TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Cytotoxicity Assay
2.3. MP Purification
2.4. Western Blot Analysis
2.5. Real Time Quantitative PCR (qRT-PCR)
2.6. Apoptosis Detection
2.7. Detection of Pgp by Flow Cytometer
2.8. UIC2 Shift Assay
2.9. Efflux Activity of Pgp by Flow Cytometer
2.10. Enzime-Linked Immunosorbent Assay (ELISA)
2.11. Anchorage Independent Growth Assay
2.12. Wound Healing Assay
2.13. Crystal Violet Incorporation Assay
2.14. Statistical Analysis
3. Results
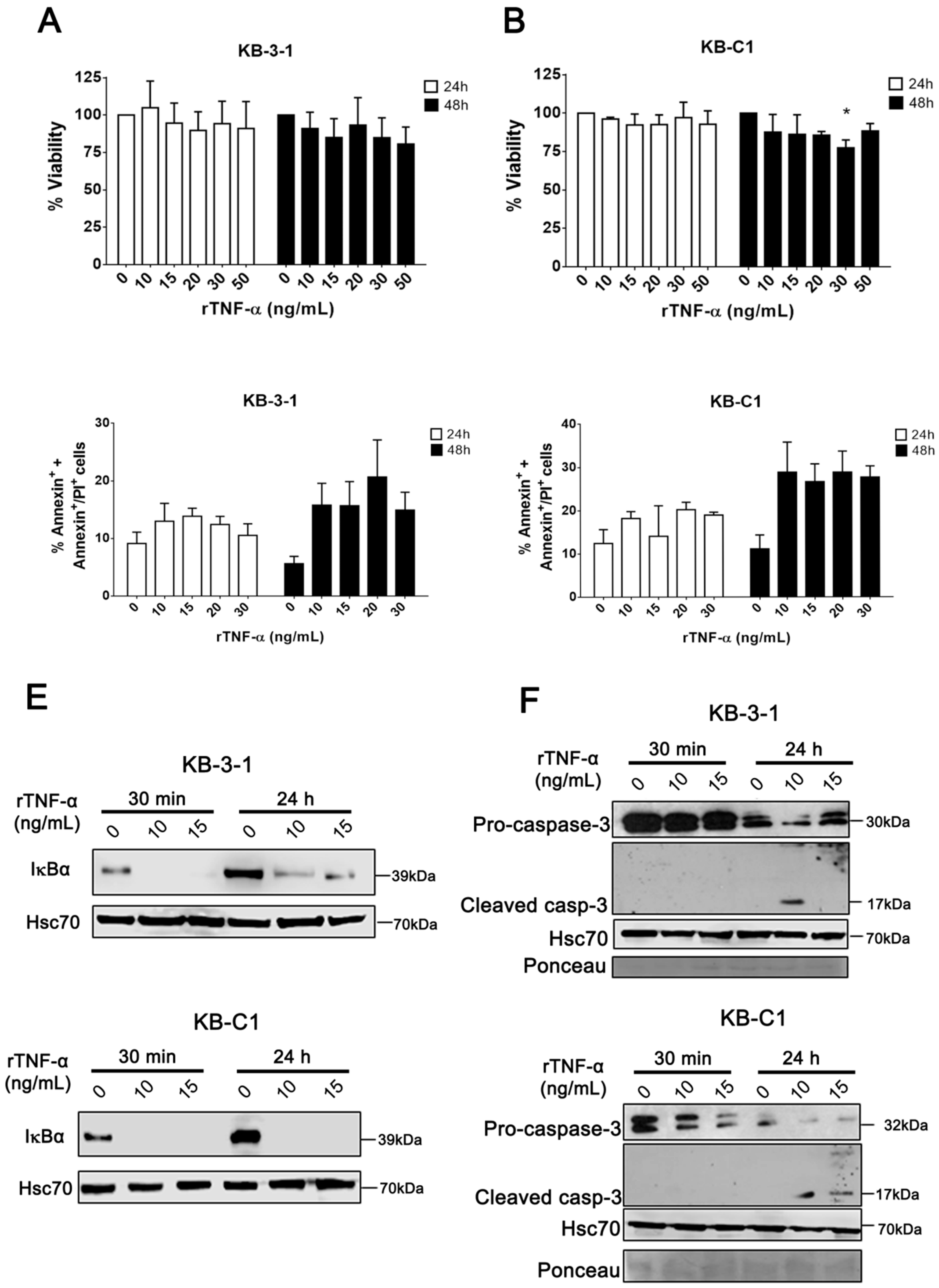
3.1. rTNF-α Induces Differential Effects on MDR Cells
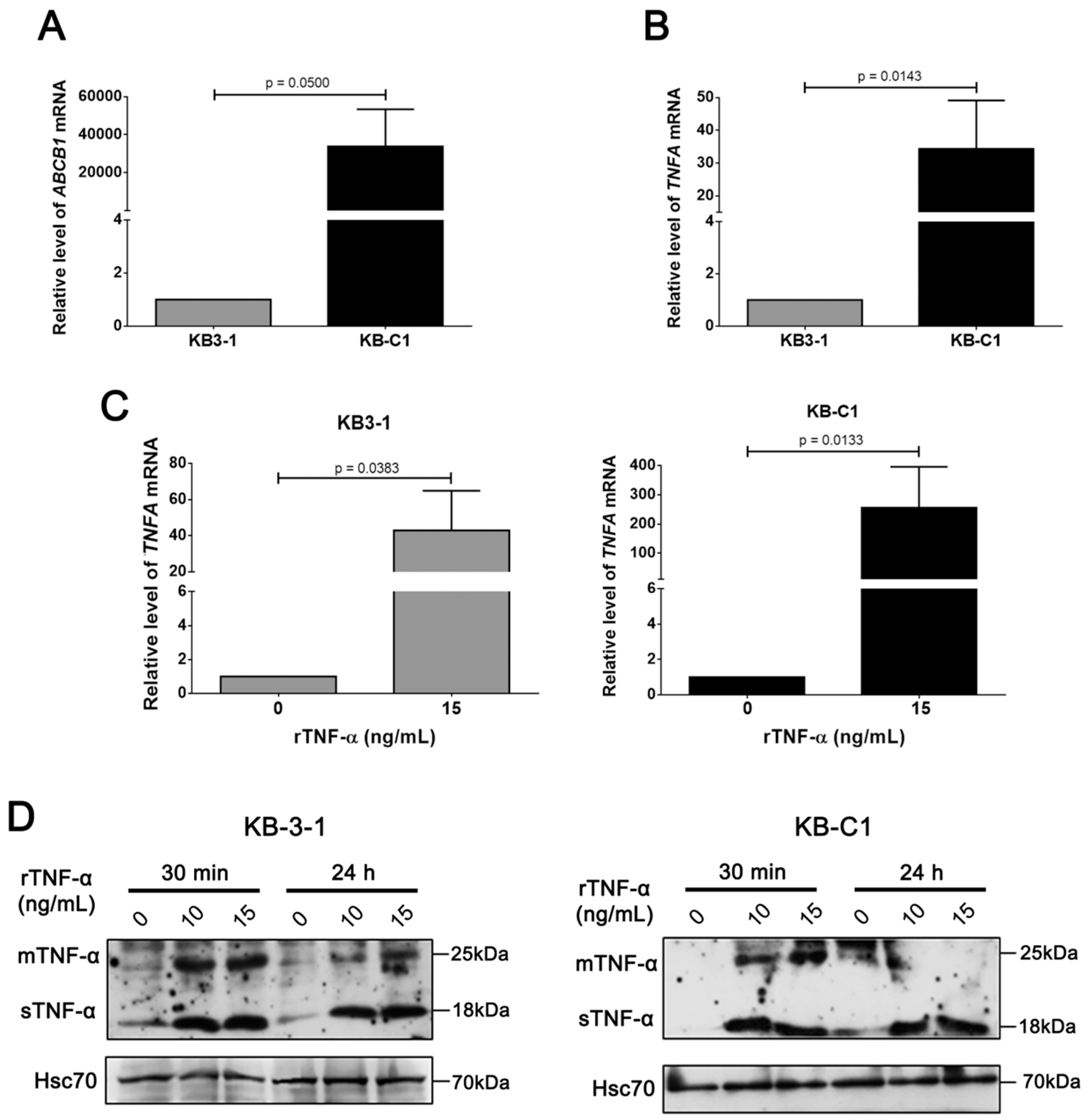
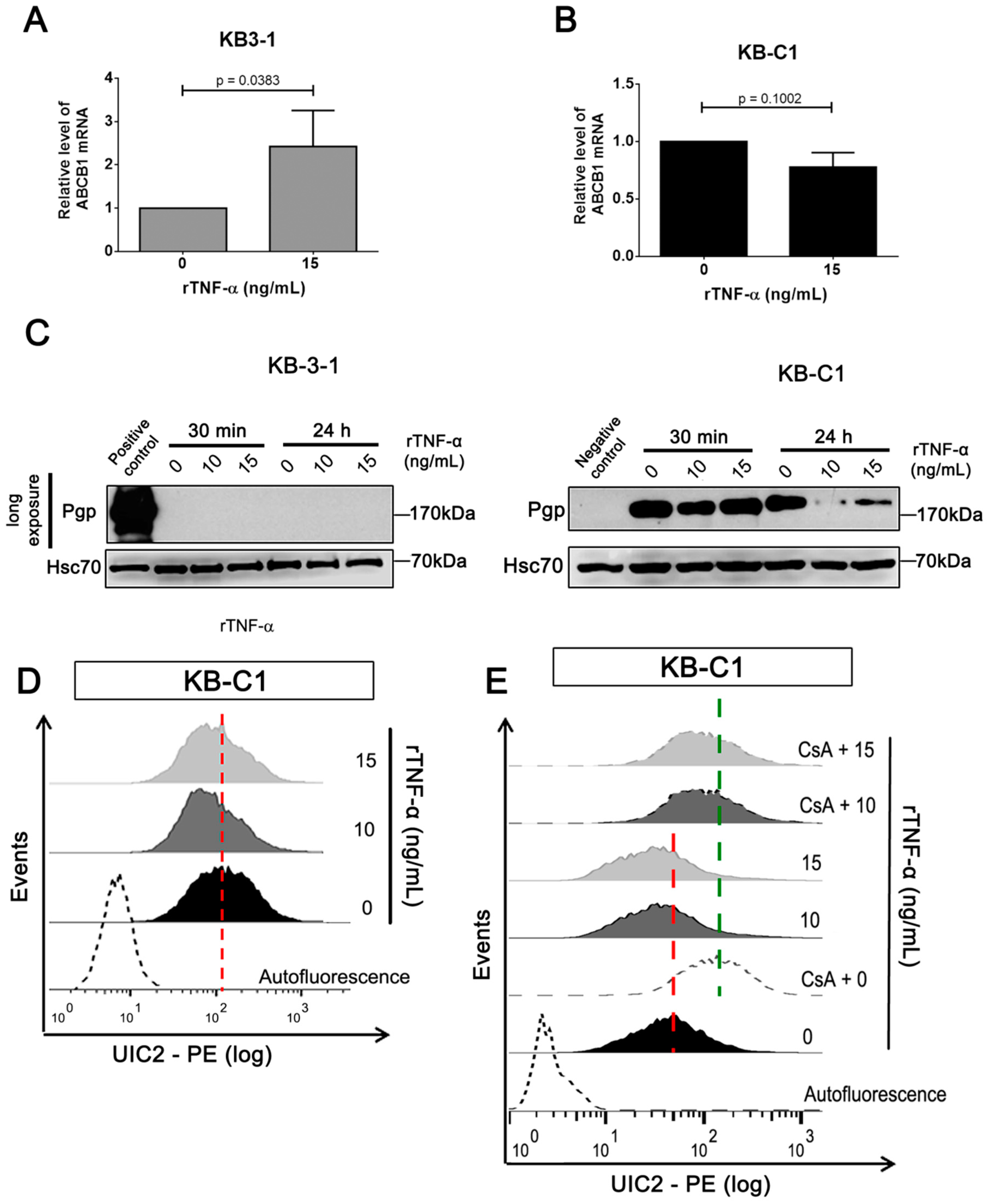
3.2. rTNF-α Modulates Pgp and Endogenous TNF-α Expression Levels in Cancer Cells
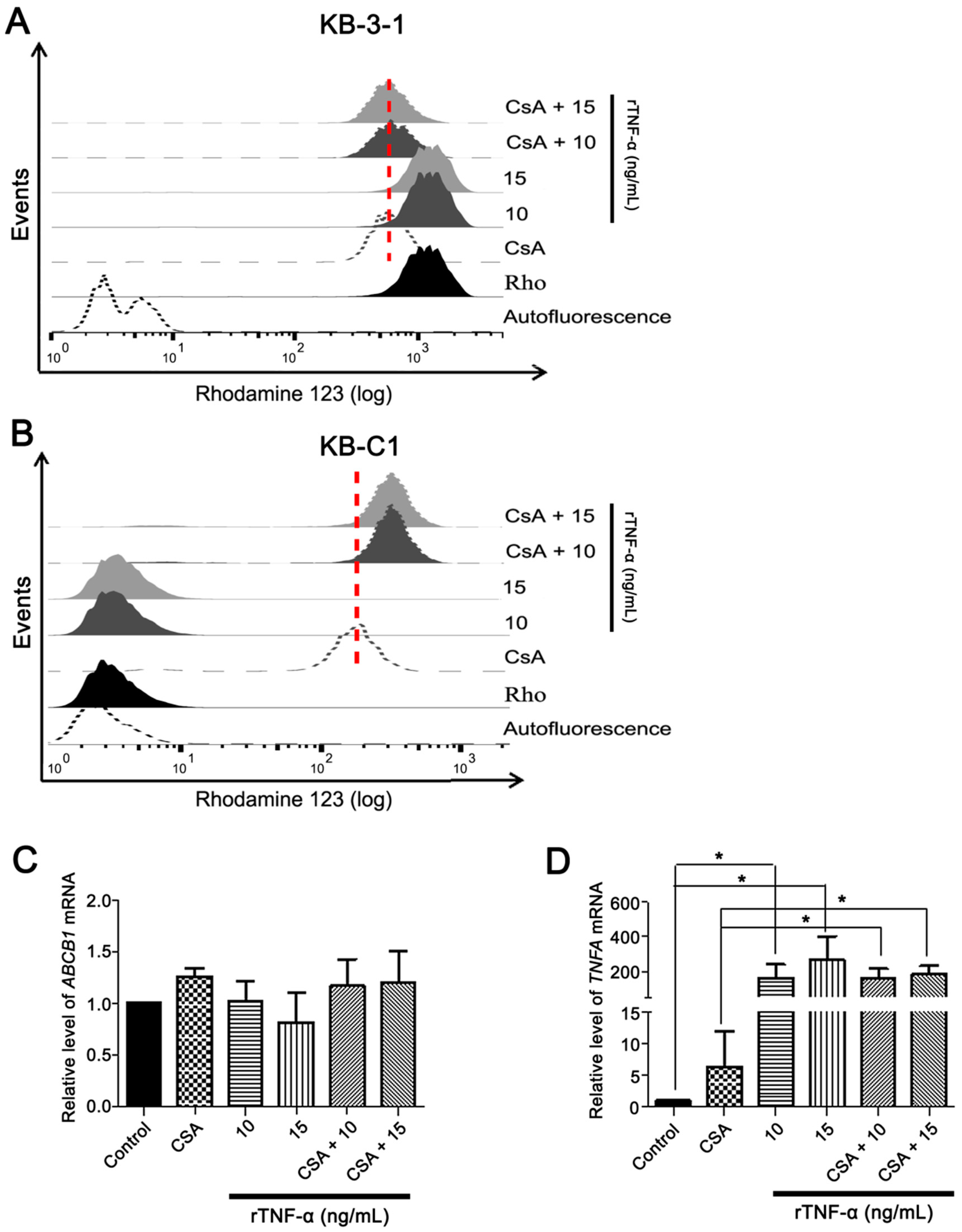
3.3. Endogenous TNF-α is Upregulated in MDR Cells Independently of Pgp Efflux Activity
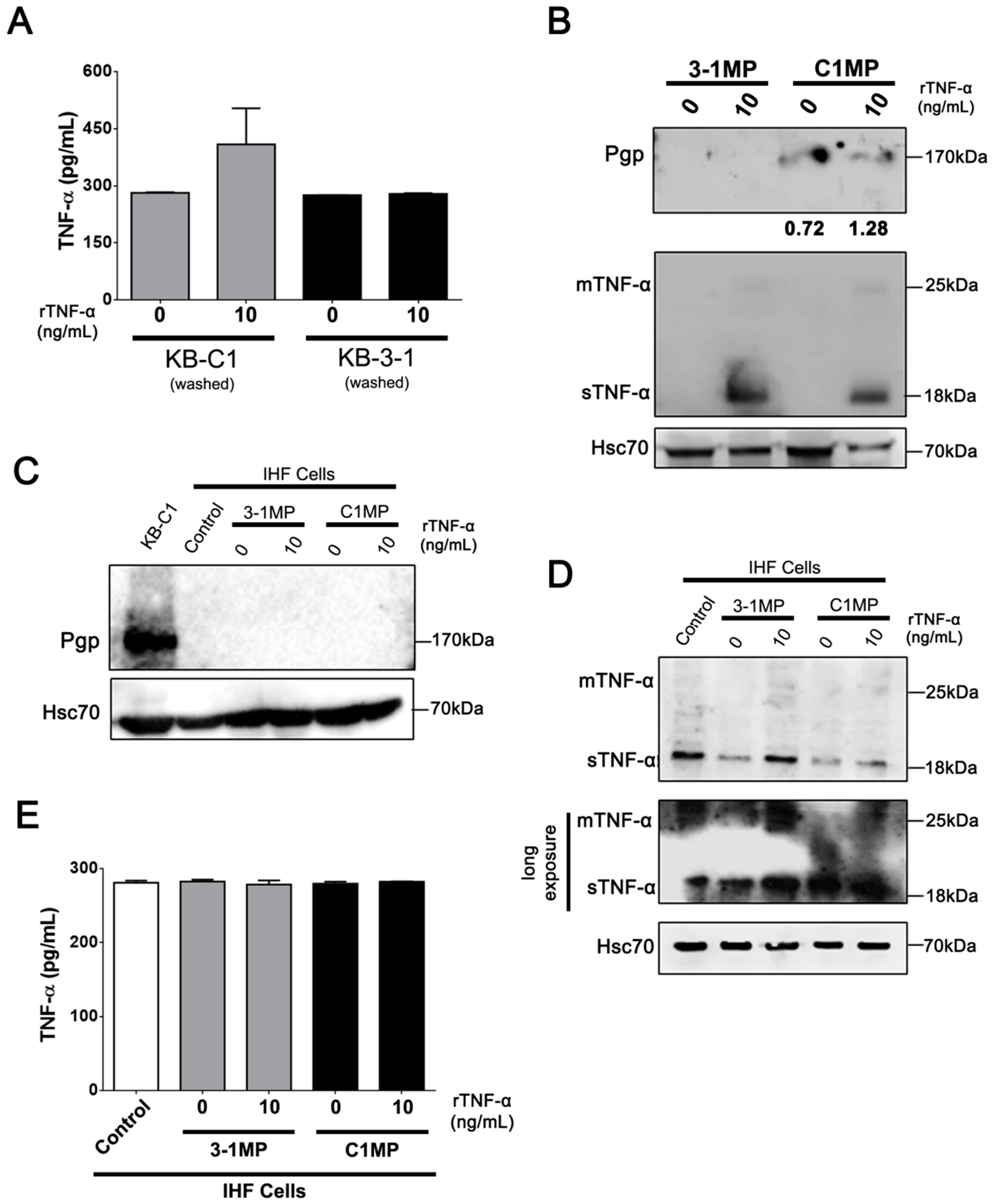
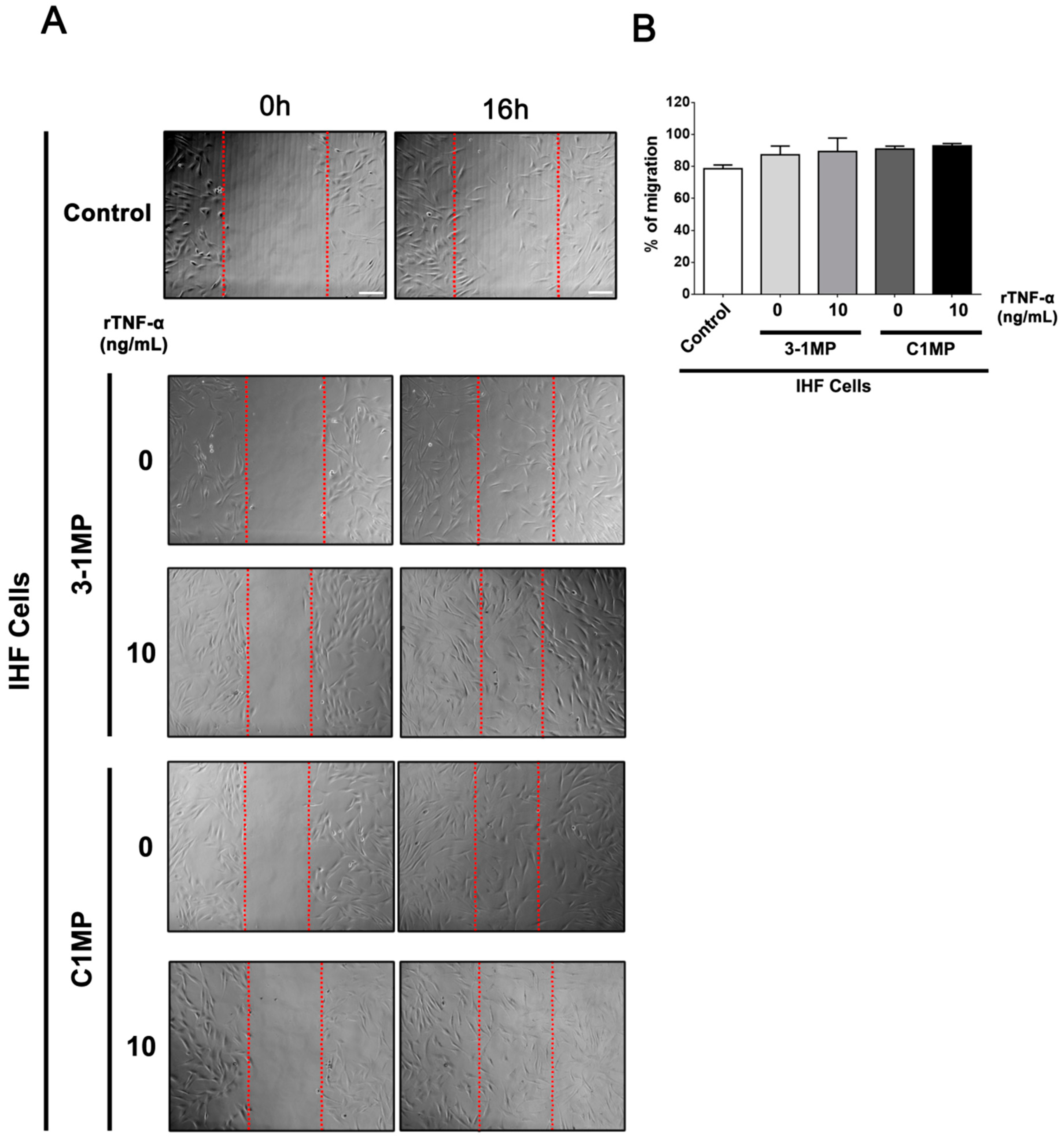
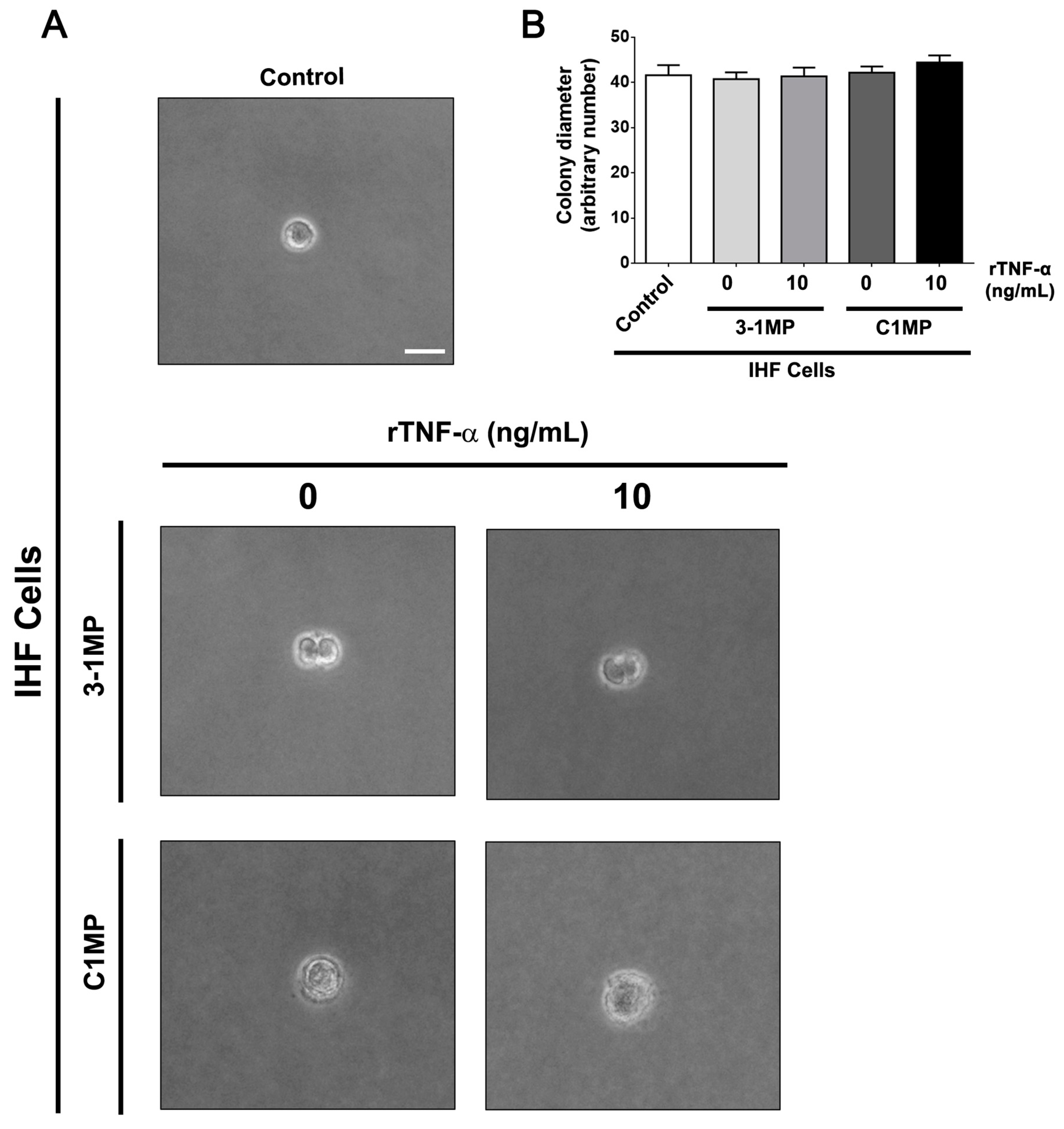
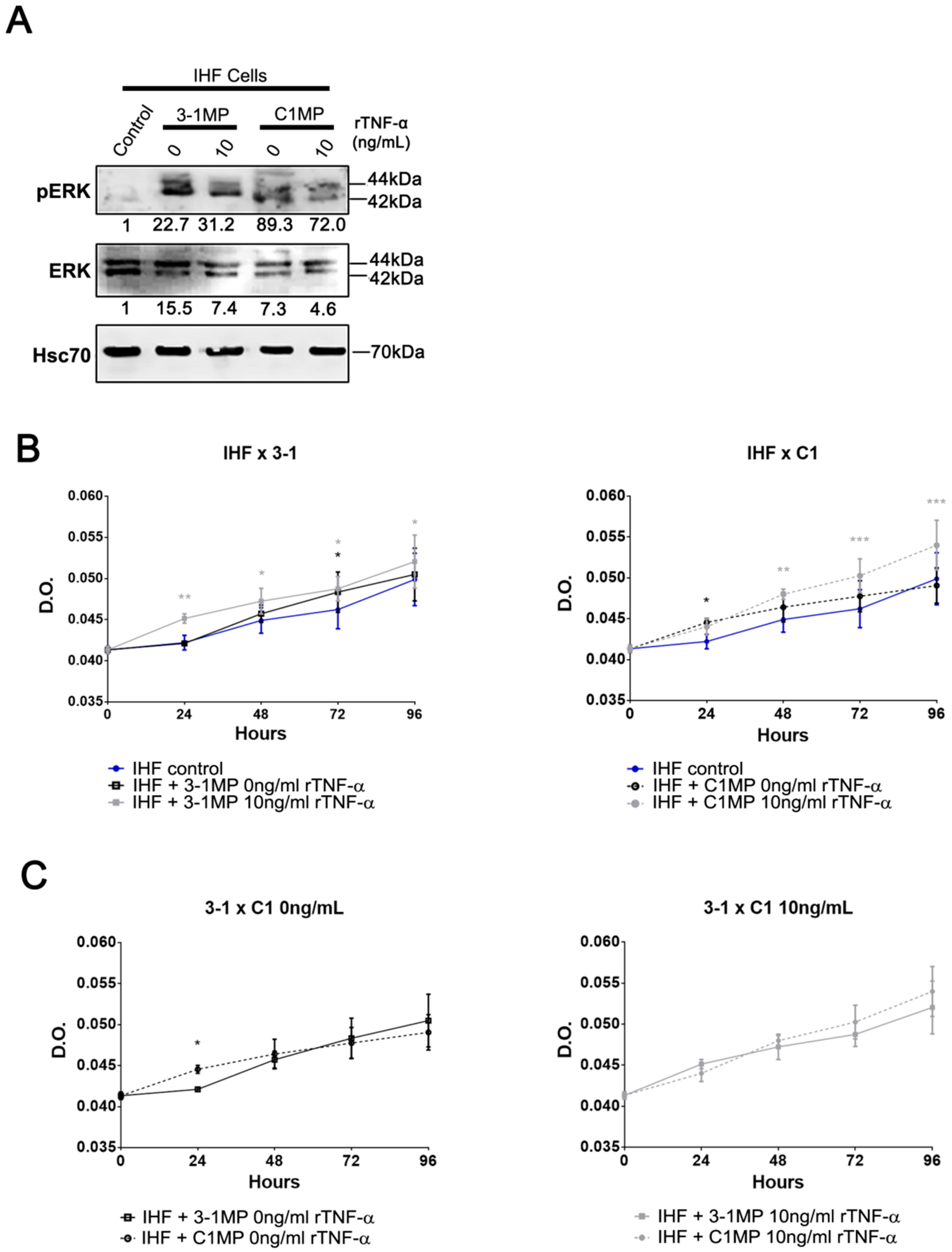
3.4. Cancer Cell-Derived MP Carry Pgp and Endogenous TNF-α and Promote Proliferation in Non-Tumor Cell Lines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug Resistance in Cancer: Role of ATP–dependent Transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. ABC Multidrug Transporters: Structure, Function and Role in Chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Raquel, M.; Vasconcelos, F.; Souza, P.; Rumjanek, V. Towards Comprehension of the ABCB1/P-Glycoprotein Role in Chronic Myeloid Leukemia. Molecules 2018, 23, 119. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Burger, H.; Nooter, K. Pharmacokinetic Resistance to Imatinib Mesylate: Role of the ABC Drug Pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the Oral Bioavailability of Imatinib. Cell Cycle 2004, 3, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Tainton, K.M.; Smyth, M.J.; Jackson, J.T.; Tanner, J.E.; Cerruti, L.; Jane, S.M.; Darcy, P.K.; Johnstone, R.W. Mutational Analysis of P-Glycoprotein: Suppression of Caspase Activation in the Absence of ATP-Dependent Drug Efflux. Cell Death Differ. 2004, 11, 1028–1037. [Google Scholar] [CrossRef]
- Gillet, J.-P.; Calcagno, A.M.; Varma, S.; Davidson, B.; Bunkholt Elstrand, M.; Ganapathi, R.; Kamat, A.A.; Sood, A.K.; Ambudkar, S.V.; Seiden, M.V.; et al. Multidrug Resistance-Linked Gene Signature Predicts Overall Survival of Patients with Primary Ovarian Serous Carcinoma. Clin. Cancer Res. 2012, 18, 3197–3206. [Google Scholar] [CrossRef]
- Bebawy, M.; Combes, V.; Lee, E.; Jaiswal, R.; Gong, J.; Bonhoure, A.; Grau, G.E.R. Membrane Microparticles Mediate Transfer of P-Glycoprotein to Drug Sensitive Cancer Cells. Leukemia 2009, 23, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.S.; Cruz, A.L.S.; Viola, J.P.B.; Maia, R.C. Microparticles Induce Multifactorial Resistance through Oncogenic Pathways Independently of Cancer Cell Type. Cancer Sci. 2014, 106, 60–68. [Google Scholar] [CrossRef]
- De Souza, P.S.; Faccion, R.S.; Bernardo, P.S.; Maia, R.C. Membrane Microparticles: Shedding New Light into Cancer Cell Communication. J. Cancer Res. Clin. Oncol. 2015, 142, 1395–1406. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front. Pharmacol. 2017, 7. [Google Scholar] [CrossRef]
- Jaiswal, R.; Johnson, M.S.; Pokharel, D.; Krishnan, S.R.; Bebawy, M. Microparticles Shed from Multidrug Resistant Breast Cancer Cells Provide a Parallel Survival Pathway through Immune Evasion. BMC Cancer 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Krasovskis, E.; Sutton, V.R.; Johnstone, R.W. The Drug Efflux Protein, P-Glycoprotein, Additionally Protects Drug-Resistant Tumor Cells from Multiple Forms of Caspase-Dependent Apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 7024–7029. [Google Scholar] [CrossRef]
- Johnstone, R.W.; Cretney, E.; Smyth, M.J. P-Glycoprotein Protects Leukemia Cells against Caspase-Dependent, but Not Caspase-Independent, Cell Death. Blood 1999, 93, 1075–1085. [Google Scholar] [PubMed]
- Ruth, A.C.; Roninson, I.B. Effects of the Multidrug Transporter P-Glycoprotein on Cellular Responses to Ionizing Radiation. Cancer Res. 2000, 60, 2576–2578. [Google Scholar]
- Zafar, M.; Shukla, Y. Death Receptors: Targets for Cancer Therapy. Exp. Cell Res. 2010, 316, 887–899. [Google Scholar]
- Tansey, M.G.; Szymkowski, D.E. The TNF Superfamily in 2009: New Pathways, New Indications, and New Drugs. Drug Discov. Today 2009, 14, 1082–1088. [Google Scholar] [CrossRef]
- Wolczyk, D.; Zaremba-Czogalla, M.; Hryniewicz-Jankowska, A.; Tabola, R.; Grabowski, K.; Sikorski, A.F.; Augoff, K. TNF-α Promotes Breast Cancer Cell Migration and Enhances the Concentration of Membrane-Associated Proteases in Lipid Rafts. Cell. Oncol. 2016, 39, 353–363. [Google Scholar] [CrossRef]
- Pal, S.; Yadav, P.; Sainis, K.B.; Shankar, B.S. TNF-α and IGF-1 Differentially Modulate Ionizing Radiation Responses of Lung Cancer Cell Lines. Cytokine 2018, 101, 89–98. [Google Scholar] [CrossRef]
- Ardestani, S.; Li, B.; Deskins, D.L.; Wu, H.; Massion, P.P.; Young, P.P. Membrane versus Soluble Isoforms of TNF-α Exert Opposing Effects on Tumor Growth and Survival of Tumor-Associated Myeloid Cells. Cancer Res. 2013, 73, 3938–3950. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhao, G.; Li, H. Forward and Reverse Signaling Mediated by Transmembrane Tumor Necrosis Factor-Alpha and TNF Receptor 2: Potential Roles in an Immunosuppressive Tumor Microenvironment. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-G.; Liu, C.; Su, K.; Yu, S.; Zhang, L.; Zhang, S.; Wang, J.; Cao, X.; Grizzle, W.; Kimberly, R.P. A Membrane Form of TNF- Presented by Exosomes Delays T Cell Activation-Induced Cell Death. J. Immunol. 2006, 176, 7385–7393. [Google Scholar] [CrossRef]
- Souza, P.S.; Madigan, J.P.; Gillet, J.P.; Kapoor, K.; Ambudkar, S.V.; Maia, R.C.; Gottesman, M.M.; Fung, K.L. Expression of the Multidrug Transporter P-Glycoprotein Is Inversely Related to That of Apoptosis-Associated Endogenous TRAIL. Exp. Cell Res. 2015, 336, 318–328. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Brimacombe, K.R.; Hall, M.D.; Auld, D.S.; Inglese, J.; Austin, C.P.; Gottesman, M.M.; Fung, K.L. A dual-fluorescence high-throughput cell line system for probing multidrug resistance. Assay Drug Dev. Technol. 2009, 7, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.; Bhatnagar, J.; Chufan, E.E.; Ambudkar, S.V. Mutations in intracellular loops 1 and 3 lead to misfolding of human P-glycoprotein (ABCB1) that can be rescued by cyclosporine A, which reduces its association with chaperone Hsp70. J. Biol. Chem. 2013, 288, 32622–32636. [Google Scholar] [CrossRef]
- Shen, D.W.; Cardarelli, C.; Hwang, J.; Cornwell, M.; Richert, N.; Ishii, S.; Pastan, I.; Gottesman, M.M. Multiple Drug-Resistant Human KB Carcinoma Cells Independently Selected for High-Level Resistance to Colchicine, Adriamycin, or Vinblastine Show Changes in Expression of Specific Proteins. J. Biol. Chem. 1986, 261, 7762–7770. [Google Scholar]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Gasparini, C.; Celeghini, C.; Monasta, L.; Zauli, G. NF-κB Pathways in Hematological Malignancies. Cell. Mol. Life Sci. 2014, 71, 2083–2102. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The Molecular Basis of Multidrug Resistance in Cancer: The Early Years of P-Glycoprotein Research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef]
- Mulvey, H.E.; Chang, A.; Adler, J.; Del Tatto, M.; Perez, K.; Quesenberry, P.J.; Chatterjee, D. Extracellular Vesicle-mediated Phenotype Switching in Malignant and Non-malignant Colon Cells. BMC Cancer 2015, 15. [Google Scholar] [CrossRef]
- Gillet, J.-P.; Gottesman, M.M. Mechanisms of Multidrug Resistance in Cancer. Methods Mol. Biol. Multi-Drug Resist. Cancer 2009. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Liu, F.; Xie, Z.H.; Cai, G.P.; Jiang, Y.Y. The Effect of Survivin on Multidrug Resistance Mediated by P-Glycoprotein in MCF-7 and Its Adriamycin Resistant Cells. Biol. Pharm. Bull. 2007, 30, 2279–2283. [Google Scholar] [CrossRef]
- Silva, K.L.; de Souza, P.S.; de Moraes, G.N.; Moellmann-Coelho, A.; da Cunha Vasconcelos, F.; Maia, R.C. XIAP and P-Glycoprotein Co-Expression Is Related to Imatinib Resistance in Chronic Myeloid Leukemia Cells. Leukemia Res. 2013, 37, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Galski, H.; Oved-Gelber, T.; Simanovsky, M.; Lazarovici, P.; Gottesman, M.M.; Nagler, A. P-Glycoprotein-Dependent Resistance of Cancer Cells toward the Extrinsic TRAIL Apoptosis Signaling Pathway. Biochem. Pharm. 2013, 86, 584–596. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging Roles of Caspase-3 in Apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, K.; Satou, Y. Caspases: Evolutionary Aspects of Their Functions in Vertebrates. J. Fish Biol. 2009, 74, 727–753. [Google Scholar] [CrossRef]
- Brentnall, M.; Weir, D.B.; Rongvaux, A.; Marcus, A.I.; Boise, L.H. Procaspase-3 Regulates Fibronectin Secretion and Influences Adhesion, Migration and Survival Independently of Catalytic Function. J. Cell Sci. 2014, 127, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Agard, N.J.; Mahrus, S.; Trinidad, J.C.; Lynn, A.; Burlingame, A.L.; Wells, J.A. Global Kinetic Analysis of Proteolysis via Quantitative Targeted Proteomics. Proc. Natl. Acad. Sci. USA 2012, 109, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.D.; Rampalli, S.; Burns, L.E.; Brunette, S.; Dilworth, F.J.; Megeney, L.A. Caspase 3/caspase-activated DNase Promote Cell Differentiation by Inducing DNA Strand Breaks. Proc. Natl. Acad. Sci. USA 2010, 107, 4230–4235. [Google Scholar] [CrossRef]
- Solier, S.; Fontenay, M.; Vainchenker, W.; Droin, N.; Solary, E. Non-Apoptotic Functions of Caspases in Myeloid Cell Differentiation. Cell Death Differ. 2017, 24, 1337–1347. [Google Scholar] [CrossRef]
- Lage, H. An overview of cancer multidrug resistance: A still unsolved problem. Cell. Mol. Life Sci. 2008. [Google Scholar] [CrossRef]
- Gao, Y.; Liao, Y.; Shen, J.K.; Feng, Y.; Choy, E.; Cote, G.; Harmon, D.; Mankin, H.J.; Hornicek, F.J.; Duan, Z. Evaluation of P-glycoprotein (Pgp) expression in human osteosarcoma by high-throughput tissue microarray. J. Orthop. Res. 2016, 34, 1606–1612. [Google Scholar] [CrossRef]
- Sedláková, I.; Laco, J.; Caltová, K.; Cervinka, M.; Tošner, J.; Rezác, A.; Špacek, J. Clinical significance of the resistance proteins LRP, Pgp, MRP1, MRP3, and MRP5 in epithelial ovarian cancer. Int. J. Gynecol. Cancer. 2015. [Google Scholar] [CrossRef]
- Reis, F.R.; Vasconcelos, F.C.; Pereira, D.L.; Moellman-Coelho, A.; Silva, K.L.; Maia, R.C. Survivin and P-glycoprotein are associated and highly expressed in late phase chronic myeloid leukemia. Oncol. Rep. 2011, 26, 471–478. [Google Scholar]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From inflammation to cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proceed. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef]
- Györffy, B.; Surowiak, P.; Kiesslich, O.; Denkert, C.; Schäfer, R.; Dietel, M.; Lage, H. Gene Expression Profiling of 30 Cancer Cell Lines Predicts Resistance towards 11 Anticancer Drugs at Clinically Achieved Concentrations. Int. J. Cancer 2006, 118, 1699–1712. [Google Scholar] [CrossRef]
- Moreira, M.A.M.; Bagni, C.; de Pinho, M.B.; Mac-Cormick, T.M.; dos Santos Mota, M.; Pinto-Silva, F.E.; Daflon-Yunes, N.; Rumjanek, V.M. Changes in Gene Expression Profile in Two Multidrug Resistant Cell Lines Derived from a Same Drug Sensitive Cell Line. Leukemia Res. 2014, 38, 983–987. [Google Scholar] [CrossRef]
- Lee, G.; Piquette-Miller, M. Cytokines Alter the Expression and Activity of the Multidrug Resistance Transporters in Human Hepatoma Cell Lines; Analysis Using RT-PCR and CDNA Microarrays. J. Pharm. Sci. 2003, 92, 2152–2163. [Google Scholar] [CrossRef]
- Iqbal, M.; Ho, M.L.; Petropoulos, S.; Moisiadis, V.G.; Gibb, W.; Matthews, S.G. Pro-Inflammatory Cytokine Regulation of P-Glycoprotein in the Developing Blood-Brain Barrier. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Walther, W.; Kobelt, D.; Bauer, L.; Aumann, J.; Stein, U. Chemosensitization by Diverging Modulation by Short-Term and Long-Term TNF-α Action on ABCB1 Expression and NF-ΚB Signaling in Colon Cancer. Int. J. Oncol. 2015, 47, 2276–2285. [Google Scholar] [CrossRef]
- Hartmann, G.; Kim, H.; Piquette-Miller, M. Regulation of the Hepatic Multidrug Resistance Gene Expression by Endotoxin and Inflammatory Cytokines in Mice. Int. Immunopharm. 2001, 1, 189–199. [Google Scholar] [CrossRef]
- Bauer, B.; Hartz, A.M.S.; Miller, D.S. Tumor Necrosis Factor and Endothelin-1 Increase P-Glycoprotein Expression and Transport Activity at the Blood-Brain Barrier. Mol. Pharm. 2006, 71, 667–675. [Google Scholar] [CrossRef]
- Heemskerk, S.; Peters, J.G.P.; Louisse, J.; Sagar, S.; Russel, F.G.M.; Masereeuw, R. Regulation of P-Glycoprotein in Renal Proximal Tubule Epithelial Cells by LPS and TNF-α. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Collart, M.A.; Baeuerle, P.; Vassalli, P. Regulation of Tumor Necrosis Factor Alpha Transcription in Macrophages: Involvement of Four Kappa B-like Motifs and of Constitutive and Inducible Forms of NF-Kappa B. Mol. Cell. Biol. 1990, 10, 1498–1506. [Google Scholar] [CrossRef]
- Liu, H.; Sidiropoulos, P.; Song, G.; Pagliari, L.J.; Birrer, M.J.; Stein, B.; Anrather, J.; Pope, R.M. TNF-α Gene Expression in Macrophages: Regulation by NF- B Is Independent of c-Jun or C/EBP. J. Immunol. 2000, 164, 4277–4285. [Google Scholar] [CrossRef] [PubMed]
- Kagoya, Y.; Yoshimi, A.; Kataoka, K.; Nakagawa, M.; Kumano, K.; Arai, S.; Kobayashi, H.; Saito, T.; Iwakura, Y.; Kurokawa, M. Positive Feedback between NF-κB and TNF-α Promotes Leukemia-initiating Cell Capacity. J. Clin. Investig. 2014, 124, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Gray-Schopfer, V.C.; Karasarides, M.; Hayward, R.; Marais, R. Tumor Necrosis Factor-α Blocks Apoptosis in Melanoma Cells When BRAF Signaling Is Inhibited. Cancer Res. 2007, 67, 122–129. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Yan, D.; Qin, N.; Zhang, H.; Liu, T.; Yu, M.; Jiang, X.; Feng, W.; Wang, J.; Yin, B.; Zhang, T.; et al. Expression of TNF-α Leader Sequence Renders MCF-7 Tumor Cells Resistant to the Cytotoxicity of Soluble TNF-α. Breast Cancer Res. Treat. 2009, 116, 91–102. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, X.; Niu, L.; Lin, G.; Huang, J.; Zhou, W.; Gan, H.; Wang, J.; Jiang, X.; Yin, B.; et al. Targeting Transmembrane TNF-α Suppresses Breast Cancer Growth. Cancer Res. 2013, 73, 4061–4074. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Li, B.; Li, Q.; Gao, L.; Li, D.; Gong, Q.; Zhu, L.; Wang, J.; Wang, N.; et al. Transmembrane TNF-A Preferentially Expressed by Leukemia Stem Cells and Blasts Is a Potent Target for Antibody Therapy. Blood 2015, 126, 1433–1442. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, G.; Yan, Y.; Li, X.; Hu, Y.; Wang, J.; Yin, B.; Wu, Y.; Li, Z.; Yang, X. Transmembrane TNF-alpha Promotes Chemoresistance in Breast Cancer Cells. Oncogene 2018, 37, 3456–3470. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Ren, H.; Ma, J.; Sun, X.; Li, N.; Liu, C.; Huang, K.; Xu, M.; Ming, L. Molecular Correlates and Prognostic Value of TmTNF-α Expression in Colorectal Cancer of 5-Fluorouracil-Based Adjuvant Therapy. Cancer Biol. Ther. 2016, 17, 684–692. [Google Scholar] [CrossRef]
- Alokail, M.S.; Al-Daghri, N.M.; Mohammed, A.K.; Vanhoutte, P.; Alenad, A. Increased TNF α, IL-6 and ErbB2 mRNA expression in peripheral blood leukocytes from breast cancer patients. Med. Oncol. 2014. [Google Scholar] [CrossRef]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: Prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res. Ther. 2015. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Dai, Z.; Wu, C.; Ji, Y.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Hong, H.; Jiang, L.; Lin, Y.; He, C.; Zhu, G.; Du, Q.; Wang, X.; She, F.; Chen, Y. TNF-alpha promotes lymphangiogenesis and lymphatic metastasis of gallbladder cancer through the ERK1/2/AP-1/VEGF-D pathway. BMC Cancer 2016, 16, 240. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Lopes-Rodrigues, V.; Di Luca, A.; Mleczko, J.; Meleady, P.; Henry, M.; Pesic, M.; Cabrera, D.; Van Liempd, S.; Lima, R.T.; O’Connor, R.; et al. Identification of the Metabolic Alterations Associated with the Multidrug Resistant Phenotype in Cancer and Their Intercellular Transfer Mediated by Extracellular Vesicles. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells Release Subpopulations of Exosomes with Distinct Molecular and Biological Properties. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, H.; Zhu, L.; Lin, F.; Hu, Z.; Jing, R.; Zhang, W.; Zhao, C.; Hong, X.; Zhong, J.; et al. Microvesicles Packaging IL-1β and TNF-α Enhance Lung Inflammatory Response to Mechanical Ventilation in Part by Induction of Cofilin Signaling. Int. Immunopharm. 2018, 63, 74–83. [Google Scholar] [CrossRef]
- Hsu, D.; Paz, P.; Villaflor, G.; Rivas, A.; Mehta-Damani, A.; Angevin, E.; Zitvogel, L.; Le Pecq, J. Exosomes as a Tumor Vaccine: Enhancing Potency Through Direct Loading of Antigenic Peptides. J. Immunother. 2003, 26, 440–450. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lechman, E.R.; Bianco, N.; Menon, R.; Keravala, A.; Nash, J.; Mi, Z.; Watkins, S.C.; Gambotto, A.; Robbins, P.D. Exosomes Derived from IL-10-Treated Dendritic Cells Can Suppress Inflammation and Collagen-Induced Arthritis. J. Immunol. 2005, 174, 6440–6448. [Google Scholar] [CrossRef]
- Segura, E. ICAM-1 on Exosomes from Mature Dendritic Cells Is Critical for Efficient Naive T-cell Priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef]
- Martínez-Lorenzo, M.J.; Anel, A.; Alava, M.A.; Piñeiro, A.; Naval, J.; Lasierra, P.; Larrad, L. The Human Melanoma Cell Line MelJuSo Secretes Bioactive FasL and APO2L/TRAIL on the Surface of Microvesicles. Possible Contribution to Tumor Counterattack. Exp. Cell Res. 2004, 295, 315–329. [Google Scholar] [CrossRef]
- Huber, V.; Fais, S.; Iero, M.; Lugini, L.; Canese, P.; Squarcina, P.; Zaccheddu, A.; Colone, M.; Arancia, G.; Gentile, M.; et al. Human Colorectal Cancer Cells Induce T-Cell Death through Release of Proapoptotic Microvesicles: Role in Immune Escape. Gastroenterology 2005, 128, 1796–1804. [Google Scholar] [CrossRef]
- Urciuoli, E.; Giorda, E.; Scarsella, M.; Petrini, S.; Peruzzi, B. Osteosarcoma-Derived Extracellular Vesicles Induce a Tumor-like Phenotype in Normal Recipient Cells. J. Cell. Physiol. 2018, 233, 6158–6172. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Berguetti, T.; S. P. Quintaes, L.; Hancio Pereira, T.; Robaina, M.C.; Cruz, A.L.S.; C. Maia, R.; de Souza, P.S. TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles. Cells 2019, 8, 500. https://doi.org/10.3390/cells8050500
S. Berguetti T, S. P. Quintaes L, Hancio Pereira T, Robaina MC, Cruz ALS, C. Maia R, de Souza PS. TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles. Cells. 2019; 8(5):500. https://doi.org/10.3390/cells8050500
Chicago/Turabian StyleS. Berguetti, Tandressa, Lucas S. P. Quintaes, Thais Hancio Pereira, Marcela C. Robaina, André L. S. Cruz, Raquel C. Maia, and Paloma Silva de Souza. 2019. "TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles" Cells 8, no. 5: 500. https://doi.org/10.3390/cells8050500
APA StyleS. Berguetti, T., S. P. Quintaes, L., Hancio Pereira, T., Robaina, M. C., Cruz, A. L. S., C. Maia, R., & de Souza, P. S. (2019). TNF-α Modulates P-Glycoprotein Expression and Contributes to Cellular Proliferation via Extracellular Vesicles. Cells, 8(5), 500. https://doi.org/10.3390/cells8050500




