Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. BMP8B Depletion with Si-RNA-Pools
2.3. Lipid Analysis
2.4. Quantitative Real-Time PCR Analysis
2.5. Protein Analysis
2.6. Analysis of Cell Proliferation
2.7. Statistical Analysis
3. Results
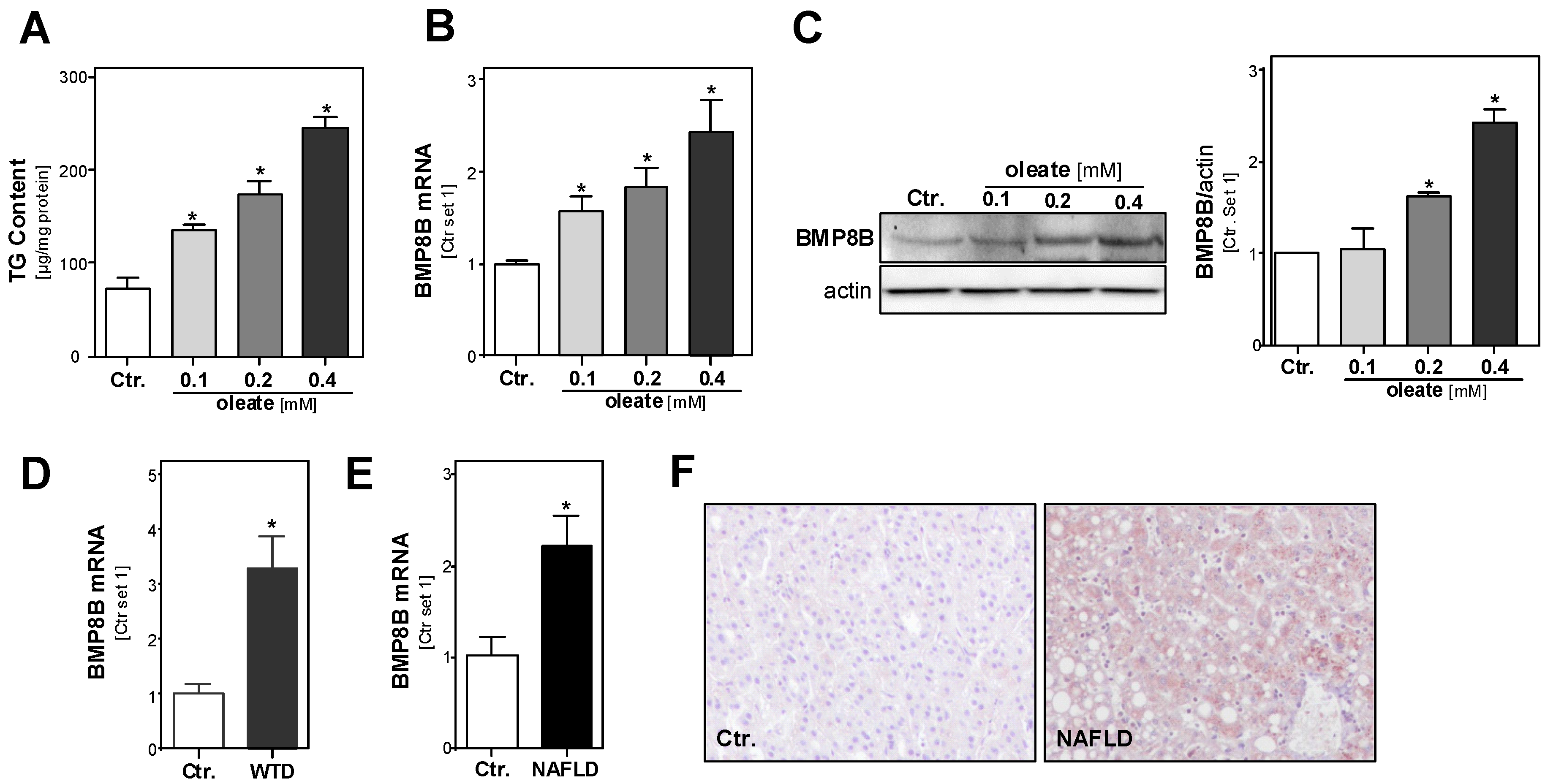
3.1. Effect of Steatosis on Hepatic BMP8B Expression
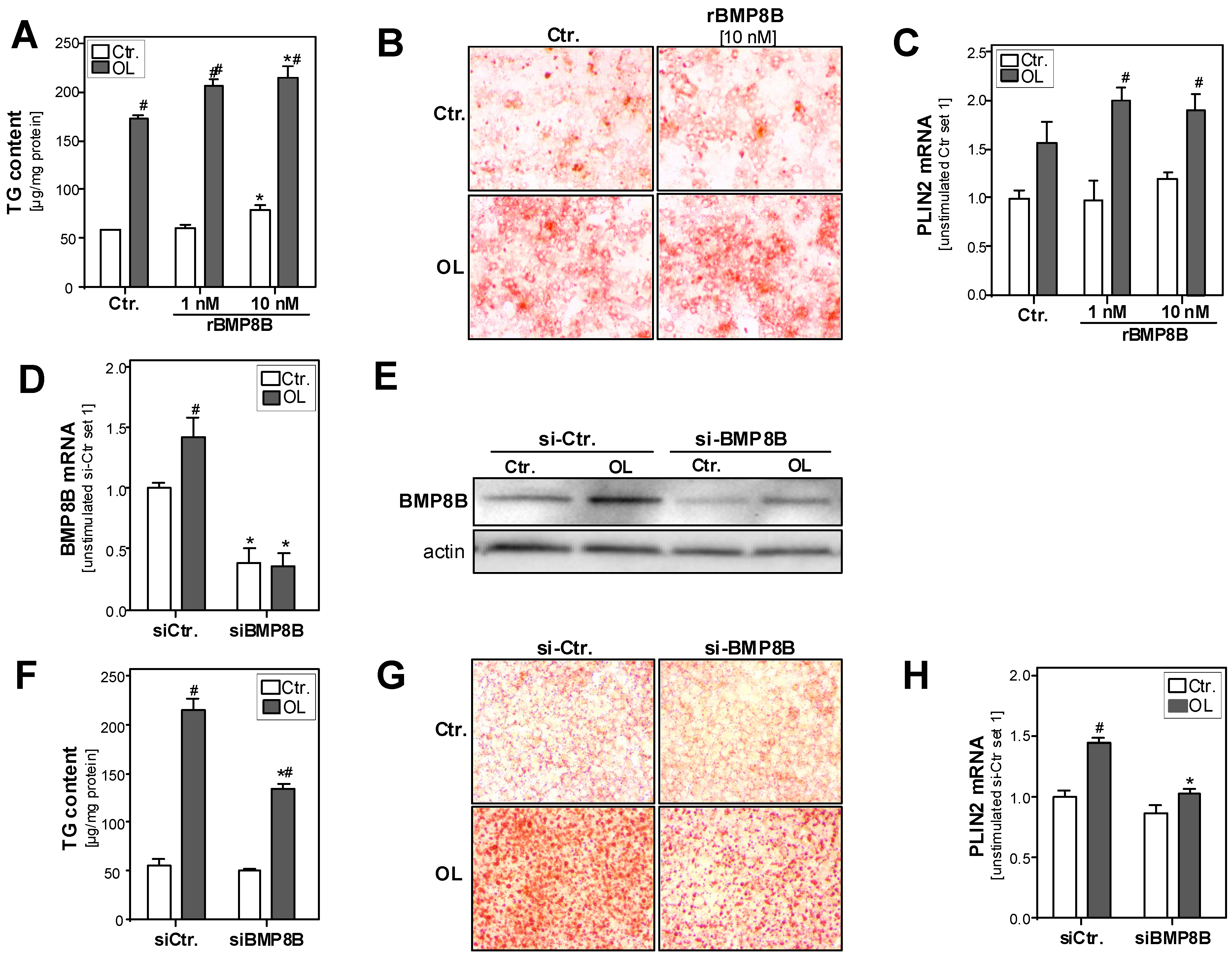
3.2. Effect of the BMP8B on Steatosis in Hepatocytes
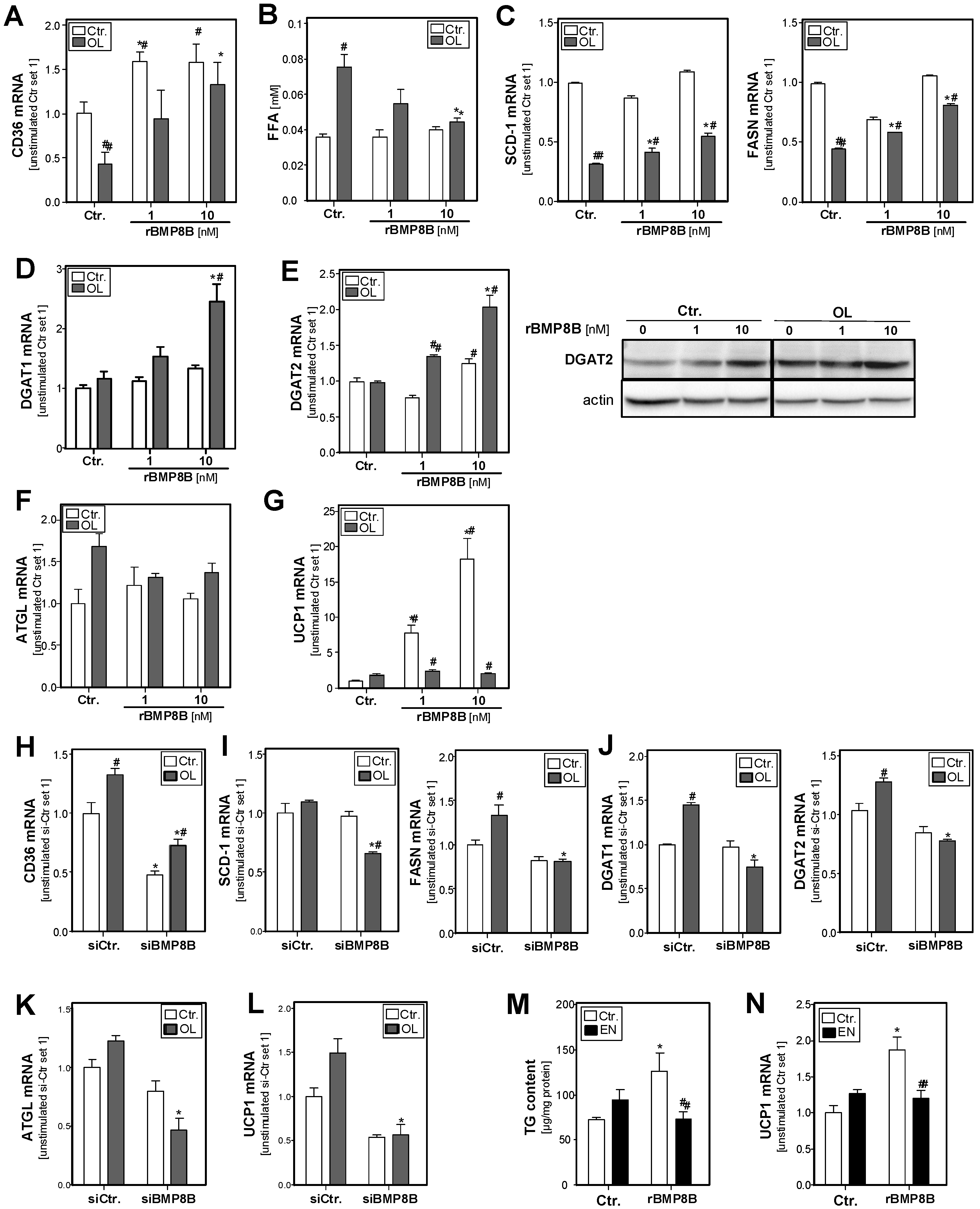
3.3. Effect of the BMP8B on Lipid Metabolism in Hepatocytes
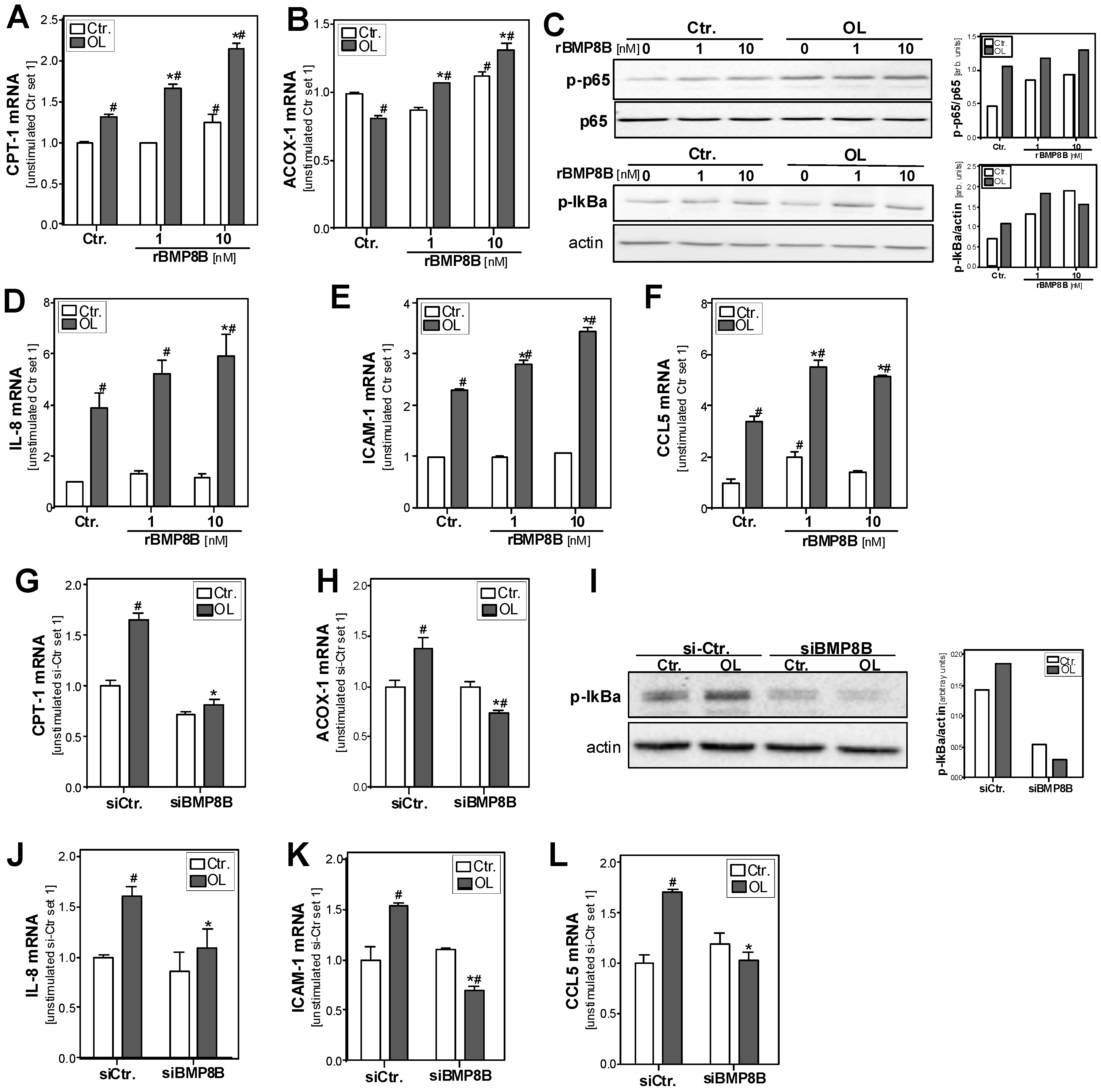
3.4. Effect of the BMP8B on Steatosis-Induced Pro-Inflammatory Gene Expression in Hepatocytes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of nafld and nash: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Powell, E.E.; Cooksley, W.G.; Hanson, R.; Searle, J.; Halliday, J.W.; Powell, L.W. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology 1990, 11, 74–80. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P. Pathology of non-alcoholic fatty liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37 (Suppl. 1), 85–89. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. Tgf-β and bmp signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Addante, A.; Sánchez, A. Bmp signalling at the crossroad of liver fibrosis and regeneration. Int. J. Mol. Sci. 2017, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Grgurevic, L.; Christensen, G.L.; Schulz, T.J.; Vukicevic, S. Bone morphogenetic proteins in inflammation, glucose homeostasis and adipose tissue energy metabolism. Cytokine Growth Factor Rev. 2016, 27, 105–118. [Google Scholar] [CrossRef]
- Martins, L.; Seoane-Collazo, P.; Contreras, C.; Gonzalez-Garcia, I.; Martinez-Sanchez, N.; Gonzalez, F.; Zalvide, J.; Gallego, R.; Dieguez, C.; Nogueiras, R.; et al. A functional link between ampk and orexin mediates the effect of bmp8b on energy balance. Cell Rep. 2016, 16, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vazquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. Bmp8b increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Gonzalez, F.; Ferno, J.; Dieguez, C.; Rahmouni, K.; Nogueiras, R.; Lopez, M. The brain and brown fat. Ann. Med. 2015, 47, 150–168. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Deng, K.; Labosky, P.A.; Liaw, L.; Hogan, B.L. The gene encoding bone morphogenetic protein 8b is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 1996, 10, 1657–1669. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, W.; Ding, Y.; Liu, T.; Liu, W.; Qin, Y.; Xia, W.; Xu, J.; Zhang, Y.; Zou, X. Bmp8b mediates the survival of pancreatic cancer cells and regulates the progression of pancreatic cancer. Oncol. Rep. 2014, 32, 1861–1866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mima, K.; Fukagawa, T.; Kurashige, J.; Takano, Y.; Uchi, R.; Ueo, H.; Matsumura, T.; Ishibashi, M.; Sawada, G.; Takahashi, Y.; et al. Gene expression of bone morphogenic protein 8b in the primary site, peripheral blood and bone marrow of patients with gastric cancer. Oncol. Lett. 2013, 6, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wisnieski, F.; Leal, M.F.; Calcagno, D.Q.; Santos, L.C.; Gigek, C.O.; Chen, E.S.; Artigiani, R.; Demachki, S.; Assumpcao, P.P.; Lourenco, L.G.; et al. Bmp8b is a tumor suppressor gene regulated by histone acetylation in gastric cancer. J. Cell. Biochem. 2017, 118, 869–877. [Google Scholar] [CrossRef]
- Hellerbrand, C.; Bumes, E.; Bataille, F.; Dietmaier, W.; Massoumi, R.; Bosserhoff, A.K. Reduced expression of cyld in human colon and hepatocellular carcinomas. Carcinogenesis 2007, 28, 21–27. [Google Scholar] [CrossRef]
- Weiss, T.S.; Jahn, B.; Cetto, M.; Jauch, K.W.; Thasler, W.E. Collagen sandwich culture affects intracellular polyamine levels of human hepatocytes. Cell Prolif. 2002, 35, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Erwin Thasler, W.; Hellerbrand, C. Establishment of a p-nitrophenol oxidation-based assay for the analysis of cyp2e1 activity in intact hepatocytes in vitro. Toxicol. Mech. Methods 2019, 29, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Wobser, H.; Dorn, C.; Weiss, T.S.; Amann, T.; Bollheimer, C.; Buttner, R.; Scholmerich, J.; Hellerbrand, C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009, 19, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Riener, M.O.; Kirovski, G.; Saugspier, M.; Steib, K.; Weiss, T.S.; Gabele, E.; Kristiansen, G.; Hartmann, A.; Hellerbrand, C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2010, 3, 505–514. [Google Scholar]
- Pellegrinelli, V.; Peirce, V.J.; Howard, L.; Virtue, S.; Turei, D.; Senzacqua, M.; Frontini, A.; Dalley, J.W.; Horton, A.R.; Bidault, G.; et al. Adipocyte-secreted bmp8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nat. Commun. 2018, 9, 4974. [Google Scholar] [CrossRef] [PubMed]
- Gaidhu, M.P.; Fediuc, S.; Anthony, N.M.; So, M.; Mirpourian, M.; Perry, R.L.; Ceddia, R.B. Prolonged aicar-induced amp-kinase activation promotes energy dissipation in white adipocytes: Novel mechanisms integrating hsl and atgl. J. Lipid Res. 2009, 50, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Koch, A.; Fritz, V.; Hartmann, A.; Bosserhoff, A.K.; Hellerbrand, C. Wild type kirsten rat sarcoma is a novel microrna-622-regulated therapeutic target for hepatocellular carcinoma and contributes to sorafenib resistance. Gut 2018, 67, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Engelmann, J.C.; Saugspier, M.; Koch, A.; Hartmann, A.; Muller, M.; Spang, R.; Bosserhoff, A.; Hellerbrand, C. Increased expression of c-jun in nonalcoholic fatty liver disease. Z. Gastroenterol. 2014, 94, 394–408. [Google Scholar] [CrossRef]
- Hannus, M.; Beitzinger, M.; Engelmann, J.C.; Weickert, M.T.; Spang, R.; Hannus, S.; Meister, G. Sipools: Highly complex but accurately defined sirna pools eliminate off-target effects. Nucleic Acids Res. 2014, 42, 8049–8061. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Thasler, W.E.; Patsenker, E.; Muller, S.; Stickel, F.; Muller, M.; Seitz, H.K.; Cederbaum, A.I.; Hellerbrand, C. Identification of cytochrome cyp2e1 as critical mediator of synergistic effects of alcohol and cellular lipid accumulation in hepatocytes in vitro. Oncotarget 2015, 6, 41464–41478. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Seitz, T.; Freese, K.; Frank, J.; Weiskirchen, R.; Abdel-Tawab, M.; Behnam, D.; Hellerbrand, C. Therapeutic application of micellar solubilized xanthohumol in a western-type diet-induced mouse model of obesity, diabetes and non-alcoholic fatty liver disease. Cells 2019, 8, 359. [Google Scholar] [CrossRef]
- Sommer, J.; Mahli, A.; Freese, K.; Schiergens, T.S.; Kuecuekoktay, F.S.; Teufel, A.; Thasler, W.E.; Muller, M.; Bosserhoff, A.K.; Hellerbrand, C. Analysis of molecular mechanisms of 5-fluorouracil-induced steatosis and inflammation in vitro and in mice. Oncotarget 2017, 8, 13059–13072. [Google Scholar] [CrossRef] [PubMed]
- Mahli, A.; Koch, A.; Czech, B.; Peterburs, P.; Lechner, A.; Haunschild, J.; Müller, M.; Hellerbrand, C. Hepatoprotective effect of oral application of a silymarin extract in carbon tetrachloride-induced hepatotoxicity in rats. Clin. Phytoscience 2015, 1, 5. [Google Scholar] [CrossRef]
- Mahli, A.; Saugspier, M.; Koch, A.; Sommer, J.; Dietrich, P.; Lee, S.; Thasler, R.; Schulze-Luehrmann, J.; Luehrmann, A.; Thasler, W.E.; et al. Erk activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut 2018, 67, 746–756. [Google Scholar] [PubMed]
- Mahli, A.; Koch, A.; Fresse, K.; Schiergens, T.; Thasler, W.E.; Schonberger, C.; Bergheim, I.; Bosserhoff, A.; Hellerbrand, C. Iso-alpha acids from hops (humulus lupulus) inhibit hepatic steatosis, inflammation, and fibrosis. Lab. Investig. 2018, 98, 1614–1626. [Google Scholar] [CrossRef]
- Paglialunga, S.; Dehn, C.A. Clinical assessment of hepatic de novo lipogenesis in non-alcoholic fatty liver disease. Lipids Health Dis. 2016, 15, 159. [Google Scholar] [CrossRef]
- Liu, J.; Yang, P.; Zuo, G.Q.; He, S.; Tan, W.; Zhang, X.Y.; Su, C.X.; Zhao, L.; Wei, L.; Chen, Y.; et al. Long-chain fatty acid activates hepatocytes through cd36 mediated oxidative stress. Lipids Health Dis. 2018, 17. [Google Scholar] [CrossRef]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in nafld/nash. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L.; Baeuerle, P.A. The er-overload response: Activation of nf-kappa b. Trends Biochem. Sci. 1997, 22, 63–67. [Google Scholar] [CrossRef]
- Videla, L.A.; Tapia, G.; Rodrigo, R.; Pettinelli, P.; Haim, D.; Santibanez, C.; Araya, A.V.; Smok, G.; Csendes, A.; Gutierrez, L.; et al. Liver nf-kappab and ap-1 DNA binding in obese patients. Obesity 2009, 17, 973–979. [Google Scholar] [CrossRef]
- Chavez-Tapia, N.C.; Rosso, N.; Tiribelli, C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yukawa, T.; Uetake, S.; Yamauchi, M. Serum intercellular adhesion molecule-1 in patients with nonalcoholic steatohepatitis: Comparison with alcoholic hepatitis. Alcohol. Clin. Exp. Res. 2007, 31, S83–S87. [Google Scholar] [CrossRef] [PubMed]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. Nf-kappab controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef]
- Kirovski, G.; Gabele, E.; Dorn, C.; Moleda, L.; Niessen, C.; Weiss, T.S.; Wobser, H.; Schacherer, D.; Buechler, C.; Wasmuth, H.E.; et al. Hepatic steatosis causes induction of the chemokine rantes in the absence of significant hepatic inflammation. Int. J. Clin. Exp. Pathol. 2010, 3, 675–680. [Google Scholar] [PubMed]
- Soria, G.; Ofri-Shahak, M.; Haas, I.; Yaal-Hahoshen, N.; Leider-Trejo, L.; Leibovich-Rivkin, T.; Weitzenfeld, P.; Meshel, T.; Shabtai, E.; Gutman, M.; et al. Inflammatory mediators in breast cancer: Coordinated expression of tnfalpha & il-1beta with ccl2 & ccl5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011, 11, 130. [Google Scholar]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of nafld development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A. Weight loss: Cornerstone in the treatment of non-alcoholic fatty liver disease. Minerva Gastroenterol. E Dietol. 2010, 56, 159–167. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahli, A.; Seitz, T.; Beckröge, T.; Freese, K.; Thasler, W.E.; Benkert, M.; Dietrich, P.; Weiskirchen, R.; Bosserhoff, A.; Hellerbrand, C. Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro. Cells 2019, 8, 457. https://doi.org/10.3390/cells8050457
Mahli A, Seitz T, Beckröge T, Freese K, Thasler WE, Benkert M, Dietrich P, Weiskirchen R, Bosserhoff A, Hellerbrand C. Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro. Cells. 2019; 8(5):457. https://doi.org/10.3390/cells8050457
Chicago/Turabian StyleMahli, Abdo, Tatjana Seitz, Tobias Beckröge, Kim Freese, Wolfgang Erwin Thasler, Matthias Benkert, Peter Dietrich, Ralf Weiskirchen, Anja Bosserhoff, and Claus Hellerbrand. 2019. "Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro" Cells 8, no. 5: 457. https://doi.org/10.3390/cells8050457
APA StyleMahli, A., Seitz, T., Beckröge, T., Freese, K., Thasler, W. E., Benkert, M., Dietrich, P., Weiskirchen, R., Bosserhoff, A., & Hellerbrand, C. (2019). Bone Morphogenetic Protein-8B Expression is Induced in Steatotic Hepatocytes and Promotes Hepatic Steatosis and Inflammation In Vitro. Cells, 8(5), 457. https://doi.org/10.3390/cells8050457





