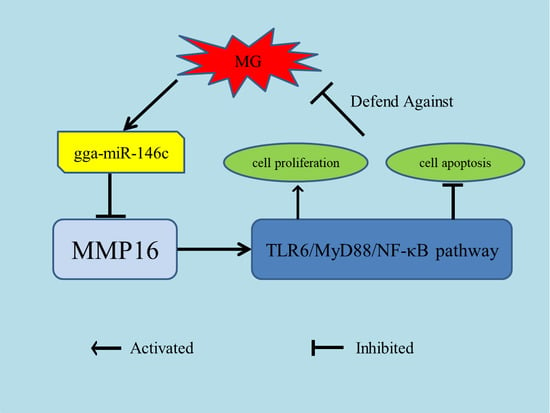
gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Declaration
2.2. Mycoplasma Strains
2.3. Cell infection Experiments
2.4. gga-miR-146c Target Gene Prediction
2.5. RNA Oligonucleotides and DNA Primers
2.6. Dual-Luciferase Reporter Assay
2.7. RNA Extraction and RT-qPCR
2.8. Immunofluorescence
2.9. ELISA
2.10. Western-Blot Analysis
2.11. Cell Proliferation, Cycle, and Apoptosis
2.12. Statistical Analysis
3. Results
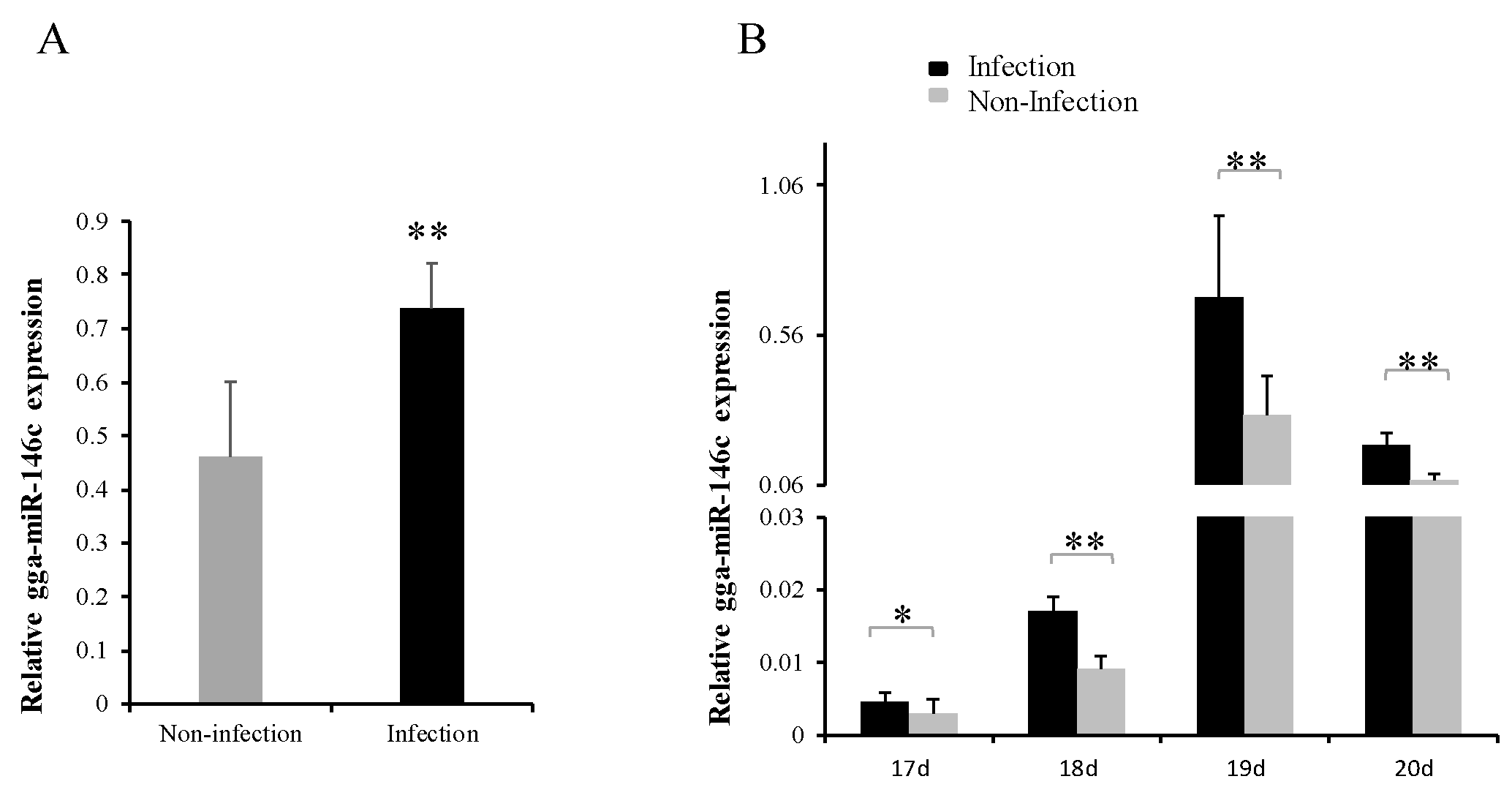
3.1. gga-miR-146c Expression Was Upregulated after MG Infection
3.2. gga-miR-146c Contains Conserved Target Site of MMP16
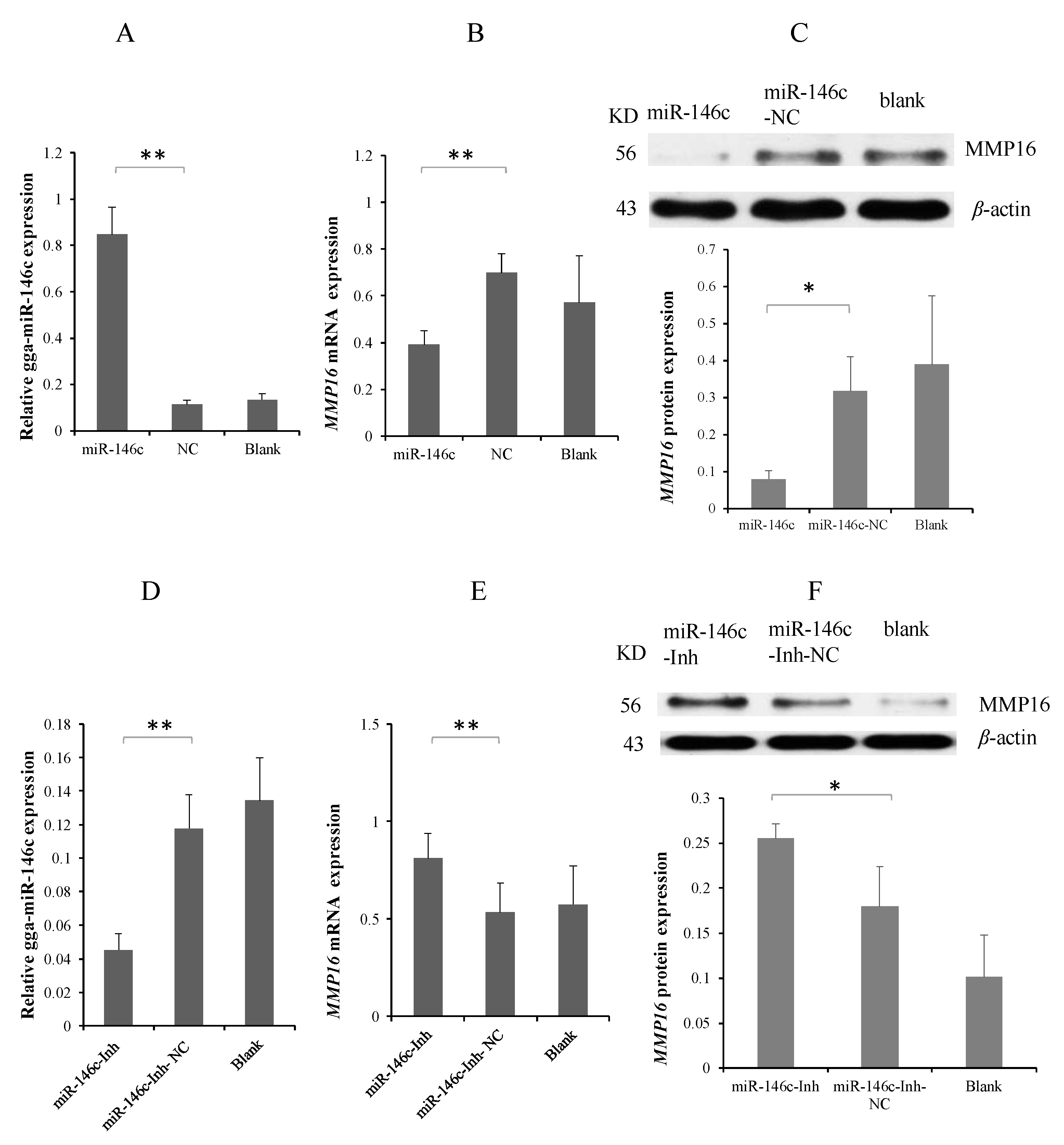
3.3. MMP16 Is a Direct Target Gene of gga-miR-146c
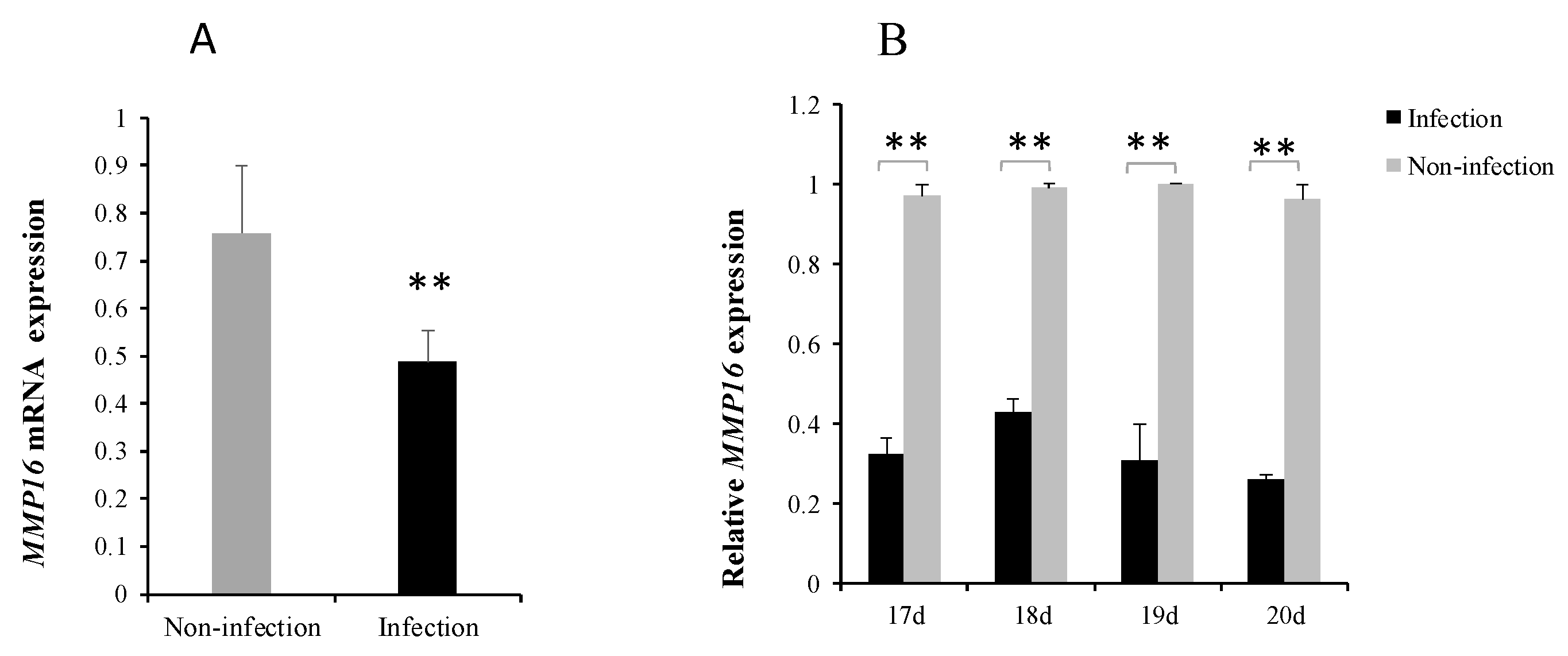
3.4. MG Infection Downregulates MMP16 Expression
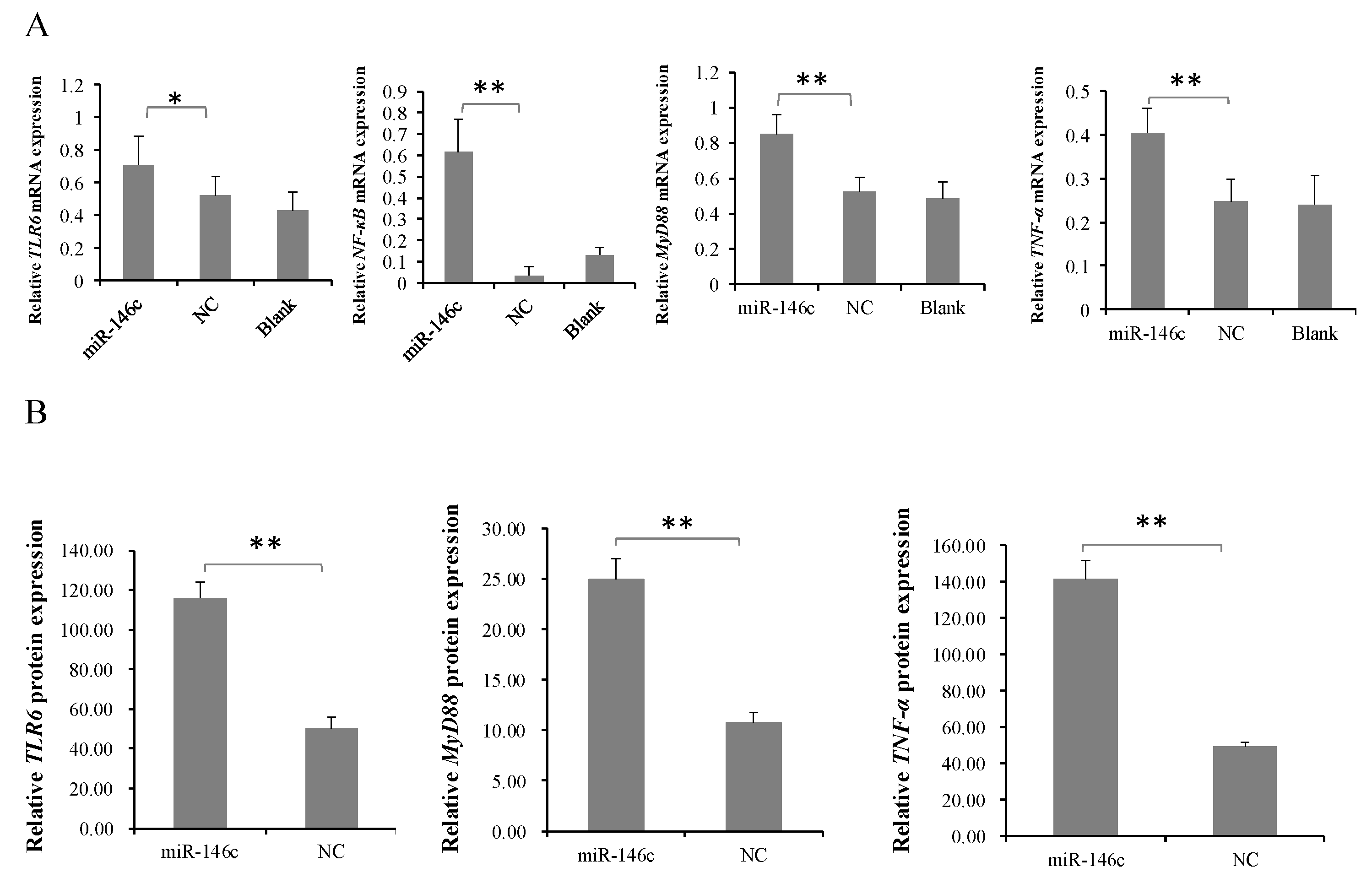
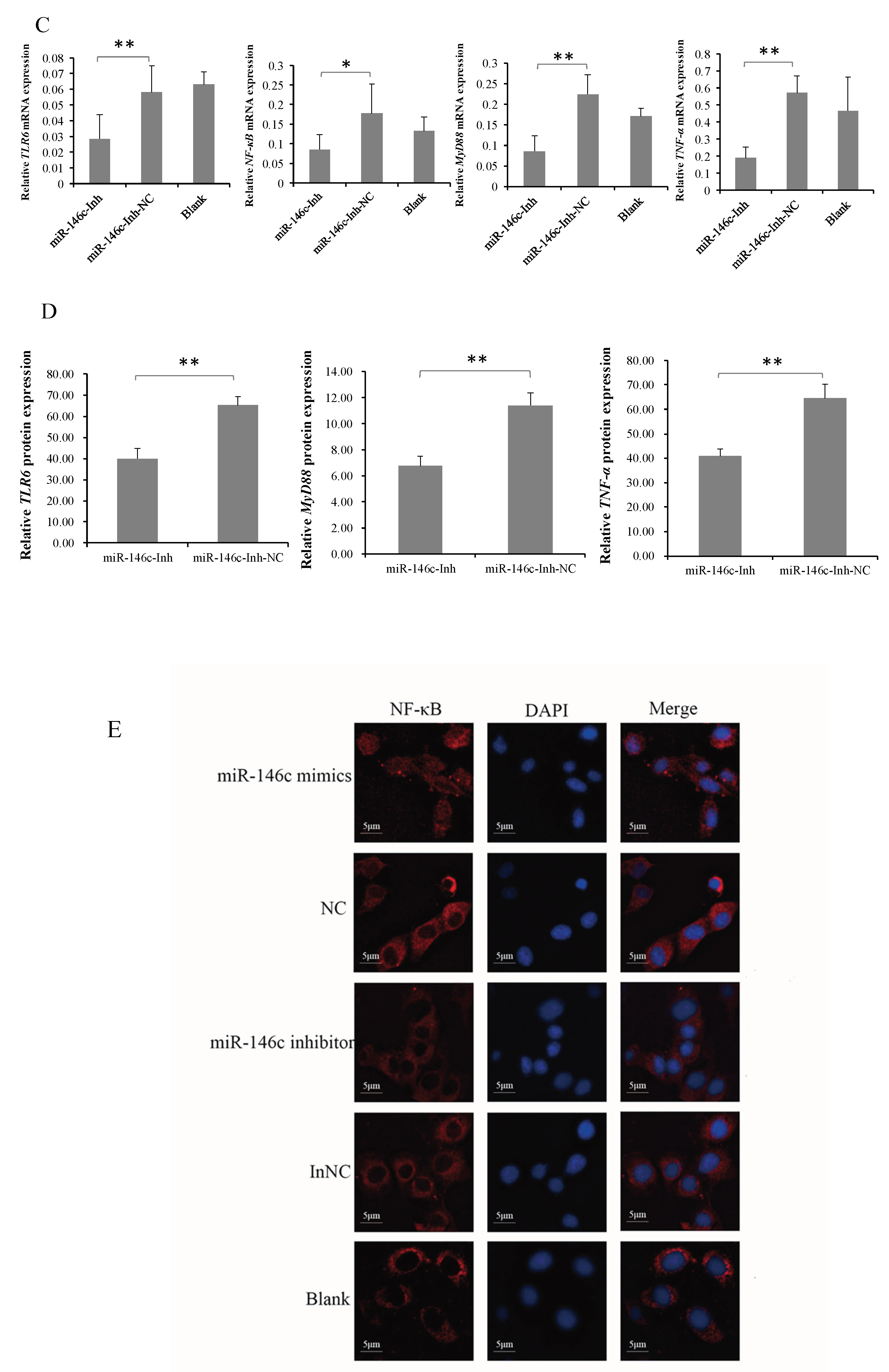
3.5. gga-miR-146c Is of Importance in NF-κB Pathway Regulation
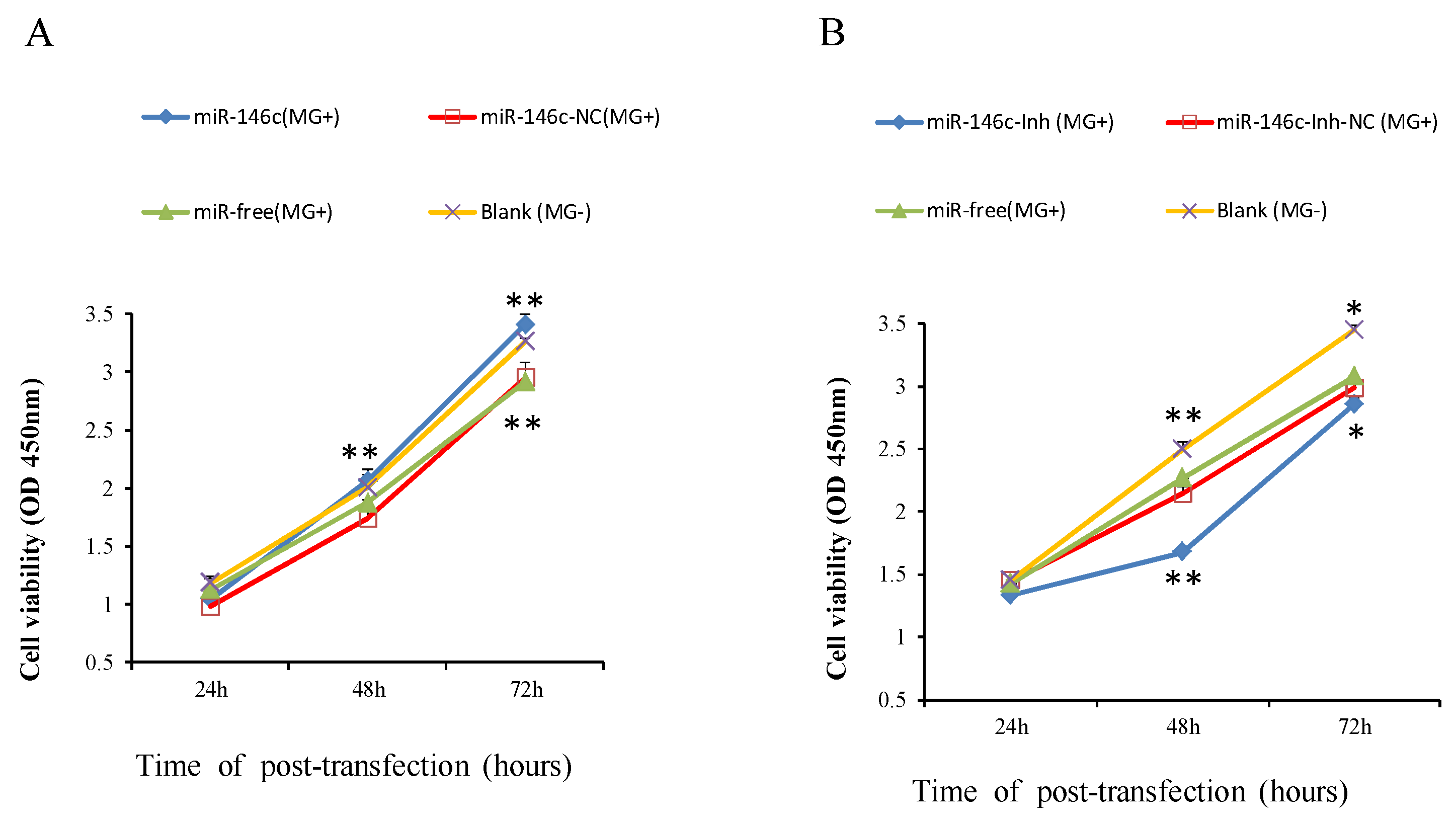
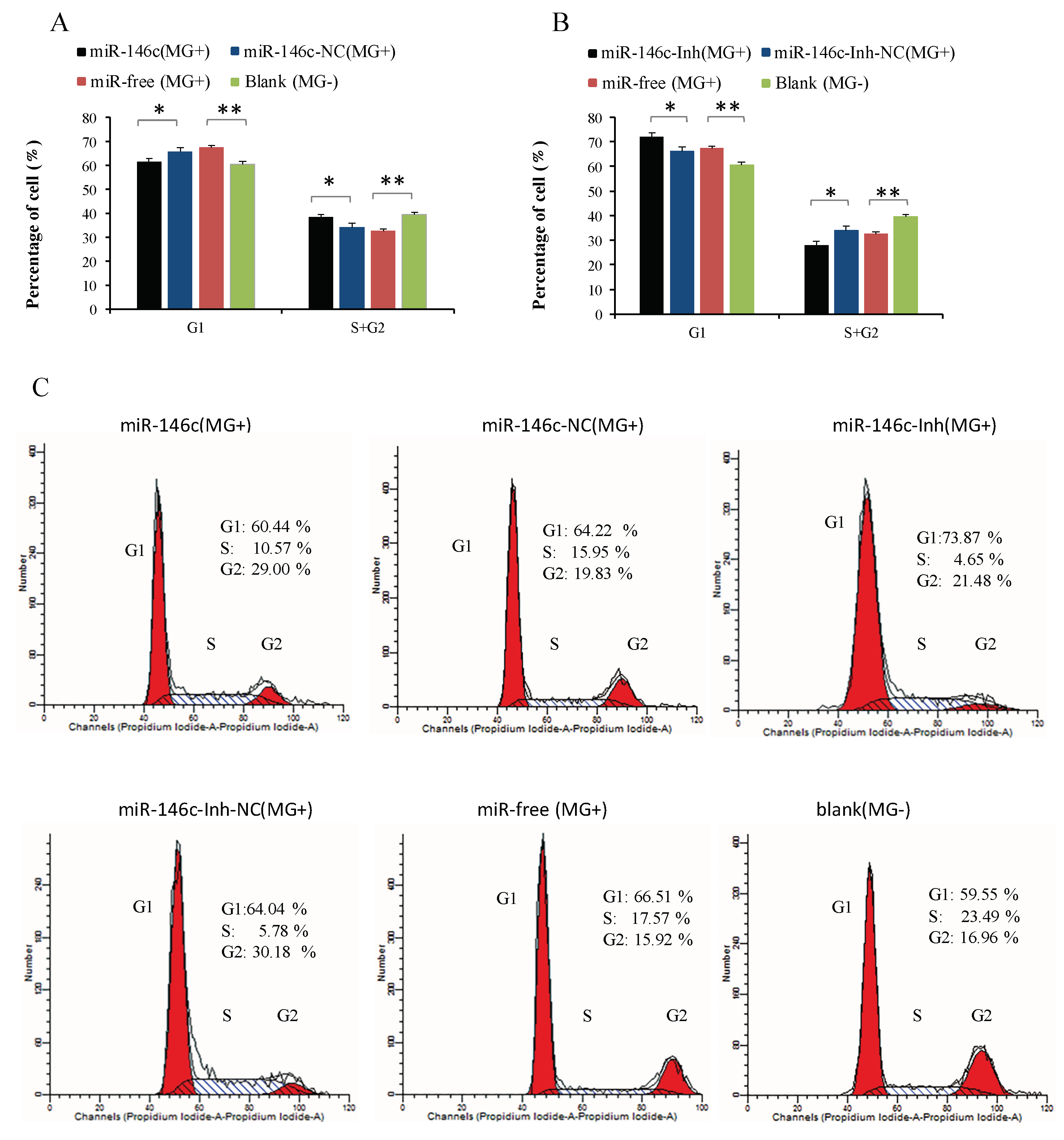
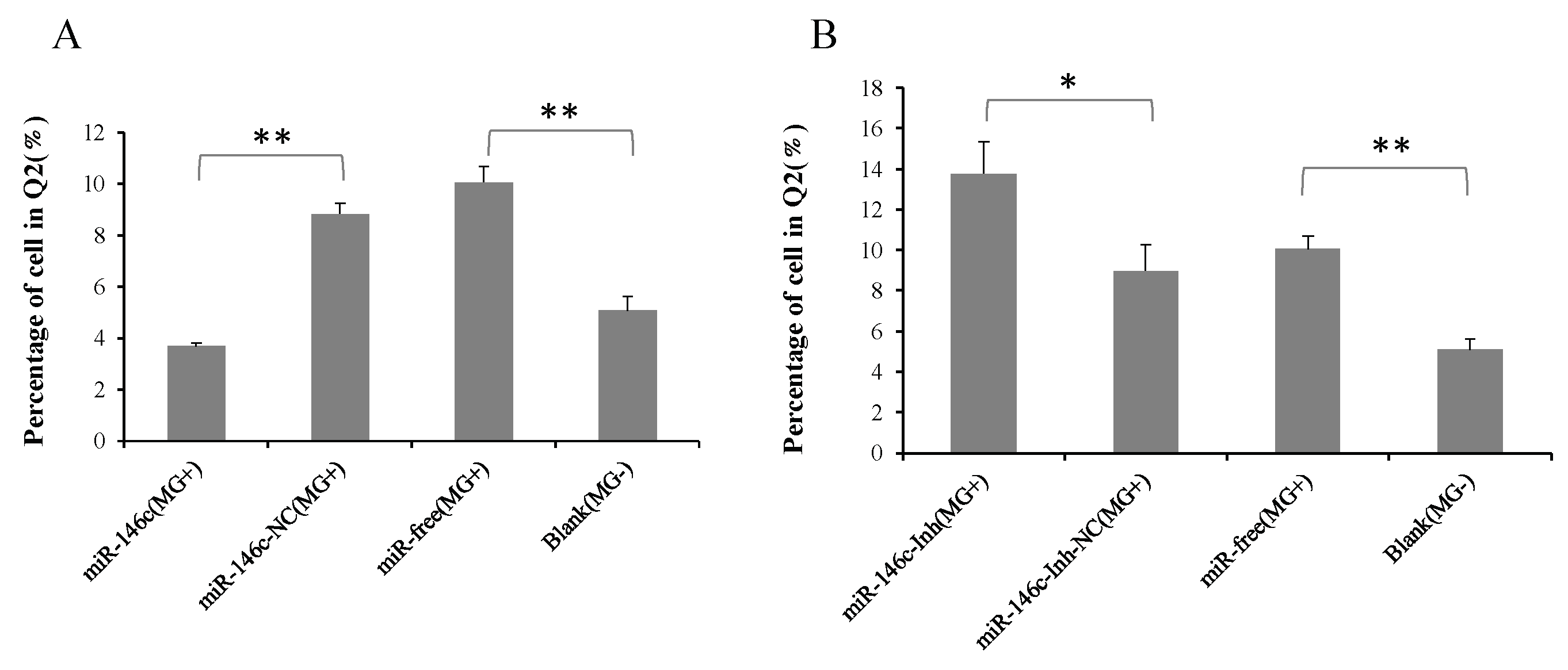
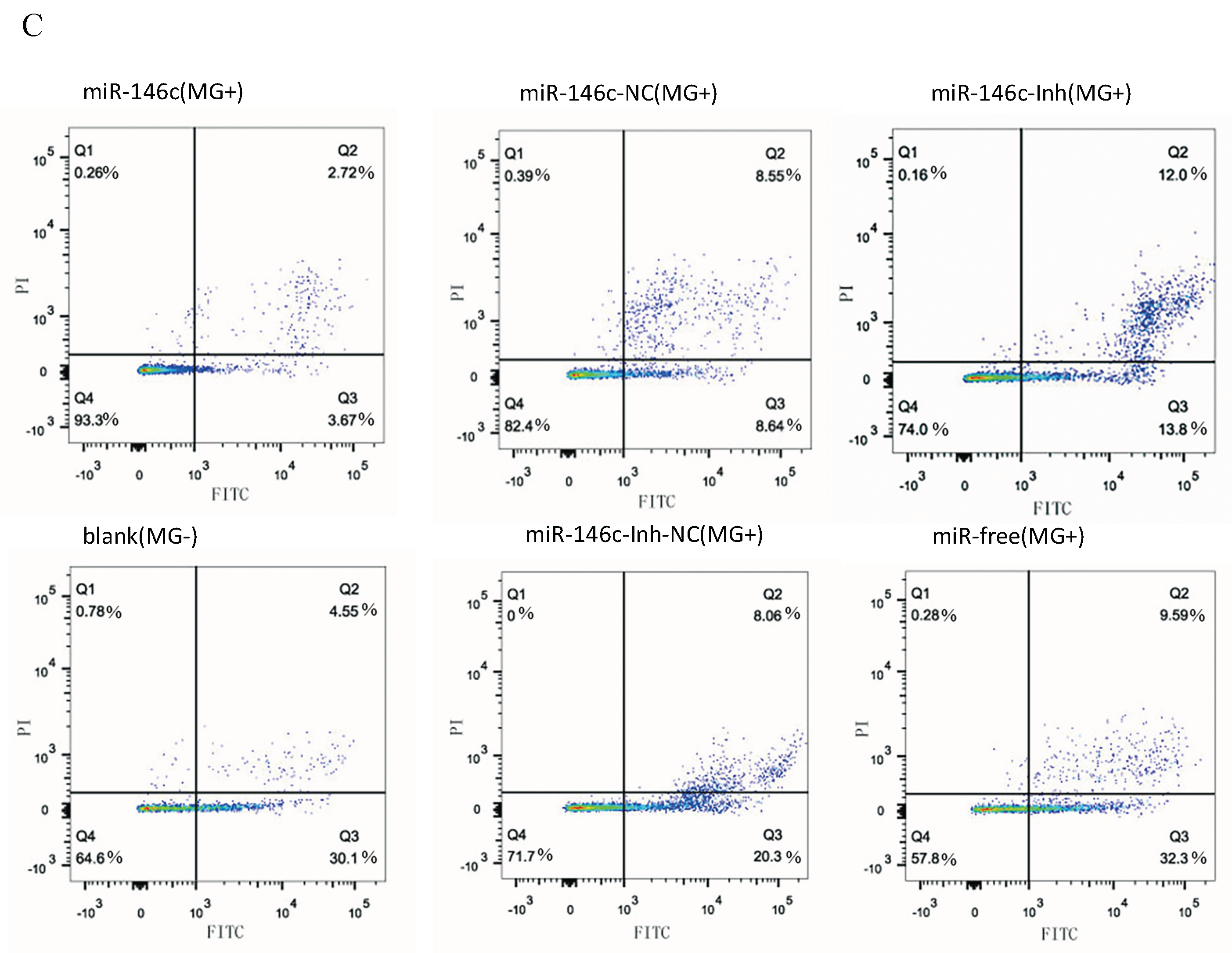
3.6. Upregulation of gga-miR-146c Promotes Proliferation and Cycle Progression by Repressing Apoptosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gambarini, M.L.; Kunz, T.L.; Oliveira Filho, B.D.; Porto, R.N.; Oliveira, C.M.; Brito, W.M.; Viu, M.A. Granular Vulvovaginitis Syndrome in Nelore pubertal and post pubertal replacement heifers under tropical conditions: role of Mycoplasma spp., Ureaplasma diversum and BHV-1. Trop. Anim. Health Prod. 2009, 41, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.; Ayling, R. Mycoplasma in cattle. Vet. Rec. 2016, 178, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Feline hemotropic mycoplasmas. J. Vet. Emerg. Crit. Care (San Antonio) 2010, 20, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.R.; Nettles, V.F.; Couvillion, C.E.; Yoder, H.W., Jr. Infectious sinusitis in wild turkeys. Avian Dis. 1982, 26, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Stipkovits, L.; Egyed, L.; Palfi, V.; Beres, A.; Pitlik, E.; Somogyi, M.; Szathmary, S.; Denes, B. Effect of low-pathogenicity influenza virus H3N8 infection on Mycoplasma gallisepticum infection of chickens. Avian Pathol. 2012, 41, 51–57. [Google Scholar] [CrossRef]
- Quirk, M. Antibiotic-resistant bacteria in food animals on the rise. Lancet Infect. Dis. 2001, 1, 293. [Google Scholar] [CrossRef]
- Hofacre, C.L.; White, D.G.; Maurer, J.J.; Morales, C.; Lobsinger, C.; Hudson, C. Characterization of antibiotic-resistant bacteria in rendered animal products. Avian Dis. 2001, 45, 953–961. [Google Scholar] [CrossRef]
- Witter, R.L. Control strategies for Marek’s disease: a perspective for the future. Poult. Sci. 1998, 77, 1197–1203. [Google Scholar] [CrossRef]
- Pennycott, T.W.; Dare, C.M.; Yavari, C.A.; Bradbury, J.M. Mycoplasma sturni and Mycoplasma gallisepticum in wild birds in Scotland. Vet. Rec. 2005, 156, 513–515. [Google Scholar] [CrossRef]
- O’Reilly, S. MicroRNAs in fibrosis: opportunities and challenges. Arthritis Res. Ther. 2016, 18, 11. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Rao, D.S.; Chaudhuri, A.A.; Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010, 10, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Lee, M.Y.; Araldi, E.; Landskroner-Eiger, S.; Fernandez-Fuertes, M.; Sahraei, M.; Quiles Del Rey, M.; van Solingen, C.; Yu, J.; Fernandez-Hernando, C.; et al. VEGF-Induced Expression of miR-17-92 Cluster in Endothelial Cells Is Mediated by ERK/ELK1 Activation and Regulates Angiogenesis. Circ. Res. 2016, 118, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Li, X.; Zhao, C.; Han, B.; Qu, L.; Song, J.; Liu, C.; Yang, N. Chicken gga-miR-181a targets MYBL1 and shows an inhibitory effect on proliferation of Marek’s disease virus-transformed lymphoid cell line. Poult. Sci. 2015, 94, 2616–2621. [Google Scholar] [CrossRef]
- Lin, J.; Xia, J.; Chen, Y.T.; Zhang, K.Y.; Zeng, Y.; Yang, Q. H9N2 avian influenza virus enhances the immune responses of BMDCs by down-regulating miR29c. Vaccine 2017, 35, 729–737. [Google Scholar] [CrossRef]
- Li, H.; Shang, H.; Shu, D.; Zhang, H.; Ji, J.; Sun, B.; Li, H.; Xie, Q. gga-miR-375 plays a key role in tumorigenesis post subgroup J avian leukosis virus infection. PLoS ONE 2014, 9, e90878. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Wang, Y.S.; Meng, K.; Pan, Q.X.; Wang, X.L.; Xia, X.X.; Zhu, Y.M.; Bi, Z.W.; Zhang, H.B.; Luo, K. gga-miR-2127 downregulates the translation of chicken p53 and attenuates chp53-mediated innate immune response against IBDV infection. Vet. Microbiol. 2017, 198, 34–42. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, Y.; Wang, Z.; Hou, Y.; Bi, D.; Sun, J.; Peng, X. Chicken gga-miR-19a Targets ZMYND11 and Plays an Important Role in Host Defense against Mycoplasma gallisepticum (HS Strain) Infection. Front. Cell. Infect. Microbiol. 2016, 6, 102. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Hou, Y.; Zhang, K.; Peng, X. gga-miR-99a targets SMARCA5 to regulate Mycoplasma gallisepticum (HS strain) infection by depressing cell proliferation in chicken. Gene 2017, 627, 239–247. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Bi, D.; Hou, Y.; Zhao, Y.; Sun, J.; Peng, X. Gga-miR-101-3p Plays a Key Role in Mycoplasma gallisepticum (HS Strain) Infection of Chicken. Int. J. Mol. Sci. 2015, 16, 28669–28682. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Yang, J.P.; Hori, M.; Sanda, T.; Okamoto, T. Identification of a novel inhibitor of nuclear factor-kappaB, RelA-associated inhibitor. J. Biol. Chem. 1999, 274, 15662–15670. [Google Scholar] [CrossRef]
- Lecellier, C.H.; Dunoyer, P.; Arar, K.; Lehmann-Che, J.; Eyquem, S.; Himber, C.; Saib, A.; Voinnet, O. A cellular microRNA mediates antiviral defense in human cells. Science 2005, 308, 557–560. [Google Scholar] [CrossRef]
- Zheng, C.Z.; Shu, Y.B.; Luo, Y.L.; Luo, J. The role of miR-146a in modulating TRAF6-induced inflammation during lupus nephritis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1041–1048. [Google Scholar]
- Jang, S.Y.; Chae, M.K.; Lee, J.H.; Lee, E.J.; Yoon, J.S. Role of miR-146a in the Regulation of Inflammation in an In Vitro Model of Graves’ Orbitopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4027–4034. [Google Scholar] [CrossRef]
- Meisgen, F.; Xu Landen, N.; Wang, A.; Rethi, B.; Bouez, C.; Zuccolo, M.; Gueniche, A.; Stahle, M.; Sonkoly, E.; Breton, L.; et al. MiR-146a negatively regulates TLR2-induced inflammatory responses in keratinocytes. J. Investig. Dermatol. 2014, 134, 1931–1940. [Google Scholar] [CrossRef]
- Liang, G.; Malmuthuge, N.; Guan, Y.; Ren, Y.; Griebel, P.J.; Guan, L.L. Altered microRNA expression and pre-mRNA splicing events reveal new mechanisms associated with early stage Mycobacterium avium subspecies paratuberculosis infection. Sci. Rep. 2016, 6, 24964. [Google Scholar] [CrossRef]
- Liu, P.; Yang, F.; Zhuang, Y.; Xiao, Q.; Cao, H.; Zhang, C.; Wang, T.; Lin, H.; Guo, X.; Hu, G. Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens. Oncotarget 2017, 8, 1993–2007. [Google Scholar] [CrossRef]
- Curtale, G.; Mirolo, M.; Renzi, T.A.; Rossato, M.; Bazzoni, F.; Locati, M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc. Natl. Acad. Sci. USA 2013, 110, 11499–11504. [Google Scholar] [CrossRef]
- Peng, X.; Gao, Q.S.; Zhou, L.; Chen, Z.H.; Lu, S.; Huang, H.J.; Zhan, C.Y.; Xiang, M. MicroRNAs in avian influenza virus H9N2-infected and non-infected chicken embryo fibroblasts. Genet. Mol. Res. 2015, 14, 9081–9091. [Google Scholar] [CrossRef]
- Lian, L.; Qu, L.; Chen, Y.; Lamont, S.J.; Yang, N. A systematic analysis of miRNA transcriptome in Marek’s disease virus-induced lymphoma reveals novel and differentially expressed miRNAs. PLoS ONE 2012, 7, e51003. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Zhang, K.; Yuan, B.; Peng, X. Identification of differentially expressed miRNAs through high-throughput sequencing in the chicken lung in response to Mycoplasma gallisepticum HS. Comp. Biochem. Physiol. Part D Genomics Proteomics 2017, 22, 146–156. [Google Scholar] [CrossRef]
- Bi, D.; Ji, X. The isolation and identification of the mycoplasma gallisepticum. Acta Vet. Zootechnol. Sin. 1988, 1, 146–148. [Google Scholar]
- Bi, D.; Xu, Q. Study on pathogenicity of HS strain Mycoplasma gallisepticum. Chin. J. Anim. Poult. Infect. Dis. 1997, 5, 24–26. [Google Scholar]
- Calus, D.; Maes, D.; Vranckx, K.; Villareal, I.; Pasmans, F.; Haesebrouck, F. Validation of ATP luminometry for rapid and accurate titration of Mycoplasma hyopneumoniae in Friis medium and a comparison with the color changing units assay. J. Microbiol. Methods 2010, 83, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, C.; Hu, Q.; Sun, J.; Peng, X. Roles of Toll-like receptors 2 and 6 in the inflammatory response to Mycoplasma gallisepticum infection in DF-1 cells and in chicken embryos. Dev. Comp. Immunol. 2016, 59, 39–47. [Google Scholar] [CrossRef]
- Indikova, I.; Much, P.; Stipkovits, L.; Siebert-Gulle, K.; Szostak, M.P.; Rosengarten, R.; Citti, C. Role of the GapA and CrmA cytadhesins of Mycoplasma gallisepticum in promoting virulence and host colonization. Infect. Immun. 2013, 81, 1618–1624. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Zhao, J.; Yang, S.; Wang, L.; Cheng, G.; Liu, D.; Xiao, J.; Liu, Z.; Zhao, Z. MiRNA-29c regulates the expression of inflammatory cytokines in diabetic nephropathy by targeting tristetraprolin. Sci. Rep. 2017, 7, 2314. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Podyminogin, R.L.; Diercks, A.H.; Treuting, P.M.; Peschon, J.J.; Rodriguez, D.; Gundapuneni, M.; Weiss, M.J.; Aderem, A. miR-144 attenuates the host response to influenza virus by targeting the TRAF6-IRF7 signaling axis. PLoS Pathog. 2017, 13, e1006305. [Google Scholar] [CrossRef]
- Ji, J.; Shang, H.; Zhang, H.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Temporal changes of microRNA gga-let-7b and gga-let-7i expression in chickens challenged with subgroup J avian leukosis virus. Vet. Res. Commun. 2017. [Google Scholar] [CrossRef]
- Dai, Z.; Ji, J.; Yan, Y.; Lin, W.; Li, H.; Chen, F.; Liu, Y.; Chen, W.; Bi, Y.; Xie, Q. Role of gga-miR-221 and gga-miR-222 during Tumour Formation in Chickens Infected by Subgroup J Avian Leukosis Virus. Viruses 2015, 7, 6538–6551. [Google Scholar] [CrossRef]
- Han, B.; Lian, L.; Li, X.; Zhao, C.; Qu, L.; Liu, C.; Song, J.; Yang, N. Chicken gga-miR-130a targets HOXA3 and MDFIC and inhibits Marek’s disease lymphoma cell proliferation and migration. Mol. Biol. Rep. 2016, 43, 667–676. [Google Scholar] [CrossRef]
- Lian, L.; Zhang, D.; Wang, Q.; Yang, N.; Qu, L. The inhibitory effects of gga-miR-199-3p, gga-miR-140-3p, and gga-miR-221-5p in Marek’s disease tumorigenesis. Poult. Sci. 2015, 94, 2131–2135. [Google Scholar] [CrossRef]
- Khorrami, S.; Zavaran Hosseini, A.; Mowla, S.J.; Soleimani, M.; Rakhshani, N.; Malekzadeh, R. MicroRNA-146a induces immune suppression and drug-resistant colorectal cancer cells. Tumour Biol. 2017, 39, 1010428317698365. [Google Scholar] [CrossRef]
- Huang, B.Y.; Hu, P.; Zhang, D.D.; Jiang, G.M.; Liu, S.Y.; Xu, Y.; Wu, Y.F.; Xia, X.; Wang, Y. C-type natriuretic peptide suppresses mesangial proliferation and matrix expression via a MMPs/TIMPs-independent pathway in vitro. J. Recept. Signal Transduct. Res. 2017, 37, 355–364. [Google Scholar] [CrossRef]
- Mali, A.V.; Joshi, A.A.; Hegde, M.V.; Kadam Sh, S. Enterolactone Suppresses Proliferation, Migration and Metastasis of MDA-MB-231 Breast Cancer Cells Through Inhibition of uPA Induced Plasmin Activation and MMPs-Mediated ECM Remodeling. Asian Pac. J. Cancer Prev. 2017, 18, 905–915. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Chen, Y.; Xie, X.; Zhang, Y.; Ma, C.; Yang, Q.; Han, Y.; Zhu, C.; Xiong, Y.; et al. Matrix Metalloproteinase 9 Facilitates Hepatitis B Virus Replication through Binding with Type I Interferon (IFN) Receptor 1 To Repress IFN/JAK/STAT Signaling. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Rodas, P.I.; Perez, D.; Jaffret, C.; Gonzalez, Y.; Carreno, C.; Tapia, C.V.; Osorio, E.; Velasquez, L.A.; Christodoulides, M. Modified profile of matrix metalloproteinase-2 and -9 production by human Fallopian tube epithelial cells following infection in vitro with Neisseria gonorrhoeae. J. Infect. Dis. 2016. [Google Scholar] [CrossRef]
- Tatti, O.; Gucciardo, E.; Pekkonen, P.; Holopainen, T.; Louhimo, R.; Repo, P.; Maliniemi, P.; Lohi, J.; Rantanen, V.; Hautaniemi, S.; et al. MMP16 Mediates a Proteolytic Switch to Promote Cell-Cell Adhesion, Collagen Alignment, and Lymphatic Invasion in Melanoma. Cancer Res. 2015, 75, 2083–2094. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, L.; Sun, C.; Cheng, D.; Yu, S.; Wang, Q.; Yan, Y.; Kang, C.; Jin, S.; et al. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013, 339, 260–269. [Google Scholar] [CrossRef]
- Astarci, E.; Erson-Bensan, A.E.; Banerjee, S. Matrix metalloprotease 16 expression is downregulated by microRNA-146a in spontaneously differentiating Caco-2 cells. Dev. Growth Differ. 2012, 54, 216–226. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Z.; Zhang, Y.; Chen, Y.; Li, Z. MicroRNA-145 Suppresses Osteosarcoma Metastasis via Targeting MMP16. Cell. Physiol. Biochem. 2015, 37, 2183–2193. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef]
- Peteranderl, C.; Sznajder, J.I.; Herold, S.; Lecuona, E. Inflammatory Responses Regulating Alveolar Ion Transport during Pulmonary Infections. Front. Immunol. 2017, 8, 446. [Google Scholar] [CrossRef]
- Nishiguchi, M.; Matsumoto, M.; Takao, T.; Hoshino, M.; Shimonishi, Y.; Tsuji, S.; Begum, N.A.; Takeuchi, O.; Akira, S.; Toyoshima, K.; et al. Mycoplasma fermentans lipoprotein M161Ag-induced cell activation is mediated by Toll-like receptor 2: role of N-terminal hydrophobic portion in its multiple functions. J. Immunol. 2001, 166, 2610–2616. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Ozata, D.M.; Li, X.; Lee, L.; Liu, J.; Warsito, D.; Hajeri, P.; Hultman, I.; Fotouhi, O.; Marklund, S.; Ahrlund-Richter, L.; et al. Loss of miR-514a-3p regulation of PEG3 activates the NF-kappa B pathway in human testicular germ cell tumors. Cell Death Dis. 2017, 8, e2759. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Cheng, N.; Wang, K.; Li, B.; Jiang, X.; Sun, S. Hepatitis C virus-induced up-regulation of microRNA-155 promotes hepatocarcinogenesis by activating Wnt signaling. Hepatology 2012, 56, 1631–1640. [Google Scholar] [CrossRef]
- Wang, P.; Cao, J.; Liu, S.; Pan, H.; Liu, X.; Sui, A.; Wang, L.; Yao, R.; Liu, Z.; Liang, J. Upregulated microRNA-429 inhibits the migration of HCC cells by targeting TRAF6 through the NF-kappaB pathway. Oncol. Rep. 2017, 37, 2883–2890. [Google Scholar] [CrossRef]
- Gu, Y.; Ampofo, E.; Menger, M.D.; Laschke, M.W. miR-191 suppresses angiogenesis by activation of NF-kappaB signaling. FASEB J. 2017. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Rosillo, M.A.; Sanchez-Fidalgo, S.; Utrilla, J.; Martin-Lacave, I.; Alarcon-de-la-Lastra, C. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-kappaB and MAPK activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, S.; Abbasirad, N.; Movahed, A.; Khazaei, H.A.; Pishjoo, M.; Rezaei, N. TLR9-based immunotherapy for the treatment of allergic diseases. Immunotherapy 2017, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef]
- Yeh, D.W.; Huang, L.R.; Chen, Y.W.; Huang, C.F.; Chuang, T.H. Interplay between Inflammation and Stemness in Cancer Cells: The Role of Toll-Like Receptor Signaling. J. Immunol. Res. 2016, 2016, 4368101. [Google Scholar] [CrossRef]
- Ghosh, S.; Dass, J.F. Study of pathway cross-talk interactions with NF-kappaB leading to its activation via ubiquitination or phosphorylation: A brief review. Gene 2016, 584, 97–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. Toll-like receptor-6 (TLR6) deficient mice are protected from myocardial fibrosis induced by high fructose feeding through anti-oxidant and inflammatory signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 388–395. [Google Scholar] [CrossRef]
- Monaco, C.; Andreakos, E.; Kiriakidis, S.; Mauri, C.; Bicknell, C.; Foxwell, B.; Cheshire, N.; Paleolog, E.; Feldmann, M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc. Natl. Acad. Sci. USA 2004, 101, 5634–5639. [Google Scholar] [CrossRef]
- Zhong, J.H.; Li, J.; Liu, C.F.; Liu, N.; Bian, R.X.; Zhao, S.M.; Yan, S.Y.; Zhang, Y.B. Effects of microRNA-146a on the proliferation and apoptosis of human osteoarthritis chondrocytes by targeting TRAF6 through the NF-kappaB signalling pathway. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Wang, W.M.; Liu, J.C. Effect and molecular mechanism of mir-146a on proliferation of lung cancer cells by targeting and regulating MIF gene. Asian Pac. J. Trop. Med. 2016, 9, 806–811. [Google Scholar] [CrossRef]
- Fang, J.; Barker, B.; Bolanos, L.; Liu, X.; Jerez, A.; Makishima, H.; Christie, S.; Chen, X.; Rao, D.S.; Grimes, H.L.; et al. Myeloid malignancies with chromosome 5q deletions acquire a dependency on an intrachromosomal NF-kappaB gene network. Cell Rep. 2014, 8, 1328–1338. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, J.; Zhang, C.Y.; Shen, Y.Q.; Wang, H.M.; Ding, L.; Gu, Y.C.; Lou, J.T.; Zhao, X.T.; Ma, Z.L.; et al. MiR-146a-5p inhibits cell proliferation and cell cycle progression in NSCLC cell lines by targeting CCND1 and CCND2. Oncotarget 2016, 7, 59287–59298. [Google Scholar] [CrossRef]
- Cai, H.H.; Wang, H.Y.; Liu, H.R.; Sheng, Y.J.; Xi, D.G.; Xue, Y.P.; Dai, X.L.; Wang, A.D.; Huang, Q.; Dong, J. Down-regulation of miR-146b-5p promotes malignant transformation of fusion cells after co-culture of macrophages with glioma stem cells in vitro. Zhonghua Yi Xue Za Zhi 2017, 97, 380–386. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Kochumon, S.; Usmani, S.; Sindhu, S.; Ahmad, R. Pam3CSK4 Induces MMP-9 Expression in Human Monocytic THP-1 Cells. Cell. Physiol. Biochem. 2017, 41, 1993–2003. [Google Scholar] [CrossRef]
- Cao, L.; Wang, Z.; Ma, J.; Chen, J.; Zhu, H.; Zhou, X.; Zhu, Q.; Dong, J.; Lan, Q.; Huang, Q. Clinical characteristics and molecular pathology of skull ectopic thyroid cancer. Ann. Transl. Med. 2016, 4, 462. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.T.; Wu, C.; Wu, Z.W.; Li, Y.Y.; Yang, T.Q.; Chen, G.L.; Xie, X.S.; Huang, Y.L.; Du, Z.W.; et al. miR-132 can inhibit glioma cells invasion and migration by target MMP16 in vitro. Onco Targets Ther. 2015, 8, 3211–3218. [Google Scholar] [CrossRef]
- Cao, L.; Chen, C.; Zhu, H.; Gu, X.; Deng, D.; Tian, X.; Liu, J.; Xiao, Q. MMP16 is a marker of poor prognosis in gastric cancer promoting proliferation and invasion. Oncotarget 2016, 7, 51865–51874. [Google Scholar] [CrossRef][Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Han, Y.; Wang, Z.; Zhao, Y.; Fu, Y.; Peng, X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells 2019, 8, 501. https://doi.org/10.3390/cells8050501
Zhang K, Han Y, Wang Z, Zhao Y, Fu Y, Peng X. gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells. 2019; 8(5):501. https://doi.org/10.3390/cells8050501
Chicago/Turabian StyleZhang, Kang, Yun Han, Zaiwei Wang, Yabo Zhao, Yali Fu, and Xiuli Peng. 2019. "gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens" Cells 8, no. 5: 501. https://doi.org/10.3390/cells8050501
APA StyleZhang, K., Han, Y., Wang, Z., Zhao, Y., Fu, Y., & Peng, X. (2019). gga-miR-146c Activates TLR6/MyD88/NF-κB Pathway through Targeting MMP16 to Prevent Mycoplasma Gallisepticum (HS Strain) Infection in Chickens. Cells, 8(5), 501. https://doi.org/10.3390/cells8050501