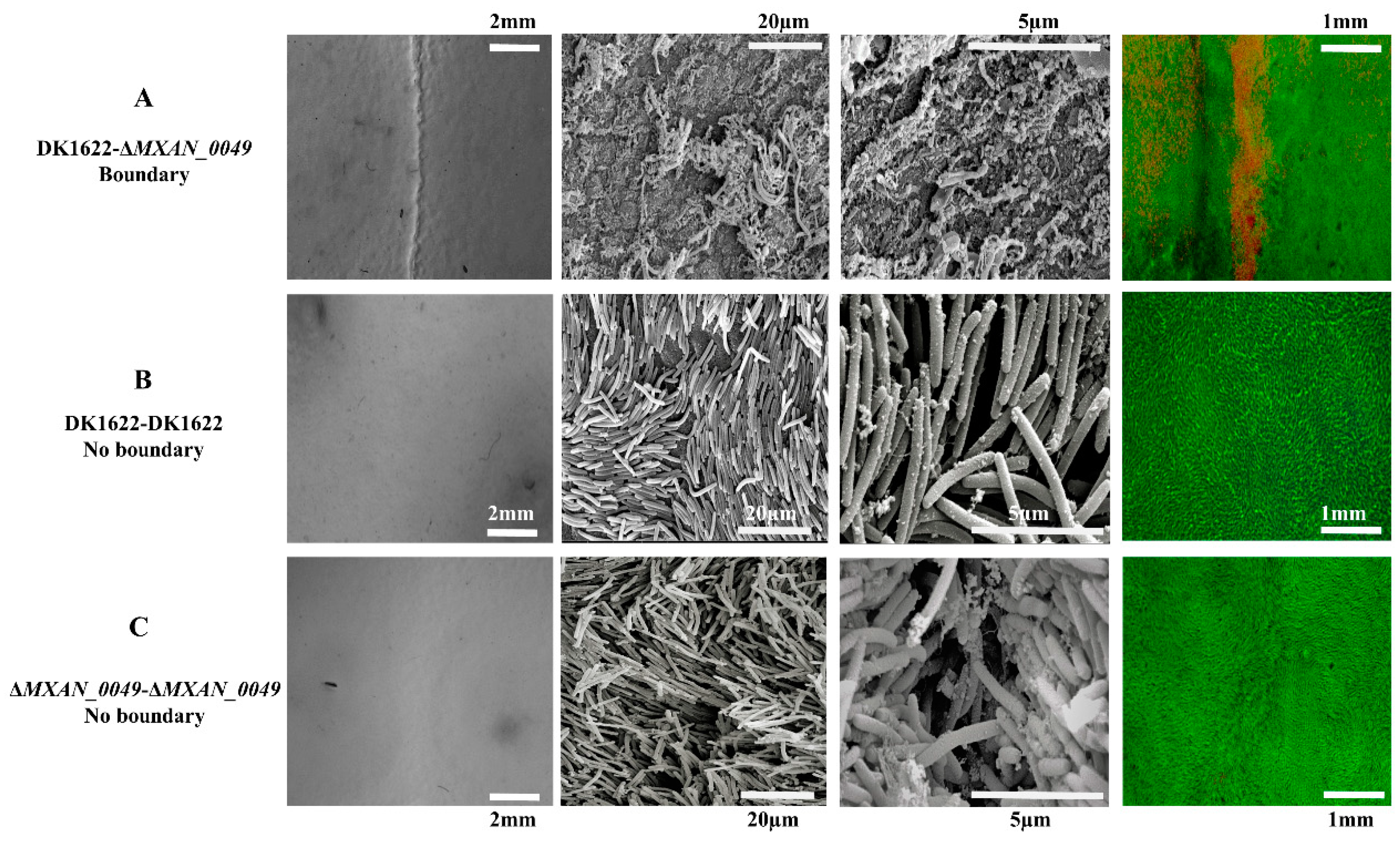
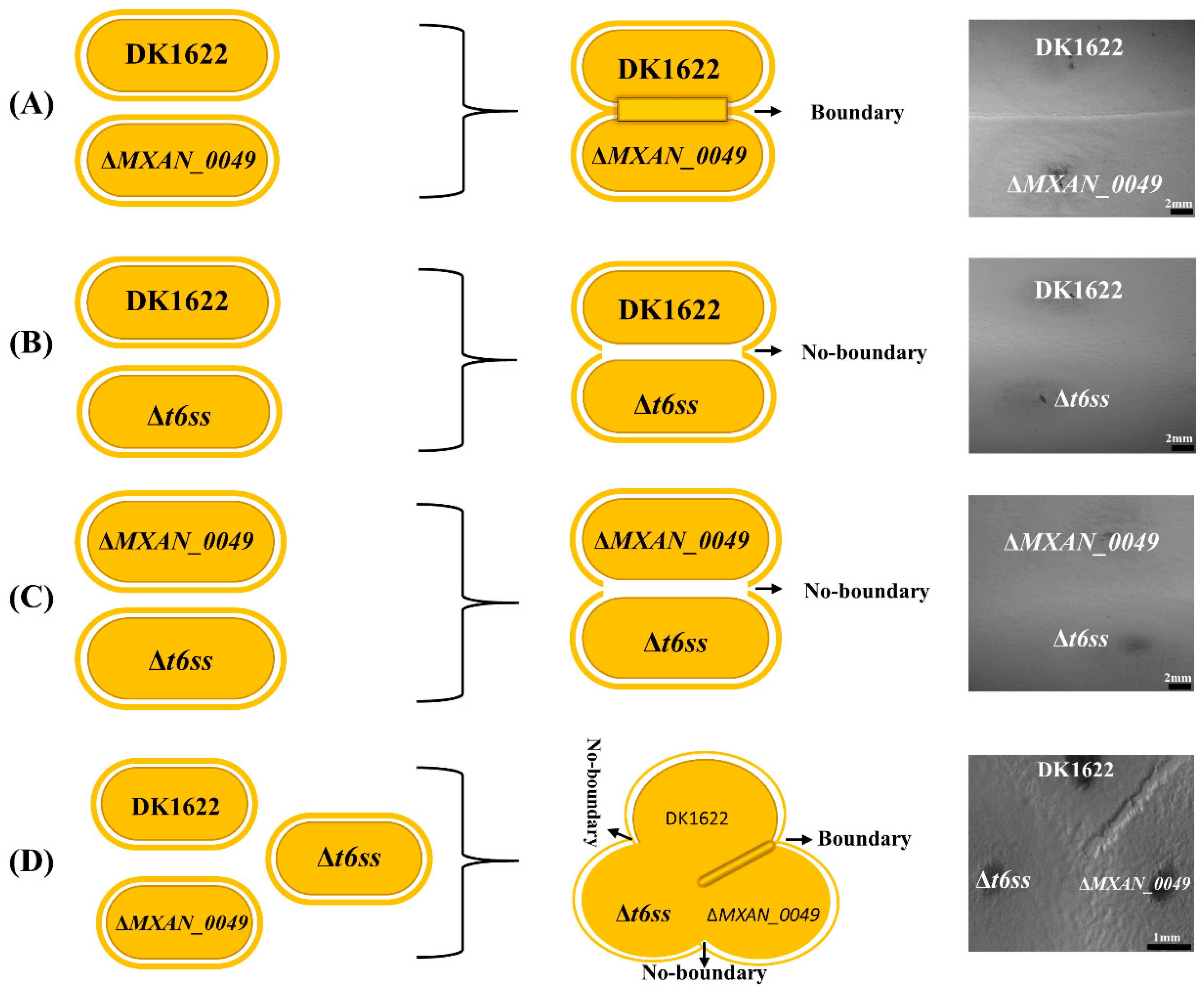
3.1. Colony Boundaries between Incompatible M. xanthus Strains under Microscope
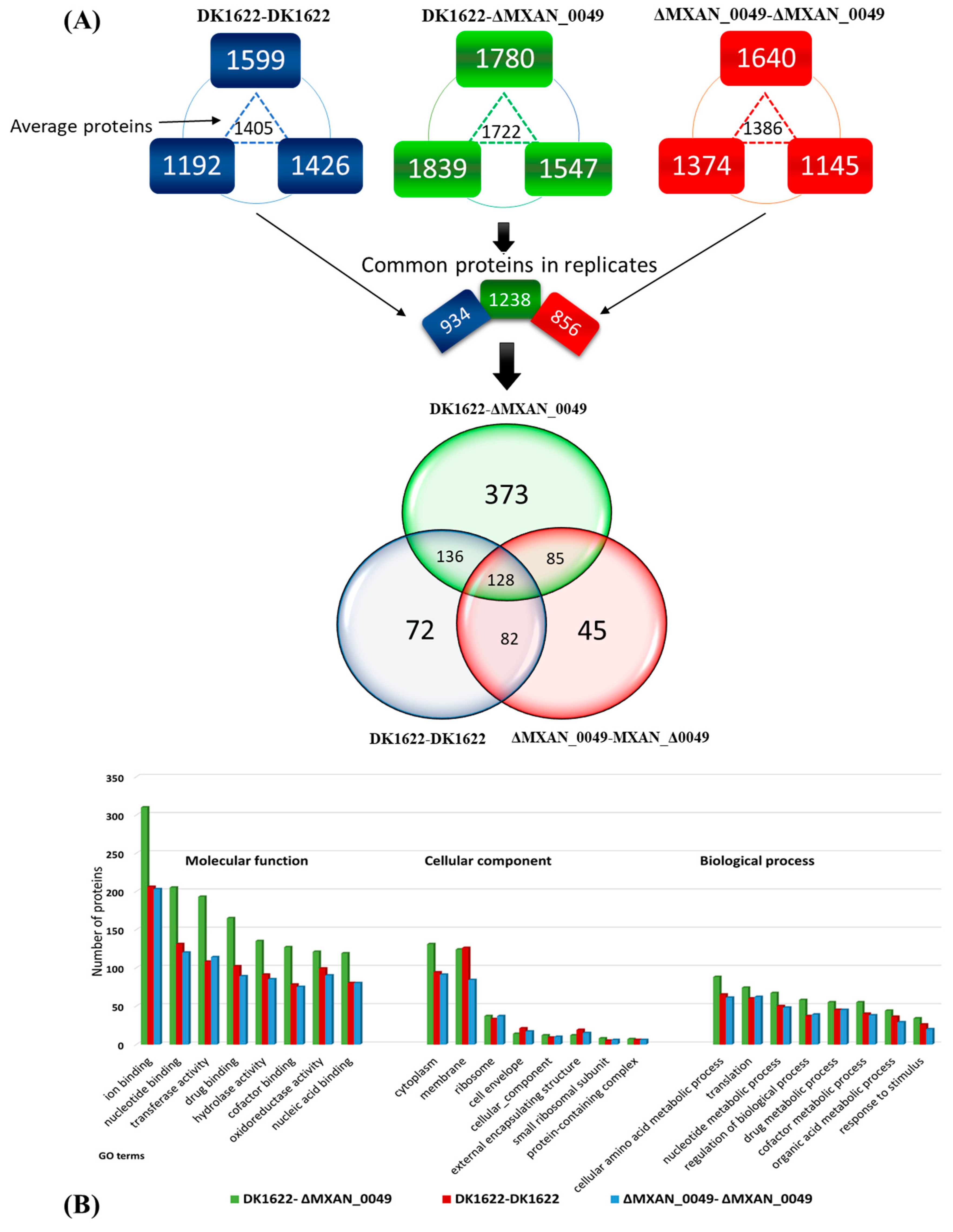
3.2. Proteomic Analysis of Colony Boundaries
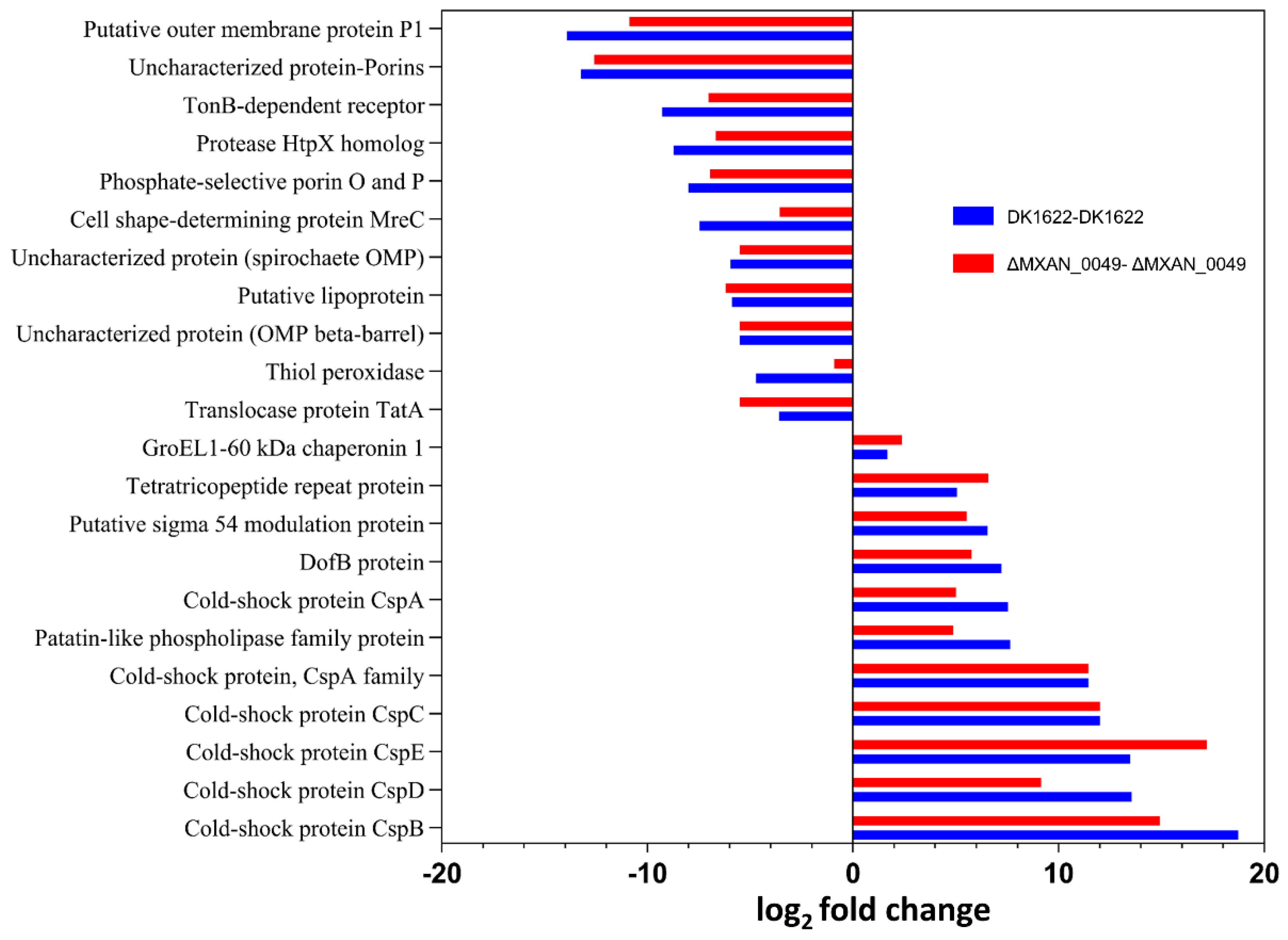
3.2.1. Significant Proteins and Their Co-Relation with Boundary Formation
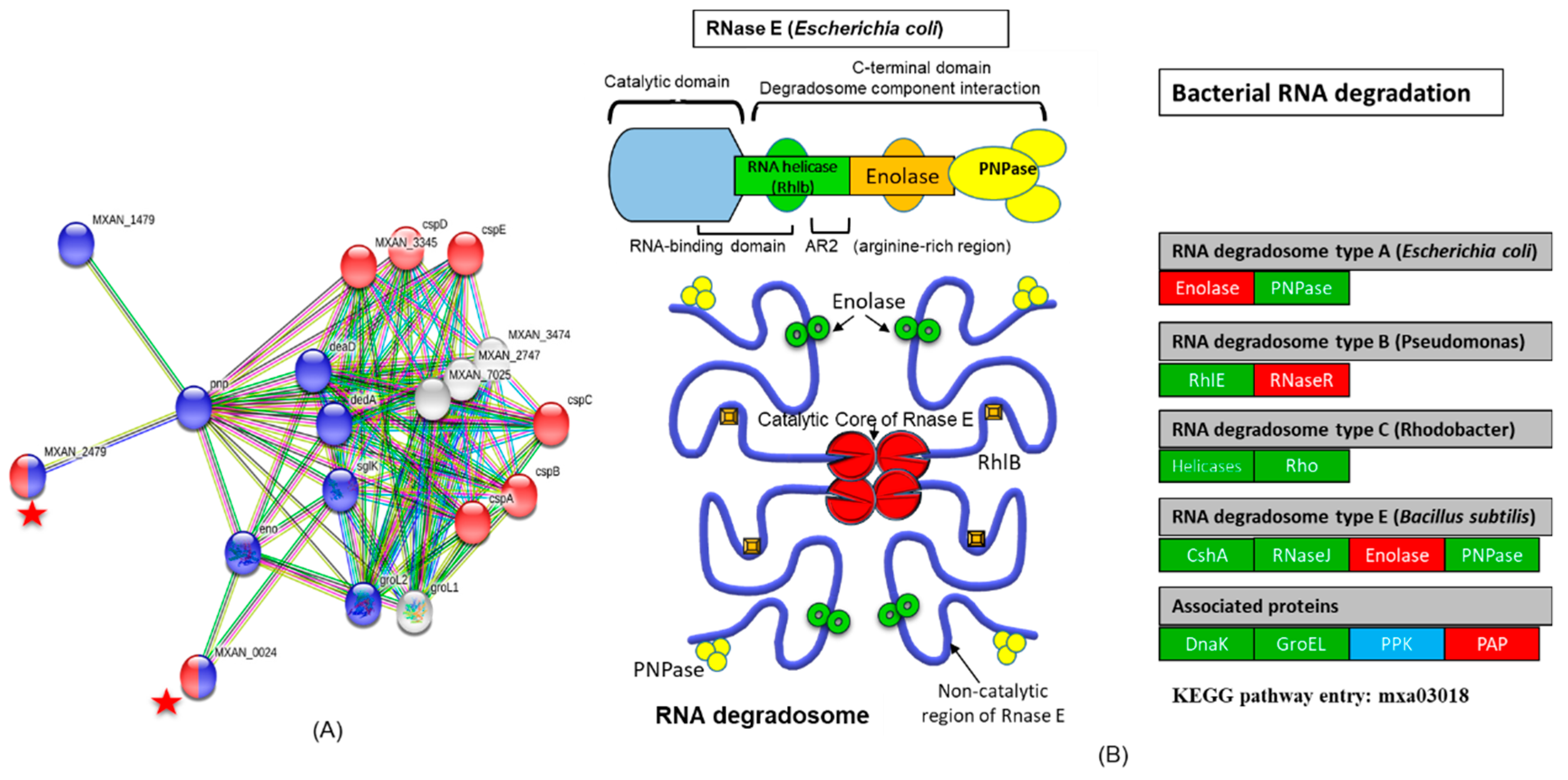
3.2.2. Interaction of Cold Shock Proteins with RNA Degradation Pathway
3.3. Co-Relationships of Boundary Formation with T6SS System
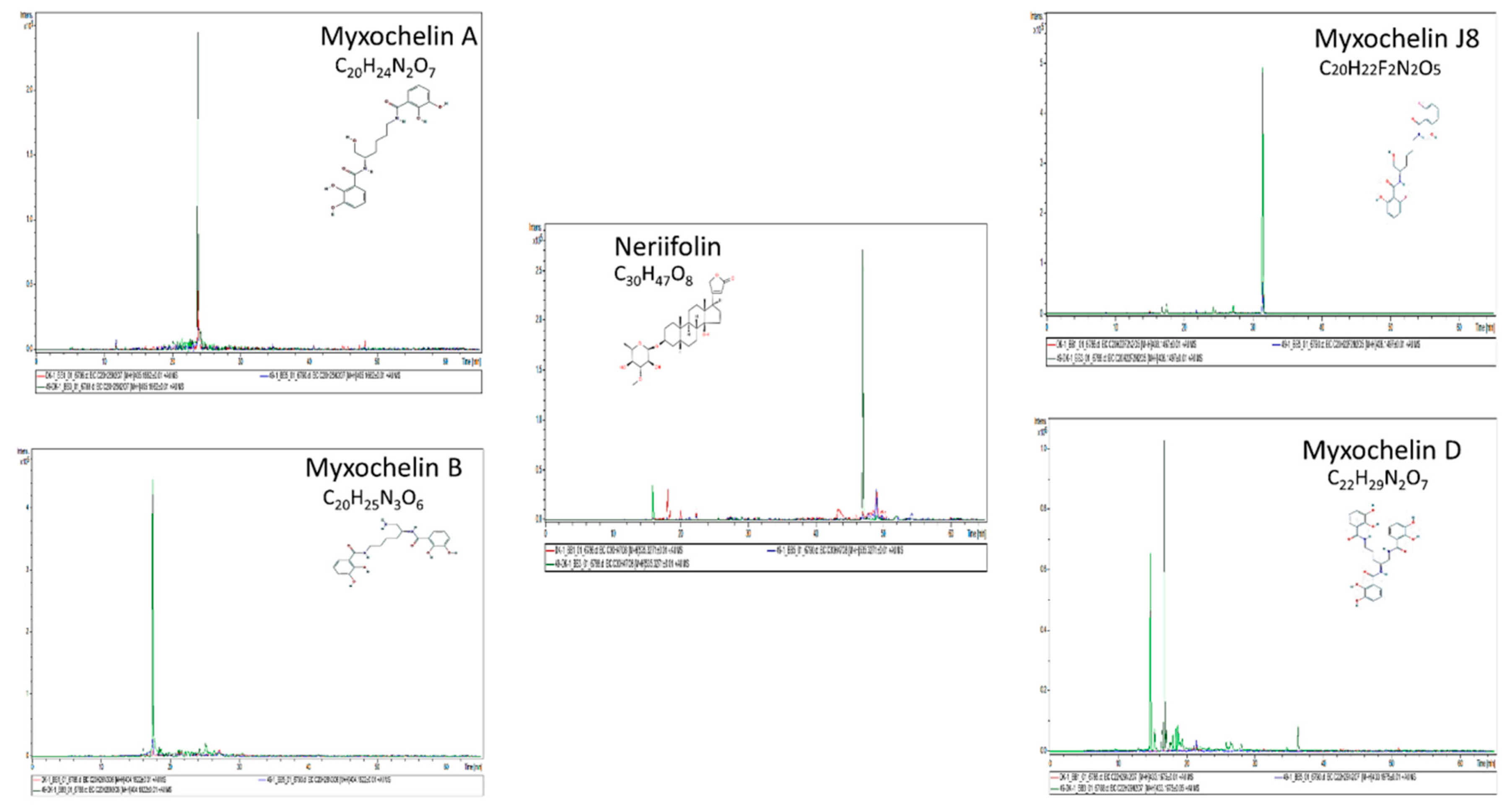
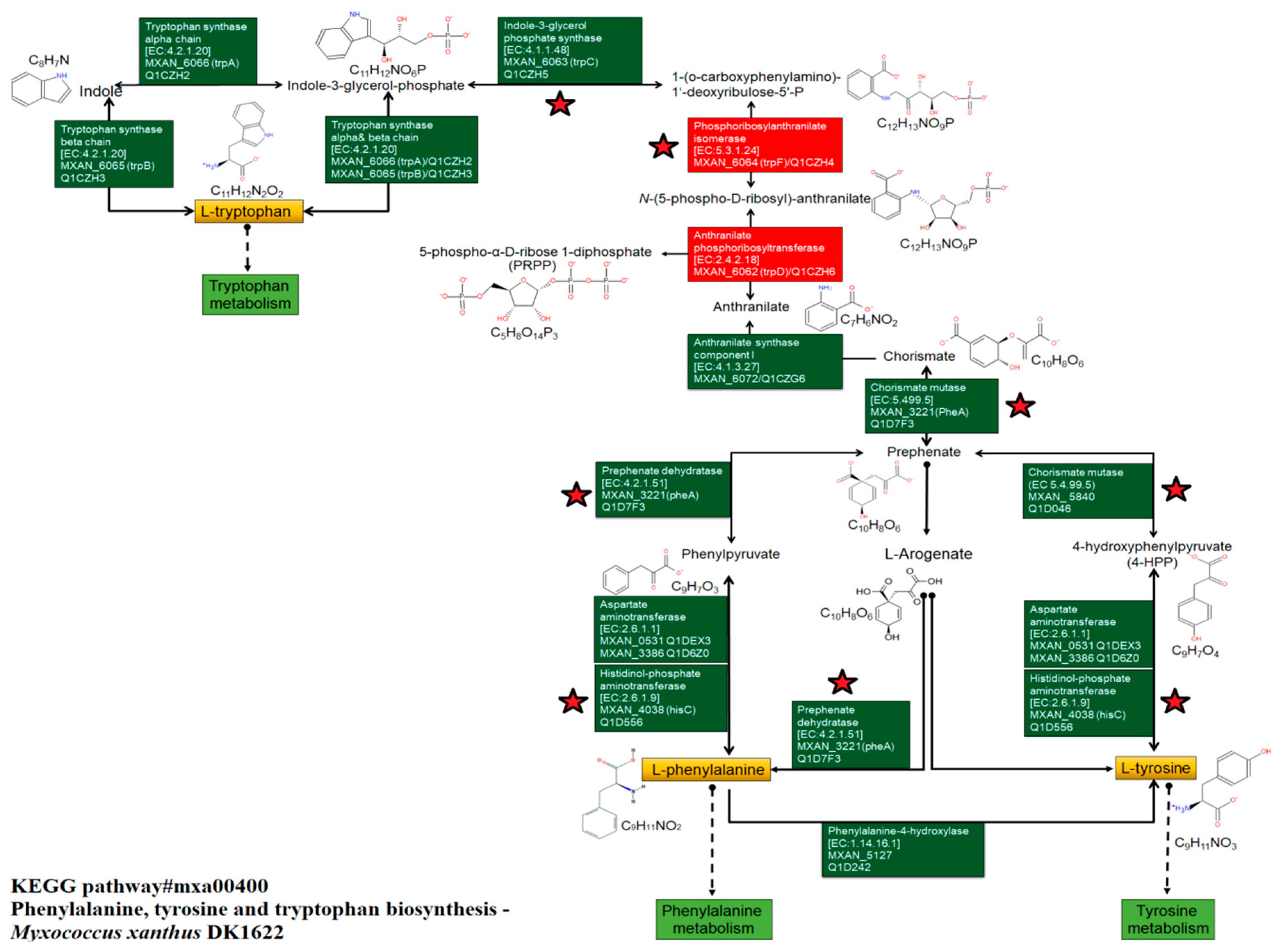
3.4. Identification of Chemical Compounds by HPLC-MS/MS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velicer, G.J.; Vos, M. Sociobiology of the Myxobacteria. Annu. Microbiol. 2009, 63, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Dienes, L. Reproductive Processes in Proteus Cultures. Exp. Boil. Med. 1946, 63, 265–270. [Google Scholar] [CrossRef]
- Gibbs, K.A.; Urbanowski, M.L.; Greenberg, E.P. Genetic determinants of self identity and social recognition in bacteria. Science 2008, 321, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, P.; Kraigher, B.; Lyons, N.A.; Kolter, R.; Mandic-Mulec, I. Kin discrimination between sympatric Bacillus subtilis isolates. Proc. Natl. Acad. Sci. USA 2015, 112, 14042–14047. [Google Scholar] [CrossRef] [PubMed]
- Lyons, N.A.; Kraigher, B.; Stefanic, P.; Mandic-Mulec, I.; Kolter, R. A Combinatorial Kin Discrimination System in Bacillus subtilis. Curr. Boil. 2016, 26, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.B.; Peterson, S.B.; Mougous, J.D. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 2014, 12, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordonez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Pukatzki, S.; Ma, A.T.; Sturtevant, D.; Krastins, B.; Sarracino, D.; Nelson, W.C.; Heidelberg, J.F.; Mekalanos, J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA 2006, 103, 1528–1533. [Google Scholar] [CrossRef]
- Benz, J.; Sendlmeier, C.; Barends, T.R.; Meinhart, A. Structural insights into the effector-immunity system Tse1/Tsi1 from Pseudomonas aeruginosa. PLoS ONE 2012, 7, e40453. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Z.; Liu, Y.; Zhou, X.W.; Anwar, M.N.; Li, Z.S.; Hu, W.; Li, Y.Z. A nuclease-toxin and immunity system for kin discrimination in Myxococcus xanthus. Env. Microbiol. 2018, 20, 2552–2567. [Google Scholar] [CrossRef]
- Patra, P.; Vassallo, C.N.; Wall, D.; Igoshin, O.A. Mechanism of Kin-Discriminatory Demarcation Line Formation between Colonies of Swarming Bacteria. Biophys. J. 2017, 113, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.T.; Wei, X.; Bucuvalas, A.; Haft, D.H.; Gerloff, D.L.; Wall, D. Cell contact-dependent outer membrane exchange in myxobacteria: Genetic determinants and mechanism. PLoS Genet. 2012, 8, e1002626. [Google Scholar]
- Vassallo, C.N.; Cao, P.; Conklin, A.; Finkelstein, H.; Hayes, C.S.; Wall, D. Infectious polymorphic toxins delivered by outer membrane exchange discriminate kin in myxobacteria. Elife 2017, 6, e29397. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.; Evangelia, G.K. Proteomics: Potential in the post-genome era. Hell. J. Cardiol. 2003, 44, 301–307. [Google Scholar]
- Mallick, P.; Kuster, B. Proteomics: A pragmatic perspective. Nat. Biotechnol. 2010, 28, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Nat, N.V.K.; Srivastava, S.; Yajima, W.; Sharma, N. Application of Proteomics to Investigate Plant-Pathogen Interactions. Curr. Proteom. 2007, 4, 28–43. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics Reviewed: A New “Omics” Platform Technology for Systems Biology and Implications for Natural Products Research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef]
- Want, E.J.; Cravatt, B.F.; Siuzdak, G. The expanding role of mass spectrometry in metabolite profiling and characterization. ChemBioChem 2005, 6, 1941–1951. [Google Scholar] [CrossRef]
- Castro-Santos, P.; Laborde, C.M.; Diaz-Pena, R. Genomics, proteomics and metabolomics: Their emerging roles in the discovery and validation of rheumatoid arthritis biomarkers. Clin. Exp. Rheumatol. 2015, 33, 279–286. [Google Scholar]
- Dworkin, M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 1996, 60, 70–102. [Google Scholar]
- Gong, Y.; Zhang, Z.; Zhou, X.W.; Anwar, M.N.; Hu, X.Z.; Li, Z.S.; Chen, X.J.; Li, Y.Z. Competitive Interactions Between Incompatible Mutants of the Social Bacterium. Myxococcus xanthus DK1622. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Hodgkin, J.; Kaiser, D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 1977, 74, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Codling, C.E.; Hann, A.C.; Maillard, J.Y.; Russell, A.D. An investigation into the antimicrobial mechanisms of action of two contact lens biocides using electron microscopy. Cont. Lens Anterior Eye 2005, 28, 163–168. [Google Scholar] [CrossRef]
- Leon, I.R.; Schwammle, V.; Jensen, O.N.; Sprenger, R.R. Quantitative assessment of in-solution digestion efficiency identifies optimal protocols for unbiased protein analysis. Mol. Cell. Proteom. 2013, 12, 2992–3005. [Google Scholar] [CrossRef]
- Sequencing Grade Modified Trypsin, Part# 9PIV5113, Revised 4/18. Promega Corporation. Available online: https://www.promega.com/-/media/files/resources/protocols/product-information-sheets/n/sequencing-grade-modified-trypsin-frozen-protocol.pdf?la=en (accessed on 14 February 2018).
- Zachara, N.E.; Vosseller, K.; Hart, G.W. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr. Protoc. Protein Sci. 2011. [Google Scholar] [CrossRef]
- Breitkopf, S.B.; Asara, J.M. Determining in vivo phosphorylation sites using mass spectrometry. Curr. Protoc. Mol. Biol. 2012. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Bonsall, R.F.; Weller, D.M. Effect of Population Density of Pseudomonas fluorescens on Production of 2,4-Diacetylphloroglucinol in the Rhizosphere of Wheat. Phytopathology 1999, 89, 470–475. [Google Scholar] [CrossRef]
- Garbeva, P.; Silby, M.W.; Raaijmakers, J.M.; Levy, S.B.; Boer, W. Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competitors. ISME J. 2011, 5, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Wang, Y.; Li, Z.F.; Gong, Y.; Zhang, P.; Hu, W.C.; Sheng, D.H.; Li, Y.Z. Increasing on-target cleavage efficiency for CRISPR/Cas9-induced large fragment deletion in Myxococcus xanthus. Microb. Cell Fact. 2017, 16, 142. [Google Scholar] [CrossRef]
- VanRheenen, S.M.; Luo, Z.-Q.; O’Connor, T.; Isberg, R.R. Members of a Legionella pneumophila Family of Proteins with ExoU (Phospholipase A) Active Sites Are Translocated to Target Cells. Infect. Immun. 2006, 74, 3597–3606. [Google Scholar] [CrossRef]
- Brugirard-Ricaud, K.; Givaudan, A.; Parkhill, J.; Boemare, N.; Kunst, F.; Zumbihl, R.; Duchaud, E. Variation in the Effectors of the Type III Secretion System among Photorhabdus Species as Revealed by Genomic Analysis. J. Bacteriol. 2004, 186, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Housley, N.A.; Winkler, H.H.; Audia, J.P. The Rickettsia prowazekii ExoU Homologue Possesses Phospholipase A1 (PLA1), PLA2, and Lyso-PLA2 Activities and Can Function in the Absence of Any Eukaryotic Cofactors In Vitro. J. Bacteriol. 2011, 193, 4634–4642. [Google Scholar] [CrossRef]
- Rahman, M.S.; Gillespie, J.J.; Kaur, S.J.; Sears, K.T.; Ceraul, S.M.; Beier-Sexton, M.; Azad, A.F. Rickettsia typhi Possesses Phospholipase A2 Enzymes that Are Involved in Infection of Host Cells. PLOS Pathog. 2013, 9, e1003399. [Google Scholar] [CrossRef][Green Version]
- Goranson, J.; Zhu, L.; Sawa, T.; Fleiszig, S.M.J.; Wu, C.; Frank, D.W.; Finck-Barbançon, V.; Wiener-Kronish, J.P.; Mende-Mueller, L.; Finck-Barbançon, V.; et al. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 1997, 25, 547–557. [Google Scholar]
- Bröms, J.E.; Edqvist, P.J.; Forsberg, A.; Francis, M.S.; Bröms, J.E. Tetratricopeptide repeats are essential for PcrH chaperone function inPseudomonas aeruginosatype III secretion. FEMS Microbiol. Lett. 2006, 256, 57–66. [Google Scholar] [CrossRef]
- Edqvist, P.J.; Bröms, J.E.; Betts, H.J.; Forsberg, Å.; Pallen, M.J.; Francis, M.S. Tetratricopeptide repeats in the type III secretion chaperone, LcrH: their role in substrate binding and secretion. Mol. Microbiol. 2006, 59, 31–44. [Google Scholar] [CrossRef]
- Chakraborty, S.; Monfett, M.; Maier, T.M.; Benach, J.L.; Frank, D.W.; Thanassi, D.G. Type IV Pili in Francisella tularensis: Roles of pilF and pilT in Fiber Assembly, Host Cell Adherence, and Virulence. Infect. Immun. 2008, 76, 2852–2861. [Google Scholar] [CrossRef]
- Chao, J.; Wong, D.; Zheng, X.; Poirier, V.; Bach, H.; Hmama, Z.; Av-Gay, Y. Protein kinase and phosphatase signaling in Mycobacterium tuberculosis physiology and pathogenesis. Biochim. Biophys. Acta. 2010, 1804, 620–627. [Google Scholar] [CrossRef]
- Cane, D.E. Introduction: Polyketide and Nonribosomal Polypeptide Biosynthesis. From Collie to Coli. Chem. Rev. 1997, 97, 2463–2464. [Google Scholar] [CrossRef]
- Wachi, M.; Doi, M.; Okada, Y.; Matsuhashi, M. New mre genes mreC and mreD, responsible for formation of the rod shape of Escherichia coli cells. J. Bacteriol. 1989, 171, 6511–6516. [Google Scholar] [CrossRef]
- Ent, F.V.D.; Leaver, M.; Bendezu, F.; Errington, J.; De Boer, P.; Löwe, J. Dimeric structure of the cell shape protein MreC and its functional implications. Mol. Microbiol. 2006, 62, 1631–1642. [Google Scholar]
- Lee, J.-C.; Stewart, G.C. Essential Nature of the mreC Determinant of Bacillus subtilis†. J. Bacteriol. 2003, 185, 4490–4498. [Google Scholar] [CrossRef]
- Yen, M.-R.; Peabody, C.R.; Partovi, S.M.; Zhai, Y.; Tseng, Y.-H.; Saier, M.H. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim. Biophys. Acta. 2002, 1562, 6–31. [Google Scholar] [CrossRef]
- Higgs, P.I.; Myers, P.S.; Postle, K. Interactions in the TonB-Dependent Energy Transduction Complex: ExbB and ExbD Form Homomultimers. J. Bacteriol. 1998, 180, 6031–6038. [Google Scholar]
- Michaux, C.; Holmqvist, E.; Vasicek, E.; Sharan, M.; Barquist, L.; Westermann, A.J.; Gunn, J.S.; Vogel, J. RNA target profiles direct the discovery of virulence functions for the cold-shock proteins CspC and CspE. Proc. Natl. Acad. Sci. USA 2017, 114, 6824–6829. [Google Scholar] [CrossRef]
- Horn, G.; Hofweber, R.; Kremer, W.; Kalbitzer, H.R. Structure and function of bacterial cold shock proteins. Cell. Mol. Life Sci. 2007, 64, 1457–1470. [Google Scholar] [CrossRef]
- Michaux, C.; Martini, C.; Shioya, K.; Lecheheb, S.A.; Budin-Verneuil, A.; Cosette, P.; Sanguinetti, M.; Hartke, A.; Verneuil, N.; Giard, J.-C. CspR, a Cold Shock RNA-Binding Protein Involved in the Long-Term Survival and the Virulence of Enterococcus faecalis. J. Bacteriol. 2012, 194, 6900–6908. [Google Scholar] [CrossRef]
- Eshwar, A.K.; Guldimann, C.; Oevermann, A.; Tasara, T. Cold-Shock Domain Family Proteins (Csps) Are Involved in Regulation of Virulence, Cellular Aggregation, and Flagella-Based Motility in Listeria monocytogenes. Front. Microbiol. 2017, 7, 453. [Google Scholar] [CrossRef]
- Schmid, B.; Klumpp, J.; Raimann, E.; Loessner, M.J.; Stephan, R.; Tasara, T. Role of Cold Shock Proteins in Growth of Listeria monocytogenes under Cold and Osmotic Stress Conditions▿. Appl. Env. Microbiol. 2009, 75, 1621–1627. [Google Scholar] [CrossRef]
- Ginalski, K.; Kinch, L.; Rychlewski, L.; Grishin, N.V. BOF: a novel family of bacterial OB-fold proteins. Febs Lett. 2004, 567, 297–301. [Google Scholar] [CrossRef]
- Agrawal, V.; Kishan, K. OB-fold: Growing Bigger with Functional Consistency. Curr. Protein Pept. Sci. 2003, 4, 195–206. [Google Scholar] [CrossRef]
- Carpousis, A.J. The RNA Degradosome of Escherichia coli: An mRNA-Degrading Machine Assembled on RNase E. Annu. Rev. Microbiol. 2007, 61, 71–87. [Google Scholar] [CrossRef]
- Chopra, I. Molecular mechanisms involved in the transport of antibiotics into bacteria. Parasitology 1988, 96, S25–S44. [Google Scholar] [CrossRef]
- Livermore, D.M. Antibiotic uptake and transport by bacteria. Scand. J. Infect. Dis. Suppl. 1990, 74, 15–22. [Google Scholar]
- Filloux, A. The rise of the Type VI secretion system. F1000Prime Rep. 2013, 5, 52. [Google Scholar] [CrossRef]
- Ho, B.T.; Dong, T.G.; Mekalanos, J.J. A view to a kill: The bacterial type VI secretion system. Cell Host Microbe 2014, 15, 9–21. [Google Scholar] [CrossRef]
- Konovalova, A.; Petters, T.; Søgaard-Andersen, L. Extracellular biology of Myxococcus xanthus. FEMS Microbiol. Rev. 2010, 34, 89–106. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Chen, S.; Tocheva, E.I.; Treuner-Lange, A.; Löbach, S.; Søgaard-Andersen, L.; Jensen, G.J. Correlated cryogenic photoactivated localization microscopy and cryo-electron tomography. Nat. Methods 2014, 11, 737–739. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Xu, H.H.; Hao, L.; Deng, Z.; Rajakumar, K.; Ou, H.-Y. SecReT6: a web-based resource for type VI secretion systems found in bacteria. Env. Microbiol. 2015, 17, 2196–2202. [Google Scholar] [CrossRef]
- Kudryashev, M.; Wang, R.Y.-R.; Brackmann, M.; Scherer, S.; Maier, T.; Baker, D.; DiMaio, F.; Stahlberg, H.; Egelman, E.H.; Basler, M. Structure of the Type VI secretion system contractile sheath. Cell 2015, 160, 952–962. [Google Scholar] [CrossRef]
- Troselj, V.; Treuner-Lange, A.; Sogaard-Andersen, L.; Wall, D. Physiological Heterogeneity Triggers Sibling Conflict Mediated by the Type VI Secretion System in an Aggregative Multicellular Bacterium. mBio 2018, 9. [Google Scholar] [CrossRef]
- Schneiker, S.; Perlova, O.; Kaiser, O.; Gerth, K.; Alici, A.; O Altmeyer, M.; Bartels, D.; Bekel, T.; Beyer, S.; Bode, E.; et al. Complete genome sequence of the myxobacterium Sorangium cellulosum. Nat. Biotechnol. 2007, 25, 1281–1289. [Google Scholar] [CrossRef]
- Wenzel, S.C.; Müller, R. The biosynthetic potential of myxobacteria and their impact in drug discovery. Drug Discov. Dev. 2009, 12, 220–230. [Google Scholar]
- Lee, C.H. LC-MS/MS Profiling-Based Secondary Metabolite Screening of Myxococcus xanthus. J. Microbiol. Biotechnol. 2009, 19, 51–54. [Google Scholar] [CrossRef]
- Reichenbach, H. Myxobacteria, producers of novel bioactive substances. J. Ind. Microbiol. Biotechnol. 2001, 27, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Korp, J.; Gurovic, M.S.V.; Nett, M.; Dickschat, J.S. Antibiotics from predatory bacteria. Beilstein J. Org. Chem. 2016, 12, 594–607. [Google Scholar] [CrossRef]
- Krug, D.; Zurek, G.; Revermann, O.; Vos, M.; Velicer, G.J.; Müller, R. Discovering the Hidden Secondary Metabolome of Myxococcus xanthus: A Study of Intraspecific Diversity. Appl. Env. Microbiol. 2008, 74, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J.; Müller, R. Myxobacterial secondary metabolites: bioactivities and modes-of-action. Nat. Prod. Rep. 2010, 27, 1276. [Google Scholar] [CrossRef]
- Yanofsky, C. RNA-based regulation of genes of tryptophan synthesis and degradation, in bacteria. RNA 2007, 13, 1141–1154. [Google Scholar] [CrossRef]
- Anderson, M.S.; Garcia, E.C.; Cotter, P.A. Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure. PLOS Pathog. 2014, 10, e1004076. [Google Scholar] [CrossRef]
- Wall, D. Kin Recognition in Bacteria. Annu. Rev. Microbiol. 2016, 70, 143–160. [Google Scholar] [CrossRef]
- Rendueles, O.; Zee, P.C.; Dinkelacker, I.; Amherd, M.; Wielgoss, S.; Velicer, G.J. Rapid and widespread de novo evolution of kin discrimination. Proc. Natl. Acad. Sci. USA 2015, 112, 9076–9081. [Google Scholar] [CrossRef]
- Mehdiabadi, N.J.; Jack, C.N.; Farnham, T.T.; Platt, T.G.; Kalla, S.E.; Shaulsky, G.; Queller, D.C.; Strassmann, J.E. Social evolution: Kin preference in a social microbe. Nature 2006, 442, 881–882. [Google Scholar] [CrossRef]
- Strassmann, J.E. Kin Discrimination in Dictyostelium Social Amoebae. J. Eukaryot. Microbiol. 2016, 63, 378–383. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Hayes, C.S.; Aoki, S.K.; Low, D.A. Bacterial Contact-Dependent Delivery Systems. Annu. Genet. 2010, 44, 71–90. [Google Scholar] [CrossRef]
- Budding, A.E.; Ingham, C.J.; Bitter, W.; Vandenbroucke-Grauls, C.M.; Schneeberger, P.M. The Dienes phenomenon: Competition and territoriality in Swarming Proteus mirabilis. J. Bacteriol. 2009, 191, 3892–3900. [Google Scholar] [CrossRef]
- Vos, M.; Velicer, G.J. Social conflict in centimeter-and global-scale populations of the bacterium Myxococcus xanthus. Curr. Biol. 2009, 19, 1763–1767. [Google Scholar] [CrossRef]
- Rendueles, O.; Amherd, M.; Velicer, G.J. Positively Frequency-Dependent Interference Competition Maintains Diversity and Pervades a Natural Population of Cooperative Microbes. Curr. Biol. 2015, 25, 1673–1681. [Google Scholar] [CrossRef]
- Munson, E.L.; Pfaller, M.A.; Doern, G.V. Modification of dienes mutual inhibition test for epidemiological characterization of Pseudomonas aeruginosa isolates. J. Clin. Microbiol. 2002, 40, 4285–4288. [Google Scholar] [CrossRef]
- Bednarska, N.G.; Schymkowitz, J.; Rousseau, F.; Van Eldere, J. Protein aggregation in bacteria: The thin boundary between functionality and toxicity. Microbiology 2013, 159, 1795–1806. [Google Scholar] [CrossRef]
- Hernández-Macedo, M.L.; Barreto, E.N.; de Souza Cavalcanti, A.C.; da Silva, R.S.; dos Anjos Brandão, E.C.T.; Fernandes, R.P.M.; Talamini, V.; Diniz, L.E.C.; Fernandes, M.F. Antimicrobial potential of Actinomycetes by NRPS and PKS-I pathways. BMC Protocol. 2014, 8, P175. [Google Scholar] [CrossRef]
- Müller, C.A.; Oberauner-Wappis, L.; Peyman, A.; Amos, G.C.A.; Wellington, E.M.H.; Berg, G. Mining for Nonribosomal Peptide Synthetase and Polyketide Synthase Genes Revealed a High Level of Diversity in the Sphagnum Bog Metagenome. Appl. Environ. Microbiol. 2015, 81, 5064–5072. [Google Scholar] [CrossRef]
| Protein ID | Protein Description | Gene ID | Mean Value | Log2 (Fold Change) | |||
|---|---|---|---|---|---|---|---|
| DK1622–ΔMXAN_0049 Boundary | ΔMXAN_0049-ΔMXAN_0049 Non-Boundary | DK1622-DK1622 Non-Boundary | Boundary/ΔMXAN_0049 | Boundary/DK1622 | |||
| Q1D3Y5 | GroEL1-60 kDa chaperonin 1 | MXAN_4467 | 80.49 | 60.81 | 66.24 | 2.381892085 | 1.672802712 |
| Q1DEI3 | Cold-shock protein CspC | MXAN_0672 | 77.61 | 13.93 | 13.93 | 11.9978081 | 11.9978081 |
| Q1D1L2 | Cold-shock protein CspB | MXAN_5310 | 75.7567 | 7.07 | 1 | 14.90209495 | 18.72748517 |
| Q1D6F1 | Cold-shock protein CspD | MXAN_3582 | 64.1767 | 17.4167 | 6.96667 | 9.154352467 | 13.55123262 |
| Q1CZK1 | Cold-shock protein CspE | MXAN_6037 | 63.2333 | 16.86333 | 6.86333 | 17.19 | 13.48 |
| Q1D4F3 | Patatin-like phospholipase family protein | MXAN_4295 | 61.19 | 32.1267 | 20.9867 | 4.88 | 7.65 |
| Q1D4M7 | DofB protein | MXAN_4295 | 55.25 | 24.83 | 19.6867 | 5.77 | 7.21 |
| Q1DF46 | Putative sigma 54 modulation protein | MXAN_0457 | 37.83 | 15.91 | 13.21 | 5.54 | 6.53 |
| Q1D4P0 | Tetratricopeptide repeat protein | MXAN_4221 | 44.18 | 16.22 | 21.07 | 6.58 | 5.06 |
| Q1D730 | Cold-shock protein, CspA family | MXAN_3345 | 42.29 | 4.97 | 4.97 | 11.44 | 11.44 |
| Q1DBV4 | Cold-shock protein CspA | MXAN_1617 | 19.12 | 6.86333 | 2.94 | 4.99 | 7.54 |
| Protein ID | Protein Description | Gene ID | Mean Value | Log2 (Fold Change) | |||
|---|---|---|---|---|---|---|---|
| DK1622–ΔMXAN_0049 Boundary | ΔMXAN_0049-ΔMXAN_0049 Non-Boundary | DK1622-DK1622 Non-Boundary | Boundary/ΔMXAN_0049 | Boundary/DK1622 | |||
| Q1CYN2 | Putative lipoprotein | MXAN_6367 | 21.36 | 51.75 | 49.72 | −6.20 | −5.88 |
| Q1D854 | Sec-independent protein translocase protein TatA | MXAN_2960 | 19.57 | 44.20 | 34.06 | −5.51 | −3.60 |
| Q1D031 | Uncharacterized protein (Porin domain superfamily) | MXAN_5855 | 2.40 | 38.7 | 42.16 | −12.59 | −13.23 |
| Q1CWS0 | Putative outer membrane protein P1 | MXAN_7040 | 1.51 | 27.70 | 42.57 | −10.86 | −13.89 |
| Q1CZB5 | Glyoxalase family protein | MXAN_6123 | 22.97 | 25.49 | 42.85 | −0.66 | −4.29 |
| Q1CYA4 | Thiol peroxidase | MXAN_6496 | 17.35 | 20.27 | 36.06 | −0.91 | −4.71 |
| Q1CX48 | TonB-dependent receptor | MXAN_6911 | 3.21 | 18.12 | 26.10 | −7.03 | −9.27 |
| Q1DDT8 | Uncharacterized protein (OMP beta-barrel) | MXAN_0924 | 3.47 | 14.22 | 14.22 | −5.49 | −5.49 |
| Q1DEU3 | Protease HtpX homolog | MXAN_0561 | 1.85667 | 14.21 | 20.4367 | −6.66 | −8.72 |
| Q1D5R1 | Uncharacterized protein (spirochaete OMP) | MXAN_3830 | 1.23 | 10.56 | 11.59 | −5.51 | −5.96 |
| Q1DEU2 | Phosphate-selective porin O and P | MXAN_0562 | 1 | 13.9333 | 16.9233 | −6.93 | −8.00 |
| Compounds | Sample | Boundary/Non-Boundary | RT [min] | Calc. m/z [M + H] | Formula | Signal to-Noise (S/N) | Area | Intensity (I) | Difference with Boundary by Area (in Folds) |
|---|---|---|---|---|---|---|---|---|---|
| Phenylalanine | DK1622/ΔMXAN_0049 | Boundary | 11.5 | 166.0863 | C9H11NO2 | 6118.5 | 2.02 × 10 8 | 10,960,726 | |
| DK1622-DK1622 | Non-boundary | 11.6 | 166.0863 | C9H11NO2 | 127.5 | 1,833,551 | 212,562 | 110.2998 | |
| ΔMXAN_0049–ΔMXAN_0049 | Non-boundary | 11.6 | 166.0863 | C9H11NO2 | 244.5 | 3,740,288 | 347,508 | 54.07075 | |
| Tyrosine | DK1622/ΔMXAN_0049 | Boundary | 6.8 | 182.0812 | C9H11NO3 | 3017.4 | 53,175,768 | 5,498,402 | |
| DK1622-DK1622 | Non-boundary | N.A | N.A | C9H11NO3 | N.A | N.A | N.A | N.A | |
| ΔMXAN_0049–ΔMXAN_0049 | Non-boundary | N.A | N.A | C9H11NO3 | N.A | N.A | N.A | N.A | |
| Tryptophan | DK1622/ΔMXAN_0049 | Boundary | 14.7 | 205.0972 | C11H12N2O2 | 1761.1 | 18,269,390 | 2,708,408 | |
| DK1622-DK1622 | Non-boundary | 14.7 | 205.0972 | C11H12N2O2 | 211.8 | 2,941,254 | 391,400 | 6.211429 | |
| ΔMXAN_0049–ΔMXAN_0049 | Non-boundary | 14.8 | 205.0972 | C11H12N2O2 | 207.5 | 263,8907 | 328,542 | 6.92309 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, M.N.; Li, Z.F.; Gong, Y.; Singh, R.P.; Li, Y.-Z. Omics Studies Revealed the Factors Involved in the Formation of Colony Boundary in Myxococcus xanthus. Cells 2019, 8, 530. https://doi.org/10.3390/cells8060530
Anwar MN, Li ZF, Gong Y, Singh RP, Li Y-Z. Omics Studies Revealed the Factors Involved in the Formation of Colony Boundary in Myxococcus xanthus. Cells. 2019; 8(6):530. https://doi.org/10.3390/cells8060530
Chicago/Turabian StyleAnwar, Mian Nabeel, Zhi Feng Li, Ya Gong, Raghvendra Pratap Singh, and Yue-Zhong Li. 2019. "Omics Studies Revealed the Factors Involved in the Formation of Colony Boundary in Myxococcus xanthus" Cells 8, no. 6: 530. https://doi.org/10.3390/cells8060530
APA StyleAnwar, M. N., Li, Z. F., Gong, Y., Singh, R. P., & Li, Y.-Z. (2019). Omics Studies Revealed the Factors Involved in the Formation of Colony Boundary in Myxococcus xanthus. Cells, 8(6), 530. https://doi.org/10.3390/cells8060530





