From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hällberg, B.M.; Larsson, N.G. Making proteins in the powerhouse. Cell Metab. 2014, 20, 226–240. [Google Scholar] [CrossRef]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ito, Y.; Niikura, T.; Shao, Z.; Hata, M.; Oyama, F.; Nishimoto, I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001, 283, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, K.H.; Cohen, P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic. Biol. Med. 2016, 100, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Rackham, O.; Shearwood, A.M.; Mercer, T.R.; Davies, S.M.; Mattick, J.S.; Filipovska, A. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 2011, 17, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Rüberg, S.; Girard, M.; Cagnard, N.; Hanein, S.; Chrétien, D.; Munnich, A.; Lyonnet, S.; Henrion-Caude, A. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS ONE 2011, 6, e20746. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Skuratovskaia, D.A.; Sofronova, J.K.; Zatolokin, P.A.; Popadin, K.Y.; Vasilenko, M.A.; Litvinova, L.S.; Mazunin, I.O. Additional evidence of the link between mtDNA copy number and the body mass index. Mitochondrial DNA Part A 2018, 29, 1240–1244. [Google Scholar] [CrossRef]
- Kaaman, M.; Sparks, L.M.; van Harmelen, V.; Smith, S.R.; Sjölin, E.; Dahlman, I.; Arner, P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 2007, 50, 2526–2533. [Google Scholar] [CrossRef]
- Wachsmuth, M.; Hübner, A.; Li, M.; Madea, B.; Stoneking, M. Age-Related and Heteroplasmy-Related Variation in Human mtDNA Copy Number. PLoS Genet. 2016, 12, e1005939. [Google Scholar] [CrossRef]
- Xu, F.X.; Zhou, X.; Shen, F.; Pang, R.; Liu, S.M. Decreased peripheral blood mitochondrial DNA content is related to HbA1c, fasting plasma glucose level and age of onset in type 2 diabetes mellitus. Diabet. Med. 2012, 29, e47–e54. [Google Scholar] [CrossRef]
- Rubino, F.; Schauer, P.; Kaplan, L.; Cummings, D. Metabolic surgery to treat type 2 diabetes: Clinical outcomes and mechanisms of action. Annu. Rev. Med. 2010, 61, 393–411. [Google Scholar] [CrossRef]
- Webb, D.L.; Abrahamsson, N.; Sundbom, M.; Hellström, P.M. Bariatric surgery time to replace with GLP-1? Scand. J. Gastroenterol. 2017, 52, 635–640. [Google Scholar] [CrossRef]
- Dankel, S.N.; Staalesen, V.; Bjørndal, B.; Berge, R.K.; Mellgren, G.; Burri, L. Tissue-specific effects of bariatric surgery including mitochondrial function. J. Obes. 2011, 2011, 435245. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef]
- Karim, R.; Stanczyk, F.Z.; Brinton, R.D.; Rettberg, J.; Hodis, H.N.; Mack, W.J. Association of endogenous sex hormones with adipokines and ghrelin in postmenopausal women. J. Clin. Endocrinol. Metab. 2015, 100, 508–515. [Google Scholar] [CrossRef]
- Abdeen, G.; Le Roux, C.W. Mechanism Underlying the Weight Loss and Complications of Roux-en-Y Gastric Bypass. Review. Obes. Surg. 2016, 26, 410–421. [Google Scholar] [CrossRef]
- Ronveaux, C.C.; Tomé, D.; Raybould, H.E. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J. Nutr. 2015, 145, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lee, J.H.; Bongmba, O.Y.; Ma, X.; Zhu, X.; Sheikh-Hamad, D.; Sun, Y. The suppression of ghrelin signaling mitigates age-associated thermogenic impairment. Aging (Albany NY) 2014, 6, 1019–1032. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Astin, R.; Mcphail, M.W.; Saeed, S.; Pasha, Y.; Bear, D.E.; Constantin, D.; Velloso, C.; Manning, S.; Calvert, L.; et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018, 73, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.H.; Rees, D.J.; Andrews, Z.B.; Davies, J.S. Ghrelin mediated neuroprotection—A possible therapy for Parkinson’s disease? Neuropharmacology 2018, 136 Pt B, 317–326. [Google Scholar] [CrossRef]
- Laferrère, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef]
- Laferrère, B.; Teixeira, J.; McGinty, J.; Tran, H.; Egger, J.R.; Colarusso, A.; Kovack, B.; Bawa, B.; Koshy, N.; Lee, H.; et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Pisconti, A.; Palomba, L.; Cantoni, O.; Clementi, E.; Moncada, S.; et al. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J. Clin. Investig. 2006, 116, 2791–2798. [Google Scholar] [CrossRef]
- Karstoft, K.; Pedersen, B.K. Exercise and type 2 diabetes: Focus on metabolism and inflammation. Immunol. Cell Biol. 2016, 94, 146–150. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Skuratovskaia, D.; Zatolokin, P.; Vulf, M.; Mazunin, I.; Litvinova, L. Interrelation of chemerin and TNF-α with mtDNA copy number in adipose tissues and blood cells in obese patients with and without type 2 diabetes. BMC Med. Genom. 2019, 12 (Suppl. 2), 41. [Google Scholar] [CrossRef]
- Croft, M.; Duan, W.; Choi, H.; Eun, S.Y.; Madireddi, S.; Mehta, A. TNF superfamily in inflammatory disease: Translating basic insights. Trends Immunol. 2012, 33, 144–152. [Google Scholar] [CrossRef]
- Pahwa, R.; Adams-Huet, B.; Jialal, I. The effect of increasing body mass index on cardio-metabolic risk and biomarkers of oxidative stress and inflammation in nascent metabolic syndrome. J. Diabetes Complicat. 2017, 31, 810–813. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, W.; Liu, Z.; Li, Y.; Li, S.; Wu, J. Association between serum soluble tumor necrosis factor-α receptors and early childhood obesity. Endocr. J. 2016, 63, 581–587. [Google Scholar] [CrossRef]
- Glenn, C.L.; Wang, W.Y.; Benjafield, A.V.; Morris, B.J. Linkage and association of tumor necrosis factor receptor 2 locus with hypertension, hypercholesterolemia and plasma shed receptor. Hum. Mol. Genet. 2000, 9, 1943–1949. [Google Scholar] [CrossRef][Green Version]
- Selinsky, C.L.; Boroughs, K.L.; Halsey, W.A.; Howell, M.D. Multifaceted inhibition of anti-tumour immune mechanisms by soluble tumour necrosis factor receptor type I. Immunology 1998, 94, 88–93. [Google Scholar] [CrossRef]
- Vijgen, G.H.; Bouvy, N.D.; Hoeks, J.; Wijers, S.; Schrauwen, P.; van Marken Lichtenbelt, W.D. Impaired skeletal muscle mitochondrial function in morbidly obese patients is normalized one year after bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 936–941. [Google Scholar] [CrossRef]
- Meng, S.; Wu, S.; Liang, L.; Liang, G.; Giovannucci, E.; De Vivo, I.; Nan, H. Leukocyte mitochondrial DNA copy number, anthropometric indices, and weight change in US women. Oncotarget 2016, 7, 60676–60686. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Aljadaan, A.; Kamal, A.; Bakhiet, M. Peripheral blood mitochondrial DNA copy number as a novel potential biomarker for diabetic nephropathy in type 2 diabetes patients. Exp. Ther. Med. 2018, 16, 1483–1492. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s curse: The effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef]
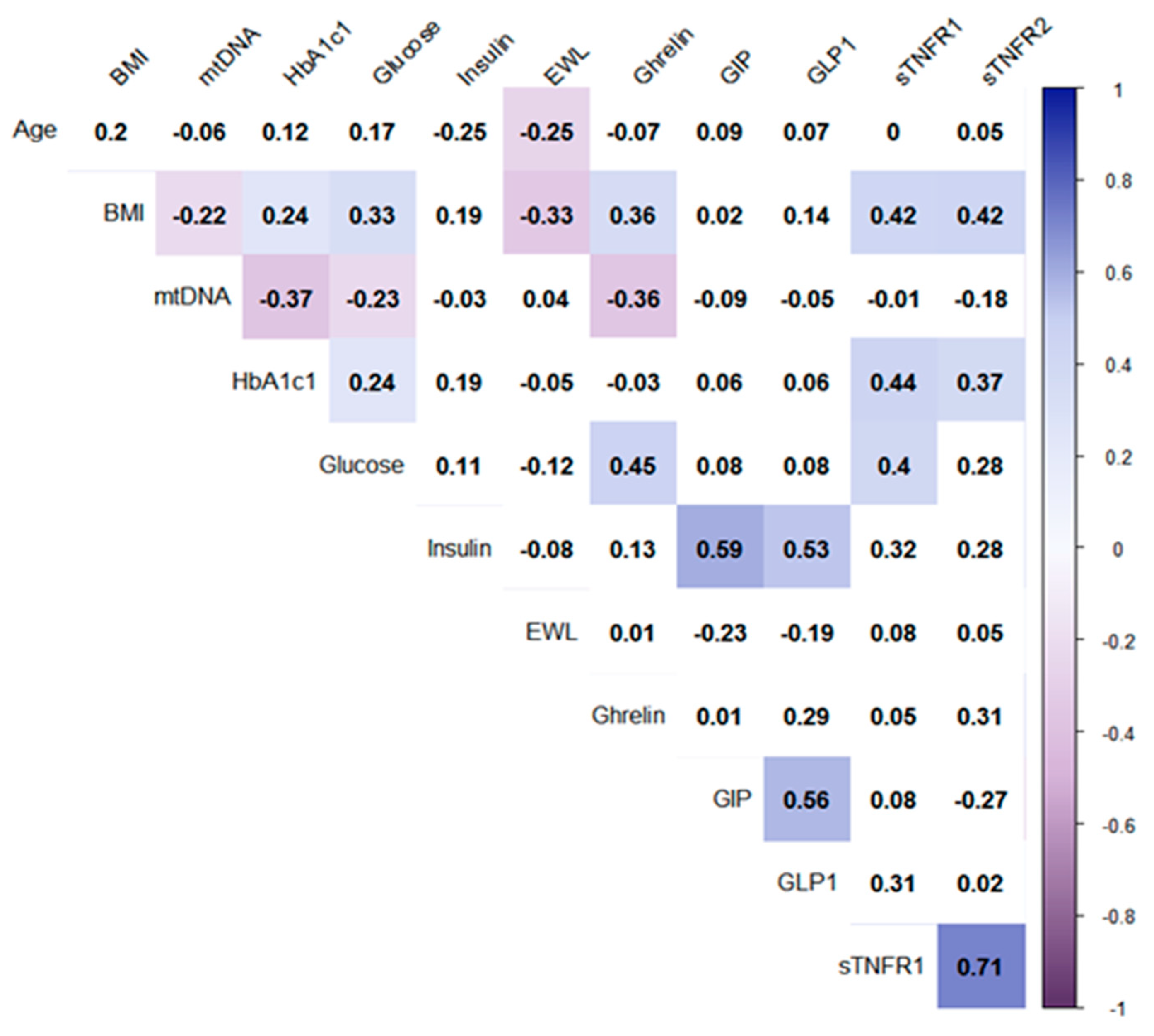
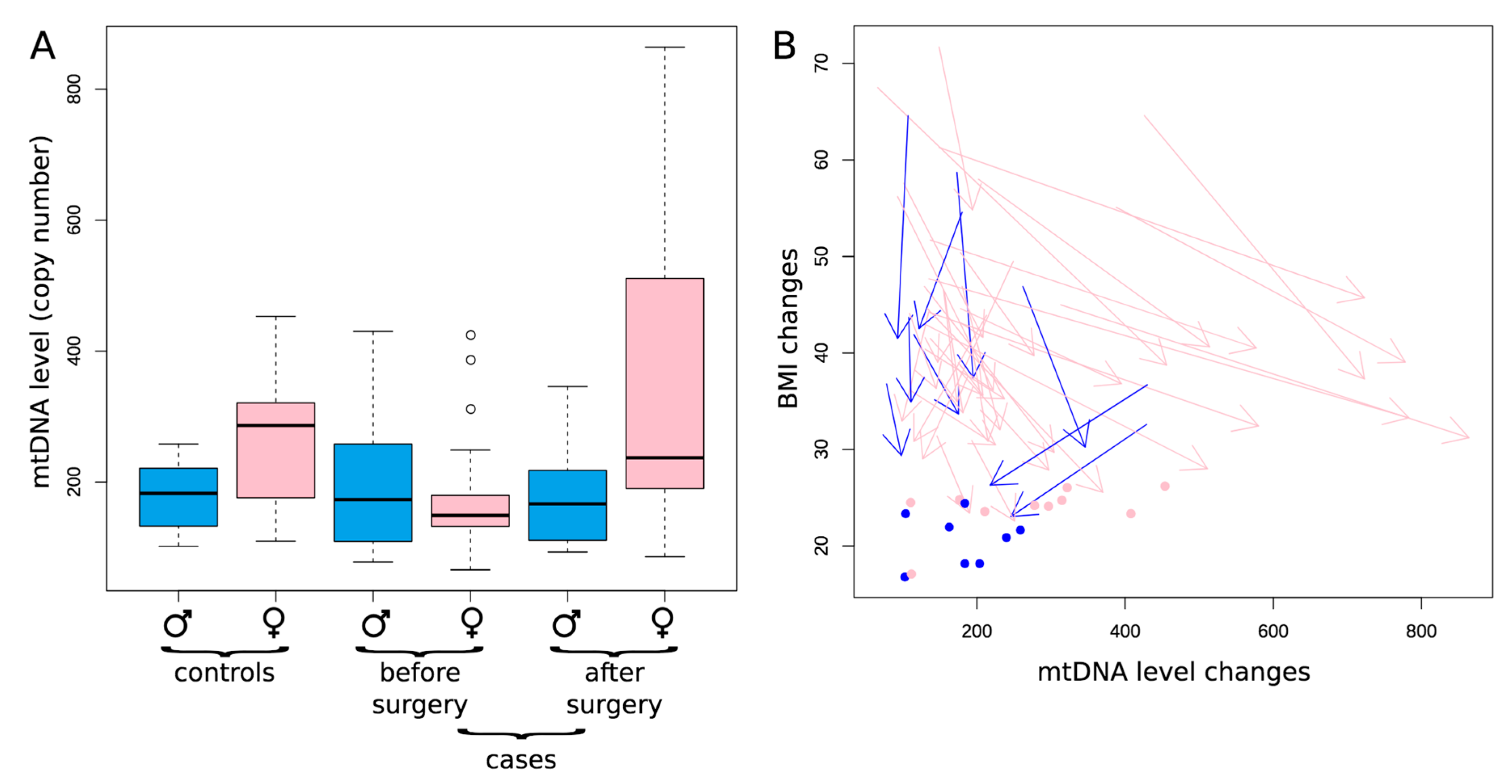
| Sample | Control Group 1 (Healthy Donors) | Control Group 2 (Patients with Obesity without T2DM) | Patients with T2DM before LSG | Patients with T2DM after LSG | Patients with T2DM before RYGB | Patients with T2DM after RYGB | |
|---|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | 5 | 6 | |
| Anthropometric and Biochemical Parameters | Age (years) | 41.8 ± 4.6 | 42.8 ± 8.3 | 48 ± 8.6 | 49 ± 8.6 | 47 ± 9.4 | 48 ± 9.4 |
| Sex (m/w) | 8/10 | 9/18 | 5/16 | 5/16 | 6/19 | 6/19 | |
| BMI (kg/m2) | 22.5 ± 3.01 | 36 ± 3.45 p1-2 < 0.001 * | 53.9 ± 8.98 p1-3 < 0.001 * p2-3 < 0.001 * | 37.11 ± 6.22 p1-4 = 0.001 * p3-4 < 0.001 * | 43.26 ± 7.0 p1-5 < 0.001 * p2-5 < 0.001 * | 31.68 ± 5.2 p1-6 = 0.001 * p5-6 < 0.001 * | |
| EWL (%) | - | - | - | 53.15 (40.08–62.24) | - | 60.20 (47.66–70.63) | |
| mtDNA (copy number) | 206.5 (165–228.2) | 164.0 (135.7–231.5) | 149.0 (115.0–180) p1-3 = 0.023 * | 194.5 (125.8–486.2) p3-4 = 0.011 * | 155 (132–202) | 231 (185.8–352) p2-6 = 0.002 * p5-6 = 0.007 * | |
| Fasting serum glucose level (mmol/L) | 4.9 (4.64–5.34) | 5.89 (5.6–6.76) | 8.1 (6.87–9.08) p1-3 < 0.001 * p2-3 < 0.001 * | 4.99 (4.7–5.26) p3-4 < 0.001 * p2-4 < 0.013 * | 8.61 (6.36–9.07) p1-5 = 0.001 * p2-5 = 0.013 * | 5.5 (5.13–6.30) p5-6 = 0.002 * | |
| Insulin (pg/mL) | 44.8 (32.49–54.48) | 119 (55.2–193.7) p1-2 = 0.002 * | 140 (105.57–454.75) p1-3 = 0.011 * | 145.13 (64.5–238.22) p1-4 = 0.041 * | 469.0 (161.4–767.3) p1-5 = 0.001 * p2-5 = 0.014 * | 60.48 (36.95–303.21) p5-6 = 0.043 * | |
| HbA1c (%) | 5.5 (3.5–5.8) | 5.81 (5.6–5.9) | 6.7 (6.12–7.9) p1-3 = 0.008 * p2-3 < 0.05 * | 5.5 (4.7–6.0) p3-4 < 0.05 * | 8.05 (6.5–9.9) p1-5 < 0.05 * p2-5 < 0.05 * | 5.7 (5.0–6.6) p5-6 < 0.05 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skuratovskaia, D.; Litvinova, L.; Vulf, M.; Zatolokin, P.; Popadin, K.; Mazunin, I. From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index. Cells 2019, 8, 430. https://doi.org/10.3390/cells8050430
Skuratovskaia D, Litvinova L, Vulf M, Zatolokin P, Popadin K, Mazunin I. From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index. Cells. 2019; 8(5):430. https://doi.org/10.3390/cells8050430
Chicago/Turabian StyleSkuratovskaia, Daria, Larisa Litvinova, Maria Vulf, Pavel Zatolokin, Konstantin Popadin, and Ilia Mazunin. 2019. "From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index" Cells 8, no. 5: 430. https://doi.org/10.3390/cells8050430
APA StyleSkuratovskaia, D., Litvinova, L., Vulf, M., Zatolokin, P., Popadin, K., & Mazunin, I. (2019). From Normal to Obesity and Back: The Associations between Mitochondrial DNA Copy Number, Gender, and Body Mass Index. Cells, 8(5), 430. https://doi.org/10.3390/cells8050430


.JPG)



