A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis
Abstract
1. Introduction
2. Methods
2.1. Study Drug
2.2. Study Objectives
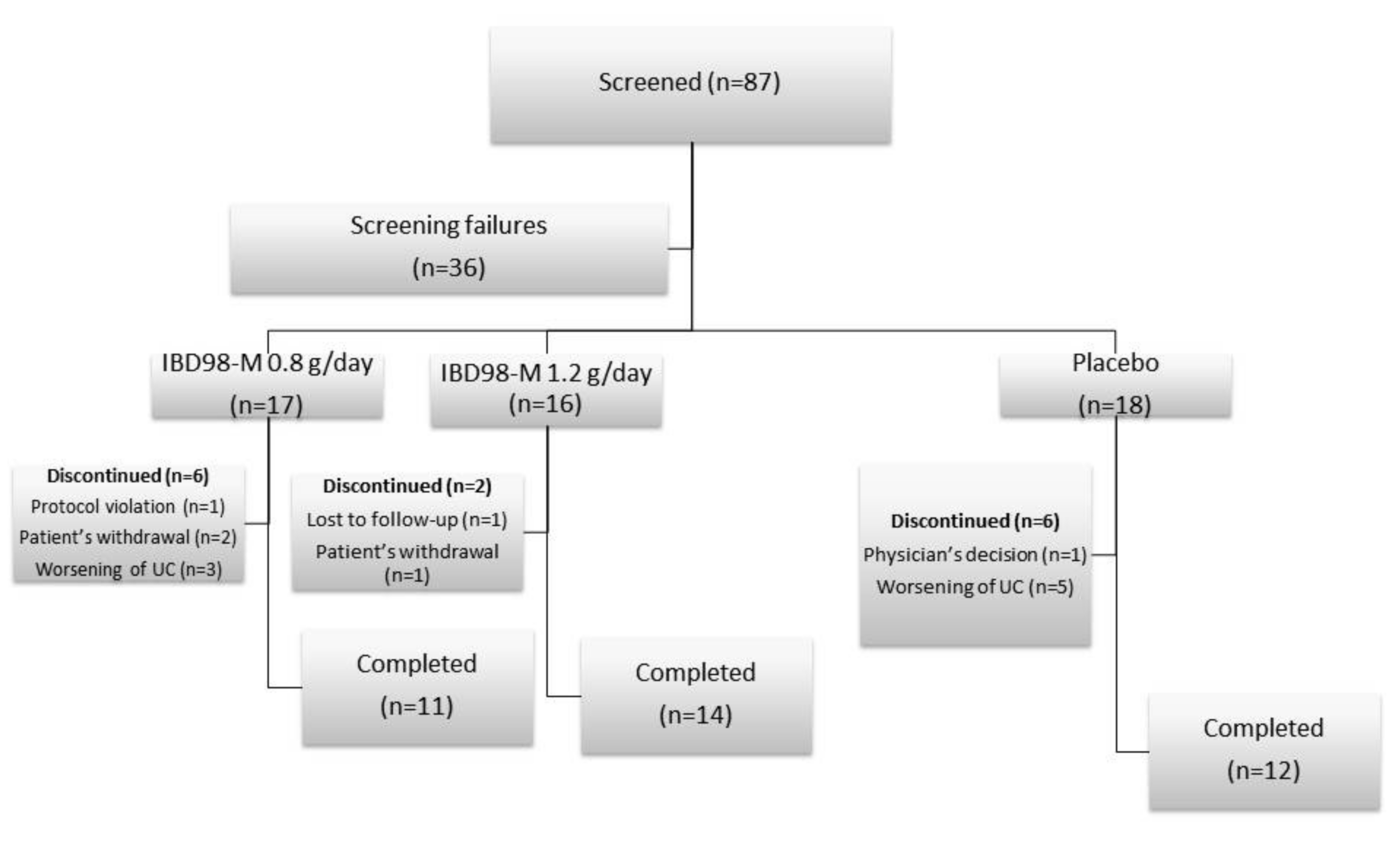
2.3. Study Design and Procedures
- IBD98-M 0.8 g/day (mesalamine 0.8 g with sodium hyaluronate 92 mg), or
- IBD98-M 1.2 g/day (mesalamine 1.2 g with sodium hyaluronate 138 mg), or
- Placebo.
- Patients in group 1 received 2 capsules of IBD98-M (200 mg of mesalamine/23 mg of sodium hyaluronate) and 1 placebo BID, a total of 800 mg of mesalamine and 92 mg of sodium hyaluronate.
- Patients in group 2 received 3 capsules of IBD98-M (200 mg of mesalamine/23 mg of sodium hyaluronate) BID, a total of 1200 mg of mesalamine and 138 mg of sodium hyaluronate.
- Patients in the placebo group received 3 placebo capsules BID.
2.4. Ethical Considerations
2.5. Statistical Methods
Sample Size Calculation
2.6. Analysis Populations
3. Results
Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Eng. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns. Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- NCT02493712. A Phase 2a, Multicenter, Randomized, Double-blind, Parallel Group, Placebo-controlled Trial of IBD98-M. Available online: https://clinicaltrials.gov/ct2/show/NCT02493712?term=IBD98M&cond=ulcerative+colitis&ran=1 (accessed on 18 March 2019).
- Mahadevan, U. Medical treatment of ulcerative colitis. Clin. Colon Rectal Surg. 2004, 17, 7–19. [Google Scholar] [CrossRef]
- Ham, M.; Moss, A.C. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev. Clin. Pharmacol. 2012, 5, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Achkar, J.P.; Khan, K.J.; Kane, S.V.; Talley, N.J.; Marshall, J.K.; Moayyedi, P. Efficacy of 5-aminosalicylates in ulcerative colitis: Systematic review and meta-analysis. Am. J. Gastroenterol. 2011, 106, 601–616. [Google Scholar] [CrossRef]
- Sutherland, L.; Macdonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Feagan, B.G.; Macdonald, J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012, 10. [Google Scholar] [CrossRef]
- Murch, S.H.; MacDonald, T.T.; Walker-Smith, J.A.; Levin, M.; Lionetti, P.; Klein, N.J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 1993, 341, 711–714. [Google Scholar] [CrossRef]
- Voinchet, V.; Vasseur, P.; Kern, J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am. J. Clin. Dermatol 2006, 7, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Sarath Krishna, N.; Meddings, R.N. Sequential hydrodistension and intravesical instillation of hyaluronic acid under general anaesthesia for treatment of refractory interstitial cystitis: A pilot study. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Brandt, F.; Bassichis, B.; Bassichis, M.; O’Connell, C.; Lin, X. Safety and effectiveness of small and large gel-particle hyaluronic acid in the correction of perioral wrinkles. J. Drugs Dermatol. 2011, 10, 982–987. [Google Scholar]
- Hess, H.; Rothhaar, J.; Thiel, W. Clinical studies of intra-articular injections of Arteparon. Retrospective study following the treatment of 754 patients. Fortschr. Med. 1982, 100, 1624–1627. [Google Scholar]
- Innocenti, M.; Moscatelli, G.; Lopez, S. Efficacy of gelclair in reducing pain in palliative care patients with oral lesions: Preliminary findings from an open pilot study. J. Pain Symptom Manage. 2002, 24, 456–457. [Google Scholar] [CrossRef]
- Morales, A.; Emerson, L.; Nickel, J.C. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. Urology 1997, 49, 111–113. [Google Scholar] [CrossRef]
- Porru, D.; Campus, G.; Tudino, D.; Valdes, E.; Vespa, A.; Scarpa, R.M.; Usai, E. Results of treatment of refractory interstitial cystitis with intravesical hyaluronic acid. Urol. Int. 1997, 59, 26–29. [Google Scholar] [CrossRef]
- Soldati, D.; Rahm, F.; Pasche, P. Mucosal wound healing after nasal surgery. A controlled clinical trial on the efficacy of hyaluronic acid containing cream. Drugs Exp. Clin. Res. 1999, 25, 253–261. [Google Scholar]
- Sturm, A.; Dignass, A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 348–353. [Google Scholar] [CrossRef]
- Fiorino, G.; Gilardi, D.; Naccarato, P.; Sociale, O.R.; Danese, S. Safety and efficacy of sodium hyaluronate (IBD98E) in the induction of clinical and endoscopic remission in subjects with distal ulcerative colitis. Dig. Liver Dis. 2014, 46, 330–334. [Google Scholar] [CrossRef]
- Guyatt, G.; Mitchell, A.; Irvine, E.J.; Singer, J.; Williams, N.; Goodacre, R.; Tompkins, C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989, 96, 804–810. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Simon, R.; Wittes, R.E.; Ellenberg, S.S. Randomized phase II clinical trials. Cancer Treat. Rep. 1985, 69, 1375–1381. [Google Scholar]
- Simon, R. Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 1989, 10, 1–10. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, K.M. On the estimation of the binomial probability in multistage clinical trials. Stat. Med. 2004, 23, 881–896. [Google Scholar] [CrossRef]
- Su, C. Outcomes of placebo therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 2006, 12, 328–333. [Google Scholar] [CrossRef]
- Intention to treat analysis and per protocol analysis: Complementary information. Prescrire. Int. 2012, 21, 304–306.
- Kim, H.-Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef]
- Morris, T.P.; Kahan, B.C.; White, I.R. Choosing sensitivity analyses for randomised trials: Principles. BMC Med. Res. Method. 2014, 14, 11. [Google Scholar] [CrossRef]
- Fong, Y.; Rue, H.; Wakefield, J. Bayesian inference for generalized linear mixed models. Biostatistics 2010, 11, 397–412. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical notes for clinical researchers: Analysis of covariance (ANCOVA). Restor. Dent. Endod. 2018, 43. [Google Scholar] [CrossRef]
| IBD98-M 0.8 g/day (n = 17) n (%) | IBD98-M 1.2 g/day (N = 16) n (%) | Placebo (N = 18) n (%) | Total (N = 51) n (%) | |
|---|---|---|---|---|
| Age (years), Mean ± SD | 36.6 ± 10.68 | 43.1 ± 12.23 | 45.8 ± 12.21 | 41.9 ± 12.14 |
| Males | 12 (70.6) | 7 (43.8) | 9 (50.0) | 28 (54.9) |
| Current smokers | 0 | 1 (6.3) | 0 | 1 (2.0) |
| BMI at Screening (kg/m2), Mean ± SD | 24.08 ± 3.104 | 25.22 ± 3.771 | 24.38 ± 4.016 | 24.54 ± 3.614 |
| Baseline UCDAI | ||||
| ≤5 | 7 (43.8) | 7 (43.8) | 9 (50.0) | 23 (45.1%) |
| 6–8 | 5 (31.3) | 4 (25.0) | 9 (50.0) | 18 (35.2%) |
| 9–10 | 4 (25.0) | 5 (31.3) | 0 (0.0) | 9 (17.7%) |
| Mean endoscopic score | 2.08 | 1.79 | 1.86 | 1.90 |
| Concomitant medications | ||||
| Agents acting on the renin-angiotensin system | 1 (5.9) | 0 | 3 (16.7) | 4 (7.8) |
| Ace inhibitors | 1 (5.9) | 0 | 2 (11.1) | 3 (5.9) |
| Ramipril | 0 | 0 | 2 (11.1) | 2 (3.9) |
| Analgesics | 5 (29.4) | 5 (31.3) | 5 (27.8) | 15 (29.4) |
| Anilides | 5 (29.4 | 5 (31.3) | 5 (27.8) | 15 (29.4) |
| Paracetamol | 5 (29.4) | 5 (31.3) | 5 (27.8) | 15 (29.4) |
| Antianemic preparations | 1 (5.9) | 1 (6.3) | 0 | 2 (3.9) |
| Iron, parenteral preparations | 1 (5.9) | 1 (6.3) | 0 | 2 (3.9) |
| Ferric carboxymaltose | 1 (5.9) | 1 (6.3) | 0 | 2 (3.9) |
| Antibacterials for systemic use | 2 (11.8) | 1 (6.3) | 2 (11.1) | 5 (9.8) |
| Penicillins, incl. Beta-lactamase inhibitors | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Amoxicillin + clavulanic acid | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Antidiarrheals, intestinal anti-inflammatory/anti-infectious agents | 6 (35.3) | 7 (43.8) | 7 (38.9) | 20 (39.2) |
| Aminosalicylic acid and similar agents | 4 (23.5) | 7 (43.8) | 7 (38.9) | 18 (35.3) |
| Mesalazine | 4 (23.5) | 7 (43.8) | 7 (38.9) | 18 (35.3) |
| Antidiarrheal microorganisms | 2 (11.8) | 0 | 2 (11.1) | 4 (7.8) |
| Corticosteroids acting locally | 2 (11.8) | 1 (6.3) | 1 (5.6) | 4 (7.8) |
| Prednisone | 2 (11.8) | 0 | 1 (5.6) | 3 (5.9) |
| Anti-inflammatory and anti-rheumatic products | 2 (11.8) | 2 (12.5) | 2 (11.1) | 6 (11.8) |
| Propionic acid derivatives | 2 (11.8) | 1 (6.3) | 2 (11.1) | 5 (9.8) |
| Ketoprofen | 2 (11.8) | 1 (6.3) | 0 | 3 (5.9) |
| Ibuprofen | 0 | 0 | 2 (11.1) | 2 (3.9) |
| Cough and cold preparations | 1 (5.9) | 0 | 2 (11.1) | 3 (5.9) |
| Other cough suppressants | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Drugs for acid related disorders | 1 (5.9) | 1 (6.3) | 2 (11.1) | 4 (7.8) |
| Proton pump inhibitors | 1 (5.9) | 1 (6.3) | 1 (5.6) | 3 (5.9) |
| Lansoprazole | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Drugs for constipation | 4 (23.5) | 6 (37.5) | 3 (16.7) | 13 (25.5) |
| Enemas | 4 (23.5) | 6 (37.5) | 2 (11.1) | 12 (23.5) |
| Fleet | 1 (5.9) | 4 (25.0) | 1 (5.6) | 6 (11.8) |
| Sodium phosphate | 2 (11.8) | 1 (0.3) | 1 (5.6) | 4 (7.8) |
| Mineral supplements | 1 (5.9) | 1 (6.3) | 1 (5.6) | 3 (5.9) |
| Calcium, combinations with vitamin d and/or other drugs | 1 (5.9) | 1 (6.3) | 1 (5.6) | 3 (5.9) |
| Calcium w/colecalciferol | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Psycholeptics | 2 (11.8) | 1 (6.3) | 1 (5.6) | 4 (7.8) |
| Benzodiazepine derivatives | 1 (5.9) | 0 | 1 (5.6) | 2 (3.9) |
| Benzodiazepine derivatives | 1 (5.9) | 1 (6.3) | 0 | 2 (3.9) |
| IBD98-M 0.8 g/day (N = 17) | IBD98-M 1.2 g/day (N = 16) | Placebo (N = 18) | ||
|---|---|---|---|---|
| Remission at Week 6 | n (%) | 1 (5.9) | 2 (12.5) | 2 (11.1) |
| 95% confidence interval | 0.1, 28.7 | 1.6, 38.3 | 1.4, 34.7 | |
| p value versus placebo | >0.999 | >0.999 | ||
| IBD98-M 0.8 g/day (N = 17) | IBD98-M 1.2 g/day (N = 16) | Placebo (N = 18) | ||
|---|---|---|---|---|
| Clinical Improvement at Week 6 | n (%) | 3 (17.6) | 5 (31.3) | 3 (16.7) |
| 95% confidence interval | 5.5, 57.2 | 12.8, 64.9 | 4.7, 50.8 | |
| p value versus placebo | >0.999 | 0.678 | ||
| IBD98-M 0.8 g/day (N = 17) | IBD98-M 1.2 g/day (N = 16) | Placebo (N = 18) | ||
|---|---|---|---|---|
| Endoscopic improvement at Week 6 | n (%) | 5 (29.4) | 2 (12.5) | 4 (22.2) |
| 95% confidence interval | 15.2, 72.3 | 1.8, 42.8 | 8.4, 58.1 | |
| p value versus Placebo | 0.683 | 0.648 | ||
| IBD98-M 0.8 g/day (N = 12) | IBD98-M 1.2 g/day (N = 14) | Placebo (N = 14) | |
|---|---|---|---|
| UCDAI at Baseline | |||
| n | 12 | 14 | 14 |
| Median (range) | 5.00 (4–10) | 5.50 (4–10) | 5.00 (4–8) |
| UCDAI at Visit 6 | |||
| n | 12 | 14 | 14 |
| Median | 4.00 (1–9) | 4.50 (0–11) | 5.00 (0–10) |
| UCDAI at Visit 6 – Baseline | |||
| n | 12 | 14 | 14 |
| Median | −1.00 (−4–3) | −1.00 (−5–1) | −1.00 (−4–6) |
| p value * | 0.837 | 0.335 |
| IBD98-M 0.8 g/day (N = 12) | IBD98-M 1.2 g/day (N = 14) | Placebo (N = 14) | |
|---|---|---|---|
| Endoscopic score at baseline | |||
| n | 12 | 14 | 14 |
| Median (range) | 2.00 (1–3) | 2.00 (1–3) | 2.00 (1–3) |
| Endoscopic score at visit 6 | |||
| n | 12 | 14 | 14 |
| Median (range) | 1.50 (0-3) | 2.00 (0–3) | 2.00 (0–3) |
| Endoscopic score at visit 6 – baseline | T | ||
| n | 12 | 14 | 14 |
| Median | 0.00 (−2–0) | 0.00 (−1–1) | 0.00 (−2–1) |
| p value * | 0.133 | 0.832 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorino, G.; Sturniolo, G.C.; Bossa, F.; Cassinotti, A.; Di Sabatino, A.; Giuffrida, P.; Danese, S. A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis. Cells 2019, 8, 523. https://doi.org/10.3390/cells8060523
Fiorino G, Sturniolo GC, Bossa F, Cassinotti A, Di Sabatino A, Giuffrida P, Danese S. A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis. Cells. 2019; 8(6):523. https://doi.org/10.3390/cells8060523
Chicago/Turabian StyleFiorino, Gionata, Giacomo Carlo Sturniolo, Fabrizio Bossa, Andrea Cassinotti, Antonio Di Sabatino, Paolo Giuffrida, and Silvio Danese. 2019. "A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis" Cells 8, no. 6: 523. https://doi.org/10.3390/cells8060523
APA StyleFiorino, G., Sturniolo, G. C., Bossa, F., Cassinotti, A., Di Sabatino, A., Giuffrida, P., & Danese, S. (2019). A Phase 2a, Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Trial of IBD98-M Delayed-Release Capsules to Induce Remission in Patients with Active and Mild to Moderate Ulcerative Colitis. Cells, 8(6), 523. https://doi.org/10.3390/cells8060523





